Abstract
Background: The rise in nutrition-related morbidity and mortality requires public health intervention programs targeting nutritional behavior. In addition to socio-economical, socio-cultural, psychological determinants, taste is one of the main factors that influence food choices. Differences in taste perception and sensitivity may be explained by genetic variations, therefore the knowledge of the extent to which genetic factors influence the development of individual taste preferences and eating patterns is important for public policy actions addressing nutritional behaviors. Our aim was to review genetic polymorphisms accounting for variability in taste and food preferences to contribute to an improved understanding of development of taste and food preferences.
Methods: The electronic databases PubMed, Scopus, and Web of Science were searched using MeSH in PubMed and free text terms for articles published between January 1, 2000 and April 13, 2018. The search strategy was conducted following the PRISMA statement. The quality of the included studies was assessed by the validated Q-Genie tool.
Results: Following the PRISMA flowchart, finally 103 articles were included in the review. Among the reviewed studies, 43 were rated to have good quality, 47 were rated to have moderate quality, and 13 were rated to have low quality. The majority of the studies assessed the association of genetic variants with the bitter taste modality, followed by articles analyzing the impact of polymorphisms on sweet and fat preferences. The number of studies investigating the association between umami, salty, and sour taste qualities and genetic polymorphisms was limited.
Conclusions: Our findings suggest that a significant association exists between TAS2R38 variants (rs713598, rs1726866, rs10246939) and bitter and sweet taste preference. Other confirmed results are related to rs1761667 (CD36) and fat taste responsiveness. Otherwise further research is essential to confirm results of studies related to genetic variants and individual taste sensitivity. This knowledge may enhance our understanding of the development of individual taste and related food preferences and food choices that will aid the development of tailored public health strategy to reduce nutrition-related disease and morbidity.
Keywords: food preference, taste preference, taste threshold, taste sensitivity, genetics, genomics
Introduction
Globalization related changes resulted in the extremely high prevalence of unhealthy dietary behaviors all over the world especially in low and middle income countries which led to the rise in morbidity (Popkin, 2006; Lachat et al., 2013; Ford et al., 2017) and mortality (Global Burden of Disease Risk Factors Collaborators, 2018) caused by diet-related noncommunicable diseases (NCDs). The unfavorable dietary patterns referred to as the “nutrition transition” can be characterized with an increased consumption of industrially processed foods with high salt, fat, and sugar content and contribute to the development of metabolic abnormalities and consequent NCDs of high public health significance (cardiovascular diseases, type 2 diabetes, and cancer) (Atrup et al., 2008; World Cancer Research Fund, 2018) and have emerged as the biggest contributor to premature mortality around the world, accounting for 11 million deaths in 2017 (Afshin et al., 2019). Understanding the determinants and drivers of food preferences and food choices is therefore essential to design and implement public health intervention programs targeting nutritional behavior.
Individual food preferences are important predictors of food intake (Drewnowski et al., 1999; Duffy et al., 2009) and are highly influenced by taste perception and taste preference (Glanz et al., 1998; Kearney et al., 2000; Kourouniotis et al., 2016). Taste is listed among the five main values (taste, health, cost, time, and social relationships) in the Food Choice Process Model, which explains the motivations behind food choice decisions (Connors et al., 2001). Sensory perceptions, such as taste sensitivity vary widely among individuals that may partly be explained by genetic polymorphisms located in genes involved in taste perception of the five basic taste qualities and the most recently identified fat taste (Malles, 2010; Running et al., 2015) modality. The magnitude of genetic predisposition to perceived intensity and preference of distinct compounds is provided by family and twin studies, expressed in terms of heritability, i.e., the degree to which genetic differences contribute to individual differences in taste perception and preference. Heritability estimates range from high to moderate for bitter tasting stimuli [0.72, 0.71, 0.34, for 6-n-propylthiouracil (PROP) (Hansen et al., 2006), phenylthiocarbamide (PTC) (Knaapila et al., 2012), and quinine hydrochloride (Hansen et al., 2006), respectively] and moderate for sweet tasting compounds (glucose: h2 = 0.31, fructose: h2 = 0.34) (Hwang et al., 2015). Accordingly food preferences that are determined by taste perception are also influenced by genetic factors with similar heritability estimates (dessert foods (0.20), vegetables (0.37–0.54), fruits (0.49–0.53), protein foods (0.48–0.78) (Breen et al., 2006; Fildes et al., 2014; Smith et al., 2016) and the correlation for fat intake (as a percentage of energy intake) was found to be 0.61 for monozygotic twins in a study conducted among subjects of French descent (Pérusse et al., 1988).
The aim of our present study was to review genetic polymorphisms accounting for variability in taste and food preferences to contribute to an improved understanding of the role of genetic polymorphisms in the development of taste and food preferences. Knowledge of the extent to which genetic and environmental factors influence the development of individual taste preferences and eating patterns is important for public policy actions addressing nutritional behavior of populations.
Methods
Search Strategy and Eligibility Criteria
Systematic searches were conducted in the electronic databases PubMed, Scopus, and Web of Science for articles published between January 1, 2000 and April 13, 2018 to identify relevant publications. The search strategy was to follow the PRISMA statement. The search terms included controlled terms, e.g., MeSH in PubMed and free text terms. The search was based on a combination of the following keywords: (“taste preference” OR “taste sensitivity” OR “taste threshold” OR “food preference” OR “bitter taste preference” OR “bitter taste sensitivity” OR “bitter taste threshold” OR “sweet taste preference” OR “sweet taste sensitivity” OR “sweet taste threshold” OR “salt taste preference” OR “salt taste sensitivity” OR “salt taste threshold” OR “sour taste preference” OR “sour taste sensitivity” OR “sour taste threshold” OR “umami taste preference” OR “umami taste sensitivity” OR “umami taste threshold” OR “fat taste preference” OR “fat taste sensitivity” OR “fat taste threshold”) AND (“genetics” OR “genomics”). The references of the selected publications were also checked for other potentially eligible studies.
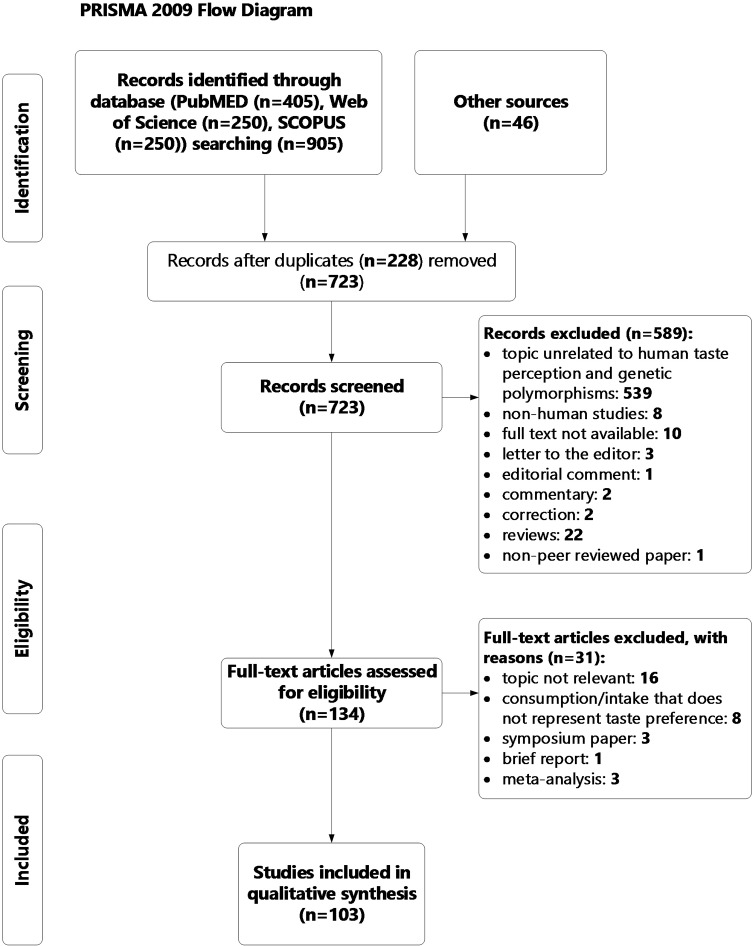
Studies were excluded: i) that were not written in English; ii) not targeting human subjects; iii); which were not published as peer reviewed in scientific journals; iv) that were not available in full-text format. Figure 1 summarizes the manuscript selection process.
Figure 1.
PRISMA flowchart of study selection process.
Quality Assessment of Primary Studies
The quality of the included studies was assessed independently by two reviewers (JD, EL) using the validated Q-Genie tool. This tool was developed by Sohani et al. (2015) based on the Strengthening the Reporting of Genetic Association Studies (STREGA) and Strengthening the Reporting of Genetic Risk Prediction Studies (GRIPS) and as well as recommendations by Human Molecular Genetics, Diabetologia, Nature Genetics, and individual research groups guidelines. This instrument is composed of 11 items formulated as questions covering the following categories: rationale for study, selection and definition of outcome of interest, selection and comparability of comparison groups, technical classification of the exposure, non-technical classification of the exposure, other source of bias, sample size and power, a priori planning of analysis, statistical methods and control for confounding, testing of assumptions and inferences for genetic analysis, and appropriateness of inferences drawn from results. Each item is scored on 7-point Likert Scale ranging from 1 (poor) to 7 (excellent). Each reviewer (JD, EL) generated an overall quality score, after reading and examining each study independently. The quality of studies could be labeled as poor (total scores ≤35 for studies with control groups and scores ≤32 for studies without control groups), moderate (total scores >35 and ≤45 for studies with control groups and scores >32 and ≤40 for studies without control groups), or good (total scores >45 for studies with control groups and scores >40 for studies without control groups) (Sohani et al., 2015). Disagreement between the reviewers on individual items were identified and solved during a consensus meeting. Detailed instructions for using the checklist are provided elsewhere (Sohani et al., 2016).
Data Extraction
First, duplicates were removed and then all the abstracts of the remaining articles were screened by two independent reviewers (JD, EL). Two authors (JD, EL) extracted data independently from the identified studies.
Results
Search Outcomes
In total, 949 publications were identified using PubMed (n = 405), Scopus (n = 250), Web of Science (n = 250), and from reference lists of all relevant articles (n = 46). After the duplicates were removed (n = 228) the abstracts of the remaining articles (n = 723) were screened by two independent reviewers (JD, EL). Any disagreement was resolved by discussion. Studies that did not meet the inclusion criteria (n = 589) were removed, resulting in 134 articles for full-text assessment for eligibility. Out of them 31 were further excluded ( Figure 1 displays the reasons). Finally, 103 articles were included in the review.
Studies Included in the Review
The majority of the studies assessed analyzed the association of genetic variants with the bitter taste modality (n = 64), followed by articles on the impact of polymorphisms on sweet (n = 28) and fat preferences (n = 22). The number of studies investigating the association between umami, salty, and sour taste perceptions and genetic polymorphisms was limited (n = 6, n = 6, and n = 4, respectively). Tables 1 – 6 summarize the findings for each modality. Due to the extensive literature and data extracted regarding bitter, sweet, and fat taste qualities, only those genetic associations are presented in Tables 1 – 3 , where the number of studies with confirmed association was more than one, except only few single publications on sweet perception, where the association could be expected on the basis of the known molecular mechanisms of taste recognition. Genetic variants with only one or no confirmed associations are presented in Supplementary Tables 1 and 2 . Tables 4 – 6 include all findings for umami, salty and sour taste modalities, respectively. Supplementary Tables 3 – 12 include the design, methodology, and study population details of each study included in the review.
Table 1.
Overview of genetic association studies related to bitter taste preferences.
| Gene | SNP | Applied tastant/method | Number of studies with confirmed association | Findings | Reference | Number of studies with no association | Reference |
|---|---|---|---|---|---|---|---|
| TAS2R38 | rs713598 | PTC, PROP, bitterness of wine/alcohol, food habits questionnaire (liking), detection threshold of methimazole, salicin | 13 (1) | Homozygotes (P49) had the lowest mean thresholds (i.e., greater sensitivity) to both PTC and PROP. The A49P variant demonstrated a strong association with PTC taster status. The variant alleles were inversely associated with bitterness perception of PROP and wine/alcohol. PP conferred to PROP sensitivity. Associated with broccoli score. PP tasters associated with aversion of bitter vegetables and preference of sweet vegetables. Associated with thioamide and salicin detection threshold | (Kim et al., 2003; Ooi et al., 2010; Wooding et al., 2010; Lucock et al., 2011; Colares-Bento et al., 2012; Allen et al., 2013a; Behrens et al., 2013; Allen et al., 2014; Bering et al., 2014; Keller et al., 2014; Mennella et al., 2014a; Carrai et al., 2017; Risso et al., 2017) | 1 | (Mennella et al., 2011a) (PROP, mixed population of children, adolescents, and adults) |
| TAS2R38 | rs1726866 | PTC, PROP, bitterness of wine, food habits questionnaire (liking), detection threshold of methimazole, salicin | 10 (1) | Homozygotes (V296) had the lowest mean thresholds (i.e., greater sensitivity) to both PTC and PROP. Individuals with “A” rather than a “V,” could perceive the bitterness of PROP increased. The variant alleles were inversely associated with bitterness perception of PROP and wine. Associated with broccoli score. Associated with thioamide and salicin detection threshold | (Kim et al., 2003; Wooding et al., 2010; Lucock et al., 2011; Mennella et al., 2011a; Allen et al., 2013a; Behrens et al., 2013; Bering et al., 2014; Robino et al., 2016; Carrai et al., 2017; Risso et al., 2017) | 2 | (Duffy et al., 2004a; Timpson et al., 2007) (AceK bitterness, bitterness of alcohol) |
| TAS2R38 | rs10246939 | PROP, bitterness of wine/alcohol, food habits questionnaire (liking), detection threshold of methimazole, salicin | 8 (1) | Individuals with a “V” in the last position were more likely to detect bitterness at the lowest concentration compared with subjects with the same diplotype but with an “I” in the last position. The variant alleles were inversely associated with bitterness perception of PROP and wine. Associated with broccoli score. Associated with thioamide and salicin detection threshold | (Lucock et al., 2011; Mennella et al., 2011a; Behrens et al., 2013; Allen et al., 2014; Bering et al., 2014; Ledda et al., 2014; Carrai et al., 2017; Risso et al., 2017) | 0 | – |
| TAS2R38 | A49P (rs713598), A262V (rs1726866) | PROP | 2 | Associated with PROP phenotype | (Timpson et al., 2007; Bering et al., 2014) | 0 | – |
| TAS2R38 | A49P (rs713598), A262V (rs1726866) | Cruciferous/Brassica vegetable intake (24-hour dietary recall, food record) | 1 (1) | Haplotype associated with cruciferous vegetable intake | (Sacerdote et al., 2007) | 1 (Popkin, 2006) | (Inoue et al., 2013) |
| TAS2R38 | A49P (rs713598), A262V (rs1726866), V296I (rs10246939) | PTC, PROP, food habits questionnaire (liking), bitterness of nonglucosinolate-generating vegetables, bitterness of alcohol, liking and bitterness perception of salad rocket, detection threshold of methimazole, salicin | 23 (4) | PAV homozygotes possessed a greater sensitivity to PTC compared with AVI. Taster PAV haplotypes inversely correlated with broccoli score and positively associated with PROP perceived bitterness. PAV/PAV subjects rated the glucosinolate-generating vegetables more bitter, than AVI/AVI subjects. Bitterness of ethanol differed significantly among haplotypes. Associated with bitterness perception and scores of salad rocket. Associated with thioamide and salicin detection threshold | (Kim et al., 2003; Duffy et al., 2004a; Mennella et al., 2005; Sandell and Breslin, 2006; Hayes et al., 2008; Duffy et al., 2010; Wooding et al., 2010; Calò C et al., 2011; Lucock et al., 2011; Cabras et al., 2012; Negri et al., 2012; Campbell et al., 2012; Melis et al., 2013; Allen et al., 2014; Bering et al., 2014; Garneau et al., 2014; Robino et al., 2014; Melis et al., 2015; Nolden et al., 2016; Bella et al., 2017; Deshaware and Singhal, 2017; Risso et al., 2017in children also, Behrens et al., 2013) | 2 | (Feeney et al., 2017) |
| TAS2R38 | A49P (rs713598), A262V (rs1726866), V296I (rs10246939) | Bitterness of berry juice samples and extracts (bilberry, crowberry) | 1 (1) | AVI/AVI subjects rated bitterness, higher than the PAV/PAV subjects | (Laaksonen et al., 2013) | 0 | – |
| TAS2R38 | A49P (rs713598), A262V (rs1726866), V296I (rs10246939) | Brassica vegetable intake (FFQ) | 1 (1) | Associated with consumption of bitter-tasting vegetables (only in children) | (Feeney et al., 2011) | 2 (1) | (Gorovic N et al., 2011; Negri et al., 2012) |
| TAS2R19 | rs10772420 | Quinine, detection and recognition thresholds, perceived bitter taste intensities of absinthin, amarogentin, cascarillin, grosheimin, quassin, and quinine, PROP bitterness of unsweetened grapefruit juice | 5 | Associated with quinine intensity ratings. A allele was associated with more intense quinine perception. Associated with grosheimin detection threshold and intensities (weak, moderate, strong, very strong). Individuals who were homozygous for the Cys299 allele rated grapefruit juice twice as bitter and liked it less as Arg299 homozygotes or heterozygotes. | (Reed et al., 2010; Hayes et al., 2011; Knaapila et al., 2012; Hayes et al., 2015; Roudnitzky et al., 2015) | 1 | (Bering et al., 2014) (PROP) |
| TAS2R19 | rs1868769 | Quinine, detection and recognition thresholds, perceived bitter taste intensities of absinthin, amarogentin, cascarillin, grosheimin, quassin, and quinine, PROP | 2 | Associated with quinine intensity ratings. Associated with grosheimin detection threshold, recognition threshold, and weak intensity | (Knaapila et al., 2012; Roudnitzky et al., 2015) | 1 | (Bering et al., 2014) (PROP) |
| TAS2R31 (formerly TAS2R44) | rs10845293 | Detection and recognition thresholds, perceived bitter taste intensities of absinthin, amarogentin, cascarillin, grosheimin, quassin, and quinine, saccharin recognition threshold, bitterness of acesulfame potassium | 6(1) | Associated with grosheimin detection threshold and intensities (weak, moderate, strong, very strong). Associated with saccharin response. Individuals with at least one TAS2R44-W35 allele were more sensitive to saccharin compared to the group homozygous for the hTAS2R44-R35 allele. Val227 homozygotes reported less bitterness from AceK than the Ala227 homozygotes (heterozygotes intermediate). Association with quinine bitterness | (Pronin et al., 2007; Roudnitzky et al., 2011; Allen et al., 2013a; Allen et al., 2013b; Hayes et al., 2015; Roudnitzky et al., 2015) | 1 | (Timpson et al., 2007) (PROP) |
| TAS2R31 (formerly TAS2R44) | rs10772423 | Detection and recognition thresholds, perceived bitter taste intensities of absinthin, amarogentin, cascarillin, grosheimin, quassin, and quinine, bitterness of acesulfame potassium, grapefruit liking | 3 | Associated with amarogentin intensity (weak), grosheimin detection threshold, recognition threshold, and intensities (weak, moderate, strong, very strong intensity). Val240 homozygotes reported less bitterness from AceK than the Ile240 homozygotes (Val/Ile heterozygotes intermediate). Association with quinine bitterness and grapefruit liking | (Allen et al., 2013b; Hayes et al., 2015; Roudnitzky et al., 2015) | 1 | (Nolden et al., 2016) (Bitterness of capsaicin, piperine, ethanol) |
| TAS2R4 | rs2234001 | Bitterness of stevioside, bitterness of unsweetened grapefruit juice, instant espresso | 2 | Bitterness of stevioside positively associated with G allele. Haplotype, allelic variation (TAS2R3, -R4, and -R5) explained variability in coffee bitterness [individuals with one or two copies of the more responsive haplotype (TGAG) experienced twice as much bitterness compared with individuals homozygous for the less responsive haplotype (CCGT), but these haplotypes did not predict coffee liking]. | (Hayes et al., 2011; Risso et al., 2017) | 4 | (Duffy et al., 2004a; Pronin et al., 2007; Timpson et al., 2007; Lucock et al., 2011) (PROP, AceK bitterness) |
| TAS2R5 | rs2227264 | Bitterness of unsweetened grapefruit juice, instant espresso, PROP | 2 | Haplotype, allelic variation (TAS2R3, -R4, and -R5) explained variability in coffee bitterness [individuals with one or two copies of the more responsive haplotype (TGAG) experienced twice as much bitterness compared with individuals homozygous for the less responsive haplotype (CCGT), the haploblock did not predict coffee liking]. Associated with PROP phenotype | (Hayes et al., 2011; Carrai et al., 2017) | 0 | – |
| TAS2R5 | rs2234012 | Bitterness of unsweetened grapefruit juice, instant espresso, intake, PROP | 2 | Haplotype, allelic variation (TAS2R3, -R4, and -R5) explained variability in coffee bitterness [individuals with one or two copies of the more responsive haplotype (TGAG) experienced twice as much bitterness compared with individuals homozygous for the less responsive haplotype (CCGT), the haploblock did not predict coffee liking]. Associated with PROP phenotype | (Hayes et al., 2011; Nolden et al., 2016) | 0 | – |
| TAS2R9 | rs3741845 | Bitterness of acesulfame potassium, bitterness of capsaicin, piperine, ethanol | 2 | Ala187 homozygotes reported less bitterness than heterozygotes and the Val187 homozygotes. | (Pronin et al., 2007; Timpson et al., 2007) | 2 | (Sandell and Breslin, 2006; Timpson et al., 2007) (PROP) |
| CA6 | rs2274333 | PROP | 4 (1) | The genotype AA and allele A were more frequent in supertasters, whereas genotype GG and allele G were more frequent in non-tasters. GG vs. AA or AG had thresholds that were more than 10-fold higher. Supertasters had a very high frequency of genotype AA and allele A, whereas non-tasters had a higher frequency of genotype GG and allele G. PROP super-tasters had a very high frequency of allele A, whereas non-tasters had a higher frequency of allele G. | (Padiglia et al., 2010; Calò C et al., 2011; Cabras et al., 2012; Melis et al., 2013) | 3 | (Bering et al., 2014; Feeney and Hayes, 2014; Risso et al., 2017) |
Number of low quality studies is presented in parentheses.
PTC, phenylthiocarbamide; PROP, 6-n-propylthiouracil; FFQ, food frequency questionnaire; AceK, acesulfame potassium.
Table 6.
Overview of genetic association studies related to sour taste preferences.
| Gene | SNP | Applied tastant/method | Number of studies with confirmed association | Findings | Reference | Number of studies with no association | Reference |
|---|---|---|---|---|---|---|---|
| TAS1R1 | rs17492553 | Citric acid | 1 | T (allele, genotype) associated with lower intensities | (Rawal et al., 2013) | 0 | – |
| TAS1R1 | rs34160967 | Citric acid | 1 | A (allele, genotype) associated with lower intensities | (Rawal et al., 2013) | 0 | – |
| TAS2R38 | A49P (rs713598), A262V (rs1726866), V296I (rs10246939) | Citric acid | 0 | – | – | 1 | (Hayes et al., 2008) |
| TAS2R38 | A49P (rs713598), A262V (rs1726866), V296I (rs10246939) | Sourness of berry juice samples and extracts (bilberry, crowberry) | 1 (1) | AVI/AVI rated sourness higher than the PAV/PAV subjects. | (Laaksonen et al., 2013) | 0 | – |
| NA | rs6466849 | Wine sourness | 1 | Variant allele associated with wine sourness | (Carrai et al., 2017) | 0 | – |
Number of low quality studies is presented in parentheses.
Table 3.
Overview of genetic association studies related to fat taste preferences.
| Gene | SNP | Applied tastant/method | Number of studies with confirmed association | Findings | Reference | Number of studies with no association | Reference |
|---|---|---|---|---|---|---|---|
| CD36 | rs1761667 | Oleic acid threshold | 3 (1) | GG vs. AA linked to lower threshold for oleic acid. Threshold higher in A-allele (obese) children than in G-allele children. | (Pepino et al., 2012; Melis et al., 2015; Mrizak et al., 2015; Sayed et al., 2015) | 1 | (Daoudi et al., 2015) |
| CD36 | rs1761667 | Perceived oiliness, fat content, and creaminess | 2 | AA vs. GA or GG perceived more creaminess (regardless of fat concentration), associated with acceptance of added fats and oils but no differences in perceived oiliness were reported. AA lowest perceived ratings of fat content. | (Keller et al., 2012; Ong et al., 2017) | 0 | – |
| CD36 | rs1527483 | Perceived oiliness, fat content, and creaminess | 2 | C/T or T/T perceived greater creaminess, oiliness, and fat content. | (Keller et al., 2012; Ong et al., 2017) | 1 | (Melis et al., 2015) |
| IZUMO1 | rs838145 | Fat intake (FFQ) | 2 | Tendency toward decreased total fat intake (A allele carriers), (MUFAs, PUFAs, omega-3 fatty acids). A variant associated with lower fat consumption. | (Tanaka et al., 2013; Søberg et al., 2017) | 0 | – |
Number of low quality studies is presented in parentheses.
MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Table 4.
Overview of genetic association studies related to umami taste preferences.
| Gene | SNP | Applied tastant/method | Number of studies with confirmed association | Findings | Reference | Number of studies with no association | Reference |
|---|---|---|---|---|---|---|---|
| TAS1R3 | rs307377 | Umami | 3 | Significant associations between allele frequency and recognition threshold for IMP. The mutation was less frequent in tasters than expected. CT subjects rated MPG/L twice as did those with CC genotype. | (Shigemura et al., 2009; Raliou et al., 2009; Chen et al., 2009) | 0 | – |
| TAS1R3 | rs76755863 | Umami | 2 | The mutation G13A was associated with non-tasters and hypotasters. For the rare allele doubling of umami taste intensity ratings. | (Raliou et al., 2009; Chen et al., 2009) | 0 | – |
| TAS1R3 | rs111615792 | Umami | 1 | For the rare allele doubling of umami taste intensity ratings. | (Chen et al., 2009) | 0 | – |
| TAS1R1 | rs34160967 | Umami | 1 | Significant associations between genotypes and recognition thresholds for MSG and M+I. The SNP was more frequent in tasters than expected. | (Shigemura et al., 2009; Raliou et al., 2009) | 2 | (Chen et al., 2009; Risso et al., 2017) |
| TAS1R1 | rs41278020 | Umami | 1 | The mutation was more frequent in non-tasters than expected. | (Raliou et al., 2009) | 1 | (Chen et al., 2009) |
| TAS1R1 | rs35118458 | Umami | 1 | The mutation tended to be more frequent in non-tasters. | (Raliou et al., 2009) | 0 | – |
| GRM1 | rs2814863 | Umami | 1 | The mutation tended to be associated with the non-taster phenotype. | (Raliou et al., 2009) | 0 | – |
For SNPs with more than one confirmed association are presented in bold.
IMP, inosine monophosphate; MPG, monopotassium glutamate; MSG, monosodium glutamate; M+I: MSG in the presence of IMP.
Quality Assessment of Primary Studies
Among the reviewed studies, 43 (41.75%) were rated to have good quality, 47 (45.63%) were rated to have moderate quality, and 13 (12.62%) were rated to have low quality.
Bitter Taste Preference
Ever since the discovery of PTC (bitter) taster status in 1931 (Fox, 1932) a variety of studies investigated this taste quality. More recent studies analyzed the related bitter tasting thiourea compound PROP rather than PTC, and other phenotyping approaches include the preference for bitter tasting foods and beverages. Investigations confirmed that three single nucleotide polymorphisms (SNPs) in the coding region of the TAS2R38 gene leading to amino-acid changes account for variation in human bitter taste perception (2728). Although this gene is the most widely studied there are 25 different taste receptor type 2 (T2Rs) genes (Chandrashekar et al., 2000; Guo and Reed, 2001) (Conte et al., 2002) involved in bitter taste perception. Moreover the bitter taste phenotype is a complex trait influenced by other genetic variants as well, such as the salivary carbonic anhydrase VI (CA6) or gustin protein, which has an effect on fungiform papillae density and maintenance (Melis et al., 2013).
As expected, the majority of studies focused on candidate genes and relevant variants, with TAS2R38 the most extensively studied (n = 40) (Kim et al., 2003; Duffy et al., 2004a; Mennella et al., 2005; Sandell and Breslin, 2006; Sacerdote et al., 2007; Timpson et al., 2007; Hayes et al., 2008; Duffy et al., 2010; Ooi et al., 2010; Wooding et al., 2010; Calò C et al., 2011; Feeney et al., 2011; Gorovic N et al., 2011; Lucock et al., 2011; Mennella et al., 2011a; Cabras et al., 2012; Campbell et al., 2012; Colares-Bento et al., 2012; Negri et al., 2012; Allen et al., 2013a; Behrens et al., 2013; Inoue et al., 2013; Laaksonen et al., 2013; Melis et al., 2013; Allen et al., 2014; Bering et al., 2014; Feeney et al., 2014; Garneau et al., 2014; Keller et al., 2014; Ledda et al., 2014; Mennella et al., 2014a; Robino et al., 2014; Melis et al., 2015; Nolden et al., 2016; Bella et al., 2017; Carrai et al., 2017; Deshaware and Singhal, 2017; Feeney et al., 2017; Risso et al., 2017), followed by TAS2R31 (n = 7) (Pronin et al., 2007; Roudnitzky et al., 2011; Allen et al., 2013a; Allen et al., 2013b; Hayes et al., 2015; Roudnitzky et al., 2015; Nolden et al., 2016), TAS2R19 (n = 6) (1835, Reed et al., 2010; Hayes et al., 2011; Roudnitzky et al., 2015), TAS2R4 (n = 6) (Roudnitzky et al., 2011; Allen et al., 2013a; Allen et al., 2014; Bering et al., 2014; Risso et al., 2017), TAS2R5 (n = 3) (Hayes et al., 2011; Nolden et al., 2016; Carrai et al., 2017), and TAS2R9 (n = 2) (Allen et al., 2013a; Allen et al., 2013b) ( Table 1 ). The association of rs227433 (CA6) with PROP phenotype was inconclusive (Padiglia et al., 2010; Calò C et al., 2011; Cabras et al., 2012; Melis et al., 2013; Bering et al., 2014; Feeney and Hayes, 2014; Risso et al., 2017) (presented in Table 1 ). The effect of other TAS2R gene polymorphisms were demonstrated by single studies only (presented in Supplementary Table 1 ). The assessment of perceived bitterness of PROP, PTC, quinine, caffeine/coffee, unsweetened grapefruit juice, berry juice samples and extracts, salad rocket, stevioside, thioamide, aloin, salicin, saccharin, methimazole, acesulfame potassium, denatonium benzoate, absinthin, amarogentin, cascarillin, grosheimin, quassin, capsaicin, piperine, gentiobiose, aspartame, rebaudioside A and D, alcohol/wine, and preference for bitter tasting foods and beverages (broccoli, artichoke, chicory, glucosinolate-generating vegetables, coffee, dark chocolate) were applied as phenotyping methods. Publications on consumption of bitter foods and drinks (Brassica/cruciferous vegetables, coffee; measured by the food frequency questionnaire, 24-h dietary recall, and the 3-day food record) only appeared among the search results and were considered for further evaluation as preference, if it was supported by background information, that the consumption was based on free choice and not influenced by other factors.
TAS2R38 rs713598, rs1726866, rs10246939 polymorphisms were associated with PTC and PROP phenotypes and with differences in perceived bitterness of bitter tasting vegetables and berries, wine/alcohol, thioamide, and salicin studies (Kim et al., 2003; Ooi et al., 2010; Wooding et al., 2010; Lucock et al., 2011; Mennella et al., 2011a; Colares-Bento et al., 2012; Behrens et al., 2013; Melis et al., 2013; Bering et al., 2014; Keller et al., 2014; Ledda et al., 2014; Mennella et al., 2014a; Robino et al., 2016; Carrai et al., 2017; Risso et al., 2017). In one study, conducted among 7–13 year old Irish children, TAS2R38 genotype had no significant impact on bitter vegetable liking, although the authors suggest that taster children might be more prone to food neophobia, and might associate green vegetables with bitter or unpleasant tastes (Feeney et al., 2014). The influence of these variants on cruciferous/Brassica vegetable intake was showed only by two poor quality studies (Sacerdote et al., 2007; Feeney et al., 2011) and three studies (out of which two were rated as low quality studies) found no association with this phenotype (Negri et al., 2012; Inoue et al., 2013).
Genetic variations located in the TAS2R19 gene were associated with perceived bitterness of phytochemicals quinine and grosheimin (Reed et al., 2010; Knaapila et al., 2012; Hayes et al., 2015; Roudnitzky et al., 2015;) and unsweetened grapefruit juice (Reed et al., 2010; Hayes et al., 2015). TAS2R31 variants correlated with grosheimin, amarogentin, saccharin, acesulfame potassium response, quinine bitterness, and grapefruit liking (Roudnitzky et al., 2011; Allen et al., 2013a; Allen et al., 2013b; Hayes et al., 2015; Roudnitzky et al., 2015). However, it is important to highlight, that the phenotypic associations of rs10772420 (TAS2R19) may be due to strong linkage disequilibrium (LD) between TAS2R19 and TAS2R31 SNPs (Hayes et al., 2015).
Sweet Taste Preference
Confirmed associations were found in case of 41 polymorphisms in 18 genes (presented in Table 2 and Supplementary Table 1 ). Variants of the TAS2R38 genes were analyzed as a haploblock and individually as well. Sugar intake/consumption (measured by the food frequency or the three-factor eating questionnaire and the 3-day weighted dietary record) was only considered as preference (regardless of the authors’ statement), if consumption was truly associated with food choice. Other phenotyping methods included the measurement of sucrose detection thresholds and intensities and preference for sweet tasting foods. Sweet taste perception is mediated by heterodimers of two G protein-coupled receptors, taste receptor type 1 member 2 (T1R2), and taste receptor type 1 member 3 (T1R3) (Nelson et al., 2001; Li et al., 2002; Zhao GQ et al., 2003).
Table 2.
Overview of genetic association studies related to sweet taste preferences.
| Gene | SNP | Applied tastant/method | Number of studies with confirmed association | Findings | Reference | Number of studies with no association | Reference |
|---|---|---|---|---|---|---|---|
| TAS1R2 | rs3935570 | Sucrose, sugar intake (FFQ) | 1 | GG or GT vs. TT had significantly higher detection thresholds [and lower suprathreshold sensitivity ratings (iAUC)] but only in individuals with BMI ≥ 25. (No effect on sugar consumption.) | (Dias et al., 2015) | 0 | – |
| TAS1R2 | rs12033832 | Sucrose, sugar intake (FFQ) | 1 | Individuals with a BMI ≥ 25: G allele carriers had significantly higher detection and lower suprathreshold sensitivity ratings (iAUC), higher intake of total sugars, sucrose, fructose, and glucose. Individuals with a BMI <25: significantly lower detection thresholds and no effect on suprathreshold taste, lower intake of total sugars, sucrose, fructose, glucose, and lactose | (Dias et al., 2015) | 2 | (Fushan et al., 2009; Han et al., 2017) |
| TAS1R2 | rs35874116 | Intake of sweet food (three factor eating questionnaire) | 2 | CC and CT vs. TT associated with higher intake of sweet foods. Overweight Val carriers consumed less sugars, sucrose, fructose, and glucose than Ile homozygotes. | (Eny et al., 2010; Han et al., 2017) | 0 | – |
| TAS1R3 | rs307355 | Sucrose | 1 | Strong association with decreased sucrose AUC scores (reduced taste sensitivity to sucrose associated with T alleles) | (Fushan et al., 2009) | 1 | (Han et al., 2017) |
| TAS1R3 | rs35744813 | Sucrose | 2 | Strong association with decreased sucrose AUC scores (reduced taste sensitivity to sucrose associated with T alleles). Adults with no T alleles preferred a lower concentration of sucrose than did those with one or two T alleles (no association in children). | (Fushan et al., 2009; Mennella et al., 2014b) | 2 | (Joseph et al., 2016; Han et al., 2017) |
| TAS2R38 | rs713598 | Intake of sweet tasting foods (3-day, weighed dietary records, test meal) | 2 | The PP/PA genotype was associated with a higher intake of (energy dense) sweet tasting foods in children. AP or PP children consumed more chocolate chip cookies at the test-meal than children who had the AA genotype. | (Keller et al., 2014; Pawellek et al., 2016) | 0 | – |
| TAS2R38 | rs713598 | Sucrose, food preference questionnaire | 3 | PP children preferred higher concentrations of sucrose in water and beverages containing more sugar than AA children (AP intermediate preference). GG subjects did not prefer sweet foods (dessert and chocolate). P allele more common in children with lower sucrose thresholds | (Lipchock et al., 2012; Joseph et al., 2016; Perna et al., 2018) | 1 | (Ooi et al., 2010) |
| TAS2R38 | rs1726866 | Sucrose | 1 | Children with one or two bitter-sensitive A alleles had lower detection thresholds (more sensitive to the taste of sucrose). | (Joseph et al., 2016) | 1 | (Timpson et al., 2007) (AceK sweetness) |
| TAS2R38 | rs10246939 | Sucrose | 1 | Children with one or two bitter-sensitive V alleles had lower detection thresholds (more sensitive to the taste of sucrose). | (Joseph et al., 2016) | 0 | – |
| TAS2R38 | A49P (rs713598), A262V (rs1726866), V296I (rs10246939) | Berry products liking | 1 | Majority of PAV/PAV and PAV/AVI children, liked the sweetened, dried bilberries with rather high sugar content. | (Suomela et al., 2012) | 0 | – |
| TAS2R38 | A49P (rs713598), A262V (rs1726866), V296I (rs10246939) | Sweet food intake (FFQ) | 1 | PAV homozygotic individuals consumed more sweet foods than did the AVI homozygotic subjects. | (Sandell et al., 2014) | 0 | – |
FFQ, food frequency questionnaire; BMI, body mass index; AUC, area under the curve; AceK, acesulfame potassium; iAUC, incremental area under the curve.
Individual ratings of the intensity of suprathreshold solutions were plotted, and the incremental area under the taste sensitivity curve (iAUC) was computed.
Recent studies suggest the involvement of the gustducin signaling molecule in bitter, sweet, and umami taste transduction (Glendinning et al., 2005) as well, still the most convincing findings of this review are linked to TAS2R38 gene polymorphisms (rs713598, rs1726866, rs10246939) (Lipchock et al., 2012; Suomela et al., 2012; Keller et al., 2014; Sandell et al., 2014; Joseph et al., 2016; Pawellek et al., 2016; Perna et al., 2018) involved in shaping bitter taster status. Results of candidate gene studies targeting TAS1R2 rs12033832 (Dias et al., 2015) and TAS1R3 polymorphisms were inconclusive (Fushan et al., 2009; Mennella et al., 2014b). TAS1R2 rs3935570 (Dias et al., 2015) and rs35874116 (Eny et al., 2010; Han et al., 2017) were associated with sweet taste preference but with limited number of studies (n = 1 and n = 2, respectively). Several GNAT3 polymorphisms also showed significant associations (Fushan et al., 2009) ( Supplementary Table 2 ). The list of other polymorphisms with possible explanatory mechanism, which were confirmed by single studies, consists of genes involved in glucose metabolism, umami perception, metabolic processes, signal transduction and neurotransmission, regulation of energy homeostasis (SLC2A2, TAS1R1, ADIPOQ, ANKK1, DRD2, OPRM1, LEP, LEPR, NPY1, respectively) (Mizuta et al., 2008; Elbers et al., 2009; Eny et al., 2010; Davis et al., 2011; Jabłoński et al., 2013; Rawal et al., 2013; Wakai et al., 2013). Further studies are essential to confirm the results of candidate gene studies and discover the pathomechanistic link between other SNPs and sweet taste perception.
Fat Taste Preference
The most recently identified primary sensory quality of the gustatory system is oleogustus (Malles, 2010; Running et al., 2015). A total of 24 SNPs in 15 genes were associated with fat taste preference (presented in Table 3 and Supplementary Table 1 ). The three TAS2R38 SNPs were investigated as a haplotype. If fat intake/consumption (measured by food frequency or diet history questionnaire) was interpreted as preference by authors, only those results were considered as preference where consumption was truly associated with voluntary diet selection.
Other phenotyping methods included the measurement of oleic acid sensory threshold, perception of creaminess, oiliness, fat content, and preference for fatty foods. The most convincing results are related to polymorphisms (rs1761667, rs15227483) in the CD36 gene (Keller et al., 2012; Pepino et al., 2012; Melis et al., 2015; Mrizak et al., 2015; Sayed et al., 2015; Ong et al., 2017). This gene encodes the fatty acid translocase that has strong affinity for dietary long-chain fatty acids (LCFA) (Baillie et al., 1996) and serves as a fat taste receptor (Laugerette et al., 2005). The effect of the rs838145 variant located in the IZUMO sperm-egg fusion 1 gene (IZUMO1) on fat preference was also confirmed by two independent studies (Tanaka et al., 2013; Søberg et al., 2017). Some of the other polymorphisms with only one confirmed association and a possible molecular link to fat sensitivity include genes involved in the regulation of lipolysis and thermogenesis, lipoprotein metabolism, neurotransmission, and signaling regulators (ADRB3, APOA2, OPRM1, RGS6, respectively) (Corella et al., 2007; Davis et al., 2011; Sibbel et al., 2011; Sasaki et al., 2013). Future research is needed to explore the effect of other genetic variants and fat taste perception.
Umami Taste Preference
Umami taste is mediated by a heterodimer complex of G-protein-coupled receptors, taste receptor type 1 member 1 (T1R1) and taste receptor type 1 member 3 (T1R3) (Nelson et al., 2001; Li et al., 2002; Zhao GQ et al., 2003) interacting with amino acids, such as monosodium glutamate (GMP) and inosine monophosphate (IMP). The effect of metabotropic glutamate receptors mGluR1 and mGluR2 has also been implicated in umami taste perception (Chaudhari and Roper, 2010; Raliou et al., 2011; Yasumatsu et al., 2012). Another candidate gene accounting for differences in umami sensitivity is GNAT3 gene that is co-expressed with TAS1R1 (Ishimaru et al., 2012) and encodes G protein alpha subunit gustducin, a taste signaling molecule involved in G-protein-coupled membrane receptors mediated taste transduction (bitter, sweet, and umami) (Glendinning et al., 2005). The number of studies investigating the association between umami taste preference and genetic variants was limited (n = 4) (presented in Table 4 ) and analyzed four candidate genes. Findings suggest that TAS1R1 and TAS1R3 polymorphisms influence taster status (Raliou et al., 2009; Chen et al., 2009; Shigemura et al., 2009), but further investigation is needed to confirm these results and elucidate the role of SNPs located in candidate genes on individual sensitivity to umami.
Salty Taste Preference
The source of salty taste found in foods is NaCl. The molecular mechanism of salty taste responsiveness is not clear, but the involvement of epithelial sodium channels (ENaCs) located in taste cell membranes in fungiform papillae and amiloride-sensitive vanilloid receptors (Trpv1) have been hypothesized in salt perception (Heck et al., 1984; Lin et al., 1999; Lyall et al., 2004). In humans there are four ENaC subunits (αβγδ) coded by SCNN1A, SCNN1B, SCNN1G, and SCNN1D genes, respectively. The number of studies investigating the association between salty taste preference and genetic polymorphisms was very low (n = 5) (presented in Table 5 ). Only one study analyzed the association between polymorphisms in putative salt receptors and differences in salt taste perception. Homozygotes of TRPV1 rs8065080 (CC genotype), SCNN1B rs239345 (AA genotype), and rs3737665 (TT genotype) perceived salt solutions as significantly weaker than heterozygotes or other allele homozygotes (Dias et al., 2013). Other findings were related to genes linked to bitter (TAS2R38, CA6) (Hayes et al., 2008; Feeney and Hayes, 2014; Deshaware and Singhal, 2017) and umami taste responsiveness (TAS1R1) (Rawal et al., 2013). Future studies are essential to confirm these findings and analyze the role of receptors involved in other taste perception pathways rather than salt.
Table 5.
Overview of genetic association studies related to salty taste preferences.
| Gene | SNP | Applied tastant/method | Number of studies with confirmed association | Findings | Reference | Number of studies with no association | Reference |
|---|---|---|---|---|---|---|---|
| TRPV1 | rs8065080 | NaCl | 1 | CC genotype significantly lower iAUCs | (Dias et al., 2013) | 0 | – |
| SCNN1B | rs239345 | NaCl | 1 | AA genotype significantly lower iAUCs | (Dias et al., 2013) | 0 | – |
| SCNN1B | rs3785368 | NaCl | 1 | TT genotype significantly lower iAUCs | (Dias et al., 2013) | 0 | – |
| CA6 | rs3737665 | NaCl, KCl | 1 | The SNP associated with differences in the perceived intensity of NaCl and KCl saltiness | (Feeney and Hayes, 2014) | 0 | – |
| CA6 | rs3765964 | NaCl | 1 | The SNP associated with differences in the perceived intensity of NaCl | (Feeney and Hayes, 2014) | 0 | – |
| CA6 | rs2274333 | KCl | 1 | The SNP associated with differences in KCl saltiness | (Feeney and Hayes, 2014) | 0 | – |
| TAS1R1 | rs17492553 | NaCl | 1 | T (allele, genotype) lower intensities | (Rawal et al., 2013) | 0 | – |
| TAS1R1 | rs34160967 | NaCl | 1 | A (allele, genotype) lower intensities | (Rawal et al., 2013) | 0 | – |
| TAS2R38 | A49P (rs713598), A262V (rs1726866), V296I (rs10246939) | NaCl | 1 | PAV/PAV higher ratings for saltiness intensity | (Deshaware and Singhal, 2017) | 1 | (Hayes et al., 2008) |
NaCl, sodium chloride; KCl, potassium chloride; iAUC, incremental area under the curve.
Individual ratings of the intensity of suprathreshold solutions were plotted, and the incremental area under the taste sensitivity curve (iAUC) was computed.
Sour Taste Preference
Sour taste perception is triggered by acidic foods and substances. The exact mechanism behind the sensitivity to this taste quality is not yet fully understood, but recent data suggest the involvement of transient receptor potential channels (TRPs), namely polycystic-kidney disease like (PKDL-like) receptors in the mediation of sour taste (Huang et al., 2006; Huque et al., 2009; Ishimaru Y et al., 2016). The number of studies investigating the association between sour taste quality and genetic polymorphisms was limited (n = 4). Phenotype was defined by using citric acid or the sourness perception of berry products and wine. The findings of reviewed studies were not related to candidate genes (PKD2L1, PKD2L3), rather to genes encoding two receptors involved in bitter and umami perception (Laaksonen et al., 2013; Carrai et al., 2017) ( Table 6 ). Exploring the effect of these variants on sour taste perception and subsequent sensitivity and implementing studies targeting candidate genes is a future direction.
Discussion
To our knowledge—after a few narrative reviews (Kim et al., 2004; Garcia-Bailo et al., 2008; Grimm and Steinle, 2011; Dotson et al., 2012; Hayes et al., 2013; Keller and Adise, 2016; Chamoun et al., 2018) that provided a comprehensive, critical, and objective analyses of the scientific knowledge regarding the genetic implications of food preference at the time of their publications—this is the first systematic review prepared by following the PRISMA guideline and using all the most relevant research databases to explore associations between genetic polymorphisms and taste preferences. Food preferences are shaped during fetal development and eating behavior evolves over time. It is a complex trait, determined by interactions of genetic and environmental factors (Birch, 1999; Ventura and Worobey, 2013). The environmental determinants include in utero exposures, early postnatal experiences, parental feeding practices, family (social, economic factors), and the wider contexts of the environment (Gibson and Cooke, 2017). Sensory properties of consumed food is an important determinant of dietary habits and taste has been considered as one of the main drivers of food choices and dietary patterns (Connors et al., 2001; Honkanen and Frewer, 2009; Kourouniotis et al., 2016). Chemical compounds in food activate specialized taste receptors that can be altered by genetic polymorphisms and consequently lead to individual taste variability and preferences. Bitter, sweet, and umami perception is linked to G-protein-coupled receptors (Nelson et al., 2001; Li et al., 2002), whereas salt and sour tastes are mediated by ion channels (Heck et al., 1984; Lin et al., 1999; Lyall et al., 2004; Huang et al., 2006; Huque et al., 2009; Ishimaru Y et al., 2016). The most recently identified fat taste modality is believed to be linked to the fatty acid transporter CD36 (Laugerette et al., 2005). There is growing interest in characterizing taste preference based on genetic variation, as well as the association between taste preference and the prevalence of different risk conditions and major diet-related NCDs. Increased understanding of interplay between taste genetics, nutrition, and diet can contribute to the development of public health strategies to improve population health through the prevention of diet-related NCDs.
The genetic components shaping human taste abilities could be a result of natural selection driven by evolutionary adaption mechanisms to avoid the consumption of plant-based toxic substances (Kalmus, 1950; Hladik and Simmen, 1996; Wooding S et al., 2004; Glendinning et al., 2005; Soranzo et al., 2005). These plant-derived toxins generally have an unpleasant bitter taste (Maga, 1990) and excessively bitter-tasting plants will be rejected by humans (Rouseff, 1990). Bitter-tasting compounds include amino acids and peptides, sulfimides (saccharin), ureas and thioureas (PROP and PTC), esters and lactones, terpenoids, and phenols and polyphenols (McBurney, 1990). The oral sensitivity to thiourea moiety (N-C = S) containing chemicals and related structures in food varies widely among individuals.
The bitter-tasting thiourea moiety is present as glucosinolates in Brassica vegetables (Hanschen et al., 2014), but other foods and beverages without the thiourea moiety (grapefruit juice, coffee, alcohol, green tea, and soy products) are also perceived as bitter for sensitive individuals (Gayathri Devi et al., 1997; Lanier et al., 2005; Dinehart et al., 2006; Sandell and Breslin, 2006). The supertaster-taster-non-taster categories (Harris H, 1949; Bartoshuk et al., 1994) are linked to combinations of three functional SNPs (rs713598, rs1726866, rs10246939) of the TAS2R38 gene. The homozygous PAV (proline–alanine–valine) haplotype defines the taster form, while the homozygous AVI (alanine-valine-isoleucine) haplotype specifies the non-taster phenotype and heterozygotes possess intermediate sensitivity to PROP and PTC (Kim et al., 2003; Kim and Drayna, 2005), accounting for 85% of the phenotypic variance in PTC perception (Wooding S et al., 2004; Bufe et al., 2005). According to the in vitro assays of Buffet et al. (2005) rs713598 has the greatest effect on bitter taste signal transduction, rs1726866 possesses weaker effects, and rs10246939 site has no detectable effect at all (Bufe et al., 2005). The bitter taste modality has been the most extensively studied and the majority of genetic association studies related to the bitter quality focused on TAS2R38 gene polymorphisms (Kim et al., 2003; Mennella et al., 2005; Sandell and Breslin, 2006; Sacerdote et al., 2007; Timpson et al., 2007; Hayes et al., 2008; Duffy et al., 2010; Ooi et al., 2010; Wooding et al., 2010; Calò C et al., 2011; Feeney et al., 2011; Gorovic N et al., 2011; Lucock et al., 2011; Mennella et al., 2011a; Cabras et al., 2012; Campbell et al., 2012; Colares-Bento et al., 2012; Negri et al., 2012; Behrens et al., 2013; Inoue et al., 2013; Laaksonen et al., 2013; Melis et al., 2013; Bering et al., 2014; Garneau et al., 2014; Keller et al., 2014; Ledda et al., 2014; Mennella et al., 2014a; Robino et al., 2014; Melis et al., 2015; Nolden et al., 2016; Robino et al., 2016; Bella et al., 2017; Carrai et al., 2017; Deshaware and Singhal, 2017; Feeney et al., 2017; Risso et al., 2017). Results of the reviewed studies were congruent, supporting the genetic determination of the bitter taster status by TAS2R38 rs713598, rs1726866, and rs10246939 SNPs (Kim et al., 2003; Mennella et al., 2005; Sandell and Breslin, 2006; Sacerdote et al., 2007; Timpson et al., 2007; Hayes et al., 2008; Duffy et al., 2010; Ooi et al., 2010; Wooding et al., 2010; Calò C et al., 2011; Feeney et al., 2011; Gorovic N et al., 2011; Lucock et al., 2011; Mennella et al., 2011a; Cabras et al., 2012; Campbell et al., 2012; Colares-Bento et al., 2012; Negri et al., 2012; Behrens et al., 2013; Inoue et al., 2013; Laaksonen et al., 2013; Bering et al., 2014; Garneau et al., 2014; Keller et al., 2014; Ledda et al., 2014; Mennella et al., 2014a; Robino et al., 2014; Melis et al., 2015; Nolden et al., 2016; Robino et al., 2016; Bella et al., 2017; Carrai et al., 2017; Deshaware and Singhal, 2017; Feeney et al., 2017; Risso et al., 2017).
Much less is known about the effect of the genetic alterations of other taste 2 receptors (TAS2Rs), which proteins also function as bitter taste receptors. Respondents for TAS2R31 receptors (formerly TAS2R44) are compounds with no common chemical substructure (acesulfame K, famotidine, diphenidol) (Meyerhof et al., 2009). Research included in our review (n = 7) focused on two polymorphisms rs10845293 (Ala227Val) and rs10772423 (Val240Ile) located in this gene (Pronin et al., 2007; Roudnitzky et al., 2011; Allen et al., 2013a; Allen et al., 2013b; Hayes et al., 2015; Roudnitzky et al., 2015; Nolden et al., 2016). The Val240Ile SNP was associated with the bitter compounds amarogentin [found in gentian (Gentiana lutea) or in Swertia chirata] (Keil et al., 2000) and grosheimin [present in artichokes (Cravotto et al., 2005)] intensities, detection and recognition threshold, quinine bitterness and grapefruit liking. Moreover, Val240 homozygotes reported less bitterness from the artificial sweetener acesulfame potassium than the Ile240 homozygotes (Pronin et al., 2007; Roudnitzky et al., 2011; Allen et al., 2013a; Allen et al., 2013b; Hayes et al., 2015; Roudnitzky et al., 2015; Nolden et al., 2016). This latter finding is in accordance with in vitro study results, whereas acesulfame K activated TAS2R43 and TAS2R44 at concentrations known to stimulate bitter taste (Kuhn et al., 2004). The same polymorphism showed no association with bitterness of capsaicin, piperine, and ethanol (Nolden et al., 2016). The bitterness perception from capsaicin and piperine is characterized by individual diversities (Green and Hayes, 2004) and the sensitivity to perceived bitterness of alcohol correlates with PROP phenotypes (Lanier et al., 2005), but based on findings of these studies it was not related to TAS2R31 genetic variants (Nolden et al., 2016).
Two polymorphisms of the TAS2R19 gene were investigated by studies (n = 6) included in the review (Reed et al., 2010; Hayes et al., 2011; Knaapila et al., 2012; Bering et al., 2014; Hayes et al., 2015; Roudnitzky et al., 2015). TAS2R19 rs10772420 codes for an arginine-to-cysteine substitution at amino acid 299 (R299C) (Allen et al., 2013a). This variant showed associations with quinine and grosheimin intensity ratings, grosheimin detection threshold, and bitterness perception of grapefruit juice (Reed et al., 2010; Hayes et al., 2011; Knaapila et al., 2012; Hayes et al., 2015; Roudnitzky et al., 2015) and no association with PROP phenotype (Bering et al., 2014). Moreover rs1868769 in the same gene was associated with quinine and grosheimin intensity ratings and detection and recognition threshold (Knaapila et al., 2012; Roudnitzky et al., 2015), but not with PROP phenotype (Bering et al., 2014). However, in in vitro studies, naringin, limonin (two main compounds responsible for the bitterness of grapefruit juice), and quinine did not activate TAS2R19 (Meyerhof et al., 2009; Thalmann et al., 2013), accordingly confirmed associations may be due to strong LD between the Arg299Cys (rs10772420) polymorphism, and other SNPs located in nearby TAS2R genes (Allen et al., 2013a; Hayes et al., 2015).
Both polymorphisms T > G rs2227264 and rs2234012 (A > G) SNPs are located in the 5’ untranslated region (5’UTR) of TAS2R5, which region typically contains sequences that regulate translation efficiency or messenger RNA stability (Hayes et al., 2011), that may account for altered protein function and consecutive variation in bitterness perception.
The gustin protein (or carbonic anhydrase VI) is secreted by the parotid, submandibular, and von Ebner glands (Henkin et al., 1975; Piras et al., 2012) and it has been identified as a trophic factor for growth and development of taste buds (Henkin et al., 1999). The rs2274333 SNP causes the amino acid substitution at position Ser90Gly in the protein sequence of carbonic anhydrase VI (Henkin et al., 1975) and is associated with formation and function of fungiform papillae (Barbarossa et al., 2015).
Due to the inconclusive findings related to the gustin gene (Padiglia et al., 2010; Calò C et al., 2011; Cabras et al., 2012; Melis et al., 2013; Bering et al., 2014; Feeney and Hayes, 2014; Risso et al., 2017), and to the low number of studies focusing on TAS2R19, TAS2R31, and TAS2R5 polymorphisms, additional work is needed to determine the effect of these variants on bitter taste perception, but otherwise the confirmed effect of TAS2R38 rs713598, rs1726866, and rs10246939 SNPs shaping bitter taste preference is notable, since studies suggest a relationship between PROP sensitivity and nutritional behavior. In particular, it has been reported that taster status shows an inverse relationship with the acceptance of bitter tasting foods. Greater sensitivity to PROP is associated with lower preference of citrus fruit (Drewnowski A et al., 1998a), Brussels sprouts, cabbage and spinach (Drewnowski et al., 1999), asparagus, and curly kale (Dinehart et al., 2006) and lower overall vegetable (Drewnowski et al., 2000; Kaminski et al., 2000; Yackinous and Guinard, 2002) and fruit consumption. In other investigations, tasters showed lower acceptance of cruciferous, green and raw vegetables and supertasters higher sensitivity to dark chocolate, black coffee, and caffeine solutions (Reed et al., 2010). Since meta-analysis results provide evidence that a higher consumption of fruit and vegetables is associated with a lower risk of all-cause mortality, particularly cardiovascular mortality (Wang et al., 2014), this genetically-determined bitter phenotype is a substantial contributor to shape healthy eating patterns.
Moreover, several studies in human nutrition have suggested that the PROP phenotype may serve as a general marker for oral sensations and food preferences, and influence dietary behavior and nutritional status (Tepper, 2008). Given the nutritional importance of dietary lipids and sugars an extensive research has investigated the impact of PROP taster status on sweet and fat consumption. Most studies focusing on the relationship between taster status and dietary fat perception (Tepper and Nurse, 1997; Kirkmeyer and Tepper, 2003; Duffy et al., 2004b; Prescott et al., 2004; Hayes and Duffy, 2007; Hayes and Duffy, 2008), but not all (Drewnowski et al., 1998b; Drewnowski et al., 2007) reported that taster individuals had a lower ability to distinguish fat content and creaminess in certain fatty foods and gave higher taste intensity ratings for linoleic acid, than non-tasters (Ebba et al., 2012). Moreover, PROP non-tasters possessed preferences for dietary fat (Forrai and Bánkövi, 1994; Tepper and Nurse, 1998; Duffy, 2000; Keller et al., 2002; Hayes and Duffy, 2007) and consumed more servings of discretionary fats and high-energy foods per day compared to tasters (Keller et al., 2002; Tepper et al., 2011). Findings to elucidate the association between PROP taster status and sweet preference and sugar intake were inconclusive. Some studies found that more sensitive individuals to PROP showed lower sweet preference (Looy et al., 1992; Duffy, 2000; Hayes and Duffy, 2007; Yeomans et al., 2007). Other investigators found that sucrose tasted sweeter to tasters (Gent and Bartoshuk, 1983), but some found no link between PROP taster status and hedonic ratings for sweet (Gent and Bartoshuk, 1983; Drewnowski et al., 1997; Drewnowski et al., 2007; Von Atzingen and Silva, 2012) and the consumption sweet beverages (Wijtzes et al., 2017). Accordingly the role of bitter-taster status in shaping dietary preferences is certainly not negligible, but more research is needed to determine its effect on nutrition, besides the intake of bitter-tasting foods. Although the focus of this review was on genetic variants affecting taste, studies examining associations with PROP/PTC were not included despite their strong linkage with TAS2R38 genotype. This may have resulted in some relevant papers not being included in the analyses and discussion.
The signal transduction of sweet taste is linked to heterodimers of two G protein-coupled receptors T1R2 and T1R3) (Pronin et al., 2007; Roudnitzky et al., 2015), which are encoded by genes clustered on chromosome 1 (Liao and Schultz, 2003). TAS1R2 is characterized by an increased level of genetic diversity, furthermore TAS1R3 is more conserved (Kim et al., 2006). Candidate gene studies of sweet preference targeted the polymorphic sites located in T1R2 and T1R3 genes involved in the signal transduction of this taste modality (Fushan et al., 2009; Eny et al., 2010; Mennella et al., 2014b; Dias et al., 2015; Joseph et al., 2016; Han et al., 2017), with results not allowing further conclusions to make, since only the effect of the functional Ile191Val (rs35874116) variation (Dias et al., 2015) and the intronic rs3935570 yielded positive findings (Eny et al., 2010; Han et al., 2017) ( Table 2 ). The most convincing results were related to variants in the bitter taste receptor gene (TAS2R38). These polymorphisms were reported to affect the sensory experience of sweet taste, changes in taste sensitivity and preference, and sweet food intake (Lipchock et al., 2012; Suomela et al., 2012; Keller et al., 2014; Sandell et al., 2014; Joseph et al., 2016; Pawellek et al., 2016; Perna et al., 2018) ( Table 2 ), with the only exclusion a study by Ooi et al. (2010). The genetically-determined taster phenotype preferred higher sucrose concentrations (Lipchock et al., 2012), had lower detection thresholds (Joseph et al., 2016), and consumed more sweet tasting foods (Suomela et al., 2012; Keller et al., 2014; Sandell et al., 2014; Pawellek et al., 2016; Perna et al., 2018) whereas genetically determined non-taster individuals did not prefer sweet foods (Ooi et al., 2010), despite that the PROP phenotype without underlying genetic investigations showed inconclusive findings with sugar preference and intake in adults (Gent and Bartoshuk, 1983; Looy et al., 1992; Drewnowski et al., 1998b; Drewnowski et al., 1997; Duffy, 2000; Hayes and Duffy, 2007; Yeomans et al., 2007; Von Atzingen and Silva, 2012; Wijtzes et al., 2017), which is probably related to other genetic variants that influence bitter perception, and also in children that may be explained by age-related changes in taste perception and preference, beyond genetic factors (reviewed in Keller and Adise, 2016).
Although less well-studied than bitter sensitivity, variation in sweet taste responsiveness may also influence food preference and intake. It has been demonstrated that a higher preference for sucrose solutions or sweet taste was associated with increased preferences for sweet desserts (Drewnowski et al., 1999), higher habitual intake of sweet foods (Holt et al., 2000; Ashi et al., 2017), an increased consumption of sweet beverages (Mahar and Duizer, 2007), total sugar consumption (Ko et al., 2015), and the sugar content of preferred sugar-rich cereals (Mennella et al., 2011b) and more sensitive individuals tended to have a lower preference for sugar than less sensitive individuals (Looy et al., 1992). As reviewed by Rippe et al. (2016) excessive sugar intake is responsible for the development metabolically based diseases such as obesity, diabetes, and cardiovascular diseases (Rippe and Angelopoulos, 2016), therefore sweet preference has a clearly important role in determining health status.
The two SNPs rs1761667 and rs1527483, located in the CD36 gene, has received much attention in the research of fat taste perception. The fatty acid translocase, coded by the CD36 gene, is involved in the transport of LCFA across cell membranes, which is first step in fat metabolism (Hajri and Abumrad, 2002). CD36 is expressed on taste cells in animals (Fukuwatar et al., 1997; Laugerette et al., 2005) and has been detected in human foliate and circumvallate papillae (Simons et al., 2011; Hochheimer et al., 2014). Results of research, except for one single study (Daoudi et al., 2015) are consistent, namely individuals with the AA genotype of rs1761667 have higher thresholds for lipid taste perception (decrease in sensitivity and consequent higher acceptance of fatty acids) than do those with GG genotypes (Keller et al., 2012; Pepino et al., 2012; Melis et al., 2015; Mrizak et al., 2015; Sayed et al., 2015. Ong et al., 2017). The intronic SNP, rs1527483, which encodes a C/T substitution, was also found to influence fat perception by two studies. Subjects with C/T or T/T genotypes perceived greater fat content of salad dressings and cream crackers, independent of fat concentration (Keller et al., 2012; Ong et al., 2017). Although, creaminess is a complex sensory characteristic consisting of both flavor and textural components, but overall, it is experienced as a positive attribute of fat containing foods (Mela, 1988). Due to the low number studies related to SNP rs1527483 (Keller et al., 2012; Ong et al., 2017) and IZUMO1 rs838145 (Tanaka et al., 2013; Søberg et al., 2017), replication is needed to confirm the influence of these SNPs on fat taste perception.
According to investigations fat hypersensitivity is associated with lower energy and fat intake (Stewart et al., 2010) and high fat food preference with high dietary fat intake (Fisher and Birch, 1995; Ricketts, 1997). Given that adiposity is a critical risk factor in course of the development of insulin resistance and the development of type 2 diabetes (reviewed in Forouhi et al., 2018) and that atherogenic dyslipidemia [low high-density lipoprotein cholesterol (HDL-C), high triglyceride-rich lipoprotein levels] which occurs with low-fat, high carbohydrate diets and increases risk of coronary heart disease (Trumbo et al., 2002), following dietary guidelines with recommended intakes is essential to ensure adequate consumption of total energy, essential fatty acids, and fat-soluble vitamins (Food and Agriculture Organization of the United Nations/World Health Organization, 2010), and prevent cardiovascular diseases and type 2 diabetes.
The number of studies investigating salty (Hayes et al., 2008; Dias et al., 2013; Rawal et al., 2013; Feeney and Hayes, 2014; Deshaware and Singhal, 2017), umami (Raliou et al., 2009; Shigemura et al., 2009; Chen et al., 2009; Risso et al., 2017), and sour (Hayes et al., 2008; Laaksonen et al., 2013; Rawal et al., 2013; Carrai et al., 2017) taste preferences was limited. The effect of these latter two taste modalities on health status is not yet known. Though the health effects, namely the correlation of salt (sodium) intake with blood pressure is clear (reviewed in Farquhar et al., 2015), and research suggest that sensory phenotypes with greater perceived saltiness from solutions liked the solution less (Hayes et al., 2010) and individuals with a preference of high salt concentrations and salty foods were found to consume more salt compared to those who did not prefer salty beverages (Shepherd et al., 1984; Takamura et al., 2014), results of our review allows us no conclusions to make on the genetic background on salt preference.
Limitations
Several limitations must be considered in interpreting the findings of this systematic review. Many of the results of genetic association studies on different taste modalities have not been replicated, and it is not possible to perform a qualitative synthesis and meta-analysis. Despite a growing body of nutrigenomics research, the overall number of studies available for this specific review was limited. Some studies had relatively small sample sizes, and several of them were conducted by the same research groups. Certain samples and study groups may have overlapped and participants were from similar backgrounds, without a representation of diverse populations or ethnic background. Considering the work carried out by different research teams, important factors such as genotyping method (s), assessment methods, ethnic composition, and genetic variant (s) evaluated, make direct comparison of findings hard and limit the generalizability of some results. Despite the mentioned limitations, this review represents the first systematic effort to compile and discuss studies on genetic background of taste perception, taste preferences, and its nutritional implications.
Conclusions
Our findings suggest that a significant association exists between TAS2R38 variants (rs713598, rs1726866, rs10246939) and bitter and sweet taste preference. Due to the limited number of studies related to other tastes (salt and sour) further research is needed to assess the possible effect of TAS2R38 genetic variants on these taste modalities. Other confirmed results are related to rs1761667 (CD36) and fat taste responsiveness. Otherwise further research is essential to confirm results of single studies or clarify inconclusive findings related to genetic variants and individual sensitivity of the gustatory pathway.
Since convincing findings of genetic association studies only exists for bitter, fat, and partly for sweet taste preference, highlighting the role of environmental factors of food preferences and dietary choices has great importance in the planning of public health intervention programs. These interventions should be tailored to change the modifiable determinants of poor dietary practices to promote healthy eating. Two major areas should be recognized for nutrition policies and programs focusing on early and environmental exposures. Early exposures include experiences in utero and during the lactation and complementary feeding period of infants (reviewed in Beckerman et al., 2017). Research shows that maternal unhealthy food intake during pregnancy and/or lactation increases the preference for high-fat and/or high-sugar diets of the offspring (Muhlhausler et al., 2017). The timing and repeated intake of bitter tasting fruits and vegetables should be the primary focus of the complementary feeding period (reviewed in Beckerman et al., 2017), since sweet and bitter-taste preference can be influenced by early childhood experiences (Bartoshuk and Beauchamp, 1994; Mela, 2001). This is essential since sweet preference is the highest during childhood and it declines with age (reviewed in Hoffman et al., 2016) and the correlation between preferences of preschool children and their consumption patterns is considerably higher than the relationship reported by adults (Birch, 1979). Environmental exposures involve the social environment, such as parents (feeding practices and social and emotional context of food), peers, community (daycare, preschool, school, etc.), media, and other environmental effects (food access and advertisements) (reviewed in Beckerman et al., 2017). Therefore, it is important to strengthen the implementation of the International Code of Marketing of Breast-milk Substitutes (World Health Organization, 2018) and introduce restrictions on marketing of unhealthy foods to children, covering all media, including digital, and to close any regulatory loopholes, as current evidence indicates that child-directed advertising has a major impact on children’s diets (Tatlow-Golden et al., 2016; World Health Organization, 2016; Emond et al., 2019). Building combined and well-coordinated interventions encompassing all these target areas is essential in managing successful nutrition programs (reviewed in Beckerman et al., 2017).
Regardless, it is still important to emphasize that further genetic research is needed to elucidate the effect of genetic variants on food preference and nutritional behavior. This knowledge may enhance our understanding of the development of individual taste and related food preferences and food choices that will aid public health intervention programs targeting unhealthy dietary behaviors.
Author Contributions
JD conducted the literature search (involved in all the phases of the study selection procedure), performed the quality assessment of primary studies, interpreted the results, and wrote the manuscript. EL was involved in the screening process of abstracts, assessing full-text articles for eligibility, data extraction, and quality assessment of included studies and contributed to drafting the discussion section of the manuscript. RÁ guided the writing of the manuscript and was involved in finalizing it.
Funding
This work was supported by the GINOP-2.3.2-15-2016-00005 project. The project is co-financed by the European Union under the European Social Fund and European Regional Development Fund, as well as by the Hungarian Academy of Sciences (TK2016-78).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.01272/full#supplementary-material
References
- Afshin A., Sur P. J., Fay K. A., Cornaby L., Ferrara G., et al. (2019). Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 393 (10184), 1958–1972. 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. L., McGeary J. E., Knopik V. S., Hayes J. E. (2013. a). Bitterness of the non-nutritive sweetener Acesulfame Potassium varies with polymorphisms in TAS2R9 and TAS2R31. Chem. Senses 38 (5), 379–389. 10.1093/chemse/bjt017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. L., McGeary J. E., Hayes J. E. (2013. b). Rebaudioside A and Rebaudioside D bitterness do not covary with Acesulfame K bitterness or polymorphisms in TAS2R9 and TAS2R31. Chemosens. Percept. 6, 3. 10.1007/s12078-013-9149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. L., McGeary J. E., Hayes J. E. (2014). Polymorphisms in TRPV1 and TAS2Rs associate with sensations from sampled ethanol. Alcohol Clin. Exp. Res. 38 (10), 2550–2560. 10.1111/acer.12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashi H., Campus G., Bertéus Forslund H., Hafiz W., Ahmed N., Lingström P. (2017). The influence of sweet taste perception on dietary intake in relation to dental caries and BMI in Saudi Arabian schoolchildren. Int. J. Dent. 4, 3–8. 10.1155/2017/4262053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atrup A., Dyerberg J., Stender S. (2008). Nutrition transition and its relationship to the development obesity and related chronic diseases. Obes. Rev. 1, 48–52. 10.1111/j.1467-789X.2007.00438.x [DOI] [PubMed] [Google Scholar]
- Baillie A. G., Coburn C. T., Abumrad N. A. (1996). Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J. Membr. Biol. 153 (1), 75–81. 10.1007/s002329900111 [DOI] [PubMed] [Google Scholar]
- Barbarossa I. T., Melis M., Mattes M. Z., Calò C., Muroni P., Crnjar R., et al. (2015). The gustin (CA6) gene polymorphism, rs2274333 (A/G), is associated with fungiform papilla density, whereas PROP bitterness is mostly due to TAS2R38 in an ethnically-mixed population. Physiol. Behav. 138, 6–12. 10.1016/j.physbeh.2014.09.011 [DOI] [PubMed] [Google Scholar]
- Bartoshuk L. M., Beauchamp G. K. (1994). Chemical senses. Annu. Rev. Psychol. 45, 419–459. 10.1146/annurev.ps.45.020194.002223 [DOI] [PubMed] [Google Scholar]
- Bartoshuk L. M., Duffy V. B., Miller I. J. (1994). PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol. Behav. 56 (6), 1165–1171. 10.1016/0031-9384(94)90361-1 [DOI] [PubMed] [Google Scholar]
- Beckerman J. P., Alike Q., Lovin E., Tamez M., Mattei J. (2017). The development and public health implications of food preferences in children. Front. Nutr. 4, 66. 10.3389/fnut.2017.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens M., Gunn H. C., Ramos P. C., Meyerhof W., Wooding S. P. (2013). Genetic, functional, and phenotypic diversity in TAS2R38-mediated bitter taste perception. Chem. Senses 38 (6), 475–484. 10.1093/chemse/bjt016 [DOI] [PubMed] [Google Scholar]
- Bella L., Methven L., Wagstaff C. (2017). The influence of phytochemical composition and resulting sensory attributes on preference for salad rocket (Eruca sativa) accessions by consumers of varying TAS2R38 diplotype. Food Chem. 222, 6–17. 10.1016/j.foodchem.2016.11.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bering A. B., Pickering G., Liang P. (2014). TAS2R38 single nucleotide polymorphisms are associated with PROP—but Not thermal—tasting: a pilot study. Chem. Percept. 7, 23. 10.1007/s12078-013-9160-1 [DOI] [Google Scholar]
- Birch L. (1979). Preschool children’s food preferences and consumption patterns. J. Nutr. Educ. 11 (4), 189–192. 10.1016/S0022-3182(79)80025-4 [DOI] [Google Scholar]
- Birch L. (1999). Development of food preferences. Annu. Rev. Nutr. 19, 41–62. 10.1146/annurev.nutr.19.1.41 [DOI] [PubMed] [Google Scholar]
- Breen F. M., Plomin R., Wardle J. (2006). Heritability of food preferences in young children. Physiol. Behav. 88 (4-5), 443–447. 10.1016/j.physbeh.2006.04.016 [DOI] [PubMed] [Google Scholar]
- Bufe B., Breslin P. A. S., Kuhn C., Reed D. R., Tharp C. D., Slack J. P., et al. (2005). The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 15 (4), 322–327. 10.1016/j.cub.2005.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero B., Popkin B. M. (2002). “Biological Factors Affecting the Nutrition Transition,” in The Nutrition Transition: Diet and Disease in the Developing World. Ed. Taylor S. L. (Elsevier Science: Food Science and Technology - International series; ), 147–191. [Google Scholar]
- Cabras T., Melis M., Castagnola M., Padiglia A., Tepper B. J., Messana I., et al. (2012). Responsiveness to 6-n-propylthiouracil (PROP) is associated with salivary levels of two specific basic proline-rich proteins in humans. PloS One 7 (2), 3–7. 10.1371/journal.pone.0030962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calò C, P. A., Zonza A., Corrias L., Contu P., Tepper B. J., Barbarossa I. T. (2011). Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiol. Behav. 5 (104), 1065–1071. 10.1016/j.physbeh.2011.06.013 [DOI] [PubMed] [Google Scholar]
- Campbell M. C., Ranciaro A., Froment A., Hirbo J., Omar S., Bodo J. M., et al. (2012). Evolution of functionally diverse alleles associated with PTC bitter taste sensitivity in Africa. Mol. Biol. Evol. 29 (4), 1141–1153. 10.1093/molbev/msr293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrai M., Campa D., Vodicka P., Flamini R., Martelli I., Slyskova J., et al. (2017). Association between taste receptor (TAS) genes and the perception of wine characteristics. Sci. Rep. 7 (1), 9239. 10.1038/s41598-017-08946-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun E., Mutch D. M., Allen-Vercoe E., Buchholz A. C., Duncan A. M., Spriet L. L., et al. (2018). A review of the associations between single nucleotide polymorphisms in taste receptors, eating behaviors, and health. Crit. Rev. Food Sci. Nutr. 58 (2), 194–207. 10.1080/10408398.2016.1152229 [DOI] [PubMed] [Google Scholar]
- Chandrashekar J., Mueller K. L., Hoon M. A., Adler E., Feng L., Guo W., et al. (2000). T2Rs function as bitter taste receptors. Cell 100 (6), 703–711. 10.1016/s0092-8674(00)80706-0 [DOI] [PubMed] [Google Scholar]
- Chaudhari N., Roper S. D. (2010). The cell biology of taste. J. Cell Biol. 190 (3), 285–296. 10.1083/jcb.201003144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. Y., Alarcon S., Tharp A., Ahmed O. M., Estrella N. L., Greene T. A., et al. (2009). Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am. J. Clin. Nutr. 90 (3), 770S–779S. 10.3945/ajcn.2009.27462N [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colares-Bento F. C., Souza V. C., Toledo J. O., Moraes C. F., Alho C. S., et al. (2012). Implication of the G145C polymorphism (rs713598) of the TAS2r38 gene on food consumption by Brazilian older women. Arch. Gerontol. Geriatr. 54 (2), e13–e18. 10.1016/j.archger.2011.05.019 [DOI] [PubMed] [Google Scholar]
- Connors M., Bisogni C. A., Sobal J., Devine C. M. (2001). Managing values in personal food systems. Appetite 36, 189–200. 10.1006/appe.2001.0400 [DOI] [PubMed] [Google Scholar]
- Conte C., Ebeling M., Marcuz A., Nef P., Andres-Barquin P. J. (2002). Identification and characterization of human taste receptor genes belonging to the TAS2R family. Cytogenet. Genome Res. 98 (1), 45–53. 10.1159/000068546 [DOI] [PubMed] [Google Scholar]
- Corella D., Arnett D. K., Tsai M. Y., Kabagambe E. K., Peacock J. M., Hixson J. E., et al. (2007). The 256TC polymorphism in the apolipoprotein A-II gene promoter is associated with body mass index and food intake in the genetics of Lipid Lowering Drugs and Diet Network Study. Clin. Chem. 53 (6), 1144–1152. 10.1373/clinchem.2006.084863 [DOI] [PubMed] [Google Scholar]
- Cravotto G., Nano G. M., Binello A., Spagliardi P., Seu G. (2005). Chemical and biological modification of cynaropicrin and grosheimin: a structure–bitterness relationship study. J. Sci. Food Agric. 85 (10), 1757–1764. 10.1002/jsfa.2180 [DOI] [Google Scholar]
- Daoudi H., Plesnik J., Sayed A., Sery O., Rouabah A., et al. (2015). Oral fat sensing and CD36 gene polymorphism in Algerian lean and obese teenagers. Nutrients 7, 11, 9096–9104. 10.3390/nu7115455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C., Zai C., Levitan R., Kaplan A., Carter J., Reid-Westoby C., et al. (2011). Opiates, overeating and obesity: a psychogenetic analysis. Int. J. Obes. 35 (10), 1347–1354. 10.1038/ijo.2010.276 [DOI] [PubMed] [Google Scholar]
- Deshaware S., Singhal R. (2017). Genetic variation in bitter taste receptor gene TAS2R38, PROP taster status and their association with body mass index and food preferences in Indian population. Gene 627, 363–368. 10.1016/j.gene.2017.06.047 [DOI] [PubMed] [Google Scholar]
- Dias A. G., Rousseau D., Duizer L., Cockburn M., Chiu W., Nielsen D., et al. (2013). Genetic variation in putative salt taste receptors and salt taste perception in humans. Chem. Senses 38 (2), 137–145. 10.1093/chemse/bjs090 [DOI] [PubMed] [Google Scholar]
- Dias A. G., Eny K. M., Cockburn M., Chiu W., Nielsen D. E., Duizer L., et al. (2015). Variation in the TAS1R2 Gene, Sweet Taste Perception and Intake of sugars. J. Nutrigenetics Nutrigenomics 8 (2), 81–90. 10.1159/000430886 [DOI] [PubMed] [Google Scholar]
- Dinehart M. E., Hayes J. E., Bartoshuk L. M., Lanier S. L., Duffy V. B. (2006). Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol. Behav. 87 (2), 304–313. 10.1016/j.physbeh.2005.10.018 [DOI] [PubMed] [Google Scholar]
- Dotson C. D., Babich J., Steinle N. I. (2012). Genetic predisposition and taste preference: Impact on food intake and risk of chronic disease. Curr. Nutr. Rep. 1 (3), 175–183. 10.1007/s13668-012-0021-3 [DOI] [Google Scholar]
- Drewnowski A, H. S., Shore A. B., Barratt-Fornell A. (1998. a). Sensory responses to 6-n-propylthiouracil (PROP) or sucrose solutions and food preferences in young women. Ann. NY Acad. Sci. 855, 797–801. [DOI] [PubMed] [Google Scholar]
- Drewnowski A., Henderson S. A., Shore A. B. (1998. b). Genetic sensitivity to 6-n-propylthiouracil and sensory responses to sugar and fat mixtures. Physiol. Behav. 63, 771–777. 10.1016/S0031-9384(97)00540-4 [DOI] [PubMed] [Google Scholar]
- Drewnowski A., Henderson S. A, and Barratt-Fornell A. (1997). Genetic sensitivity to 6-n-propylthiouracil (PROP) and hedonic responses to bitter and sweet tastes. Chem. Senses 22, 27–37. 10.1093/chemse/22.1.27 [DOI] [PubMed] [Google Scholar]
- Drewnowski A., Henderson S. A., Levine A., Hann C. (1999). Taste and food preferences as predictors of dietary practices in young women. Public Health Nutr. 2 (4), 513–519. 10.1017/s1368980099000695 [DOI] [PubMed] [Google Scholar]
- Drewnowski A., Henderson S. A., Hann C. S., Berg W. A., Ruffin M. T. (2000). Genetic taste markers and preferences for vegetables and fruit of female breast care patients. J. Am. Diet Assoc. 100 (2), 191–197. 10.1016/S0002-8223(00)00061-4 [DOI] [PubMed] [Google Scholar]
- Drewnowski A., Henderson S. A., Cockroft J. E. (2007). Genetic sensitivity to 6-n-propylthiouracil has no influence on dietary patterns, body mass indexes, or plasma lipid profiles of women. J. Am. Diet Assoc. 107 (8), 1340–1348. 10.1016/j.jada.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Duffy V. B., Davidson A. C., Kidd J. R., Kidd K. K., Speed W. C., Pakstis A. J., et al. (2004. a). Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin. Exp. Res. 28 (11), 1629–1637. 10.1097/01.ALC.0000145789.55183.D4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy V., Lucchina L., Bartoshuk L. (2004. b). “Genetic variation in taste: Potential biomarker for cardiovascular disease risk?” in Genetic Variations in Taste Sensitivity. Eds. Prescott J. Tepper B. J.(New York, NY, USA: Marcel Dekker Inc.), 195–228. [Google Scholar]
- Duffy V. B., Hayes J. E., Sullivan B. S., Faghri P. (2009). Surveying food and beverage liking: A tool for epidemiological studies to connect chemosensation with health outcomes. Ann. NY Acad. Sci. 1170, 558–568. 10.1111/j.1749-6632.2009.04593.x [DOI] [PubMed] [Google Scholar]
- Duffy V. B., Hayes J. E., Davidson A. C., Kidd J. R., Kidd K. K., Bartoshuk L. M. (2010). Vegetable intake in college-aged adults is explained by oral sensory phenotypes and TAS2R38 genotype. Chemosens. Percept. 3 (3-4), 137–148. 10.1007/s12078-010-9079-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy V. B. (2000). BLM. Food Acceptance Genet. Var. In Taste Am. Diet Assoc. 100, 647–655. 10.1016/S0002-8223(00)00191-7 [DOI] [PubMed] [Google Scholar]
- Ebba S., Abarintos R. A., Kim D. G., Tiyouh M., Stull J. C., Movalia A., et al. (2012). The examination of fatty acid taste with edible strips. Physiol. Behav. 106, 579–586. 10.1016/j.physbeh.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbers C., de Kovel C., van der Schouw Y., Meijboom J., Bauer F., Grobbee D. E., et al. (2009). Variants in neuropeptide Y receptor 1 and 5 are associated with nutrient-specific food intake and are under recent selection in Europeans. PloS One, 4 (9). 10.1371/journal.pone.0007070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond J. A., Longacre M., Drake K. M., Titus L. J., Hendricks K., MacKenzie T., et al. (2019). Exposure to child-directed TV advertising and preschoolers’ intake of advertised cereals. Am. J. Prev. Med. 56, e35–e43. 10.1016/j.amepre.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eny K. M., Wolever T. M., Corey P. N., El-Sohemy A. (2010). Genetic variation in TAS1R2 (Ile191Val) is associated with consumption of sugars in overweight and obese individuals in 2 distinct populations. Am. J. Clin. Nutr. 92 (6), 1501–1510. 10.3945/ajcn.2010.29836 [DOI] [PubMed] [Google Scholar]
- Farquhar W., Edwards D., Jurkovitz C., Weintraub W. (2015). Dietary sodium and health: more than just blood pressure. J. Am. Coll. Cardiol. 65, 1042–1050. 10.1016/j.jacc.2014.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney E. L., Hayes J. (2014). Exploring associations between taste perception, oral anatomy and polymorphisms in the carbonic anhydrase (gustin) gene CA6. Physiol. Behav. 128, 148–154. 10.1016/j.physbeh.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney E., O’Brien S., Scannell A., Markey A., Gibney E. R. (2011). Genetic variation in taste perception: does it have a role in healthy eating? Proc. Nutr. Soc. 70 (1), 135–143. 10.1017/s0029665110003976 [DOI] [PubMed] [Google Scholar]
- Feeney E. L., O’Brien S. A., Scannell A. G., Markey A., Gibney E. R. (2014). Genetic and environmental influences on liking and reported intakes of vegetables in Irish children. Food Qual. Prefer 32, 253–263. 10.1016/j.foodqual.2013.09.009 [DOI] [Google Scholar]
- Feeney E., O’Brien S., Scannell A., Markey A., Gibney E. (2017). Suprathreshold measures of taste perception in children - Association with dietary quality and body weight. Appetite 113, 116–123. 10.1016/j.appet.2017.02.026 [DOI] [PubMed] [Google Scholar]
- Fildes A., van Jaarsveld C. H., Llewellyn C. H., Fisher A., Cooke L., Wardle J. (2014). Nature and nurture in children’s food preferences. Am. J. Clin. Nutr. 99, 911–917. 10.3945/ajcn.113.077867 [DOI] [PubMed] [Google Scholar]
- Fisher J. O., Birch L. L. (1995). Fat preferences and fat consumption of 3- to 5-year-old children are related to parental adiposity. J. Am. Diet Assoc. 95, 759–764. 10.1016/S0002-8223(95)00212-X [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations/World Health Organization (2010). Fats and fatty acids in human nutrition. Report of an expert consultation. Rome, Italy: Food and Agriculture Organization of the United Nations, ISBN 978-92-5-106733-8.
- Ford N. D., Patel S. A., Narayan K. M. V. (2017). Obesity in low- and middle-income countries: burden, drivers, and emerging challenges. Annu. Rev. Public Health 38 (1), 145–164. 10.1146/annurev-publhealth-031816-044604 [DOI] [PubMed] [Google Scholar]
- Forouhi N. G., Krauss R. M., Taubes G., Willett W. (2018). Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ 361, k2139. 10.1136/bmj.k2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrai G., Bánkövi G. (1994). Taste perception for phenylthiocarbamide and food choice—A Hungarian twin study. Acta Physiol. Hung. 64, 33–40. [PubMed] [Google Scholar]
- Fox A. L. (1932). The relationship between chemical constitution and taste. Proc. Natl. Acad. Sci. U.S.A. 18, 115–120. 10.1073/pnas.18.1.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuwatar T., Kawad T., Tsuruta M., Hiraoka T., Iwanaga T., Sugimoto E., et al. (1997). Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 414 (2), 461–464. 10.1016/S0014-5793(97)01055-7 [DOI] [PubMed] [Google Scholar]
- Fushan A. A., Simons C. T., Slack J. P., Manichaikul A., Drayna D. (2009). Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr. Biol. 19 (15), 1288–1293. 10.1016/j.cub.2009.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bailo B., Toguri C., Eny K. M., El-Sohemy A. (2008). Genetic variation in taste and its influence on food selection. OMICS 13 (1), 69–80. 10.1089/omi.2008.0031 [DOI] [PubMed] [Google Scholar]
- Garneau N. L., Nuessle T. M., Sloan M. M., Santorico S. A., Coughlin B. C., Hayes J. E. (2014). Crowdsourcing taste research: genetic and phenotypic predictors of bitter taste perception as a model. Front. Integr. Neurosci. 8 (33), 2–6. 10.3389/fnint.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayathri Devi A., Henderson S., Drewnowski A. (1997). Sensory acceptance of Japanese green tea and soy products is linked to genetic sensitivity to 6-n-propylthiouracil. Nutr. Cancer 29 (2), 146–151. 10.1080/01635589709514616 [DOI] [PubMed] [Google Scholar]
- Gent J., Bartoshuk L. M. (1983). Sweetness of sucrose, neohesperidin dihydrochalcone, and saccharin is related to genetic ability to taste the bitter substance 6-n-propylthiouracil 7, 265–272. Chem. Senses. 10.1093/chemse/7.3-4.265 [DOI]
- Gibson E. L., Cooke L. (2017). Understanding food fussiness and its implications for food choice, health, weight and interventions in young children: The impact of professor Jane Wardle. Curr. Obes. Rep. 6, 46–56. 10.1007/s13679-017-0248-9 [DOI] [PubMed] [Google Scholar]
- Glanz K., Basil M., Maibach E., Goldberg J., Snyder D. (1998). Why Americans eat what they do: taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J. Am. Diet Assoc. 98, 1118–1126. 10.1016/S0002-8223(98)00260-0 [DOI] [PubMed] [Google Scholar]
- Glendinning J. I., Bloom L. D., Onishi M., Zheng K. H., Damak S., Margolskee R. F., et al. (2005). Contribution of alpha-gustducin to taste-guided licking responses of mice. Chem. Senses 30 (4), 299–316. 10.1093/chemse/bji025 [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Risk Factors Collaborators. (2018). Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1923–1994. 10.1016/S0140-6736(18)32225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovic N, A. S., Tjønneland A., Overvad K., Vogel U., Albrechtsen C., Poulsen H. E. (2011). Genetic variation in the hTAS2R38 taste receptor and brassica vegetable intake. Scand. J. Clin. Lab. Invest. 71, 274–279. 10.3109/00365513.2011.559553 [DOI] [PubMed] [Google Scholar]
- Green B. G., Hayes J. E. (2004). Individual differences in perception of bitterness from capsaicin, piperine and zingerone. Chem. Senses 29 (1), 53–60. 10.1093/chemse/bjh005 [DOI] [PubMed] [Google Scholar]
- Grimm E. R., Steinle N. I. (2011). Genetics of eating behavior: established and emerging concepts. Nutr. Rev. 69 (1), 52–60. 10.1111/j.1753-4887.2010.00361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S. W., Reed D. R. (2001). The genetics of phenylthiocarbamide perception. Ann. Hum. Biol. 28 (2), 111–142. 10.1080/03014460151056310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajri T., Abumrad N. A. (2002). Fatty acid transport accross membranes: relevance to nutrition and metabolic pathology. Ann. Rev. Nutr. 22 (1), 383–415. 10.1146/annurev.nutr.22.020402.130846 [DOI] [PubMed] [Google Scholar]
- Han P., Keast R. S. J., Roura E. (2017). Salivary leptin and TAS1R2/TAS1R3 polymorphisms are related to sweet taste sensitivity and carbohydrate intake from a buffet meal in healthy young adults. Br. J. Nutr. 118 (10), 763–770. 10.1017/s0007114517002872 [DOI] [PubMed] [Google Scholar]
- Hanschen F. S., Lamy E., Schreiner M., Rohn S. (2014). Reactivity and stability of glucosinolates and their breakdown products in foods. Angew. Chem. Int. Ed Engl. 53 (43), 11430–11450. 10.1002/anie.201402639 [DOI] [PubMed] [Google Scholar]
- Hansen J. L., Reed D. R., Wright M. J., Martin N. G., Breslin P. A. (2006). Heritability and genetic covariation of sensitivity to PROP, SOA, quinine HCl, and caffeine. Chem. Senses 31 (5), 403–413. 10.1093/chemse/bjj044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H, K. H. (1949). Chemical specificity in genetical differences of taste sensitivity. Ann. Eugenics London 15 (1), 432–459. 10.1111/j.1469-1809.1949.tb02420.x [DOI] [PubMed] [Google Scholar]
- Hayes J. E., Duffy V. B. (2007). Revisiting sugar-fat mixtures: Sweetness and creaminess vary with phenotypic markers of oral sensation. Chem. Senses 32, 225–236. 10.1093/chemse/bjl050 [DOI] [PubMed] [Google Scholar]
- Hayes J. E., Duffy V. B. (2008). Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Phys. Behav. 95 (1-2), 77–87. 10.1016/j.physbeh.2008.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. E., Bartoshuk L. M., Kidd J. R., Duffy V. B. (2008). Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem. Senses 33 (3), 255–265. 10.1093/chemse/bjm084 [DOI] [PubMed] [Google Scholar]
- Hayes J. E., Sullivan B. S., Duffy V. B. (2010). Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiol. Behav. 100 (4), 369–380. 10.1016/j.physbeh.2010.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. E., Wallace M. R., Knopik V. S., Herbstman D. M., Bartoshuk L. M., et al. (2011). Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chem. Senses 36 (3), 311–319. 10.1093/chemse/bjq132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. E., Feeney E. L., Allen A. L. (2013). Do polymorphisms in chemosensory genes matter for human ingestive behavior? Food Qual. Pref 30 (2), 202–216. 10.1016/j.foodqual.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. E., Feeney E. L., Nolden A. A., McGeary J. E. (2015). Quinine bitterness and grapefruit liking associate with allelic variants in TAS2R31. Chem. Senses 40 (6), 437–443. 10.1093/chemse/bjv027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck G. L., Mierson S., DeSimone J. A. (1984). Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Sci. (New York NY) 223 (4634), 403–405. 10.1126/science.6691151 [DOI] [PubMed] [Google Scholar]
- Henkin R. I., Lippoldt R. E., Bilstad J., Edelhoch H. (1975). A zinc protein isolated from human parotid saliva. Proc. Natl. Acad. Sci. U.S.A. 72 (2), 488. 10.1073/pnas.72.2.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin R. I., Martin B. M., Agarwal R. P. (1999). Efficacy of exogenous oral zinc in treatment of patients with carbonic anhydrase VI deficiency. Am. J. Med. Sci. 318 (6), 392–405. 10.1016/S0002-9629(15)40664-0 [DOI] [PubMed] [Google Scholar]
- Hladik C. M., Simmen B. (1996). Taste perception and feeding behavior in nonhuman primates and human populations. Evol. Anthropol. 5 (2), 58–71. [DOI] [Google Scholar]
- Hochheimer A., Krohn M., Rudert K., Riedel K., Becker S., Thirion C., et al. (2014). Endogenous gustatory responses and gene expression profile of stably proliferating human taste cells isolated from fungiform papillae. Chem. Senses 39 (4), 359–377. 10.1093/chemse/bju009 [DOI] [PubMed] [Google Scholar]
- Hoffman A. C., Salgado R. V., Dresler C., Faller R. W., Bartlett C. (2016). Flavour preferences in youth versus adults: a review. Tob Control 252, ii32. 10.1136/tobaccocontrol-2016-053192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. H. A., Cobiac L., Beaumont-Smith N. E., Easton K., Best D. J. (2000). Dietary habits and the perception and liking of sweetness among Australian and Malaysian students: A cross-cultural study. Food Qual. Prefer 11, 299–312. 10.1016/S0950-3293(99)00076-2 [DOI] [Google Scholar]
- Honkanen P., Frewer L. (2009). Russian consumers’ motives for food choice. Appetite 52, 363–371. 10.1016/j.appet.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Huang A. L., Chen X., Hoon M. A., Chandrashekar J., Guo W., Tränkner D., et al. (2006). The cells and logic for mammalian sour taste detection. Nature 442 (7105), 934–938. 10.1038/nature05084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huque T., Cowart B. J., Dankulich-Nagrudny L., Pribitkin E. A., Bayley D. L., Spielman A. I., et al. (2009). Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PloS One 4, 10, e7347. 10.1371/journal.pone.0007347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang L. D., Zhu G., Breslin P. A., Reed D. R., Martin N. G., Wright M. J. (2015). A common genetic influence on human intensity ratings of sugars and high-potency sweeteners. Twin Res. Hum. Genet. 18, 361–367. 10.1017/thg.2015.42 [DOI] [PubMed] [Google Scholar]
- Inoue H., Yamakawa-Kobayashi K., Suzuki Y., Nakano T., Hayashi H., et al. (2013). A case study on the association of variation of bitter-taste receptor gene TAS2R38 with the height, weight and energy intake in Japanese female college students. J. Nutr. Sci. Vitaminol. 59 (1), 16–21. 10.3177/jnsv.59.16 [DOI] [PubMed] [Google Scholar]
- Ishimaru Y., Abe M., Asakura T., Imai H., Abe K. (2012). Expression analysis of taste signal transduction molecules in the fungiform and circumvallate papillae of the rhesus macaque, Macaca mulatta. PloS One 7 (9), e45426. 10.1371/journal.pone.0045426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, I. H., Kubota M., Zhuang H., Tominaga M., Matsunami H. (2016). Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. U.S.A. 103 (33), 12569–12574. 10.1073/pnas.0602702103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabłoński M., Jasiewicz A., Kucharska-Mazur J., Samochowiec J., Bienkowski P., Mierzejewski P., et al. (2013). The effect of selected polymorphisms of the dopamine receptor gene DRD2 and the ANKK-1 on the preference of concentrations of sucrose solutions in men with alcohol dependence. Psychiatr. Danub 25 (4), 0–378. [PubMed] [Google Scholar]
- Joseph P. V., Reed D. R., Mennella J. A. (2016). Individual differences among children in sucrose detection thresholds: Relationship with age, gender, and bitter taste genotype. Nurs. Res. 65 (1), 3–12. 10.1097/nnr.0000000000000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmus H. (1950). Taste reactions to antithyroid substances. Sci. (New York NY) 112 (2912), 475. 10.1126/science.112.2912.475 [DOI] [PubMed] [Google Scholar]
- Kaminski L. C., Henderson S. A., Drewnowski A. (2000). Young women’s food preferences and taste responsiveness to 6-n-propylthiouracil (PROP). Phys. Behav. 68 (5), 691–697. 10.1016/S0031-9384(99)00240-1 [DOI] [PubMed] [Google Scholar]
- Kearney M., Kearney J. M., Dunne A., Gibney M. J. (2000). Sociodemographic determinants of perceived influences on food choice in a nationally representative sample of Irish adults. Public Health Nutr. 3 (2), 219–226. 10.1017/S1368980000000252 [DOI] [PubMed] [Google Scholar]
- Keil M., Härtle B., Guillaume A., Psiorz M. (2000). Production of amarogentin in root cultures of Swertia chirata. Planta Med. 66 (05), 452–457. 10.1055/s-2000-8579 [DOI] [PubMed] [Google Scholar]
- Keller K. L., Adise S. (2016). Variation in the ability to taste bitter thiourea compounds: implications for food acceptance, dietary intake, and obesity risk in children. Ann. Rev. Nutr. 36 (1), 157–182. 10.1146/annurev-nutr-071715-050916 [DOI] [PubMed] [Google Scholar]
- Keller K. L., Steinmann L., Nurse R. J., Tepper B. J. (2002). Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite 38 (1), 3–12. 10.1006/appe.2001.0441 [DOI] [PubMed] [Google Scholar]
- Keller K., Liang L., Sakimura J., May D., van Belle C., Breen C., et al. (2012). Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity 20 (5), 1066–1073. 10.1038/oby.2011.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller K. L., Olsen A., Cravener T. L., Bloom R., Chung W. K., Deng L., et al. (2014). Bitter taste phenotype and body weight predict children’s selection of sweet and savory foods at a palatable test-meal. Appetite 77, 113–121. 10.1016/j.appet.2014.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U. K., Drayna D. (2005). Genetics of individual differences in bitter taste perception: lessons from the PTC gene. Clin. Genet. 67, 275–280. 10.1111/j.1399-0004.2004.00361.x [DOI] [PubMed] [Google Scholar]
- Kim U., Jorgenson E., Coon H., Leppert M., Risch N., et al. (2003). Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Sci. (New York NY) 299 (5610), 1221–1225. 10.1126/science.1080190 [DOI] [PubMed] [Google Scholar]
- Kim U., Breslin P., Reed D., Drayna D. (2004). Genetics of human taste perception. J. Dent. Res. 83 (6), 448–453. 10.1177/154405910408300603 [DOI] [PubMed] [Google Scholar]
- Kim U. K., Wooding S., Riaz N., Jorde L. B., Drayna D. (2006). Variation in the human TAS1R taste receptor genes. Chem. Senses 31, 599–611. 10.1093/chemse/bjj065 [DOI] [PubMed] [Google Scholar]
- Kirkmeyer S. V., Tepper B. J. (2003). Understanding creaminess perception of dairy products using free-choice profiling and genetic responsivity to 6-n-propylthiouracil. Chem. Senses 28 (6), 527–536. 10.1093/chemse/28.6.527 [DOI] [PubMed] [Google Scholar]
- Knaapila A., Hwang L. D., Lysenko A., Duke F. F., Fesi B., et al. (2012). Genetic analysis of chemosensory traits in human twins. Chem. Senses 37 (9), 869–881. 10.1093/chemse/bjs070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y., Mi E., Sook I., Sook H. (2015). A study of total sugar intake by middle school students in Jeju Province. J. Nutr. Health 48, 248–257. 10.4163/jnh.2015.48.3.248 [DOI] [Google Scholar]
- Kourouniotis S., Keast R. S. J., Riddell L. J., Lacy K., Thorpe M. G., Cicerale S. (2016). The importance of taste on dietary choice, behaviour and intake in a group of young adults. Appetite 103, 1–7. 10.1016/j.appet.2016.03.015 [DOI] [PubMed] [Google Scholar]
- Kuhn C., Bufe B., Winnig M., Hofmann T., Frank O., Behrens M., et al. (2004). Bitter taste receptors for saccharin and Acesulfame K. J. Neurosci. 24 (45), 10260. 10.1523/JNEUROSCI.1225-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksonen O., Ahola J., Sandell M. (2013). Explaining and predicting individually experienced liking of berry fractions by the hTAS2R38 taste receptor genotype. Appetite 61 (1), 85–96. 10.1016/j.appet.2012.10.023 [DOI] [PubMed] [Google Scholar]
- Lachat C., Otchere S., Roberfroid D., Abdulai A., Seret F. M. A., Milesevic J., et al. (2013). Diet and physical activity for the prevention of noncommunicable diseases in low- and middle-income countries: a systematic policy review. PloS Med. 10 (6), e1001465. 10.1371/journal.pmed.1001465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier S. A., Hayes J. E., Duffy V. B. (2005). Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Phys. Behav. 83 (5), 821–831. 10.1016/j.physbeh.2004.10.004 [DOI] [PubMed] [Google Scholar]
- Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., et al. (2005). CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115 (11), 3177–3184. 10.1172/JCI25299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda M., Kutalik Z., Destito M., Souza M., Cirillo C., Zamboni A., et al. (2014). GWAS of human bitter taste perception identifies new loci and reveals additional complexity of bitter taste genetics. Hum. Mol. Genet. 23 (1), 259–267. 10.1093/hmg/ddt404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Staszewski L., Xu H., Durick K., Zoller M., Adler E. (2002). Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. U.S.A. 99, 4692–4696. 10.1073/pnas.072090199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J., Schultz P. G. (2003). Three sweet receptor genes are clustered in human chromosome 1. Mamm. Genome 14 (5), 291–301. 10.1007/s00335-002-2233-0 [DOI] [PubMed] [Google Scholar]
- Lin W., Finger T. E., Rossier B. C., Kinnamon S. C. (1999). Epithelial Na+ channel subunits in rat taste cells: Localization and regulation by aldosterone. J. Comp. Neurol. 405 (3), 406–420. [DOI] [PubMed] [Google Scholar]
- Lipchock S. V., Reed D. R., Mennella J. A. (2012). Relationship between bitter-taste receptor genotype and solid medication formulation usage among young children: a retrospective analysis. Clin. Ther. 34 (3), 728–733. 10.1016/j.clinthera.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looy H., Callaghan S., Weingarten H. P. (1992). Hedonic response of sucrose likers and dislikers to other gustatory stimuli. Physiol. Behav. 52, 219–225. 10.1016/0031-9384(92)90261-y [DOI] [PubMed] [Google Scholar]
- Lucock M., Ng X., Boyd L., Skinner V., Wai R., Tang S., et al. (2011). TAS2R38 bitter taste genetics, dietary vitamin C, and both natural and synthetic dietary folic acid predict folate status, a key micronutrient in the pathoaetiology of adenomatous polyps. Food Funct. 2 (8), 457–465. 10.1039/c1fo10054h [DOI] [PubMed] [Google Scholar]
- Lyall V., Heck G. L., Vinnikova A. K., Ghosh S., Phan T. H., Alam R. I., et al. (2004). The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J. Physiol. 558 (Pt 1), 147–159. 10.1113/jphysiol.2004.065656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga J. A. (1990). “Compound structure versus bitter taste,” in Bitterness in foods and beverages; developments in food science. Ed. Rouseff R. L.(Amsterdam, Netherlands: Elsevier; ), 35–48. [Google Scholar]
- Mahar A., Duizer M. (2007). The effect of frequency of consumption of artificial sweeteners on sweetness liking by women. J. Food Sci. 72, S714–S7S8. 10.1111/j.1750-3841.2007.00573.x [DOI] [PubMed] [Google Scholar]
- Malles R. D. (2010). “Fat Taste in Humans: Is It a Primary?” in Fat Detection: Taste, Texture, and Post Ingestive Effects. Eds. Montmayeur J. P. le Coutre J.(Boca Raton, FL: CRC Press/Taylor & Francis; ), 167–193. [PubMed] [Google Scholar]
- McBurney D. (1990). “Physiological and therapeutical aspects of bitter compounds,” in Bitterness in foods and beverages; developments in food science. Ed. Rouseff R. L.(Amsterdam, Netherlands: Elsevier; ), 15–34. [Google Scholar]
- Mela D. (1988). Sensory assessment of fat content in fluid dairy products. Appetite 10, 37–44. 10.1016/s0195-6663(88)80031-x [DOI] [PubMed] [Google Scholar]
- Mela D. J. (2001). “Development and Acquisition of Food Likes,” in Food, People and Society. Eds. Frewer L. J., Risvik E., Schifferstein H.(Berlin, Heidelberg: Springer; ). [Google Scholar]
- Melis M., Atzori E., Cabras S., Zonza A., Calo C., Muroni P., et al. (2013). The gustin (CA6) gene polymorphism, rs2274333 (A/G), as a mechanistic link between PROP tasting and fungiform taste papilla density and maintenance. PloS One 8 (9), e74151. 10.1371/journal.pone.0074151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M., Sollai G., Muroni P., Crnjar R., Barbarossa I. T. (2015). Associations between orosensory perception of oleic acid, the common single nucleotide polymorphisms (rs1761667 and rs1527483) in the CD36 gene, and 6-n-propylthiouracil (PROP) tasting. Nutrients 7 (3), 2068–2084. 10.3390/nu7032068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Pepino M. Y., Reed D. R. (2005). Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 115 (2), e216. 10.1542/peds.2004-1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Pepino M. Y., Duke F. F., Reed D. R. (2011. a). Psychophysical dissection of genotype effects on human bitter perception. Chem. Senses 36 (2), 161–167. 10.1093/chemse/bjq106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Lukasewycz L. D., Griffith J. W., Beauchamp G. K. (2011. b). Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chem. Senses 36, 345–355. 10.1093/chemse/bjq134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Reed D. R., Roberts K. M., Mathew P. S., Mansfield C. J. (2014. a). Age-related differences in bitter taste and efficacy of bitter blockers. PloS One 9 (7), e103107. 10.1371/journal.pone.0103107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella J. A., Finkbeiner S., Lipchock S. V., Hwang L. D., Reed D. R. (2014. b). Preferences for salty and sweet tastes are elevated and related to each other during childhood. PloS One 9 (3), e92201. 10.1371/journal.pone.0092201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhof W., Batram C., Kuhn C., Brockhoff A., Chudoba E., Bufe B., et al. (2009). The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 35 (2), 157–170. 10.1093/chemse/bjp092 [DOI] [PubMed] [Google Scholar]
- Mizuta E., Kokubo Y., Yamanaka I., Miyamoto Y., Okayama A., Yoshimasa Y., et al. (2008). Leptin gene and leptin receptor gene polymorphisms are associated with sweet preference and obesity. Hypertens. Res. 31 (6), 1069–1077. 10.1291/hypres.31.1069 [DOI] [PubMed] [Google Scholar]
- Mrizak I., Sery O., Plesnik J., Arfa A., Fekih M., Bouslema A., et al. (2015). The A allele of cluster of differentiation 36 (CD36) SNP 1761667 associates with decreased lipid taste perception in obese Tunisian women. Br. J. Nutr. 113 (8), 1330–1337. 10.1017/s0007114515000343 [DOI] [PubMed] [Google Scholar]
- Muhlhausler B. S., Gugushef J. R., Langley-Evans S. (2017). “Maternal junk food diets: The effects on offspring fat mass and food preferences,” in Diet, Nutrition, and Fetal Programming Nutrition and Health. Eds. Rajendram R., Preedy V., Patel V.(Cham: Humana Press; ), 227–238. [Google Scholar]
- Negri R., Di Feola M., Di Domenico S., Scala M. G., Artesi G., Valente S., et al. (2012). Taste perception and food choices. J. Pediatr. Gastroenterol. Nutr. 54 (5), 624–629. 10.1097/MPG.0b013e3182473308 [DOI] [PubMed] [Google Scholar]
- Nelson G., Hoon M. A., Chandrashekar J., Zhang Y., Ryba N. J., Zuker C. S. (2001). Mammalian sweet taste receptors. Cell 106 (3), 381–390. 10.1016/s0092-8674(01)00451-2 [DOI] [PubMed] [Google Scholar]
- Nolden A. A., McGeary J. E., Hayes J. E. (2016). Differential bitterness in capsaicin, piperine, and ethanol associates with polymorphisms in multiple bitter taste receptor genes. Physiol. Behav. 156, 117–127. 10.1016/j.physbeh.2016.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong H. H., Tan Y. N., Say Y. H. (2017). Fatty acid translocase gene CD36 rs1527483 variant influences oral fat perception in Malaysian subjects. Physiol. Behav. 168, 128–137. 10.1016/j.physbeh.2016.11.006 [DOI] [PubMed] [Google Scholar]
- Ooi S. X., Lee P. L., Law H. Y., Say Y. H. (2010). Bitter receptor gene (TAS2R38) P49A genotypes and their associations with aversion to vegetables and sweet/fat foods in Malaysian subjects. Asia Pac J. Clin. Nutr. 19 (4), 491–498. [PubMed] [Google Scholar]
- Pérusse L., Tremblay A., Leblanc C., Cloninger C. R., Reich T., et al. (1988). Familial resemblance in energy intake: contribution of genetic and environmental factors. Am. J. Clin. Nutr. 47 (4), 629–635. 10.1093/ajcn/47.4.629 [DOI] [PubMed] [Google Scholar]
- Padiglia A., Zonza A., Atzori E., Chillotti C., Calo C., Tepper B. J., et al. (2010). Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am. J. Clin. Nutr. 92 (3), 539–545. 10.3945/ajcn.2010.29418 [DOI] [PubMed] [Google Scholar]
- Pawellek I., Grote V., Rzehak P., Xhonneux A., Verduci E., Stolarczyk A., et al. (2016). Association of TAS2R38 variants with sweet food intake in children aged 1-6 years. Appetite 107, 126–134. 10.1016/j.appet.2016.07.034 [DOI] [PubMed] [Google Scholar]
- Pepino M. Y., Love-Gregory L., Klein S., Abumrad N. A. (2012). The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 53 (3), 561–566. 10.1194/jlr.M021873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna S., Riva A., Nicosanti G., Carrai M., Barale R., Vigo B., et al. (2018). Association of the bitter taste receptor gene TAS2R38 (polymorphism RS713598) with sensory responsiveness, food preferences, biochemical parameters and body-composition markers. A cross-sectional study in Italy. Int. J. Food Sci. Nut 69 (2), 245–252. 10.1080/09637486.2017.1353954 [DOI] [PubMed] [Google Scholar]
- Piras M., Tandler B., Tomassini Barbarossa I., Piludu M. (2012). Immunogold labeling of carbonic anhydrase isozyme (CA-VI) in secretory granules of human parotid glands. Acta Histochem. 114 (4), 406–408. 10.1016/j.acthis.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Popkin B. M. (2006). Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am. J. Clin. Nutr. 84 (2), 289–298. 10.1093/ajcn/84.2.289 [DOI] [PubMed] [Google Scholar]
- Prescott J., Barthosuk L. M., Prutkin J. (2004). “6-n-Propylthiouracil tasting and the perception of nontaste oral sensations,” in Genetic Variations in Taste Sensitivity. Eds. Prescott J., Tepper B. J. (New York, NY, USA: Marcel Dekker Inc.), 89–104. [Google Scholar]
- Pronin A. N., Xu H., Tang H., Zhang L., Li Q., Li X. (2007). Specific Alleles of Bitter Receptor Genes Influence Human Sensitivity to the Bitterness of Aloin and Saccharin. Curr. Biol. 17 (16), 1403–1408. 10.1016/j.cub.2007.07.046 [DOI] [PubMed] [Google Scholar]
- Raliou M., Wiencis A., Pillias A. M., Planchais A., Eloit C., Boucher Y., et al. (2009). Nonsynonymous single nucleotide polymorphisms in human tas1r1, tas1r3, and mGluR1 and individual taste sensitivity to glutamate. Am. J. Clin. Nutr. 90 (3), 789S–799S. 10.3945/ajcn.2009.27462P [DOI] [PubMed] [Google Scholar]
- Raliou M., Grauso M., Hoffmann B., Schlegel-Le-Poupon C., Nespoulous C., Débat H., et al. (2011). Human genetic polymorphisms in T1R1 and T1R3 taste receptor subunits affect their function. Chem. Senses 36 (6), 527–537. 10.1093/chemse/bjr014 [DOI] [PubMed] [Google Scholar]
- Rawal S., Hayes J. E., Wallace M. R., Bartoshuk L. M., Duffy V. B. (2013). Do polymorphisms in the TAS1R1 gene contribute to broader differences in human taste intensity? Chem. Senses 38 (8), 719–728. 10.1093/chemse/bjt040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed D. R., Zhu G., Breslin P. A., Duke F. F., Henders A. K., Campbell M. J., et al. (2010). The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum. Mol. Genet. 19, 4278–4285. 10.1093/hmg/ddq324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts C. D. (1997). Fat preferences, dietary fat intake and body composition in children. Eur. J. Clin. Nutr. 51 (11), 778–781. 10.1038/sj.ejcn.1600487 [DOI] [PubMed] [Google Scholar]
- Rippe J., Angelopoulos T. (2016). Relationship between added sugars consumption and chronic disease risk factors: Current understanding. Nutrients 8, 697. 10.3390/nu8110697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risso D. S., Giuliani C., Antinucci M., Morini G., Garagnani P., Tofanelli S., et al. (2017). A bio-cultural approach to the study of food choice: The contribution of taste genetics, population and culture. Appetite 114, 240–247. 10.1016/j.appet.2017.03.046 [DOI] [PubMed] [Google Scholar]
- Robino A., Mezzavilla M., Pirastu N., Dognini M., Tepper B. J. (2014). Gasparini P. A Population-based approach to study the impact of PROP perception on food liking in populations along the Silk Road. PloS One 9 (3), e91716. 10.1371/journal.pone.0091716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robino A., Mezzavilla M., Pirastu N., La Bianca M., Gasparini P., Carlino D., et al. (2016). Understanding the role of personality and alexithymia in food preferences and PROP taste perception. Physiol. Behav. 157, 72–78. 10.1016/j.physbeh.2016.01.022 [DOI] [PubMed] [Google Scholar]
- Roudnitzky N., Bufe B., Thalmann S., Kuhn C., Gunn H. C., Xing C., et al. (2011). Genomic, genetic and functional dissection of bitter taste responses to artificial sweeteners. Hum. Mol. Genet. 20 (17), 3437–3449. 10.1093/hmg/ddr252 [DOI] [PubMed] [Google Scholar]
- Roudnitzky N., Behrens M., Engel A., Kohl S., Thalmann S., Hübner S., et al. (2015). Receptor polymorphism and genomic structure interact to shape bitter taste perception. PloS Genet. 11 (9), e1005530. 10.1371/journal.pgen.1005530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouseff R. (1990). “Bitterness in food products: an overview,” in Bitterness in foods and beverages; developments in food science. Ed. Rouseff R. L.(Amsterdam, Netherlands: Elsevier; ), 1–14. [Google Scholar]
- Running C. A., Craig B. A., Mattes R. D. (2015). Oleogustus: the unique taste of fat. Chem. Senses 7, 507–516. 10.1093/chemse/bjv036 [DOI] [PubMed] [Google Scholar]
- Søberg S., Sandholt C., Jespersen N., Toft U., Madsen A., von Holstein-Rathlou S., et al. (2017). FGF21 Is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 25 (5), 1045–53e6. 10.1016/j.cmet.2017.04.009 [DOI] [PubMed] [Google Scholar]
- Sacerdote C., Guarrera S., Smith G., Grioni S., Krogh V., Masala G., et al. (2007). Lactase persistence and bitter taste response: instrumental variables and mendelian randomization in epidemiologic studies of dietary factors and cancer risk. Am. J. Epidemiol. 166 (5), 576–581. 10.1093/aje/kwm113 [DOI] [PubMed] [Google Scholar]
- Sandell M. A., Breslin P. A. (2006). Variability in a taste-receptor gene determines whether we taste toxins in food. Curr. Biol. 16 (18), R792–R794. 10.1016/j.cub.2006.08.049 [DOI] [PubMed] [Google Scholar]
- Sandell M., Hoppu U., Mikkilä V., Mononen N., Kähönen M., Männistö S., et al. (2014). Genetic variation in the hTAS2R38 taste receptor and food consumption among Finnish adults. Genes Nutr. 9 (6), 433. 10.1007/s12263-014-0433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Yamada K., Namba H., Yoshinaga M., Du D., Uehara Y. (2013). Angiotensinogen gene polymorphisms and food-intake behavior in young, normal female subjects in Japan. Nutr. (Burbank Los Angeles County Calif) 29 (1), 60–65. 10.1016/j.nut.2012.03.013 [DOI] [PubMed] [Google Scholar]
- Sayed A., Šerý O., Plesnik J., Daoudi H., Rouabah A., Rouabah L., et al. (2015). CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int. J. Obes. 39 (6), 920–924. 10.1038/ijo.2015.20 [DOI] [PubMed] [Google Scholar]
- Shepherd R., Farleigh C. A., Land D. G. (1984). The relationship between salt intake and preferences for different salt levels in soup. Appetite 5, 281–290. 10.1016/s0195-6663(84)80001-x [DOI] [PubMed] [Google Scholar]
- Shigemura N., Shirosaki S., Sanematsu K., Yoshida R., Ninomiya Y. (2009) Genetic and molecular basis of individual differences in human umami taste perception. PloS One 4, 2–7. 10.1371/journal.pone.0006717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbel S. P., Talbert M. E., Bowden D. W., Haffner S. M., Taylor K. D., Chen Y. D., et al. (2011). RGS6 variants are associated with dietary fat intake in Hispanics: the IRAS Family Study. Obesity 19 (7), 1433–1438. 10.1038/oby.2010.333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons P. J., Kummer J. A., Luiken J. J. F. P., Boon L. (2011). Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 113 (8), 839–843. 10.1016/j.acthis.2010.08.006 [DOI] [PubMed] [Google Scholar]
- Smith A. D., Fildes A., Cooke L., Herle M., Shakeshaft N., Plomin R., et al. (2016). Genetic and environmental influences on food preferences in adolescence. Am. J. Clin. Nutr. 104, 446–453. 10.3945/ajcn.116.133983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohani Z. N., Meyre D., de Souza R. J., Joseph P. G., Gandhi M., Dennis B. B., et al. (2015). Assessing the quality of published genetic association studies in meta-analyses: the quality of genetic studies (Q-Genie) tool. BMC Genet. 16, 50. 10.1136/bmjopen-2015-010403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohani Z. N., Sarma S., Alyass A., de Souza R. J., Robiou-du-Pont S., et al. (2016). Empirical evaluation of the Q-Genie tool: a protocol for assessment of effectiveness. BMJ Open 6 (6), e010403. 10.1136/bmjopen-2015-010403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soranzo N., Bufe B., Sabeti P. C., Wilson J. F., Weale M. E., Marguerie R., et al. (2005). Positive selection on a high-sensitivity allele of the human bitter-taste receptor TAS2R16. Curr. Biol. 15 (14), 1257–1265. 10.1016/j.cub.2005.06.042 [DOI] [PubMed] [Google Scholar]
- Stewart J. E., Feinle-Bisset C., Golding M., Delahunty C., Clifton P. M., Keast R. S. (2010). Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 104, 145–152. 10.1017/S0007114510000267 [DOI] [PubMed] [Google Scholar]
- Suomela J. P., Vaarno J., Sandell M., Lehtonen H. M., Tahvonen R., Viikari J., et al. (2012). Children’s hedonic response to berry products: Effect of chemical composition of berries and hTAS2R38 genotype on liking. Food Chem. 135 (3), 1210–1219. 10.1016/j.foodchem.2012.05.079 [DOI] [PubMed] [Google Scholar]
- Takamura K., Okayama M., Takeshima T., Fujiwara S., Harada M., Murakami J., et al. (2014). Influence of salty food preference on daily salt intake in primary care. Int. J. Gen. Med. 7, 205–210. 10.2147/IJGM.S60997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Ngwa J. S., van Rooij F. J., Zillikens M. C., Wojczynski M. K., Frazier-Wood A. C., et al. (2013). Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am. J. Clin. Nutr. 97 (6), 1395–1402. 10.3945/ajcn.112.052183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatlow-Golden M., Boyland E., Jewell J., Zalnieriute M., Handsley E., et al. (2016). Tackling food marketing to children in a digital world: trans-disciplinary perspectives. Children’s rights, evidence of impact, methodological challenges, regulatory options and policy implications for the WHO European Region. .
- Tepper B. J., Nurse R. J. (1997). Fat perception is related to PROP taster status. Physiol. Behav. 61 (6), 949–954. 10.1016/s0031-9384(96)00608-7 [DOI] [PubMed] [Google Scholar]
- Tepper B. J., Nurse R. J. (1998). PROP taster status is related to fat perception and preference. Ann. NY Acad. Sci. 855, 802–804. 10.1111/j.1749-6632.1998.tb10662.x [DOI] [PubMed] [Google Scholar]
- Tepper B. J., Neilland M., Ullrich N. V., Koelliker Y., Belzer L. M. (2011). Greater energy intake from a buffet meal in lean, young women is associated with the 6-n-propylthiouracil (PROP) non-taster phenotype. Appetite 56, 104–110. 10.1016/j.appet.2010.11.144 [DOI] [PubMed] [Google Scholar]
- Tepper B. J. (2008). Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 28, 367–388. 10.1146/annurev.nutr.28.061807.155458 [DOI] [PubMed] [Google Scholar]
- Thalmann S., Behrens M., Meyerhof W. (2013). Major haplotypes of the human bitter taste receptor TAS2R41 encode functional receptors for chloramphenicol. Biochem. Biophys. Res. Commun. 435 (2), 267–273. 10.1016/j.bbrc.2013.04.066 [DOI] [PubMed] [Google Scholar]
- Timpson N. J., Heron J., Day I. N., Ring S. M., Bartoshuk L. M., Horwood J. (2007). Refining associations between TAS2R38 diplotypes and the 6-n-propylthiouracil (PROP) taste test: findings from the Avon Longitudinal Study of Parents and Children. BMC Genet. 8, 51. 10.1186/1471-2156-8-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbo P., Schlicker S., Yates A. A., Poos M. (2002). Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet Assoc. 120, 1621–1630. 10.1016/s0002-8223(02)90346-9 [DOI] [PubMed] [Google Scholar]
- Ventura A. K., Worobey J. (2013). Early influences on the development of food preferences. Curr. Biol. 23, R401–R408. 10.1016/j.cub.2013.02.037 [DOI] [PubMed] [Google Scholar]
- Von Atzingen M. C. B. C., Silva M. E. M. P. (2012). 6-n-propyltiouracil (PROP) taster status in Brazilian adults. Food Sci. Techn. 32, 673–678. 10.1590/S0101-20612012005000108 [DOI] [Google Scholar]
- Wakai K., Matsuo K., Matsuda F., Yamada R., Takahashi M., Kawaguchi T., et al. (2013). Genome-wide association study of genetic factors related to confectionery intake: Potential roles of the ADIPOQ gene. Obesity 21 (11), 2413–2419. 10.1002/oby.20316 [DOI] [PubMed] [Google Scholar]
- Wang X., Ouyang Y., Liu J., Zhu M., Zhao G., Bao W., et al. (2014). Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349, g4490. 10.1136/bmj.g4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijtzes A. I., Jaansen W., Bouthoorn S. H., Kiefte-de Jong J. C., Jansen P. W., Franco O. H., et al. (2017). PROP taster status, food preferences and consumption of high-calorie snacks and sweet beverages among 6-year-old ethnically diverse children. Matern. Child Nutr. 13, 5–8. 10.1111/mcn.12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooding S., Gunn H., Ramos P., Thalmann S., Xing C., Meyerhof W. (2010). Genetics and bitter taste responses to goitrin, a plant toxin found in vegetables. Chem. Senses 35 (8), 685–692. 10.1093/chemse/bjq061 [DOI] [PubMed] [Google Scholar]
- Wooding S, K. U., Bamshad M. J., Larsen J., Jorde L. B., Drayna D. (2004). Natural selection and molecular evolution in PTC, a bitter-taste receptor gene. Am. J. Hum. Genet. 74 (4), 637–646. 10.1086/383092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Cancer Research Fund (2018). Diet, nutrition, physical activity and cancer: a global perspective (London, United Kingdom: World Cancer Research Fund/American Institute for Cancer Research; ). [Google Scholar]
- World Health Organization: (2016). Report of the commission on ending childhood obesity (Geneva, Switzerland: World Health Organization; ). (9241565330). [Google Scholar]
- World Health Organization: (2018). Marketing of breast-milk substututes: National implementation of the international code. Status report 2018. Geneva: World Health Organization. Contract No.: Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Yackinous C. A., Guinard J. X. (2002). Relation between PROP (6-n-propylthiouracil) taster status, taste anatomy and dietary intake measures for young men and women. Appetite 38, 201–209. 10.1006/appe.2001.0481 [DOI] [PubMed] [Google Scholar]
- Yasumatsu K., Ogiwara Y., Takai S., Yoshida R., Iwatsuki K., Torii K., et al. (2012). Umami taste in mice uses multiple receptors and transduction pathways. J. Physiol. 590 (5), 1155–1170. 10.1113/jphysiol.2011.211920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans M. R., Tepper B. J., Rietzschel J., Prescott J. (2007). Human hedonic responses to sweetness: role of taste genetics and anatomy. Physiol. Behav. 91 (2-3), 264–273. 10.1016/j.physbeh.2007.03.011 [DOI] [PubMed] [Google Scholar]
- Zhao GQ, Z. Y., Hoon M. A., Chandrashekar J., Erlenbach I., Ryba N. J. (2003). Zuker CS The receptors for mammalian sweet and umami taste. Cell 115 (3), 255–266. 10.1016/s0092-8674(03)00844-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.