Abstract
Introduction:
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma and is a clinically heterogenous disease. Treatment pathways for DLBCL are diverse and integrate established and novel therapies.
Areas covered:
We review the cost burden of DLBCL and cost-effectiveness of DLBCL management including precision and cellular medicine. We utilized Medical Subject Heading (MeSH) terms and keywords to search the National Library of Medicine online MEDLINE database (PubMed) for articles related to cost, cost burden, and cost-of-illness of DLBCL and cost-effectiveness of DLBCL management strategies published in English as of June 2019.
Expert opinion:
Available and developing DLBCL therapies offer improved outcomes and often curative treatment at considerable financial expense, and the total cost burden for DLBCL management is substantial for patients and the healthcare system. In the era of personalized medicine, CAR T cells and targeted therapies provide exciting avenues for current and future DLBCL care and can further increase treatment cost. Determinations of cost and cost-effectiveness in DLBCL treatment pathways should continue to guide care providers and systems in identifying cost reduction strategies to provide appropriate therapies to the greatest number of patients in treating DLBCL.
Keywords: B-cell lymphoma, Cost, Cost-effectiveness, Diffuse large B-cell lymphoma, DLBCL, NHL, Non-Hodgkin Lymphoma
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common form of non-Hodgkin lymphoma (NHL) and is a clinically heterogeneous disease [1]. An estimated 72,400 new NHL cases are expected to occur in the USA in 2019 with DLBCL representing roughly 40% of NHL diagnoses [2,3]. As with other mature B-cell lymphoid cancers, DLBCL is more common in adults with incidence increasing rapidly after 50 years of age. The standard-care regimen of rituximab plus doxorubicin, cyclophosphamide, vincristine, and prednisone (R-CHOP) given every 21 days is curative for more than half of DLBCL cases, while patients who remain refractory to treatment will have a median overall survival (OS) of < 10 months, indicating significant unmet needs for high-risk groups [4]. Advances in the identification of DLBCL subtypes with prognostic and predictive value are providing new options for future clinical risk-stratification [5–9], and novel precision and cellular therapies present promising avenues for nuanced care in treating high-risk DLBCL patient populations [10–12]. Evaluation of the cost burden of DLBCL—including current and developing therapies—is an essential component in determining the optimal treatment strategies and future research goals during this period of rapid advancement in the history of DLBCL care.
Current treatment guidelines
Current guidelines recommend R-CHOP plus or minus involved site radiation therapy as first-line therapy to patients with Ann Arbor stage I–II disease [13]. Patients with Ann Arbor stage III–IV disease may receive R-CHOP with interim restaging or are recommended to enroll in a clinical trial. Randomized controlled trials (RCTs) designed to improve on R-CHOP as first-line therapy for high-risk DLBCL subgroups are currently ongoing and compare R-CHOP with R-CHOP plus an additional targeted therapy (R-CHOP-X) [14], though prior RCTs comparing standard care with R-CHOP-X have shown recurrent negative results [15–17]. The recent identification of DLBCL genetic subgroups with differential outcomes shows promise for the incorporation of upfront genetic testing in future first-line DLBCL RCTs [3,6–9,18,19]. Following first-line therapy, all patients undergo end-of-treatment restaging to assess response to therapy [13]. Patients exhibiting a complete response proceed with clinical observation that more commonly occurs without surveillance imaging [20–22]. Patients with relapsed or refractory disease regardless of initial staging are evaluated for second-line and, if needed, subsequent therapies including hematopoietic cell transplant (HCT), salvage and later-line chemotherapy, and the recently FDA-approved chimeric antigen receptor (CAR) T-cell therapy [23]. For patients who are not HCT candidates, polatuzumab was recently approved for relapsed DLBCL [24].
DLBCL outcomes
Without treatment, DLBCL outcomes are poor with a median OS under one year [25]. However, DLBCL patients who remain event-free 24 months after diagnosis following first-line treatment with rituximab plus an anthracycline-based chemotherapy experience a normal life expectancy [26]. Patients who are primary refractory, refractory to second-line or greater therapy, or who relapse within one year following autologous HCT will experience poor outcomes with median OS of 7.1 months, 6.1 months, and 6.2 months, respectively [4]. Though duration of follow-up has been limited, CAR T-cell therapy is yielding promising improvement in survival for patients who have failed prior therapy [27,28]. Improving outcomes for high-risk groups will rely on development of novel therapies and likely involve strategies selecting therapy based on suspected genomic susceptibility and sequential evaluation of responsiveness.
Rationale
The cost burden of cancer treatment is considerable. US expenditures on cancer management are projected to rise as the US population ages and as integration of costly novel therapies continues [29,30]. The National Cancer Institute places lymphoma among the top five cancer sites by US national expenditure with an estimated 2018 cost in US dollars (USD) in excess of $14 billion [30]. Given the increased incidence of DLBCL among older populations and the need for development of new technologies for treating high-risk DLBCL subgroups, the cost of DLBCL management will continue to place a significant financial burden on patients and the healthcare system. The objective for this review article is to provide an up-to-date summary of the cost burden of DLBCL including the cost of accepted and developing treatment methods in order to advise future treatment selection and to inform efforts to reduce costs of DLBCL care.
Methods
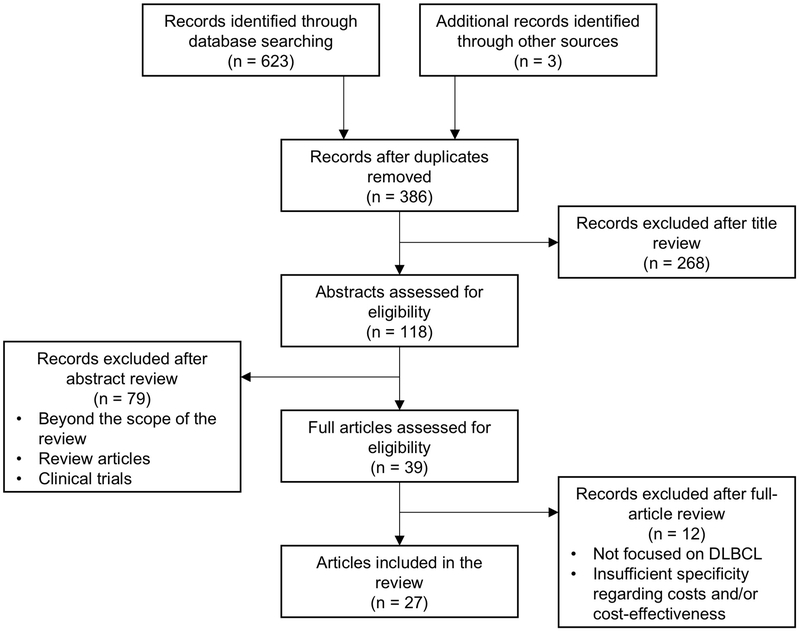
In constructing this literature review, the authors utilized Medical Subject Heading (MeSH) terms and keywords to search the National Library of Medicine online MEDLINE database (PubMed) for articles related to cost, cost burden, and cost-of-illness of DLBCL and cost-effectiveness of DLBCL treatment methods published in English as of June 2019 (Figure 1). Reference lists for identified articles were then evaluated for the inclusion of additional studies. In estimating current costs, priority was given to more recent articles. All cost estimates were converted to USD as needed using contemporaneous exchange rates [31] and were adjusted for inflation to 2019 USD using the US Bureau of Labor Statistics Consumer Price Index [32].
Figure 1.
Flowchart of article selection.
Database searches included searching the National Library of Medicine online MEDLINE database (PubMed) using the Medical Subject Heading (MeSH) terms “lymphoma, large B-cell, diffuse,” “rituximab,” “precision medicine,” “therapy,” “stem cell transplantation,” “receptors, chimeric antigen,” “Supplementary Concept: axicabtagene ciloleucel,” “Supplementary Concept: tisagenlecleucel,” “costs and cost analysis,” “cost-benefit analysis,” “cost of illness,” “Subheading: economics,” and “Subheading: therapy” and the keywords “DLBCL,” “R-CHOP,” “rituximab,” “precision therapy,” “precision medicine,” “stem cell transplantation,” “chimeric antigen receptor,” “axicabtagene ciloleucel,” “tisagenlecleucel,” “surveillance,” “cost,” “cost burden,” “cost-effectiveness,” and “cost of illness.”
1. Total cost burden of DLBCL
Multiple studies have evaluated the total per-patient cost of DLBCL management from diagnosis through full treatment pathways and follow-up (Table 1) [33–36]. Total-cost studies represent expenditures identified across multiple countries (Canada, the United Kingdom, and the United States) and multiple types of healthcare systems. In some cases, studies evaluated cost and cost-effectiveness of discrete components of the DLBCL treatment pathway. Some also estimated total cost burden to facilitate comparisons between therapeutic subgroups (e.g., total cost burden of patients experiencing relapse versus patients who do not experience relapse). Estimates of total cost burden focused on discrete components of the DLBCL treatment pathway are addressed in the corresponding section of the review discussing that aspect of DLBCL management. Of note, all DLBCL total-cost studies predated the FDA approval of CAR T cells and polatuzumab in the treatment of relapsed or refractory DLBCL.
Table 1.
Studies estimating the total cost burden of DLBCL.
| Study | Location | Intervention of interest | Indication | Mean total cost (2019 USD) | Conclusion |
|---|---|---|---|---|---|
| Wang, et al. [33] | United Kingdom | Total treatment pathway | DLBCL | Per-patient: $31,499 | Considerable variability with range $5,017 (palliative care alone) to $135,493 (patients receiving autologous HCT) |
| Morrison, et al. [34] | USA | Total treatment pathway | DLBCL | PPPM: $12,804 | Significant cost burden, particularly in the first year after DLBCL diagnosis |
| Ren, et al. [35] | USA | Total treatment pathway | DLBCL | PPPM: $16,751 | Considerable healthcare costs and resource utilization |
| Costa, et al. [36] | Canada | Total treatment pathway | DLBCL | Per-patient: first 6 months following diagnosis, $25,743; first 6 months following relapse, $16,337 | Greatest cost observed in first 6 months after diagnosis or disease progression with significant drop in cost in subsequent time intervals |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; HCT, hematopoietic cell transplant; ICER, incremental cost-effectiveness ratio; PPPM, per-patient-per-month; USD, US dollars
1.1. Estimates of total cost
While average per-patient expenses related to DLBCL care are not as high as those of some cancers [29], total costs of DLBCL treatment still place a significant cost burden on patients and the healthcare system. Total overall costs span a wide range and depend on treatment modality, patient age, and time horizon. The total cost burden of DLBCL is significant on both the individual patient level and healthcare-system levels, and the range of total cost estimates was consistently wide across studies evaluating full treatment pathways. A recent simulation model of the full DLBCL treatment pathway using patient-level data from a population-based cohort estimated a mean total cost per patient of $31,499 (95% confidence interval [CI] $31,466–$31,531) [33]. When assessing cost according to patient subgroups, estimates of mean total cost ranged from $5,017 for patients managed with palliative care alone to $135,493 for patients receiving autologous HCT as part of second-line therapy [33]. This study also evaluated the aggregate annual expenditure related to DLBCL treatment for the UK healthcare system, estimating a total National Health Service cost burden of US $151–$158 million for new and existing DLBCL patients—roughly 1/6 of the annual UK expenditure on hematological diseases altogether [33].
Multiple retrospective, observational studies using DLBCL treatment claims data have evaluated total per-patient-per-month (PPPM) costs of DLBCL care [34,35]. One recent study incorporating Optum claims information from 2007–2013 identified a mean total PPPM cost of $12,804 (SD $12,400) with median total PPPM cost of $8,790 (interquartile range [IQR] $4,611–$17,459) [34]. A similar study utilizing US MarketScan claims data found higher overall costs with mean PPPM of $16,751 (SD $21,485) [35]. A third study estimating time-dependent total costs developed a linear regression model to estimate the cost burden of DLBCL at six-month intervals [36]. Over the first 6 months following diagnosis, mean total cost of care was estimated to be $25,743 and was observed to drop significantly thereafter. A similar pattern occurred following relapse, with patients who relapsed incurring a mean total cost of $16,337 in the first six months following second-line therapy with a rapid decline in total fees in subsequent six-month intervals. Distributions of time-dependent costs in these three studies all exhibited rightward skew, consistent with prior observations that a small percentage of patients is contributing to the majority of the cost burden for DLBCL care [37]. While studies that utilize claims data to determine healthcare utilization and estimate healthcare expenditures provide meaningful estimates of costs of care from the system perspective, they omit out-of-pocket costs to patients and may be driven by few patients who experience high-cost care.
1.2. Significant contributors to total cost
Drug cost was consistently among the most significant contributors to overall DLBCL cost burden [33–36]. Unit costs for key components common to DLBCL management pathways are available in multiple studies [33,37]. The largest contributors to healthcare resource utilization in the first year following DLBCL diagnosis were outpatient or office visits, radiation therapy, and inpatient admissions [35]. Regarding variations in expenditure over time, the total cost burden of DLBCL management declined from year one to year two with decreased frequency of drug administration, hospital admissions, and outpatient services [34]. In terms of PPPM expenditure, immunochemotherapy was the largest monthly contributor to treatment cost. Multiple studies also reported cost of inpatient hospitalization and outpatient charges as considerable contributors to overall expenses, both in terms of total cost and PPPM cost [34–36], consistent with prior research evaluating cost of NHL care [38]. In studies evaluating the overall cost of DLBCL treatment pathways, HCT led to the highest total per-patient expenses.
Assessments of clinical characteristics in relation to total cost burden showed age to be a significant determinant of total expenditure per patient, with younger patients exhibiting both higher total expenses and higher total survival [33]. Notably, cost differences between younger and older patients were minimal when cost assessment was limited to the initial five years following diagnosis and first treatment, indicating that older patients were responsive to therapy [33], a finding consistent with observed treatment response rates among older DLBCL patients [39].
2. Costs associated with first-line management of DLBCL
Since the early 2000s with the addition of the monoclonal antibody rituximab to CHOP, R-CHOP has been the standard first-line therapy for DLBCL [40]. Recent studies involving treatment patterns in the management of DLBCL indicate that R-CHOP is by far the most common initial therapy, selected as first-line treatment in roughly two-thirds of patients [34,35]. Additional regimens used in the first-line setting include bendamustine-based, gemcitabine-based, and lenalidomide-based regimens and others, though each of these regimens may be used in fewer than 10% of patients for first-line care [35]. The present review addresses costs and cost-effectiveness of first-line therapy using R-CHOP. Additionally, we discuss integration of precision therapies in the first-line treatment of DLBCL and associated issues of cost.
2.1. R-CHOP
2.1.1. Background and clinical use
The 2002 LNH-98.5 study first demonstrated improved complete response rate, event-free survival, and OS for R-CHOP versus CHOP in DLBCL patients aged 60–80 years with no substantial increase in toxicity [41–43]. Improved outcomes were corroborated in subsequent studies [44] and for younger age groups [45,46], confirming R-CHOP as the consensus first-line therapy for DLBCL regardless of age at presentation. Roughly 60% of patients will experience a cure on R-CHOP [47]. R-CHOP is administered for 6–8 cycles given every 21-days and may require G-CSF for supportive treatment of neutropenia particularly for patients ≥ 60 years old. Infusion occurs predominantly in outpatient offices and clinics, though increasing numbers of DLBCL patients receive R-CHOP infusion in the outpatient hospital setting [48]. Of note, multiple studies have conducted cost analyses of G-CSF therapies for febrile neutropenia prophylaxis in first-line or later-line chemotherapy in NHL with conflicting conclusions regarding cost-effectiveness [49–56]. In-depth evaluation of these studies is beyond the scope of this review.
2.1.2. Cost burden and cost-effectiveness
Following the addition of rituximab to CHOP in the first-line treatment of DLBCL, multiple studies investigated the total cost burden of R-CHOP and its cost-effectiveness in comparison with CHOP (Table 2) [37,57–63]. Estimates of mean total cost burden for patients receiving R-CHOP as front-line therapy incorporating all elements of the treatment pathway range from $27,659 to $69,633 depending on time horizon [37,57,60,62,63]. Mean total drug costs related to R-CHOP range from $14,543 [62] to $28,726 [59], while one study estimated the mean total costs of administration including hospitalization at approximately $45,000 [57]. Increased costs related to R-CHOP administration were associated with hospitalization. Some data suggest that altering the location where R-CHOP infusion is delivered may reduce total costs [48].
Table 2.
Cost and cost-effectiveness studies comparing R-CHOP and CHOP in DLBCL treatment.
| Study | Location | Indication | Mean total cost (2019 USD) | ICER (2019 USD) | Conclusion |
|---|---|---|---|---|---|
| Lee, et al. [37] | Canada | Untreated DLBCL | R-CHOP, $46,812; CHOP, $34,871 | – | Cost of rituximab was the greatest contributor to cost difference between total treatment pathways |
| Best, et al. [57] | France | Untreated DLBCL | R-CHOP, $65,826; CHOP, $45,161 | $19,235/QALY | Cost-effective |
| Groot, et al. [58] | The Netherlands | Untreated DLBCL, stages II–IV | R-CHOP: age < 60 years, $53,480; age ≥ 60 years, $56,610; CHOP: age < 60 years, $37,380; age ≥ 60 years, $35,830 | Age < 60 years, $18,052/QALY; age ≥ 60 years, $23,152/QALY | Cost-effective |
| Hornberger, et al. [59] | USA | Untreated DLBCL, stages II–IV, ages 60–80 years, ECOG 0–2 | R-CHOP, $56,699; CHOP, $41,928 | $26,931/QALY | Cost-effective |
| Ferrara, et al. [60] | Italy | Untreated DLBCL, ages 18–60 with ≤ 1 IPI risk factor | R-CHOP, $37,037; CHOP, $38,239 | R-CHOP was the dominant therapy | Cost-effective |
| Johnston, et al. [61] | Canada | Untreated DLBCL | R-CHOP: age < 60 years, $52,635; age ≥ 60 years, $48,721; CHOP: age < 60 years, $41,762; age ≥ 60 years, $39,721 | Age < 60 years, $21,746/QALY; age ≥ 60 years, $6,648/QALY | Cost-effective |
| Griffiths, et al. [62] | USA | Untreated DLBCL, ages ≥ 66 years | R-CHOP, $27,659 | $74,753/LY | Not cost-effective |
| Khor, et al. [63] | Canada | Untreated DLBCL | R-CHOP, $86,082; CHOP, $69,633 | Ages < 60 years, $32,094/LY; 60–79 years, $81,346/LY; ≥ 80 years, $111,119/LY | Cost-effective for patients aged < 60 years; may not be cost-effective among older patients |
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; DLBCL, diffuse large B-cell lymphoma; ICER, incremental cost-effectiveness ratio; LY, life year; QALY, quality-adjusted life year; R, rituximab; USD, US dollars
In most evaluations of cost-effectiveness of R-CHOP in comparison with CHOP, R-CHOP has been shown to be cost-effective regardless of age group [57–61], though two studies incorporating population-based, retrospective cohorts suggest that R-CHOP may not be cost-effective in treating very elderly patients [62,63]. Studies indicating diminished cost-effectiveness in older patients attribute higher incremental cost-effectiveness ratio (ICER) values to increased survival associated with R-CHOP leading to greater subsequent non-cancer costs due to non-cancer comorbidities [62,63]. Despite these considerations, R-CHOP is universally accepted as the standard of care therapy for all patients with DLBCL unless there are cardiac or other concerns about ability to tolerate this regimen.
2.2. Precision therapy
2.2.1. Background
The potential benefits of precision medicine therapeutics, which rely on molecularly selective agents and clearly defined biomarker targets, have captured the attention of the medical community. Specifically, targeted therapies may reduce adverse drug effects and increase therapeutic efficacy making these therapies a special focus in oncologic research [14]. The National Cancer Institute especially promotes precision medicine research and has established two large clinical trials utilizing precision medicine approaches, National Cancer Institute-Molecular Profiling-based Assignment of Cancer Therapy (NCI-MPACT) and National Cancer Institute-Molecular Analysis for Therapy Choice (NCI-MATCH) [64].
2.2.2. Precision medicine in DLBCL
Diffuse large B-cell lymphoma (DLBCL) presents a specific case where targeted therapies could be especially important. Gene expression profiling (GEP) was first used in 2000 to subtype DLBCL by cell-of-origin (COO) [5]. This work identified at least two biologically distinct subgroups: germinal center B-cell-like (GCB)-DLBCL and activated B-cell-like (ABC)-DLBCL. ABC-DLBCLs tend to have a more aggressive course and inferior outcomes [65]. Currently, GEP is minimally used in clinical practice and only recently part of clinical trials because of lack of certified, expression-based standardized testing, time requirements for testing, and uncertain costs. Consequently, COO subtypes are clinically assessed with immunohistochemical (IHC) classification systems as substitutes for GEP. While IHC is easier than GEP to use in a clinical setting, IHC is much less accurate and frequently does not replicate the prognostic significance that GEP conveys [66].
Despite difficulties associated with GEP and IHC classification approaches, both have been implemented in RCTs for DLBCL as COO subtype-specific therapies are investigated as potential routes for precision medicine. Unfortunately, current strategies for subtype-specific treatments are not viable options [67]. For example, results from early phase trials suggested that adding bortezomib, a proteasome inhibitor, to R-CHOP (VR-CHOP) could improve poor outcomes in non-GCB-DLBCL patients, but more recent data from a randomized trial showed no difference in OS between R-CHOP and VR-CHOP in this population [16], and subsequent phase 3 evidence from the REMoDL-B study indicated no subtype-specific improvement in progression-free survival (PFS) with the addition of bortezomib [68]. Another RCT added ibrutinib, a Bruton tyrosine kinase inhibitor, to R-CHOP and has also not shown a survival benefit by subtype [69]. The most recent data presented from the randomized phase 3 trial of lenalidomide + R-CHOP vs. R-CHOP from the ROBUST trial demonstrated no benefit for the experimental arm in patients with ABC-DLBCL [70]. However, data from a randomized phase 2 trial with slightly different dosing of lenalidomide demonstrated benefit with a one-sided p-value making interpretation of this approach more complicated [71]. The substantial genetic heterogeneity in DLBCL potentially explains subtype-directed therapy’s lack of success because even the most common aberrations are found infrequently [72–75]. While targeting COO subtype is an intuitive strategy for precision medicine in DLBCL, turning to more precise molecular hallmarks like those seen in double- and triple-hit lymphomas and others may be a necessary turning point in trial design [76,77].
2.2.3. Genetic subgroups and precision medicine in DLBCL
The advent of next-generation sequencing has also presented special opportunities for precision medicine strategies in treating DLBCLs [78]. Recent sequencing efforts have identified DLBCL’s driving genetic aberrations and the aberrations which are preferentially distributed among DLBCL subtypes [79]. Identifying potential biomarkers is key to selecting precision medicine therapies, and molecular hallmarks based on mutations are increasingly accepted as nascent biomarkers [80,81]. Efforts to identify potentially targetable mutations in DLBCL are underway [6–8,11]. Recent sequencing analyses could help identify new therapeutic targets and advance precision medicine efforts by associating genetic aberrations with dysregulated molecular pathways. For DLBCL, including sequencing results in prognostic modeling and therapeutic decision-making could define new subgroups based on actionable mutations, potentially allowing for more precise therapeutic strategies.
Based on these new genetic subgroups and targeted therapeutics, clinical DLBCL trials could potentially use up-front genetic testing. However, the development of new modalities for Clinical Laboratory Improvement Amendments (CLIA)-certified genomics and treatment strategies based on testing are needed to make targeted therapies a new norm in DLBCL treatment. Because the genetic heterogeneity of DLBCL and relatively low mutation frequency, a potential strategy to incorporate genetic testing in clinical trials should separate patients into various treatment arms based on genetic subgroups, a master protocol known as an umbrella trial. Rigorous trial design for precision medicine must take external considerations into account by standardizing sequencing methods and informatics pipelines across multi-center collaborations to increase reproducibility [82]. Moreover, DLBCL precision trials with genetic biomarkers require quick turnaround for the genomic sequencing due to the disease’s aggressive nature and the frequent need for urgent treatment. Based on the potential trials’ results, clinicians will need to create collaborative relationships with bioinformaticians to balance clinical, tumor microenvironment, and genetic prognostic factors when determining treatment strategies by subtype or patient while also generating clinically relevant reports containing this streamlined data for the electronic health record [83–85].
2.2.4. Costs associated with integration of precision therapy
While precision therapies can potentially benefit DLBCL patients, the clinical advantages must be balanced against the economic costs of treatment. Consequently, cost-effectiveness analyses may be crucial to bring novel therapeutics into clinical practice. Patients can incur costs during their diagnostic process and during their treatment process. Blombery et al. note the importance of accurate diagnostic process because improper treatment intensification would ratchet up the costs from delivery of supportive care for managing treatment toxicity [86,87]. Developing comprehensive, rigorously evaluated, and multifaceted prognostic platforms are paramount for reducing cost burdens associated with the diagnostic process. Clinicians hoping to employ precision strategies based on COO subtypes must carefully decide between various IHC or GEP methods, especially when using subtype in first-line treatment considerations. Nowakowski et al. reviewed the difference between IHC and GEP as diagnostic tools and noted that while IHC is a more clinically palatable tool, GEP is more accurate and has better prognostic and potentially predictive ability [88]. However, new GEP technologies have expanded the options for assessing COO subtype in the clinical setting [88].
In this vein, King et al. analyzed the impact of real time expert central pathology review in a phase II open-label study for DLBCL patients (ClinicalTrials.gov identifier ) and found that real time pathology review was much more efficient compared to traditional review (Table 3) [87]. The real time review process added a step 0 review so that ineligible patients were rejected before enrollment instead of rejecting patients at the end as a traditional review process would. The eligibility notification was delivered in an average of 1 working day (range 0–4) and saved costs associated with prevention of lost study slots. Treatments for DLBCL would especially benefit from the potential for more accurate pathology analysis given DLBCL’s high molecular heterogeneity. Despite its clear benefits, real time review still has limitations, such as increased time and work for pathologists to ensure a rapid process.
Table 3.
Cost and cost-effectiveness studies for precision medicine in DLBCL treatment.
| Study | Location | Intervention of interest | Indication | Comparator therapy | ICER (2019 USD) | Conclusion | Comments |
|---|---|---|---|---|---|---|---|
| King, et al. [87] | USA | Real-time EPCR | Cell-of-origin testing | Retrospective EPCR alone | – | Real-time EPCR may reduce costs in precision-medicine therapy and trials | – |
| Chen, et al. [89] | USA | R2-CHOP for ABC-DLBCL; R-CHOP for GCB-DLBCL | Untreated DLBCL | R-CHOP for ABC- and GCB-DLBCL | $16,006/QALY | Cost-effective when there is a significant efficacy difference between subtypes | Based on phase 2 evidence; subsequent phase 3 results did not indicate a subtype-specific benefit for R2-CHOP |
| R2-CHOP for ABC- and GCB-DLBCL | Untreated DLBCL | R-CHOP for ABC- and GCB-DLBCL | Not reported given significant increase in cost with this intervention | Not cost-effective | Based on phase 2 evidence; subsequent phase 3 results did not indicate a subtype-specific benefit for R2-CHOP |
Abbreviations: ABC, activated B-cell-like; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; DLBCL, diffuse large B-cell lymphoma; EPCR, expert central pathology review; GCB, germinal center B-cell-like; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life years; R, rituximab; R2, rituximab plus lenalidomide; USD, US dollars
Chen et al. evaluated the cost-effectiveness of three DLBCL management strategies: R-CHOP for all patients, lenalidomide plus R-CHOP (R2-CHOP) for ABC patients and R-CHOP for GCB patients, and R2-CHOP for all patients (Table 3) [89], utilizing data from a phase 2 trial of R2-CHOP [90]. This analysis preceded the availability of results from the randomized studies described above. The base case analysis for subtype-specified treatment demonstrated a favorable ICER of $16,006/quality-adjusted life year (QALY) compared with R-CHOP. These authors noted that the cost-effectiveness of subtype driven therapies is predicated on the therapies’ asymmetric survival benefit for each subtype. In other words, subtype-based treatment is most cost-effective as a front-line approach when there is a significant differential efficacy in GCB and ABC subtypes, so rigorously investigating the benefit of treatment in both subtypes is paramount. Chen et al. also found that the more accurate subtyping method is preferred as the difference in survival grows, even at higher test costs. While phase 3 results ultimately failed to demonstrate a subtype-specific benefit for R2-CHOP, this study provides baseline evidence for important considerations related to the economics of precision medicine treatment strategies. Additional data from precision medicine RCTs are needed to corroborate findings and inform the use of novel therapies for DLBCL.
3. Costs associated with relapsed and refractory DLBCL
Standard therapy for patients with relapsed and refractory DLBCL involves second-line chemotherapy and determination of transplant eligibility followed by high-dose therapy and autologous HCT for transplant candidates [13]. For DLBCL patients who continue to exhibit refractory disease or who relapse following second-line chemotherapy or autologous HCT, additional therapy options include allogeneic HCT and CAR T-cell therapy. Topics reviewed here include cost burden associated with second-line chemotherapy, cost and cost-effectiveness of HCT, and cost and cost-effectiveness of CAR T cells in DLBCL care.
3.1. Second-line chemotherapy
3.1.1. Background
Recommended second-line immunochemotherapy regimens in DLBCL treatment differ pending transplant eligibility [13]. Regimens for patients proceeding to transplant include ICE (ifosfamide, carboplatin, and etoposide), DHAP (dexamethasone, cisplatin, and cytarabine), and others, while regimens for non-candidates include bendamustine, rituximab monotherapy, and others [13,91]. Addition of rituximab is commonly included in second-line therapy regardless of chemotherapy regimen [92].
3.1.2. Cost burden
Limited research has been conducted to evaluate the cost of relapsed and refractory DLBCL beginning with second-line chemotherapy (Table 4) [93], though available studies indicate high costs for relapsed and refractory patients. A 2016 study evaluated the total monthly costs of second-line chemotherapy in the outpatient setting over a two-year period using SEER-Medicare data [92]. Average PPPM costs were roughly $4,800 and $5,800 for the refractory and relapsed populations, respectively. Total expenditures amounted to $116,342 for the refractory population and $139,194 for the relapsed group over the 24-month period with lower costs observed in the refractory cohort due to increased early mortality in this group. A recent study using Medicare claims for cost estimation compared total monthly costs between relapsed and non-relapsed cohorts [94]. Price comparisons began at 60 days following completion of first-line treatment. Initial PPPM costs were comparable in each group at roughly $3,600 and $4,600 for non-relapsed and relapsed cohorts, respectively. PPPM costs for the relapsed cohort then rose to approximately $11,000 PPPM following start of second-line therapy to end of follow-up, indicating significant increase in expenses due to relapse. Drug costs and outpatient fees were the largest contributors to cost for patients with relapsed DLBCL.
Table 4.
Cost and cost-effectiveness studies for relapsed/refractory DLBCL treatment beginning with second-line chemotherapy.
| Study | Location | Intervention of interest | Indication | Mean total cost (2019 USD) | Conclusion |
|---|---|---|---|---|---|
| Danese, et al. [92] | USA | Total treatment pathway from second-line chemotherapy | Relapsed DLBCL in older patients | PPPM: $5,800; per-patient: $139,194 | Second-line therapy incurs considerable expense |
| Refractory DLBCL in older patients | PPPM: $4,800; per-patient: $116,342 | Second-line therapy incurs considerable expense; higher initial mortality rate for refractory disease leads to lower overall costs | |||
| Huntington, et al. [94] | USA | Total treatment pathway from 60 days following first-line therapy | Non-relapsed DLBCL in older patients | PPPM: $3,600 | Post-first-line treatment PPPM expenses for non-relapsed and relapsed patients are comparable prior to initiation of second-line therapy in the relapsed population |
| Relapsed DLBCL in older patients | PPPM: $4,600 prior to initiation of second-line therapy; $11,000 after initiation of second-line therapy | Significant increase in expenses due to relapse |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; PPPM, per-patient-per-month; USD, US dollars
3.2. Hematopoietic cell transplant
3.2.1. Background and clinical use
The PARMA [95] and CORAL [96] trials, conducted in the pre-rituximab and rituximab eras, respectively, demonstrated survival benefits for chemosensitive NHL patients after relapse and confirmed autologous HCT as standard therapy in the relapsed or refractory population. However, 1/3 of DLBCL patients undergoing autologous HCT may experience relapse or progression, and autologous HCT may not be indicated for patients with refractory disease [97]. In these patient groups, allogeneic HCT represents an additional treatment option that may provide a cure [97]. Clinical use of HCT involves the mobilization and collection of stem cells from the patient or donor followed by a conditioning regimen of chemotherapy and transplantation. Analyses of cost burden indicate that these therapies come with considerable expense in all phases of care from stem cell collection through long-term follow-up.
3.2.2. Cost and cost-effectiveness across hematologic malignancies
Multiple cost and cost-effectiveness studies have been conducted since the 1990s to evaluate the use of autologous HCT and allogeneic HCT in hematologic malignancies, and many of those studies have been reviewed in depth [98,99]. Estimates for costs of both modalities include wide ranges depending on time horizon and types of direct medical costs included in evaluation. In one review, costs of initial transplant hospitalization were reported as low as $87,000 and $166,000 for autologous HCT and allogeneic HCT, respectively, and were shown to increase considerably over longer follow-up periods [98]. Higher costs were driven by length of hospitalization, disease severity, and posttransplant complications, and allogeneic HCT was consistently more expensive than autologous HCT [98,99]. The Milliman actuarial group recently estimated total costs per patient from pretransplant care through 180 days following discharge of approximately $429,000 and $934,000 for autologous HCT and allogeneic HCT, respectively, across all indications for HCT [100]. Differences in total cost between autologous HCT and allogeneic HCT were due to substantially greater costs of hospital transplant admission, posttransplant care 180 days following discharge, and stem cell procurement seen in allogeneic HCT.
Cost-effectiveness studies across hematologic malignancies have been inconsistent in their conclusions and vary based on donor source and treatment pathways selected for comparison [98,99]. Additional studies have investigated cost and cost-effectiveness of more nuanced aspects of HCT administration in hematologic malignancies including drugs used in stem cell mobilization [101–106]; management of complications [107] including chemotherapy-induced nausea and vomiting [108], mucositis [109], and neutropenia [110–112]; and chemotherapy regimens prior to transplant [113,114]. Evaluation of these studies is beyond the scope of the present review.
3.2.3. Cost and cost-effectiveness in DLBCL
Few cost analyses of autologous HCT and allogeneic HCT have focused on DLBCL (Table 5). A 2012 study utilized a Markov model to evaluate the cost-effectiveness of G-CSF with plerixafor (G+P) versus G-CSF alone for stem cell mobilization in DLBCL patients undergoing autologous HCT [115]. Total lifetime cost for autologous HCT was roughly $75,000 using G-CSF and $104,000 in the G+P cohort. Cost-effectiveness comparisons yielded an ICER of approximately $16,000/QALY gained using G+P, indicating cost-effectiveness of this mobilization strategy in treating DLBCL. A 2018 investigation of immediate and long-term cost burden associated with allogeneic HCT for patients with DLBCL indicated high costs over a three-year follow-up period [116]. During the first year after allogeneic HCT administration, average per-patient costs totaled $490,787 (SD $254,333) with an average of 38 inpatient days and 68 office visits per patient. Inpatient expenses including allogeneic HCT administration were the largest contributor to total cost. Expenses were lower in years two and three but remained significant, reaching $78,563 (SD $57,331) in the third year after allogeneic HCT. The largest contributor to total cost in years two and three was office visit fees. These studies confirm the substantial expenses associated with HCT for adult patients with DLBCL.
Table 5.
Cost and cost-effectiveness studies for hematopoietic cell transplant in DLBCL treatment.
| Study | Location | Intervention of interest | Indication | Comparator therapy | Mean total cost (2019 USD) | ICER (2019 USD) | Conclusion |
|---|---|---|---|---|---|---|---|
| Kymes, et al. [115] | USA | G+P | Stem cell mobilization for autologous HCT in patients with relapsed DLBCL | G-CSF alone | G+P, $104,000; G-CSF alone, $75,000 | $16,000/QALY | Cost-effective |
| Maziarz, et al. [116] | USA | Allogeneic HCT | Refractory or relapsed DLBCL after chemotherapy or autologous HCT | – | First year after HCT administration, per-patient: $490,787 | – | Significant economic burden following allogeneic HCT |
Abbreviations: G+P, G-CSF plus plerixafor; G-CSF, granulocyte-colony stimulating factor; HCT, hematopoietic cell transplant; DLBCL, diffuse large B-cell lymphoma; ICER, incremental cost-effectiveness ratio; USD, US dollars; QALY, quality-adjusted life year
3.3. CAR T-cell therapy
3.3.1. Background
CAR T-cell therapy enables the targeted destruction of cancer cells using a patient’s own T-lymphocytes [117,118]. CAR T-cell therapy was first approved for the management of a hematologic malignancy in 2017 with the Food and Drug Administration (FDA) approval of tisagenlecleucel in the treatment of pediatric B-cell acute lymphoblastic leukemia [119,120]. Following promising results from phase I and phase II studies in adult patients with DLBCL [27,28,121], the FDA approved CAR T-cell therapy for treatment of relapsed or refractory DLBCL after two or more failed systemic therapies [23]. The ZUMA-1 trial was a multicenter phase II study for the treatment of refractory large B-cell lymphomas with axicabtagene ciloleucel, an anti-CD19 CAR T-cell therapy [27]. ZUMA-1 demonstrated high levels of durable response among patients with refractory disease. The JULIET trial, an international phase II study of the anti-CD19 CAR T-cell therapy tisagenlecleucel in relapsed or refractory DLBCL, also showed high rates of durable response [28]. A trial investigating a third therapy, lisocabtagene maraleucel, is ongoing and is demonstrating durable responses across high-risk hematologic malignancies including DLBCL [122]. Multiple review articles discuss the success and use of CAR T-cells in lymphoma [123,124].
3.3.2. Clinical use
CAR T cells are indicated for DLBCL patients who fail two or more systemic therapies, have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, and have no prior CNS disease or major comorbidities [118]. In clinical application, CAR T-cell therapy is provided as a one-time administration in three primary clinical scenarios: post-autologous HCT relapse, first salvage treatment failure, and partial response to first salvage treatment [118]. The treatment requires multiple stages, beginning with leukapheresis to collect a patient’s T cells for preparation prior to infusion [118]. Patients then receive a lymphodepleting chemotherapy regimen of cyclophosphamide and fludarabine or bendamustine followed by infusion of CAR T cells [125,126]. A recent evaluation of treatment with lisocabtagene maraleucel showed the average number of hospital days for patients receiving infusion ranging between 9.3 and 15.6 days [127]. Major adverse reactions associated with CAR T-cell therapy include cytokine release syndrome (CRS) and neurological toxicities, necessitating administration in a certified healthcare facility capable of managing these toxicities [118,128]. In addition, intravenous immunoglobulin (IVIG) may be required following lymphodepleting chemotherapy due to potential for B-cell aplasia [129].
3.3.3. Cost burden
CAR T cells have received significant attention as the most expensive cancer drug yet developed [130]. Tisagenlecleucel was first introduced for treatment of pediatric acute leukemia with a single-dose price of $475,000 [131]. By comparison, common anticancer drugs typically cost on the order of $4,000–$10,000 per month [132]. Treatment with axicabtagene ciloleucel or tisagenlecleucel for relapsed or refractory DLBCL now costs $373,000 [118], still considerably more expensive than other notable, high-priced drugs such as the hepatitis C therapies sofosbuvir ($84,000 for a 12-week course) and sofosbuvir + ledipasvir ($94,500 for a 12-week course) [133]. Moving forward, CAR T-cell therapy is projected to comprise nearly half of all drug sales for DLBCL care despite limiting indications to a small set of eligible patients [134]. Costs of hospitalization and management of toxicities are not included in the infusion fee and may add an additional $150,000–$200,000 to the overall expense [128]. When all associated costs are considered, estimates for total cost range from $500,000–$1.5 million depending on the complexity of the case [135].
3.3.4. Cost-effectiveness
Initial evaluations of cost-effectiveness for CAR T cells pertained to treatment of pediatric acute leukemia [136–142]. In general, these studies found CAR T-cell therapy to be cost-effective compared to standard pediatric ALL therapy but ultimately concluded that long-term follow-up data regarding duration of response is required for robust estimates of cost-effectiveness [131,138,139]. Multiple studies of cost-effectiveness in DLBCL have since reached similar conclusions (Table 6), suggesting that CAR T-cell therapy in the treatment of relapsed or refractory DLBCL is potentially cost-effective given presently available data though long-term follow-up is required for reliable estimates [142–147]. In adults, cost-effectiveness in practice may depend on other factors including: insurance contracting, patient age, comorbidities and other factors that influence non-relapse mortality.
Table 6.
Cost and cost-effectiveness studies for CAR T cells in DLBCL treatment.
| Study | Location | Intervention of interest | Indication | Comparator therapy | Mean total cost (2019 USD) | ICER (2019 USD) | Conclusion | Comments |
|---|---|---|---|---|---|---|---|---|
| Tice, et al. [142] | USA | Axicabtagene ciloleucel | R/R B-cell lymphoma in patients ineligible for autologous HCT | Salvage chemotherapy | Axicabtagene ciloleucel, $617,000; chemotherapy, $155,000 | $136,000/ QALY | Cost-effective assuming a cure for patients who exhibited complete response after five years | Authors advise collecting additional information regarding long-term response rates |
| Roth, et al. [143] | USA | Axicabtagene ciloleucel | R/R large B-cell lymphoma | Salvage chemotherapy | Axicabtagene ciloleucel, $550,000; chemotherapy, $170,000 | $58,000/QALY | Cost-effective | Funded by the manufacturer of axicabtagene ciloleucel |
| NICE [144] | United Kingdom | Axicabtagene ciloleucel | DLBCL after ≥ 2 systemic therapies | Salvage chemotherapy ± rituximab | Total discounted cost not reported due to confidential commercial arrangement | In excess of $63,000/QALY | Potentially cost-effective given available data | Authors advise additional data are required regarding long-term outcomes and usage of IVIG |
| NICE [145] | United Kingdom | Tisagenlecleucel | R/R DLBCL after ≥ 2 systemic therapies | Salvage chemotherapy | Total discounted cost not reported due to confidential commercial arrangement | Range: $55,000/QALY to $70,000/QALY | Potentially cost-effective given available data | Significant uncertainty; authors advise that additional data regarding outcomes and IVIG are required for more robust estimates |
| Whittington, et al. [146] | USA | Axicabtagene ciloleucel | Refractory DLBCL | Chemotherapy | Axicabtagene ciloleucel: public payers, $459,700–$554,700; commercial, $554,000–$648,900; chemotherapy: public, $108,600–$151,200; commercial, $114,500–$157,000 | Public: $82,400–$230,900/QALY; commercial: $100,400–$289,000/QALY | Additional outcomes data are required for more precise estimates | Wide ranges in cost and ICER values are due to differences in long-term survival estimates from the five utilized models |
| Lin, et al. [147] | USA | Axicabtagene ciloleucel | R/R adult DLBCL | Salvage chemotherapy and HCT | Axicabtagene ciloleucel, $659,000–$677,000; chemotherapy and HCT, $175,000 | $133,000–$200,000/QALY | Potentially cost-effective given available data | Significant uncertainty in estimates using presently available data |
| Tisagenlecleucel | R/R adult DLBCL | Salvage chemotherapy and HCT | Tisagenlecleucel, $538,000–$547,000; chemotherapy and HCT, $175,000 | $174,000–$348,000/QALY | Potentially cost-effective given available data | Significant uncertainty in estimates using presently available data |
Abbreviations: CAR, chimeric antigen receptor; DLBCL, diffuse large B-cell lymphoma; HCT, hematopoietic cell transplant; ICER, incremental cost-effectiveness ratio; IVIG, intravenous immunoglobulin; NICE, National Institute for Health and Care Excellence; QALY, quality-adjusted life year; R/R, relapsed/refractory; USD, US dollars
The Institute for Clinical and Economic Review conducted a cost-effectiveness analysis for CAR T-cell therapy in B-cell malignancies using Markov modeling [142]. In the Markov model, treatment with axicabtagene ciloleucel was compared with chemotherapy for relapsed or refractory adult B-cell lymphoma in patients ineligible for autologous HCT. A price-per-unit mark-up of $100,000 was used in this study for a total drug cost of $473,000. In the base case, the axicabtagene ciloleucel arm exhibited a total discounted cost of $617,000 compared with $155,000 for salvage chemotherapy. Axicabtagene ciloleucel yielded a base-case ICER of roughly $112,000/LY gained and $136,000/QALY gained. In this analysis, axicabtagene ciloleucel met the cost-effectiveness threshold of $150,000/QALY gained assuming a cure for patients remaining in complete response to CAR T-cell therapy after five years. Model results were observed to yield ICER values in excess of $150,000/QALY when assumptions regarding durable response did not hold. Significant determinants of model results related to outcome discount rate, whether patients were alive and responding to treatment, drug cost mark-up, the standardized mortality ratio, and duration of administration of IVIG as prophylaxis during prolonged B-cell aplasia. Overall, the authors found CAR T-cell therapy cost-effective in this population with the caveat that additional information regarding long-term response rates is needed.
In a study comparing axicabtagene ciloleucel to salvage chemotherapy using a decision model, the base-case analysis identified lifetime costs for CAR T-cell therapy at roughly $550,000 and salvage chemotherapy at approximately $170,000 [143]. In this study, Axicabtagene ciloleucel exhibited an ICER of $58,000/QALY gained. The authors note that the significant differences in ICER observed in this study in comparison with the study from the Institute for Clinical and Economic Review were due to survival modeling methods. The decision model exhibited sensitivity to percentage of patients in long-term remission, outcome discount rate, and drug cost. Notably, this study was funded by Kite, the pharmaceutical company producing axicabtagene ciloleucel.
The National Institute for Health and Care Excellence (NICE), a division of the UK Department of Health, published cost-effectiveness analyses of both axicabtagene ciloleucel [144] and tisagenlecleucel [145]. The ICER for axicabtagene ciloleucel was estimated at in excess of $63,000/QALY gained. The review committee noted that cost-effectiveness estimates for axicabtagene ciloleucel encompass a wide range of potential values given limited data regarding long-term follow-up. NICE ultimately concluded that the range of ICER values demonstrates potential cost-effectiveness for axicabtagene ciloleucel with the need for additional data regarding outcomes and usage of IVIG. ICERs for tisagenlecleucel ranged between roughly $55,000–$70,000/QALY gained with significant uncertainty regarding underlying model values. The committee advised once again that there was a need for additional data collection pertaining to outcomes and usage of immunoglobulin. It remains unclear whether the exclusion of this or other downstream costs were the reason for the dramatic difference in ICERs between this study and the previously discussed studies.
A recent study utilized five separate survival models to develop outcomes and cost-effectiveness estimates for axicabtagene ciloleucel in comparison with chemotherapy [146]. Assessments of cost-effectiveness were determined based on payer status with ICER estimates of $82,400–$230,900/QALY gained and $100,400–$289,000/QALY gained for public and commercial payers, respectively. Wide ranges for estimates of ICER were due to different long-term survival estimates from the five models employed, and the authors conclude that additional outcomes data are required for more precise estimates.
A 2019 study using Markov models evaluated cost-effectiveness of both axicabtagene ciloleucel and tisagenlecleucel in comparison with salvage chemotherapy and stem-cell transplant over a range of possible long-term CAR T-cell treatment responses [147]. ICER estimates for axicabtagene ciloleucel ranged from $133,000/QALY gained with an assumed 5-year PFS of 40% to $164,000/QALY gained assuming a 5-year PFS of 30%. For tisagenlecleucel, model estimates of ICER ranged from $174,000/QALY gained under an assumed 35% 5-year PFS to $230,000/QALY gained assuming a 25% 5-year PFS. All model estimates carried wide-ranging 95% uncertainty intervals with potential to either meet the $150,000/QALY threshold or to exceed it considerably. The variation in findings from these studies illustrate the challenges in applying cost-effectiveness analysis to novel cancer therapies particularly when follow-up time is short for expensive therapies with an expected likelihood of cure.
3.3.5. Remaining financial challenges
Significant financial challenges associated with the cost of CAR T cells remain. Alternate pricing techniques have been proposed to manage the expense of CAR T cells including indication-specific pricing, pricing that incorporates the present uncertainty of long-term outcomes, and pricing based on treatment response [147–149]. Notably, the outcomes-based rebate offered by Novartis for tisagenlecleucel will likely not increase the value of this drug [131,138]. Many additional CAR T-cell therapies are being developed for indications spanning oncology [134], and future production of competing CAR T-cell therapies for treatment of DLBCL may affect drug pricing [149]. One-way uncertainty analysis indicates that decreasing costs associated with other aspects of CAR T-cell therapy including costs of drug administration, treatment related to toxicities, and follow-up are unlikely to affect overall cost-effectiveness of CAR T cells [131]. Reducing uncertainty in the estimates of cost-effectiveness and the implementation of truly value-based pricing for CAR T cells will require long-term data regarding duration of response [131].
Presently, the Centers for Medicaid and Medicare Services (CMS) has not yet stipulated a standard, nationwide plan for the coverage of CAR T-cell therapy, though methods for national coverage of CAR T cells have been proposed [150,151]. Additionally, CMS has planned an increase in reimbursement rates for CAR T-cell therapy through new technology add-on payments from $186,500 per treatment to $242,450 per treatment [152]. Reimbursements at this scale will remain insufficient to cover the estimated $500,000–$1.5 million per-patient cost and will continue to place considerable financial strain on hospitals with potential to dissuade treatment centers from providing CAR T-cell therapy to patients relying on Medicare [135]. Other concerns related to reimbursement include difficulty in standardizing billing codes for CAR T-cell therapies [153].
The cost of CAR T cells also may limit global drug availability, affecting economically disadvantaged countries in particular where high-priced anticancer treatments can be especially unaffordable for patients in need [91,128]. Proposed technologies to increase CAR T cell availability include universal cell banks in lieu of autologous therapy [149,154], and early trials incorporating this approach have demonstrated promising results [155].
4. Costs associated with surveillance imaging
4.1. Background and clinical guidelines
Relapse in DLBCL is associated with inferior outcomes [4], and past methods for the early detection of disease recurrence involved repeated cross-sectional imaging in asymptomatic patients [156]. However, modern investigations of long-term surveillance indicate that surveillance imaging is not associated with survival benefit or adequate detection of relapse [20,22]. In 2013, the Choosing Wisely medical stewardship initiative recommended limiting routine computed tomography (CT) scans in long-term follow-up of patients with aggressive lymphomas [157], and current clinical guidelines advise use of CT imaging for two years following completion of treatment [13] or not at all [158] absent clinical indication. Despite quality improvement campaigns and modern clinical guidelines, practice patterns may continue to incorporate excessive use of surveillance CT imaging [159].
4.2. Cost and cost-effectiveness
Cost and cost-effectiveness evaluations indicate that surveillance imaging is not cost-effective even at time horizons matching current clinical guidelines (Table 7) [160]. A 2015 study developed a Markov model to compare cost and cost-effectiveness of clinical follow-up with no imaging, clinical follow-up with biannual CT, and clinical follow-up with biannual positron-emission tomography (PET)/CT [160]. Total estimated per-patient costs involving the full treatment pathway were $42,084 with no imaging, $45,723 with biannual CT, and $46,778 with biannual PET/CT, resulting in an added per-patient cost between $3,500 and $5,000 with biannual imaging for two years. In comparison with clinical follow-up alone, follow-up with biannual imaging yielded ICERs in excess of $180,000/QALY gained. By these metrics, imaging surveillance is not cost-effective in the posttreatment follow-up of asymptomatic DLBCL patients. Additional methods of disease surveillance are being investigated including detection of circulating tumor DNA (ctDNA) [156], and consideration of novel methods will require similar scrutiny regarding survival benefits and cost-effectiveness prior to clinical integration.
Table 7.
Cost and cost-effectiveness studies for surveillance imaging in DLBCL treatment.
| Study | Location | Intervention of interest | Indication | Comparator therapy | Mean total cost (2019 USD) | ICER (2019 USD) | Conclusion | Comments |
|---|---|---|---|---|---|---|---|---|
| Huntington, et al. [160] | USA | Routine follow-up including biannual CT | DLBCL patients in first remission | Routine follow-up with no imaging | Total per-patient costs: biannual CT, $45,723; no imaging, $42,084 | $181,000/QALY | Not cost-effective | Conclusions were robust across one-way and probabilistic sensitivity analyses |
| Routine follow-up including biannual PET/CT | DLBCL patients in first remission | Routine follow-up with no imaging | Total per-patient costs: biannual PET/CT, $46,778; no imaging, $42,084 | $186,000/QALY | Not cost-effective | Conclusions were robust across one-way and probabilistic sensitivity analyses |
Abbreviations: CT, computed tomography; DLBCL, diffuse large B-cell lymphoma; ICER, incremental cost-effectiveness ratio; PET, positron-emission tomography; QALY, quality-adjusted life year; USD, US dollars
5. Conclusion
DLBCL carries a substantial cost burden for patients and for the healthcare system. Total cost estimates are wide ranging and depend in large part on treatment modality. Standard first-line care alone incurs considerable expense. Patients with relapsed/refractory disease will require additional costly therapies including HCT and CAR T cells, leading to dramatic increases in per-patient fees and financial strain on hospitals. Exciting advances in DLBCL treatment and surveillance including novel precision therapies and ctDNA have potential to improve outcomes, but these technological advances may yield greater costs as well. DLBCL prevalence is likely to increase with the ageing population, and determination of cost-effective first-line and later-line therapies and surveillance modalities in DLBCL will require continued economic evaluation to limit the significant financial burden placed on patients and hospitals in the treatment of DLBCL.
6. Expert opinion
6.1. Applications to current and future practice
For a subset of DLBCL patients with high-risk disease, presently available therapies may offer improved outcomes or cure at great financial cost to patients, payers, and the healthcare system. The economic analyses addressed in this review should act alongside patient-level clinical characteristics and tumor biology to inform practice patterns at all stages of the DLBCL treatment pathway. Expensive therapies that prove curative for a given patient will likely be cost-effective at accepted willingness-to-pay thresholds, but clinical outcomes are uncertain in practice. Physicians should weigh potential patient-level treatment responses against societal financial burden. We have seen the implementation of cost-based quality improvement through the Choosing Wisely campaign. Future cost-based initiatives and clinical guidelines should similarly reflect the economic realities of expensive treatments and surveillance in management of DLBCL. Nevertheless, providing care for individual DLBCL patients requires identifying and implementing treatment strategies with the greatest likelihood of benefit to the individual.
6.2. Key areas for improvement
As investigation into targeted therapies for DLBCL subgroups continues, it remains unclear in what circumstances precision medicine approaches will prove to be cost-effective for this biologically heterogeneous disease. Subgroup-specific therapy will incur additional costs at multiple stages along the diagnostic and treatment pathways as clinicians identify appropriate patients for available therapies, and these additional steps will require evaluation from clinical and cost-based viewpoints. Ongoing research into genetic subgroups provides promising targets for future precision therapies, and current cost-based efforts should focus on anticipating expenses and streamlining diagnostic steps to reduce costs in associated clinical trials and future clinical workflow.
At present, we lack sufficient long-term follow-up data for robust evaluations of novel and expensive cellular therapies. Multiple estimations of cost-effectiveness for CAR T cells indicate the potential for cost-effectiveness in eligible patients, but current methods for evaluation rely on unproven assumptions to simulate a wide range of potential outcomes. We will not truly know the cost-effectiveness of currently available and future CAR T-cell therapies until we have reliable data regarding duration of durable response. While we wait for long-term follow-up, we should work to resolve remaining financial challenges related to pricing, reimbursement, and availability of CAR T-cell therapies.
6.3. Potential for future research
As the number of approved personalized treatment options expands in the coming years, issues of cost-effectiveness will become increasingly nuanced through the comparison of similar therapies targeting discrete subsets of the DLBCL patient population. In five years, we expect that individual precision therapeutics will require subgroup-specific cost and cost-effectiveness analyses for subtyping and treatment. Results of long-term follow-up with CAR T-cell therapy will enable true evaluation of cost-effectiveness. Additionally, answers to current financial challenges associated with CAR T cells may lead us to reevaluate our assumptions regarding cost and availability, necessitating additional studies of cost burden and cost-effectiveness. Surveillance techniques currently in development such as ctDNA will similarly require evaluations of scientific efficacy and cost-effectiveness in a clinical setting to identify preferred methods for interim and long-term follow-up. This remains an exciting time for novel treatment in the management of DLBCL, and economic analyses will play an important role in the development and selection of therapies in the next era of DLBCL care.
Article highlights:
The total cost burden of DLBCL is substantial for patients and the healthcare system.
Cost and cost-effectiveness analysis of novel precision therapies will be an essential component of their eventual clinical integration.
Treatment for relapsed and refractory DLBCL adds considerable expense through costly interventions such as hematopoietic cell transplant and chimeric antigen receptor (CAR) T-cell therapy.
Early CAR T-cell therapy cost-effectiveness evaluations indicate potential for cost-effectiveness despite high drug costs. Long-term follow-up data regarding duration of durable response is required for robust estimates, and additional financial challenges remain.
Routine surveillance imaging adds significant cost with no demonstrated survival benefit and is not a cost-effective form of long-term surveillance.
Acknowledgments
Funding
This work was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR002378, TL1TR002382, and by National Cancer Institution award K24CA208132 to CR Flowers. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of Interest
RA Harkins discloses that this work was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR002378 and TL1TR002382. CR Flowers discloses that this work was supported in part by the National Cancer Institution award K24CA208132. Dr. Flowers reports consulting from: Abbvie, Bayer, Celgene (unpaid), Denovo Biopharma, Genentech/Roche (unpaid), Gilead, OptumRx, Karyopharm, Pharmacyclics/ Janssen, Spectrum. Dr. Flowers reports research funding from: Abbvie, Acerta, Celgene, Gilead, Genentech/Roche, Janssen Pharmaceutical, Millennium/Takeda, Pharmacyclics, TG Therapeutics, Burroughs Wellcome Fund, Eastern Cooperative Oncology Group, National Cancer Institute, V Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Contributor Information
R. Andrew Harkins, Emory University School of Medicine, 100 Woodruff Circle, Atlanta, GA 30322.
Sharvil P. Patel, Emory University, 201 Dowman Drive, Atlanta, GA 30322
Christopher R. Flowers, Director, Emory University Lymphoma Program, Scientific Director, Winship Research Informatics Shared Resource, Emory University School of Medicine, Winship Cancer Institute, Department of Hematology and Oncology, 1365 Clifton Road NE B4016, Atlanta, GA 30322
References
- 1.Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA: a cancer journal for clinicians. 2016. September 12. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019. January;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 3.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA: a cancer journal for clinicians. 2019. June 11. [DOI] [PubMed] [Google Scholar]
- 4.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017. October 19;130(16):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000. February 3;403(6769):503–11. [DOI] [PubMed] [Google Scholar]
- 6.Reddy A, Zhang J, Davis NS, et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell. 2017. October 5;171(2):481–494. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitz R, Wright GW, Huang DW, et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med. 2018. April 12;378(15):1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nature medicine. 2018. May;24(5):679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arthur SE, Jiang A, Grande BM, et al. Genome-wide discovery of somatic regulatory variants in diffuse large B-cell lymphoma. Nature communications. 2018. October 1;9(1):4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heward JA, Kumar EA, Korfi K, et al. Precision medicine and lymphoma. Curr Opin Hematol. 2018. July;25(4):329–334. [DOI] [PubMed] [Google Scholar]
- 11.Patel SP, Harkins RA, Lee MJ, et al. Using Informatics Tools to Characterize Precision Medicine Treatments for Diffuse Large B-Cell Lymphoma (DLBCL) [Abstract]. Blood. 2018;132.29866817 [Google Scholar]
- 12.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017. December 28;377(26):2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCCN. National Comprehensive Cancer Center Clinical Practice Guidelines in Oncology (NCCN Guidelines): B-Cell Lymphomas, Version 3.2019. 2019. [Google Scholar]
- 14.Vaidya R, Witzig TE. Prognostic factors for diffuse large B-cell lymphoma in the R(X)CHOP era. Annals of oncology : official journal of the European Society for Medical Oncology. 2014. November;25(11):2124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitolo U, Trneny M, Belada D, et al. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017. November 1;35(31):3529–3537. [DOI] [PubMed] [Google Scholar]
- 16.Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized Phase II Study of R-CHOP With or Without Bortezomib in Previously Untreated Patients With Non-Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017. November 1;35(31):3538–3546. [DOI] [PubMed] [Google Scholar]
- 17.Younes A, Zinzani PL, Sehn LH, et al. A randomized, double-blind, placebo-controlled phase 3 study of ibrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in subjects with newly diagnosed nongerminal center B-cell subtype of diffuse large B-cell lymphoma (DLBCL). Journal of Clinical Oncology. 2014;32(15_suppl):TPS8615–TPS8615. [Google Scholar]
- 18.Staudt LM. Precision medicine based on the genetic taxonomy of DLBCL. Plenary Session 1: Are We Ready for Lymphoma MATCH Trials? American Association for Cancer Research2018. p. https://webcast.aacr.org/s/2018lym/PL01;jsessionid=9A4C05B787ED1034B60F428371A3957B.
- 19.Younes A How to design a MATCH trial for lymphoma. Plenary Session 1: Are We Ready for Lymphoma MATCH Trials? American Association for Cancer Research2018. p. https://webcast.aacr.org/s/2018lym/PL01;jsessionid=9A4C05B787ED1034B60F428371A3957B.
- 20.Cohen JB, Behera M, Thompson CA, et al. Evaluating surveillance imaging for diffuse large B-cell lymphoma and Hodgkin lymphoma. Blood. 2017. February 2;129(5):561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson CA, Charlson ME, Schenkein E, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Annals of oncology : official journal of the European Society for Medical Oncology. 2010. November;21(11):2262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson CA, Ghesquieres H, Maurer MJ, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014. November 1;32(31):3506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCI. FDA Approves Second CAR T-Cell Therapy for Lymphoma 2018. [cited 2019]. Available from: https://www.cancer.gov/news-events/cancer-currents-blog/2018/tisagenlecleucel-fda-lymphoma [DOI] [PubMed]
- 24.Sehn LH, Herrera AF, Matasar MJ, et al. Polatuzumab Vedotin (Pola) Plus Bendamustine (B) with Rituximab (R) or Obinutuzumab (G) in Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL): Updated Results of a Phase (Ph) Ib/II Study. 2018;132(Suppl 1):1683–1683. [Google Scholar]
- 25.Flowers CR, Sinha R, Vose JM. Improving outcomes for patients with diffuse large B-cell lymphoma. CA: a cancer journal for clinicians. 2010. Nov-Dec;60(6):393–408. [DOI] [PubMed] [Google Scholar]
- 26.Maurer MJ, Ghesquieres H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014. April 1;32(10):1066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017. December 28;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019. January 3;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 29.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. Journal of the National Cancer Institute. 2011. January 19;103(2):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NCI. NCI Cancer Trends Progress Report: Financial Burden of Cancer Care 2019. [cited 2019]. Available from: https://progressreport.cancer.gov/after/economic_burden
- 31.XE. XE Current and Historical Rate Tables [cited 2019]. Available from: https://www.xe.com/currencytables/
- 32.BLS. US Bureau of Labor Statistics Consumer Price Index Inflation Calculator [cited 2019]. Available from: https://www.bls.gov/data/inflation_calculator.htm
- 33.Wang HI, Smith A, Aas E, et al. Treatment cost and life expectancy of diffuse large B-cell lymphoma (DLBCL): a discrete event simulation model on a UK population-based observational cohort. The European journal of health economics : HEPAC : health economics in prevention and care. 2017. March;18(2):255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison VA, Bell JA, Hamilton L, et al. Economic burden of patients with diffuse large B-cell and follicular lymphoma treated in the USA. Future oncology (London, England). 2018. October;14(25):2627–2642. [DOI] [PubMed] [Google Scholar]
- 35.Ren J, Asche CV, Shou Y, et al. Economic burden and treatment patterns for patients with diffuse large B-cell lymphoma and follicular lymphoma in the USA. Journal of comparative effectiveness research. 2019. April;8(6):393–402. [DOI] [PubMed] [Google Scholar]
- 36.Costa S, Scott DW, Steidl C, et al. Real-world costing analysis for diffuse large B-cell lymphoma in British Columbia. Current oncology (Toronto, Ont). 2019. April;26(2):108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee RC, Zou D, Demetrick DJ, et al. Costs associated with diffuse large B-cell lymphoma patient treatment in a Canadian integrated cancer care center. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2008. Mar-Apr;11(2):221–30. [DOI] [PubMed] [Google Scholar]
- 38.Kutikova L, Bowman L, Chang S, et al. Medical costs associated with non-Hodgkin’s lymphoma in the United States during the first two years of treatment. Leukemia & lymphoma. 2006. August;47(8):1535–44. [DOI] [PubMed] [Google Scholar]
- 39.Juul MB, Jensen PH, Engberg H, et al. Treatment strategies and outcomes in diffuse large B-cell lymphoma among 1011 patients aged 75 years or older: A Danish population-based cohort study. Eur J Cancer. 2018. August;99:86–96. [DOI] [PubMed] [Google Scholar]
- 40.Flowers CR, Armitage JO. A decade of progress in lymphoma: advances and continuing challenges. Clinical lymphoma, myeloma & leukemia. 2010. December;10(6):414–23. [DOI] [PubMed] [Google Scholar]
- 41.Coiffier B, Lepage E, Brière J, et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. New England Journal of Medicine. 2002;346(4):235–242. [DOI] [PubMed] [Google Scholar]
- 42.Feugier P, Hoof AV, Sebban C, et al. Long-Term Results of the R-CHOP Study in the Treatment of Elderly Patients With Diffuse Large B-Cell Lymphoma: A Study by the Groupe d’Etude des Lymphomes de l’Adulte. Journal of Clinical Oncology. 2005;23(18):4117–4126. [DOI] [PubMed] [Google Scholar]
- 43.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010. September 23;116(12):2040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP Versus CHOP Alone or With Maintenance Rituximab in Older Patients With Diffuse Large B-Cell Lymphoma. Journal of Clinical Oncology. 2006;24(19):3121–3127. [DOI] [PubMed] [Google Scholar]
- 45.Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. The Lancet Oncology. 2006. May;7(5):379–91. [DOI] [PubMed] [Google Scholar]
- 46.Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. The Lancet Oncology. 2011. October;12(11):1013–22. [DOI] [PubMed] [Google Scholar]
- 47.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125(1):22–32. [DOI] [PubMed] [Google Scholar]
- 48.Byfield SD, Becker LK, Small A. Differences In Treatment Patterns and Costs Among Diffuse Large B-Cell Lymphoma Patients Treated In The Clinic Vs. The Hospital Outpatient Setting. Blood. 2013;122(21):1751–1751. [Google Scholar]
- 49.Kim J, Malin JL, Doan QV, et al. Cost-Effectiveness of Primary Prophylaxis with Pegfilgrastim Versus Filgrastim in Non-Hodgkin’s Lymphoma Patients Receiving CHOP-21. Blood. 2007;110(11):5166–5166. [Google Scholar]
- 50.Lyman G, Lalla A, Barron R, et al. Cost-effectiveness of pegfilgrastim versus 6-day filgrastim primary prophylaxis in patients with non-Hodgkin’s lymphoma receiving CHOP-21 in United States. Current medical research and opinion. 2009. February;25(2):401–11. [DOI] [PubMed] [Google Scholar]
- 51.Lathia N, Isogai PK, De Angelis C, et al. Cost-effectiveness of filgrastim and pegfilgrastim as primary prophylaxis against febrile neutropenia in lymphoma patients. Journal of the National Cancer Institute. 2013. August 7;105(15):1078–85. [DOI] [PubMed] [Google Scholar]
- 52.Hill G, Barron R, Fust K, et al. Primary vs secondary prophylaxis with pegfilgrastim for the reduction of febrile neutropenia risk in patients receiving chemotherapy for non-Hodgkin’s lymphoma: cost-effectiveness analyses. Journal of medical economics. 2014. January;17(1):32–42. [DOI] [PubMed] [Google Scholar]
- 53.Wang XJ, Tang T, Farid M, et al. Routine Primary Prophylaxis for Febrile Neutropenia with Biosimilar Granulocyte Colony-Stimulating Factor (Nivestim) or Pegfilgrastim Is Cost Effective in Non-Hodgkin Lymphoma Patients undergoing Curative-Intent R-CHOP Chemotherapy. PloS one. 2016;11(2):e0148901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fust K, Li X, Maschio M, et al. Cost-Effectiveness Analysis of Prophylaxis Treatment Strategies to Reduce the Incidence of Febrile Neutropenia in Patients with Early-Stage Breast Cancer or Non-Hodgkin Lymphoma. PharmacoEconomics. 2017. April;35(4):425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan KK, Siu E, Krahn MD, et al. Cost-utility analysis of primary prophylaxis versus secondary prophylaxis with granulocyte colony-stimulating factor in elderly patients with diffuse aggressive lymphoma receiving curative-intent chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012. April 1;30(10):1064–71. [DOI] [PubMed] [Google Scholar]
- 56.Doorduijn JK, Buijt I, van der Holt B, et al. Economic evaluation of prophylactic granulocyte colony stimulating factor during chemotherapy in elderly patients with aggressive non-Hodgkin’s lymphoma. Haematologica. 2004. September;89(9):1109–17. [PubMed] [Google Scholar]
- 57.Best JH, Hornberger J, Proctor SJ, et al. Cost-effectiveness analysis of rituximab combined with chop for treatment of diffuse large B-cell lymphoma. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2005. Jul-Aug;8(4):462–70. [DOI] [PubMed] [Google Scholar]
- 58.Groot MT, Lugtenburg PJ, Hornberger J, et al. Cost-effectiveness of rituximab (MabThera) in diffuse large B-cell lymphoma in The Netherlands. European journal of haematology. 2005. March;74(3):194–202. [DOI] [PubMed] [Google Scholar]
- 59.Hornberger JC, Best JH. Cost utility in the United States of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone for the treatment of elderly patients with diffuse large B-cell lymphoma. Cancer. 2005. April 15;103(8):1644–51. [DOI] [PubMed] [Google Scholar]
- 60.Ferrara F, Ravasio R. Cost-effectiveness analysis of the addition of rituximab to CHOP in young patients with good-prognosis diffuse large-B-cell lymphoma. Clinical drug investigation. 2008;28(1):55–65. [DOI] [PubMed] [Google Scholar]
- 61.Johnston KM, Marra CA, Connors JM, et al. Cost-effectiveness of the addition of rituximab to CHOP chemotherapy in first-line treatment for diffuse large B-cell lymphoma in a population-based observational cohort in British Columbia, Canada. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2010. Sep-Oct;13(6):703–11. [DOI] [PubMed] [Google Scholar]
- 62.Griffiths RI, Gleeson ML, Mikhael J, et al. Comparative effectiveness and cost of adding rituximab to first-line chemotherapy for elderly patients diagnosed with diffuse large B-cell lymphoma. Cancer. 2012. December 15;118(24):6079–88. [DOI] [PubMed] [Google Scholar]
- 63.Khor S, Beca J, Krahn M, et al. Real world costs and cost-effectiveness of Rituximab for diffuse large B-cell lymphoma patients: a population-based analysis. BMC cancer. 2014. August 12;14:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coyne GO, Takebe N, Chen AP. Defining precision: The precision medicine initiative trials NCI-MPACT and NCI-MATCH. Current problems in cancer. 2017. May-Jun;41(3):182–193. [DOI] [PubMed] [Google Scholar]
- 65.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002. June 20;346(25):1937–47. [DOI] [PubMed] [Google Scholar]
- 66.Read JA, Koff JL, Nastoupil LJ, et al. Evaluating cell-of-origin subtype methods for predicting diffuse large B-cell lymphoma survival: a meta-analysis of gene expression profiling and immunohistochemistry algorithms. Clinical lymphoma, myeloma & leukemia. 2014. December;14(6):460–467. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koff JL, Flowers CR. Prognostic modeling in diffuse large B-cell lymphoma in the era of immunochemotherapy: Where do we go from here? Cancer. 2017. September 1;123(17):3222–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davies A, Cummin TE, Barrans S, et al. Gene-expression profiling of bortezomib added to standard chemoimmunotherapy for diffuse large B-cell lymphoma (REMoDL-B): an open-label, randomised, phase 3 trial. The Lancet Oncology. 2019. May;20(5):649–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Younes A, Sehn LH, Johnson P, et al. Randomized Phase III Trial of Ibrutinib and Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Non-Germinal Center B-Cell Diffuse Large B-Cell Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019. May 20;37(15):1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vitolo U, Witzig TE, Gascoyne RD, et al. ROBUST: First report of phase III randomized study of lenalidomide/R-CHOP (R2-CHOP) vs placebo/R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma. Hematological Oncology. 2019;37(S2):36–37. [Google Scholar]
- 71.Nowakowski GS, Hong F, Scott DW, et al. ADDITION OF LENALIDOMIDE TO R-CHOP (R2CHOP) IMPROVES OUTCOMES IN NEWLY DIAGNOSED DIFFUSE LARGE B-CELL LYMPHOMA (DLBCL): FIRST REPORT OF ECOG-ACRIN1412 A RANDOMIZED PHASE 2 US INTERGROUP STUDY OF R2CHOP VS R-CHOP. Hematological Oncology. 2019;37(S2):37–38. [Google Scholar]
- 72.Lohr JG, Stojanov P, Lawrence MS, et al. Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing. Proc Natl Acad Sci U S A. 2012. March 6;109(10):3879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011. July 27;476(7360):298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasqualucci L, Trifonov V, Fabbri G, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nature genetics. 2011. July 31;43(9):830–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Grubor V, Love CL, et al. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci U S A. 2013. January 22;110(4):1398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010. July 10;28(20):3360–5. [DOI] [PubMed] [Google Scholar]
- 77.Ennishi D, Jiang A, Boyle M, et al. Double-Hit Gene Expression Signature Defines a Distinct Subgroup of Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019. January 20;37(3):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siu LL, Conley BA, Boerner S, et al. Next-Generation Sequencing to Guide Clinical Trials. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015. October 15;21(20):4536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pasqualucci L The genetic basis of diffuse large B-cell lymphoma. Curr Opin Hematol. 2013. July;20(4):336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang B, Mehran RJ, Heymach JV, et al. Predictive biomarkers in precision medicine and drug development against lung cancer. Chin J Cancer. 2015. July 2;34(7):295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ribrag V, Soria J, Michot J, Schmitt A, Postel-Vinay S, Bijou F, Thomson B, Keilhack H, Blakemore SJ, Reyderman L, Kumar P, Fine G, McDonald A, Ho PT , Italiano A Phase 1 Study of Tazemetostat (EPZ-6438), an Inhibitor of Enhancer of Zeste-Homolog 2 (EZH2): Preliminary Safety and Activity in Relapsed or Refractory Non-Hodgkin Lymphoma (NHL) Patients [[Abstract]]. Blood. 2015;126(23):473. [Google Scholar]
- 82.Vermaat JS, Pals ST, Younes A, et al. Precision medicine in diffuse large B-cell lymphoma: hitting the target. Haematologica. 2015. August;100(8):989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bowdin S, Gilbert A, Bedoukian E, et al. Recommendations for the integration of genomics into clinical practice. Genet Med. 2016. November;18(11):1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He J, Abdel-Wahab O, Nahas MK, et al. Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood. 2016. June 16;127(24):3004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warner JL, Jain SK, Levy MA. Integrating cancer genomic data into electronic health records. Genome Med. 2016. October 26;8(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blombery P, Lickiss J, Dickinson M. The price of success-health economics of personalized diffuse large B-cell lymphoma treatment. Leukemia & lymphoma. 2018. July;59(7):1517–1519. [DOI] [PubMed] [Google Scholar]
- 87.King RL, Nowakowski GS, Witzig TE, et al. Rapid, real time pathology review for ECOG/ACRIN 1412: a novel and successful paradigm for future lymphoma clinical trials in the precision medicine era. Blood Cancer J. 2018. February 28;8(3):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nowakowski GS, Feldman T, Rimsza LM, et al. Integrating precision medicine through evaluation of cell of origin in treatment planning for diffuse large B-cell lymphoma. Blood Cancer J. 2019. May 16;9(6):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Q, Staton AD, Ayer T, et al. Exploring the potential cost-effectiveness of precision medicine treatment strategies for diffuse large B-cell lymphoma. Leukemia & lymphoma. 2018. July;59(7):1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide Combined With R-CHOP Overcomes Negative Prognostic Impact of Non–Germinal Center B-Cell Phenotype in Newly Diagnosed Diffuse Large B-Cell Lymphoma: A Phase II Study. Journal of Clinical Oncology. 2015;33(3):251–257. [DOI] [PubMed] [Google Scholar]
- 91.Dahi PB, Moskowitz CH, Giralt SA, et al. Novel agents may positively impact chemotherapy and transplantation in subsets of diffuse large B-cell lymphoma. Expert review of hematology. 2019. March 18:1–12. [DOI] [PubMed] [Google Scholar]
- 92.Danese MD, Griffiths RI, Gleeson ML, et al. Second-line therapy in diffuse large B-cell lymphoma (DLBCL): treatment patterns and outcomes in older patients receiving outpatient chemotherapy. Leukemia & lymphoma. 2017. May;58(5):1094–1104. [DOI] [PubMed] [Google Scholar]
- 93.Slawsky KA, Goss TF, Shinkle R, et al. Economic burden of relapsed/refractory diffuse large B-cell non-Hodgkin’s lymphoma (DLBCL): A review of the literature. Journal of Clinical Oncology. 2010;28(15_suppl):e18564–e18564. [Google Scholar]
- 94.Huntington S, Keshishian A, McGuire M, et al. Costs of relapsed diffuse large B-cell lymphoma among Medicare patients. Leukemia & lymphoma. 2018. December;59(12):2880–2887. [DOI] [PubMed] [Google Scholar]
- 95.Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995. December 7;333(23):1540–5. [DOI] [PubMed] [Google Scholar]
- 96.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010. September 20;28(27):4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Epperla N, Hamadani M. Hematopoietic cell transplantation for diffuse large B-cell and follicular lymphoma: Current controversies and advances. Hematology/oncology and stem cell therapy. 2017. December;10(4):277–284. [DOI] [PubMed] [Google Scholar]
- 98.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012. August 23;120(8):1545–51. [DOI] [PubMed] [Google Scholar]
- 99.Preussler JM, Denzen EM, Majhail NS. Costs and cost-effectiveness of hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012. November;18(11):1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bentley TS, Phillips SJ. Milliman Research Report: 2017 U.S. organ and tissue transplant cost estimates and discussion. 2017. [Google Scholar]
- 101.Hubel K, Ostermann H, Glass B, et al. Plerixafor in non-Hodgkin’s lymphoma patients: a German analysis of time, effort and costs. Bone marrow transplantation. 2019. January;54(1):123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tichopad A, Vitova V, Koristek Z, et al. Cost-effectiveness of hematopoietic stem cell mobilization strategies including plerixafor in multiple myeloma and lymphoma patients. Journal of clinical apheresis. 2013. December;28(6):395–403. [DOI] [PubMed] [Google Scholar]
- 103.Micallef IN, Sinha S, Gastineau DA, et al. Cost-effectiveness analysis of a risk-adapted algorithm of plerixafor use for autologous peripheral blood stem cell mobilization. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013. January;19(1):87–93. [DOI] [PubMed] [Google Scholar]
- 104.Costa LJ, Kramer C, Hogan KR, et al. Pegfilgrastim- versus filgrastim-based autologous hematopoietic stem cell mobilization in the setting of preemptive use of plerixafor: efficacy and cost analysis. Transfusion. 2012. November;52(11):2375–81. [DOI] [PubMed] [Google Scholar]
- 105.Watts NL, Marques MB, Peavey DB, et al. Mobilization of Hematopoietic Progenitor Cells for Autologous Transplantation Using Pegfilgrastim and Plerixafor: Efficacy and Cost Implications. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2019. February;25(2):233–238. [DOI] [PubMed] [Google Scholar]
- 106.Sung AD, Grima DT, Bernard LM, et al. Outcomes and costs of autologous stem cell mobilization with chemotherapy plus G-CSF vs G-CSF alone. Bone marrow transplantation. 2013. November;48(11):1444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cho SK, McCombs J, Punwani N, et al. Complications and hospital costs during hematopoietic stem cell transplantation for non-Hodgkin lymphoma in the United States. Leukemia & lymphoma. 2019. March 8:1–7. [DOI] [PubMed] [Google Scholar]
- 108.Nakamura A, Kojima Y, Miyazawa K, et al. Clinical Impact of Aprepitant in Patients Receiving High-Dose Chemotherapy prior to Autologous Peripheral Blood Stem Cell Transplantation: A Cost-Effectiveness Analysis. Oncology. 2017;93(5):302–308. [DOI] [PubMed] [Google Scholar]
- 109.Nooka AK, Johnson HR, Kaufman JL, et al. Pharmacoeconomic analysis of palifermin to prevent mucositis among patients undergoing autologous hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014. June;20(6):852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ianotto JC, Ngo Sack F, Couturier MA, et al. Biosimilars of filgrastim in autologous stem cell transplant: reduction in granulocyte-colony stimulating factor costs, but similar effects on bone marrow recovery. Leukemia & lymphoma. 2014. January;55(1):74–7. [DOI] [PubMed] [Google Scholar]
- 111.Sheth V, Gore A, Jain R, et al. Pegfilgrastim: More Cost Effective and Equally Efficacious Option as Compared to Filgrastim in Autologous Stem Cell Transplant. Indian journal of hematology & blood transfusion : an official journal of Indian Society of Hematology and Blood Transfusion. 2019. January;35(1):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sebban C, Lefranc A, Perrier L, et al. A randomised phase II study of the efficacy, safety and cost-effectiveness of pegfilgrastim and filgrastim after autologous stem cell transplant for lymphoma and myeloma (PALM study). European journal of cancer (Oxford, England : 1990). 2012. March;48(5):713–20. [DOI] [PubMed] [Google Scholar]
- 113.Reid RM, Baran A, Friedberg JW, et al. Outpatient administration of BEAM conditioning prior to autologous stem cell transplantation for lymphoma is safe, feasible, and cost-effective. Cancer medicine. 2016. November;5(11):3059–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheung MC, Hay AE, Crump M, et al. Gemcitabine/dexamethasone/cisplatin vs cytarabine/dexamethasone/cisplatin for relapsed or refractory aggressive-histology lymphoma: cost-utility analysis of NCIC CTG LY.12. Journal of the National Cancer Institute. 2015. July;107(7). [DOI] [PubMed] [Google Scholar]
- 115.Kymes SM, Pusic I, Lambert DL, et al. Economic evaluation of plerixafor for stem cell mobilization. The American journal of managed care. 2012. January;18(1):33–41. [PMC free article] [PubMed] [Google Scholar]
- 116.Maziarz RT, Hao Y, Guerin A, et al. Economic burden following allogeneic hematopoietic stem cell transplant in patients with diffuse large B-cell lymphoma. Leukemia & lymphoma. 2018. May;59(5):1133–1142. [DOI] [PubMed] [Google Scholar]
- 117.Chavez JC, Bachmeier C, Kharfan-Dabaja MA. CAR T-cell therapy for B-cell lymphomas: clinical trial results of available products. Therapeutic advances in hematology. 2019;10:2040620719841581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chow VA, Shadman M, Gopal AK. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood. 2018. August 23;132(8):777–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O’Leary MC, Lu X, Huang Y, et al. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019. February 15;25(4):1142–1146. [DOI] [PubMed] [Google Scholar]
- 120.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018. February 1;378(5):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 Results of ZUMA-1: A Multicenter Study of KTE-C19 Anti-CD19 CAR T Cell Therapy in Refractory Aggressive Lymphoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2017. January 4;25(1):285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abramson JS, Gordon LI, Palomba ML, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. Journal of Clinical Oncology. 2018;36(15_suppl):7505–7505. [Google Scholar]
- 123.Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nature reviews Clinical oncology. 2018. January;15(1):31–46. [DOI] [PubMed] [Google Scholar]
- 124.Havard R, Stephens DM. Anti-CD19 Chimeric Antigen Receptor T Cell Therapies: Harnessing the Power of the Immune System to Fight Diffuse Large B Cell Lymphoma. Current hematologic malignancy reports. 2018. December;13(6):534–542. [DOI] [PubMed] [Google Scholar]
- 125.Corporation NP. Prescribing Information: Kymriah [cited 2019]. Available from: https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/kymriah.pdf
- 126.Kite Pharma I. Prescribing Information: Yescarta [cited 2019]. Available from: https://www.gilead.com/-/media/files/pdfs/medicines/oncology/yescarta/yescarta-pi.pdf
- 127.Palomba ML, Garcia J, Wang L, et al. TRANSCEND: Lisocabtagene Maraleucel (liso-cel; JCAR017) Healthcare Resource Utilization in Patients with Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL). Blood. 2018;132(Suppl 1):3545–3545. [Google Scholar]
- 128.Chabannon C, Kuball J, McGrath E, et al. CAR-T cells: the narrow path between hope and bankruptcy? Bone marrow transplantation. 2017. December;52(12):1588–1589. [DOI] [PubMed] [Google Scholar]
- 129.Stadtmauer E, Mangan PA. CAR T-Cell Therapy: On the Verge of Breakthrough in Many Hematologic Malignancies. Journal of the advanced practitioner in oncology. 2017. April;8(3):228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cavallo J Weighing the Cost and Value of CAR T-Cell Therapy: A Roundtable Discussion With Carl H. June, MD; Sagar Lonial, MD; David G. Maloney, MD, PhD; and Pascal Touchon The ASCO Post 2018. [cited 2019]. Available from: https://www.ascopost.com/issues/may-25-2018/weighing-the-cost-and-value-of-car-t-cell-therapy/
- 131.Flowers CR, Ramsey SD. What Can Cost-Effectiveness Analysis Tell Us About Chimeric Antigen Receptor T-Cell Therapy for Relapsed Acute Lymphoblastic Leukemia? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018. October 2:Jco2018793570. [DOI] [PubMed] [Google Scholar]
- 132.Prasad V, De Jesus K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nature reviews Clinical oncology. 2017. June;14(6):381–390. [DOI] [PubMed] [Google Scholar]
- 133.Costantini S, Walensky RP. The Costs of Drugs in Infectious Diseases: Branded, Generics, and Why We Should Care. The Journal of infectious diseases. 2019. March 19. [DOI] [PubMed] [Google Scholar]
- 134.Yip A, Webster RM. The market for chimeric antigen receptor T cell therapies. Nature reviews Drug discovery. 2018. March;17(3):161–162. [DOI] [PubMed] [Google Scholar]
- 135.Helwick C NCCN Roundtable Tackles Issues With Innovative Immunotherapies The ASCO Post 2019. [cited 2019]. Available from: https://www.ascopost.com/issues/may-25-2019/nccn-roundtable-tackles-issues-with-innovative-immunotherapies/
- 136.Snider J, Brauer M, Hao Y, et al. The Economic Value of CTL019 Therapy for Pediatric Patients with Relapsed and Refractory Acute Lymphoblastic Leukemia in the United Kingdom. Blood. 2017;130(Suppl 1):1330–1330. [Google Scholar]
- 137.Hao Y, Eldjerou LK, Yang H, et al. Cost-Effectiveness Analysis of CTL019 for the Treatment of Pediatric and Young Adult Patients with Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia in the United States. Blood. 2017;130(Suppl 1):609–609. [Google Scholar]
- 138.Lin JK, Lerman BJ, Barnes JI, et al. Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Relapsed or Refractory Pediatric B-Cell Acute Lymphoblastic Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018. September 13:Jco2018790642. [DOI] [PubMed] [Google Scholar]
- 139.Sarkar RR, Gloude NJ, Schiff D, et al. Cost-Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Pediatric Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. Journal of the National Cancer Institute. 2018. December 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term Survival and Value of Chimeric Antigen Receptor T-Cell Therapy for Pediatric Patients With Relapsed or Refractory Leukemia. JAMA pediatrics. 2018. December 1;172(12):1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.NICE. National Insitute for Health and Care Excellence: Tisagenlecleucel for treating relapsed or refractory B-cell acute lymphoblastic leukaemia in people aged up to 25 years 2018 [cited 2019]. Available from: https://www.nice.org.uk/guidance/ta554/chapter/3-Committee-discussion#costeffectiveness-estimate
- 142.Tice JA, Walsh JME, Otuonye I, et al. Institute for Clinical and Economic Review: Chimeric Antigen Receptor T-Cell Therapy for BCell Cancers: Effectiveness and Value. 2018.
- 143.Roth JA, Sullivan SD, Lin VW, et al. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. Journal of medical economics. 2018. December;21(12):1238–1245. [DOI] [PubMed] [Google Scholar]
- 144.NICE. National Institute for Health and Care Excellence: Axicabtagene ciloleucel for treating diffuse large B-cell lymphoma and primary mediastinal large B-cell lymphoma after 2 or more systemic therapies 2019 [cited 2019]. Available from: https://www.nice.org.uk/guidance/ta559/chapter/3-Committee-discussion#cost-effectiveness
- 145.NICE. National Institute for Health and Care Excellence: Tisagenlecleucel for treating relapsed or refractory diffuse large B-cell lymphoma after 2 or more systemic therapies 2019 [cited 2019]. Available from: https://www.nice.org.uk/guidance/ta567/chapter/3-Committee-discussion#cost-effectiveness
- 146.Whittington MD, McQueen RB, Ollendorf DA, et al. Long-term Survival and Cost-effectiveness Associated With Axicabtagene Ciloleucel vs Chemotherapy for Treatment of B-Cell Lymphoma. JAMA network open. 2019. February 1;2(2):e190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin JK, Muffly LS, Spinner MA, et al. Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Multiply Relapsed or Refractory Adult Large B-Cell Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019. June 3:Jco1802079. [DOI] [PubMed] [Google Scholar]
- 148.Bach PB, Giralt SA, Saltz LB. FDA Approval of Tisagenlecleucel: Promise and Complexities of a $475000 Cancer Drug. Jama. 2017. November 21;318(19):1861–1862. [DOI] [PubMed] [Google Scholar]
- 149.Worcester S CAR T-cell therapy: Moving from cost to value 2017. Available from: https://www.mdedge.com/hematologynews/nhlhub/article/152563/all/car-t-cell-therapy-moving-cost-value
- 150.CMS. CMS proposes Coverage with Evidence Development for Chimeric Antigen Receptor (CAR) T-cell Therapy 2019. [cited 2019]. Available from: https://www.cms.gov/newsroom/press-releases/cms-proposes-coverage-evidence-development-chimeric-antigen-receptor-car-t-cell-therapy
- 151.CMS. CMS STATEMENT: Delay in Final Chimeric Antigen Receptor (CAR) T-cell therapy National Coverage Determination 2019. [cited 2019]. Available from: https://www.cms.gov/newsroom/press-releases/cms-statement-delay-final-chimeric-antigen-receptor-car-t-cell-therapy-national-coverage
- 152.CMS. Fiscal Year (FY) 2020 Medicare Hospital Inpatient Prospective Payment System (IPPS) and Long Term Acute Care Hospital (LTCH) Prospective Payment System Proposed Rule and Request for Information [cited 2019]. Available from: https://www.cms.gov/newsroom/fact-sheets/fiscal-year-fy-2020-medicare-hospital-inpatient-prospective-payment-system-ipps-and-long-term-acute
- 153.Atilla E, Kilic P, Gurman G. Cellular therapies: Day by day, all the way. Transfusion and apheresis science : official journal of the World Apheresis Association : official journal of the European Society for Haemapheresis. 2018. April;57(2):187–196. [DOI] [PubMed] [Google Scholar]
- 154.Baruch EN, Berg AL, Besser MJ, et al. Adoptive T cell therapy: An overview of obstacles and opportunities. Cancer. 2017. June 1;123(S11):2154–2162. [DOI] [PubMed] [Google Scholar]
- 155.Zhao J, Song Y, Liu D. Clinical trials of dual-target CAR T cells, donor-derived CAR T cells, and universal CAR T cells for acute lymphoid leukemia. Journal of hematology & oncology. 2019;12(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cohen JB, Kurtz DM, Staton AD, et al. Next-generation surveillance strategies for patients with lymphoma. Future oncology (London, England). 2015;11(13):1977–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely(R) campaign: five hematologic tests and treatments to question. Blood. 2013. December 5;122(24):3879–83. [DOI] [PubMed] [Google Scholar]
- 158.Cheson BD. Staging and response assessment in lymphomas: the new Lugano classification. Chinese clinical oncology. 2015. March;4(1):5. [DOI] [PubMed] [Google Scholar]
- 159.Cheung MC, Mittmann N, Earle CC, et al. Are We Choosing Wisely in Lymphoma? Excessive Use of Surveillance CT Imaging in Patients With Diffuse Large B-cell Lymphoma (DLBCL) in Long-term Remission. Clinical lymphoma, myeloma & leukemia. 2018. January;18(1):e27–e34. [DOI] [PubMed] [Google Scholar]
- 160.Huntington SF, Svoboda J, Doshi JA. Cost-effectiveness analysis of routine surveillance imaging of patients with diffuse large B-cell lymphoma in first remission. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015. May 1;33(13):1467–74. [DOI] [PubMed] [Google Scholar]