Abstract
The Th2 cytokines interleukin 4 (IL-4) and IL-13 and the heterodimeric IL-4 receptor (IL-4R) complexes that they interact with play a key role in the pathogenesis of allergic disorders. Dupilumab is a humanized IgG4 monoclonal antibody that targets the IL-4 receptor alpha chain (IL-4Rα), common to both IL-4R complexes: type 1 (IL-4Rα/γc; IL-4 specific) and type 2 (IL-4Rα/IL-13Rα1; IL-4 and IL-13 specific). In this review we detail the current state of knowledge of the different signaling pathways coupled to the IL-4R complexes, examine the possible mechanisms of Dupilumab action and survey its clinical efficacy in different allergic disorders. The development of Dupilumab and the widening spectrum of its clinical applications is relevant to the current emphasis on precision medicine approaches to the blockade of pathways involved in allergic diseases.
Introduction
The ongoing epidemic of allergic diseases is a major public health problem affecting individuals in the developed and developing countries 1,2. While these diseases, including atopic dermatitis, asthma, food allergy and others, represent a heterogenous set of disorders affecting different target tissues, they do share fundamental mechanisms of allergic inflammation 1,3. Concerted immunological and genetic studies have delineated allergic inflammatory pathways common to these disorders and driving pathogenesis, key among which is the interleukin 4 receptor (IL-4R) pathway 4,5. The centrality of this pathway in allergic inflammation stems from the critical role played by its ligands IL-4 and IL-13 in orchestrating the allergic response 6. The IL-4/IL-13/IL-4R axis promotes T helper cells type 2 (TH2) differentiation, which mediate the pro-allergic adaptive immune response 7,8. It also activates effector pathways in target tissues including the lung, skin and gut that give rise to the expression of the respective disease attributes 5. These unique properties of the IL-4R axis made it an especially appealing target for precision medicine interventions that aim to interrupt the allergic inflammatory response and attenuate or abrogate disease chronicity and severity. While several biologics targeted at the IL-4R axis have been developed and tested, Dupilumab, a human monoclonal antibody that binds interleukin 4 (IL-4) receptor alpha subunit, has emerged as one of the most successful therapies targeting this axis 8. In this review, we will survey the biology of the IL-4R axis in driving allergic inflammation and its antagonism by Dupilumab as relates to different allergic diseases
IL-4Rα Signaling and mechanism of action.
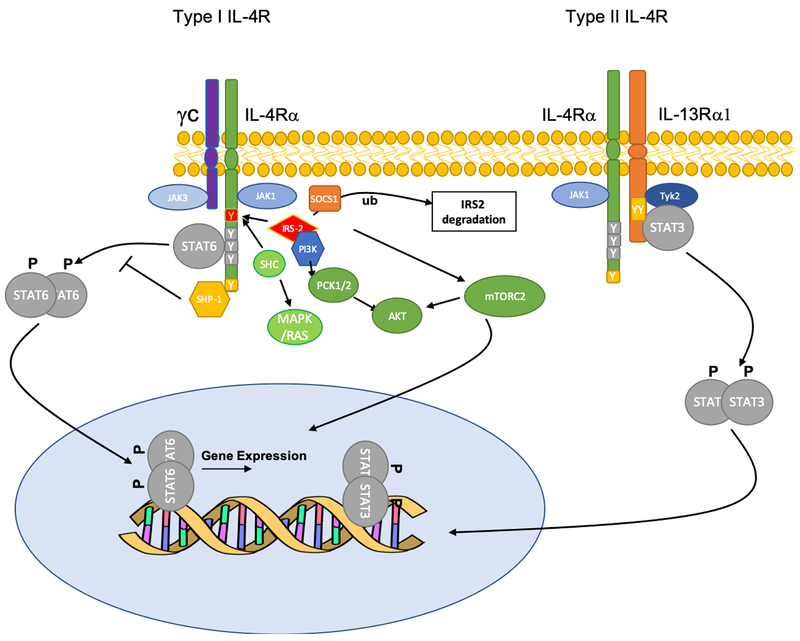
The IL-4R complex is a heterodimeric structure composed of a common subunit, the IL-4Rα, which pairs with distinct auxiliary subunits to mediate the action of IL-4 and IL-13 in different tissues 9. IL-4Rα pairs with the γc chain to form the IL-4R type I, which is expressed on hematopoietic cells and binds IL-4 exclusively 9. Further, it associates with the low affinity binding receptor for IL-13, IL-13Rα1, to form a high affinity IL-13- and IL-4-binding type II heterodimeric complex that is expressed on both hematopoietic and non-hematopoietic cells, such as the airway epithelium (Figure. 1) 7,10. The canonical signaling pathway via the IL-4R complexes has been established 5,9. Once IL-4 or IL-13 binds to the receptors, it triggers the trans-phosphorylation and activation of receptor subunit-associated Janus family protein kinases (JAKs), including JAK1, JAK3 and JAK2, associated with the IL-4Rα, γc and IL-13Rα1 chains, respectively. JAK activation initiates a cascade of phosphorylation of specific tyrosine residues in the cytoplasmic domain of the IL-4Rα 11. A trio of these tyrosine residues (human IL-4Rα Y575,/Y603/Y633 and their mouse counterpart) act as a cassette whose phosphorylation enables the recruitment of the transcription factor signal transducer and activator of transcription 6 (STAT6) through the SH2 domain of the latter, leading to its activation and the initiation of transcription programs regulated by it 12,13. Furthermore, it was found that Box1 region and the C-terminal tail of IL-13R alpha 1 were critical for binding to Tyk2 and thus activation of STAT3 14. IL13 binding to IL-13Rα1 in the IL-4R complex induces the phosphorylation of Tyk2 and thus the phosphorylation of STAT3 15.
Figure 1.
Signal transduction via the type I and II IL-4R complexes. IL-4R type I receptor, composed of IL-4Rα/γc heterodimers, binds IL-4 exclusively while the type II, composed of IL-4Rα/IL-13Rα1, binds both IL-4 and IL-13. Ligand binding triggers the trans-phosphorylation and activation of receptor subunit-associated JAK kinases, including JAK1/JAK3 (type I receptor) and JAK1/Tyk2 (type II receptor). JAK activation initiates a cascade of phosphorylation of specific tyrosine residues in the cytoplasmic domain of the IL-4Rα, from which emanates different signaling pathways including STAT6, IRS/PI3K/mTORC2/AKT, SHC/MAPK and Shp-1. Additional pathways implicated in receptor signaling include STAT3 activation via IL-13Ra1 and IRS2 regulation by Socs1/Ubiquitin (Ub).
In addition to STAT6 activation, the IL-4R complexes activate other signaling pathways that contribute to the regulation of allergic responses. One pathway involves the binding of the insulin receptor substrate 1/2 (IRS1/2) proteins at Y500 of the human IL-4Rα, which then act to link the IL-4R with several downstream signaling pathways, most notably the Phosphoinositide 3-kinases (PI3K)-AKR mouse thymoma kinase (AKT) axis, relevant to M2 macrophage activation 16–21 (Figure. 1). The latter appears to proceed via a PI3K-mTORC2 axis that activates the transcription factor IRF4 22. In turn, IRS2-dependent signaling is negatively regulated by suppressor of cytokine signaling 1 (Socs1)-dependent ubiquitination 21 (Figure 1). Notwithstanding these results, a negative regulatory function of the IRS-2 pathway in regulating the allergic response has been demonstrated using in vivo genetic approaches. In one approach, mice with a mutation that inactivates the equivalent IRS-docking site in the murine IL-4Rα (Y497F) were developed and studied for allergic responses 23. This mutation impaired T cell proliferation in response to IL-4 but did not affect TH2 cytokine secretion in vitro. Surprisingly, it was found that mice homozygous for the IL-4Rα-F497 mutation exhibited enhanced allergic inflammation, including IgE production, airway hyperresponsiveness, eosinophilic inflammation, and mucus production, suggesting a significant contribution of this pathway to the regulation of allergic inflammation in vivo. A similar phenotype of enhanced airway inflammation was also noted in IRS2–/– mice, implicating IRS2, which is the differentially expressed in hematopoietic cells versus IRS1, in the negative regulatory function of IL-4Rα-Y497 24. Thus, the precise role of this pathway in modulating the allergic response remains to be fully delineated (Figure. 1).
A third signaling branch emanates at a carboxyl-terminal tyrosine (Y713) of IL-4Rα, which defines an immunotyrosine inhibitory motif (ITIM) that binds the Src homology 2 domain containing protein tyrosine phosphatase-1 (SHP-1) to negatively regulate STAT6 signaling 25. Mice with a point mutation in the equivalent tyrosine at Y709 exhibit augmented allergic responses, with heightened susceptibility to food allergy and allergic airway inflammation, a phenotype that is recapitulated in mice with targeted deletion of Ptpn6, encoding SHP-1, in their CD4 T cells 26.
While activation of the canonical IL-4R/STAT6 axis by IL-4 and IL-13 critically promotes allergic inflammation, additional pathways linked to the IL-4R have been described that maybe of clinical relevance. The IL-4R type II has been invoked to recruit and activate STAT3 in response to IL-4/IL-13 engagement 14. More broadly, differences in signaling between the type I and type II receptor have been noted. By comparing allergic inflammatory responses of mice with targeted deletion of Il4ra versus Il13ra1, it could be deduced that while the IL-4R type I is critical for TH2 cell responses and alternative macrophage and fibroblast activation, the type II receptor was essential for allergen-induced airway hyperreactivity and mucus hypersecretion 27. Differences in signaling pathways linked to the respective receptor complexes, the abundances of individual receptor components and the kinetics of receptor complex assembly all contribute to signaling differences between the two receptor complexes 28.
IL-4R signaling in effector mechanisms
IL-4R is critical to TH2 cell differentiation 29. Moreover, in the absence of IL-4R signaling, reduced IL-10 production is due to the lack in expansion of an IL-10+ TH2 population, rather than a global defect in the production of IL-10 by CD4+ T cells. Thus, the evolution of TH2 dominance is achieved at the expense of TH1 cell development, normally restrained by IL-10 in an IL-4R-dependent manner 30. The role of IL-4Rα is not specific to effector immune cells. It is very important for the development of dendritic cells (DCs). IL-4 and IL4R are very important for the maturation and functionality of DCs 31. Furthermore, deletion of IL-4Rα on CD11c cells, rendered the mice more susceptible to helminths infection. DCs showed increased parasite loads in DCs lacking IL-4Rα. Moreover, IL-4Rα-deficient DCs produced less IL-12 but increased levels of IL-10 due to impaired DC instruction, with increased mRNA expression of IL-23p19 and activin A, cytokines previously implicated in promoting TH2 responses. Abrogation of IL-4Rα signaling on DCs is severely detrimental to the host, leading to rapid disease progression in Schistosoma model of parasite infection, with increased survival of parasites in infected DCs due to reduced killing effector functions 32.
Furthermore, innate lymphoid cells (ILCs) are central to the pathogenesis of Asthma and food allergy by virtue of their copious production of Th2 cytokines, including IL-13 and IL-4 33,34. In turn, IL-4 produced by eosinophils and basophils is a key regulator of natural helper cells, a subtype of ILC2 involved in asthma pathogenesis 35,36. In addition, neuropeptide neuromedin U (NMU) has been identified as a regulator of type 2 innate immunity37. It activates ILC2 to produce high amounts of IL-4 that in turn affect Th2 cell function and activity 37.
On the other hand, lipid mediators alter the activity and functionality of ILC2s in the context of Asthma. Thus, prostaglandin (PG)D2 and PGI2 increase IL-13 production from human peripheral blood ILC2s in the presence of IL-33 and IL-25 and also showed that lipoxin A4 could inhibit ILC2 activation 38,39. Moreover, mouse lung ILC2s were reported to be rapidly and robustly activated by the cysteinyl leukotriene (CysLT) leukotriene (LT) D4 in vitro and in vivo 40,41.
In addition to that, IL-4R have an important role as well in the amplification of IgE- and histamine-induced vascular endothelium (VE) dysfunction, fluid extravasation, and the severity of anaphylaxis through a VE IL-4Rα/ABL1–dependent mechanism 42. These studies implicate an important contribution by the VE compartment in the severity of anaphylaxis and identify a new pathway for therapeutic intervention of IgE/IL-4-mediated reactions.
IL-4R signaling in Tolerance Modulation
While the emphasis in signaling via the IL-4R has been on the role it plays in promoting allergic effector pathways, more recent studies have uncovered a fundamental role for this pathway in modulating immune tolerance by subverting allergen-specific regulatory T (Treg) cell responses. Such subversion is critical to diseases of chronic allergic inflammation such as asthma, and maybe reversed by treatment with an anti-IL-4R antibody such as Dupilumab.
The paradigm for the regulation of TH cell responses by Treg cells is for the latter to appropriate partial or “aborted” forms of the transcriptional programs of the target TH cells by expressing the relevant master transcription factors, such as T-box 21 (Tbx21) transcription factor for TH1 cells and interferon regulatory factor 4 (IRF4) and GATA binding protein 3 (GATA3) for TH2 cells. However, in the context of a chronic allergic inflammatory response, such a restrained acquisition of the target TH2 cell attribute breaks down, leading to the complete subversion of Treg cells into TH2-like cells. In the case of TH2 inflammation, the IL-4R plays a cardinal role in both outcomes. Thus, Treg cell-specific deletion of the Il4ra in mice, encoding IL-4Rα, results in ineffective regulation of an acute anti-helminth response, in line with the requirement for this pathway for the Treg cells to acquire some, but not all ,attributes of a TH2 cell to allow their homing to TH2-inflamed tissues without losing their regulatory identity 43.
In contrast to the above, chronic sustained signaling via the IL-4R/STAT6 axis disrupts tolerance by facilitating the complete subversion of Treg cells into TH2 cell-like cells. A case in point are studies on immune tolerance in allergic inflammation in mice bearing an IL-4Rα chain variant (Il4raF709), in which the tyrosine 709 residue in the IL-4Rα c-terminal ITIM motif was mutated into phenylalanine, leading to its disruption. This variant drives Treg cells into a TH2 cell-like phenotype by the excessive activation of the STAT6, leading to high GATA3 and IRF4 expression and IL-4 production. The subverted TH2-like Treg cells play a critical role in disease pathogenesis under conditions of TH2-high inflammation induced, including paradigms of food allergy (FA) and allergic airway inflammation induced in Il4raF709 mice 44. A similar phenotype is seen in human subjects with FA, indicating that this subversion is operative in human allergic disorders 44–46. A more sharply restricted Treg cell TH2 cell-like response can aid in providing protection against helminths by generating TH2 cell-like ex-Treg cells that add to the TH2 response 47.
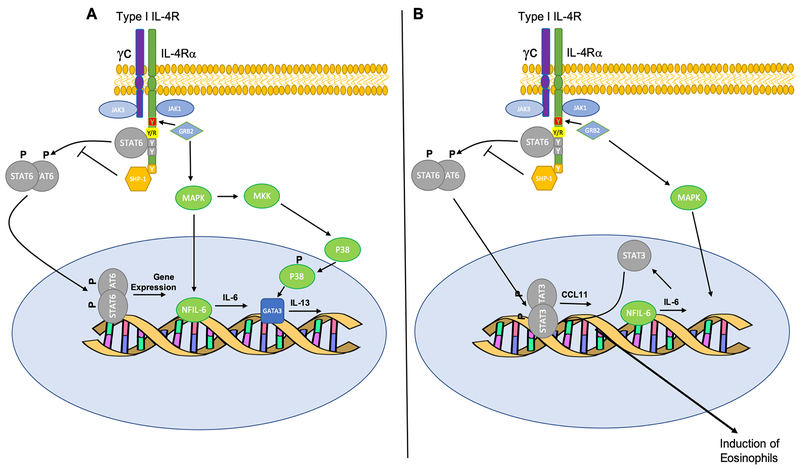
A distinct mechanism of Treg cell subversion in allergic disease involves an IL-4Rα chain variant that is particularly common in populations of African descent, including African-Americans. This variant, in which the glutamine residue at position 576 in the cytoplasmic domain is changed into to arginine (R576), is strongly associated with severe asthma and heightened asthma susceptibility 2,8,48–51. The R576 substitution enables the binding of the adaptor growth factor receptor bound protein 2 (GRB2) to the adjacent Y575 once the latter is phosphorylated by the JAKs, leading to mitogen activated kinase-like protein (MAPK) activation and IL6 gene induction. This unique attribute allows signaling via the IL4Rα-R576 to induce dual activation of STAT6 and STAT3, the latter through an autocrine/paracrine IL-6 production loop. In particular, the IL-6-STAT3 axis destabilizes allergen-specific regulatory T (Treg) cells by promoting their reprograming into pathogenic “TH17 cell-like” Treg cells, or fully differentiated TH17 cells, that lack regulatory function, express the transcription factor ROR-γt and secrete IL-17 52,53. Thus, the IL4Rα-R576 drives mixed TH2/TH17 cell inflammation, associated with severe allergic inflammation and steroid resistance (Figure 2A and B).
Figure 2. Signaling via the IL-4RαR576 variant.
(A) The normal wildtype allele of the IL-4Rα (Q576R) showing the activation STAT6 and TH2 induction. (B) The R576 substitution enables the binding of the adaptor GRB2 to the adjacent Y575 once the latter is phosphorylated by the JAKs, leading to MAPK activation and IL6 gene induction. This unique attribute allows signaling via the IL4Rα-R576 to induce dual activation of STAT6 and STAT3, leading to destabilization of Treg cells into a TH17 cell-like phenotype and mixed TH2/TH17 cell inflammation. Dual STAT6/STAT3 activation also results in heightened expression of CCL11, leading to exaggerated tissue eosinophilia.
Targeting the IL-4Rα with Dupilumab
Dupilumab is an IgG4 human monoclonal antibody (mAb) that binds IL-4Ra 54,55. Dupilumab inhibits IL-4R signaling induced by both IL-4 and IL-13, and down-regulates TH2 inflammation in a variety of allergic disorders, including atopic dermatitis, asthma and possibly other allergic diseases 54. Surprisingly, there is little available in vitro or in vivo data on the precise mechanism of action of Dupilumab. In patent applications, Dupilumab was shown to inhibit IgE production by ex-vivo B cells induced by IL-4 treatment. An in vivo mouse preclinical models, Dupilumab was noted to inhibit IL-25 induced allergic airway inflammation and eosinophilic esophagitis (EoE) and in a peanut allergy-associated EoE in conjunction with the suppression of IL-25- and peanut-induced IgE production 56,57.
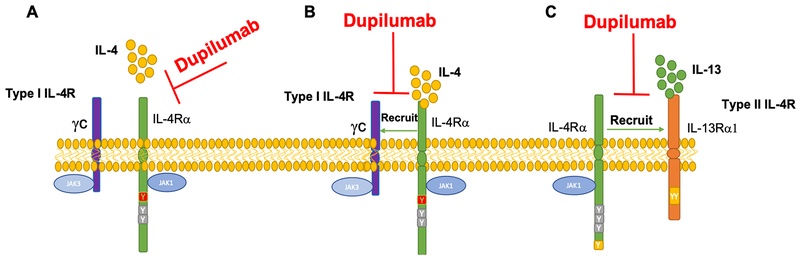
In theory, an IL-4Rα subunit antibody could either inhibit the binding of IL-4 to the type I receptor complex, or inhibit the assembly of the type II receptor complex by preventing the recruitment of the IL-4Rα subunit by the IL-13Rα1 upon the binding of the latter to IL-13. The differential impact of an IL-4Rα subunit antibody on the respective receptor complexes may well be influenced by the abundance of the IL-4Rα and IL-13Rα1 subunits in the target cells 28. The initial binding of IL-4 and IL-13 with the IL-4Rα and IL-13Rα1 subunits, acts as a “driver” event for the subsequent recruitment of the second receptor complex subunit, referred to as a “signaling trigger” event, a terminology akin to that previously proposed for the assembly of γc heterodimeric receptor complexes 58–60 (Figure 3A–C). Accordingly, if Dupilumab were to preferentially suppress IL-4 binding to the IL-4Rα subunit, then its effects would primarily manifest as suppressive of Th2 cell differentiation but not necessarily IL-13 driven TH2 inflammation in target tissues. The reverse would be true if Dupilumab primarily affected the association of IL-4Rα and IL-13Rα1 subunit (Figure 3A–C). Where these distinctions become highly relevant is in explaining the observed resistance of some patients with allergic tissue inflammation, such as asthma, eczema and EoE, to disease suppression by Dupilumab, as discussed further below. Thus, if Dupilumab differentially impacted the assembly of the type I receptor, the “leakage” of type II receptor signaling may explain to some extent the resistance of some patients to therapy. In reverse, a preference for the type II receptor may impact the capacity of Dupilumab to directly act on TH2 cell-like Treg cells, which express the type I receptor, to restore their function. Overall, the suppression of allergic tissue inflammation by Dupilumab, such as seen in eczema, would suggest prominent inhibition of IL-4R type II receptor signaling 61. Nevertheless, the issues raised in this section are best clarified by future studies on the interaction of Dupilumab with the respective receptor complexes.
Figure 3.
Potential mechanisms of action of Dupilumab in targeting the IL-4R complex. (A) Dupilumab may inhibit IL-4 binding to IL-4Rα, and/or (B) inhibit the recruitment of γc to IL-4Rα chain and/or (C) inhibit the recruitment of the IL-4Rα to IL-13Rα1.
Dupilumab in skin disease
Dupilumab has been approved by the food and drug administration (FDA) in the united states for the treatment of moderate-to-severe atopic dermatitis in uncontrolled patients 55,62. In a 16 week, randomized, placebo-controlled phase III study, the efficacy of Dupilumab in treating severe AD was investigated. Patients were treated with Dupilumab 300 mg weekly, 300 mg every 2 weeks or placebo for 16 weeks. Disease severity measured by the Eczema Area and Severity Index (EASI) significantly decreased in the Dupilumab treated groups; 69–72% in the 300 mg weekly treatment group, and 67–72% in the 300 mg every other week group as compared to the placebo group 63. In another study, after one year of treatment 64% of the patients in the Dupilumab 300 mg weekly group and 65% of patients in the 300 mg every other week group reached the endpoint of EASI-75 as compared to 22% of the patients in the placebo group 64. Furthermore, in a third study AD patients with history of treatment failure with cyclosporine A or with contraindications to this drug were included to receive Dupilumab. 59% of the patients in the Dupilumab weekly group and 65% of the patients in the Dupilumab every other week group reached the primary endpoint of EASI-75, compared to 29.6% of patients who received placebo 65.
At the molecular level, Dupilumab therapy lowered the signature of more than 800 genes affected in AD, including TH2 chemokines, T-cell proliferation and dendritic cell (DC) genes 66. Furthermore, Dupilumab was well tolerated and had very low toxicity profile 67,68. Main side effect to Dupilumab in treating AD is conjunctivitis 69–71. Other side effects include injection-site reactions 72, and localized herpes simplex infection 73. Of note, data from three randomized, double-blinded, placebo-controlled phase III trials revealed that transient blood eosinophilia was observed in a small subset of patients, with less than 1% of the patients showing high grade eosinophilia 74. It has been suggested that inhibition by Dupilumab of eosinophil recruitment from blood to inflamed skin tissues may account for this increase 74.
Dupilumab in Asthma
In 2018, the FDA has approved Dupilumab to be the newest drug among the biologics family for treating asthma. Currently Dupilumab is used for the treatment of eosinophilic asthma 3. Main indications to use Dupilumab is to have blood eosinophils > 150 cell/ul or FeNO > 25 ppb 75. There have been some clinical trials investigating the role of Dupilumab in Asthma. In a placebo-controlled study, 47.7% of patients receiving 200 mg of Dupilumab every 2 weeks had less annualized rate of severe asthma exacerbations compared to placebo. similar results were seen with the Dupilumab dose of 300 mg every 2 weeks. Moreover, patients with higher blood eosinophils (> 300 cell/cubic mm), the annualized rate of severe asthma exacerbations was 65.8% lower with Dupilumab compared to placebo 76. In another phase III clinical trial, 70.1% of patients receiving Dupilumab had a change in glucocorticoid levels compared to 42% in placebo group 77. Overall, Dupilumab treatment reduced the dose of oral-glucocorticoids, and decreased severe exacerbations and increased the patients FEV1 77.
There are as well two ongoing studies to evaluate the efficacy and safety of Dupilumab treatment of moderate-to-severe asthma. LIBERTY ASTHMA TRAVERSE (NCT02134028) is an open-label extension trial. Dupilumab is being administered every 2 weeks for a maximum of 60 or 108 weeks depending on the enrollment date, and the primary endpoint is the evaluation of any adverse events. The study is expected to be completed in October 2019 78. The second ongoing study, VOYAGE (NCT02948959), is specifically evaluating Dupilumab every 2 weeks (vs placebo) in children aged 6 to 12. The treatment period will be 52 weeks, and the main endpoint will be the annualized rate of severe exacerbation events during this treatment period. The results are expected by January 202179.
It is now appreciated that the IL-4Rα genotype influences the attributes and severity of the tissue inflammation. Specifically, and as detailed above, asthmatics with the IL-4RαR576 receptor variant mount a vigorous TH2/TH17 cell response, related to the to the subversion of the lung Treg cell response towards to a TH17 cell-like phenotype 53. To investigate the role of Dupilumab different forms/polymorphisms of IL-4Rα, a new study has been set to elucidate the effect of IL-4RαR576 Polymorphism on Response to Dupilumab in Adolescents and Adults With Asthma (I-DAG) 80. In this study Patients will be genotyped and divided into the wild type allele carriers (Q576/Q576), heterozygous allele carriers (Q576/R576), or mutant allele carriers (R576/R576). The mutant allele is associated with more severe disease 53. The patients will receive either Dupilumab subcutaneously or placebo. This study addresses the fundamental mechanism by which the IL-4Rα-R576 variant drives the TH2/TH17 disease endotype and the influence of this variant on response to Dupilumab therapy. It is expected that asthmatics bearing this endotype will be likely to favorably respond to Dupilumab therapy by virtue of its prevention of iTreg cell reprogramming into TH17-like cells, potentially leading to their long-term stability and potential for sustained immune tolerance 53,80. It is noteworthy that there have been cases with chronic eosinophilic pneumonia (CEP) after treatment with Dupilumab 81. Also, and similar to the studies on Dupilumab in AD discussed earlier, treatment of asthmatics with Dupilumab in phase III trials was associated with increased eosinophilia in a subset of patients 76,77. Overall, the role of eosinophilia suppression in the mechanism of action of Dupilumab in asthmatic (and AD) patients requires further investigation.
Dupilumab in Chronic Rhinosinusitis with Nasal Polyposis
Chronic Rhinosinusitis is a TH2 inflammation characterized by increase of IL-5, IL-13 and eosinophils numbers in the polyps 82. In 2013, a phase II clinical study throughout 13 different centers in both USA and Europe was conducted to assess the efficacy of Dupilumab in inhibiting Chronic Rhinosinusitis. Dupilumab was able decrease to the least squares (LS) mean in compared to placebo group 83. At the molecular level, type 2 biomarkers (e.g. Eotaxin-2, total IgE, pulmonary and activation‐regulated chemokine and IL‐13) were significantly reduced in the patients receiving Dupilumab compared to the placebo group 84,85. Another pathway relevant to chronic rhinosinusitis and nasal polyps involves ALOX15, encoding 15-Lipoxygenase A, whose expression is strictly dependent on IL-4 and IL-13 86,87. Loss of function mutation in ALOX15 protects against nasal polyps and chronic rhinosinusitis. Metabolites of ALOX15 activate macrophages toward a M2 phenotype. suggesting that Dupilumab may protect against chronic rhinosinusitis in part by suppressing the IL-4/13-ALOX15/M2 macrophages axis 88.
Dupilumab for Eosinophilic Esophagitis (EoE)
EoE is the inflammation of the esophagus presented with influx of eosinophils. The excess number of eosinophils implicate a TH2 mechanism to play an important role in the disease pathogenesis 89,90. Calpain 14 (CAPN14) is an esophagus-specific intracellular epithelial protease that is induced by IL-13. Different variants of this protein are considered high risk factors for EoE. It is thought that CAPN14 impair epithelial barrier function in through desmoglein 1 91. Thus, it makes EoE an ideal target for Dupilumab. In 2017, a phase II clinical trial for Dupilumab in EoE was conducted. The primary endpoint of the study was the change from baseline to week 10 in the Straumann Dysphagia Instrument (SDI) score, a patient-reported measure of swallowing difficulty on a 0–9-point scale, with 9 indicating more severe symptoms. Patients received either Dupilumab 300 mg weekly following a 600-mg loading dose or placebo. At week 10, patients who received dupilumab reported a significant improvement in the ability to swallow with a 3-point reduction in their SDI score (45% improvement) compared with 1.3 points (19% improvement) for those patients who received placebo 92,93. This study revealed that Dupilumab significantly improved different features of EoE including dysphagia, endoscopic features, histology, and esophageal distensibility 93.
Dupilumab for Food Allergy
Food allergy is the result of immune-sensitization to different food allergens. It is characterized by increased permeability of epithelial cells, skewing of dendritic cell response, downregulation of Treg promotion of TH2 inflammatory response and production of cytokines IL4, IL 5, IL13, recruitment of mast cells, eosinophils and basophils and induction of food antigen-specific IgE 94.
Abdel-Gadir et al et al. found that treatment of lymphocytes peanut-allergic patients with anti-IL-4Rα mAb augmented the suppressive function of peanut-reactive Treg cells, highlighting the potential disease modifying effect of anti-IL4 therapy 46. Recently, Rial MJ et al. have presented a case report showing a very successful therapy of newly developed food allergy to corn and nuts using Dupilumab 95. This is the first report treating a patient with Dupilumab for a food allergy indication.
Currently, there are two ongoing studies to evaluate the safety and efficacy of Dupilumab in food allergy, one involving the use of Dupilumab as an adjunct therapy in peanut oral immunotherapy 96, while the other tests Dupilumab as a monotherapy in peanut allergy 97.
Conclusion
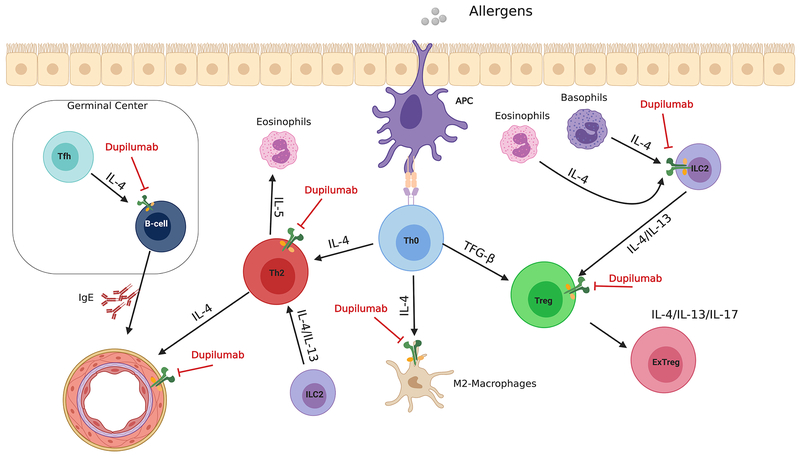
Dupilumab is the first biologic that effectively addresses the pathophysiology of TH2 allergic diseases, combining therapeutic efficacy with low incidence of adverse events. Dupilumab is already approved by the FDA for the treatment of AD, moderate to severe Asthma and showed very promising results for the treatment of Chronic Rhinosinusitis and EoE. It has potentially multiple sites of action that remain to be fully established (Figure. 4). It can target fundamental mechanisms in TH2 cell inflammatory diseases by blocking TH2 cell differentiation, IgE production by B cells, alternative macrophage activation and other hallmarks of allergic inflammatory diseases. Furthermore, it can act on the vascular endothelium to potentially reduce cellular trafficking in inflamed tissues and attenuate vascular leakage associated with anaphylaxis. Defining the spectrum of action of Dupilumab in different allergic disorders and its capacity to differentially impact pathways of IL-4 and IL-13 signaling in lymphoid and non-lymphoid tissues will further expand the potential for IL-4R blockade in the treatment of TH2 cellular inflammatory disorders.
Figure 4.
Potential sites of action of Dupilumab in inhibiting allergic inflammation. Dupilumab can act to inhibit TH2 cell differentiation, the transformation of Treg cells into ex-Treg cells in the context of allergic inflammation, and IgE production by B cells, driven by T follicular helper (TFH)- derived IL-4. It can also prevent IL-4-related vascular endothelium dysfunction. Furthermore, it can inhibit ILC2 induction via eosinophils and basophils.
Acknowledgements.
This work was supported by NIH NIAID grants 1R01AI1269151 and 5R01AI065617 to T.A.C.
Footnotes
Data Sharing Statement
Data sharing is not applicable to this Review as no new data were created or analyzed in this survey.
References
- 1.Kabesch M Early origins of asthma (and allergy). Mol Cell Pediatr. 2016;3(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasegawa K, Tsugawa Y, Brown DF, Camargo CA Jr., Childhood asthma hospitalizations in the United States, 2000–2009. J Pediatr. 2013;163(4):1127–1133 e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skolnik NS, Carnahan SP. Primary care of asthma: new options for severe eosinophilic asthma. Curr Med Res Opin. 2019:1. [DOI] [PubMed] [Google Scholar]
- 4.Shamoun L, Skarstedt M, Andersson RE, Wagsater D, Dimberg J. Association study on IL-4, IL-4Ralpha and IL-13 genetic polymorphisms in Swedish patients with colorectal cancer. Clin Chim Acta. 2018;487:101–106. [DOI] [PubMed] [Google Scholar]
- 5.Chatila TA. Interleukin-4 receptor signaling pathways in asthma pathogenesis. Trends Mol Med. 2004;10(10):493–499. [DOI] [PubMed] [Google Scholar]
- 6.Keegan AD, Zamorano J, Keselman A, Heller NM. IL-4 and IL-13 Receptor Signaling From 4PS to Insulin Receptor Substrate 2: There and Back Again, a Historical View. Front Immunol 2018;9:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiaramonte MG, Mentink-Kane M, Jacobson BA, et al. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197(6):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard TD, Koppelman GH, Xu J, et al. Gene-gene interaction in asthma: IL4RA and IL13 in a Dutch population with asthma. Am J Hum Genet. 2002;70(1):230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. [DOI] [PubMed] [Google Scholar]
- 10.Wood N, Whitters MJ, Jacobson BA, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197(6):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300(5625):1527–1528. [DOI] [PubMed] [Google Scholar]
- 12.Ryan JJ, McReynolds LJ, Huang H, Nelms K, Paul WE. Characterization of a mobile Stat6 activation motif in the human IL-4 receptor. J Immunol. 1998;161(4):1811–1821. [PubMed] [Google Scholar]
- 13.Ma B, Wu Y, Chen B, et al. Cyanidin-3-O-beta-glucoside attenuates allergic airway inflammation by modulating the IL-4Ralpha-STAT6 signaling pathway in a murine asthma model. Int Immunopharmacol. 2019;69:1–10. [DOI] [PubMed] [Google Scholar]
- 14.Umeshita-Suyama R, Sugimoto R, Akaiwa M, et al. Characterization of IL-4 and IL-13 signals dependent on the human IL-13 receptor alpha chain 1: redundancy of requirement of tyrosine residue for STAT3 activation. Int Immunol. 2000;12(11):1499–1509. [DOI] [PubMed] [Google Scholar]
- 15.Roy B, Bhattacharjee A, Xu B, Ford D, Maizel AL, Cathcart MK. IL-13 signal transduction in human monocytes: phosphorylation of receptor components, association with Jaks, and phosphorylation/activation of Stats. J Leukoc Biol. 2002;72(3):580–589. [PubMed] [Google Scholar]
- 16.Keegan AD, Nelms K, White M, Wang LM, Pierce JH, Paul WE. An IL-4 receptor region containing an insulin receptor motif is important for IL-4-mediated IRS-1 phosphorylation and cell growth. Cell. 1994;76(5):811–820. [DOI] [PubMed] [Google Scholar]
- 17.Byles V, Covarrubias AJ, Ben-Sahra I, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun. 2013;4:2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Festuccia WT, Pouliot P, Bakan I, Sabatini DM, Laplante M. Myeloid-specific Rictor deletion induces M1 macrophage polarization and potentiates in vivo pro-inflammatory response to lipopolysaccharide. PLoS One. 2014;9(4):e95432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallowell RW, Collins SL, Craig JM, et al. mTORC2 signalling regulates M2 macrophage differentiation in response to helminth infection and adaptive thermogenesis. Nat Commun. 2017;8:14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pruett W, Yuan Y, Rose E, Batzer AG, Harada N, Skolnik EY. Association between GRB2/Sos and insulin receptor substrate 1 is not sufficient for activation of extracellular signal-regulated kinases by interleukin-4: implications for Ras activation by insulin. Mol Cell Biol. 1995;15(3):1778–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormick SM, Gowda N, Fang JX, Heller NM. Suppressor of Cytokine Signaling (SOCS)1 Regulates Interleukin-4 (IL-4)-activated Insulin Receptor Substrate (IRS)-2 Tyrosine Phosphorylation in Monocytes and Macrophages via the Proteasome. J Biol Chem. 2016;291(39):20574–20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SC, Smith AM, Everts B, et al. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity. 2016;45(4):817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blaeser F, Bryce PJ, Ho N, et al. Targeted inactivation of the IL-4 receptor alpha chain I4R motif promotes allergic airway inflammation. J Exp Med. 2003;198(8):1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasgupta P, Dorsey NJ, Li J, et al. The adaptor protein insulin receptor substrate 2 inhibits alternative macrophage activation and allergic lung inflammation. Sci Signal. 2016;9(433):ra63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tachdjian R, Al Khatib S, Schwinglshackl A, et al. In vivo regulation of the allergic response by the IL-4 receptor alpha chain immunoreceptor tyrosine-based inhibitory motif. J Allergy Clin Immunol. 2010;125(5):1128–1136 e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson DJ, Pao LI, Dhanji S, Murakami K, Ohashi PS, Neel BG. Shp1 regulates T cell homeostasis by limiting IL-4 signals. J Exp Med. 2013;210(7):1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramalingam TR, Pesce JT, Sheikh F, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaPorte SL, Juo ZS, Vaclavikova J, et al. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132(2):259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka Y, Hamano S, Gotoh K, et al. T helper type 2 differentiation and intracellular trafficking of the interleukin 4 receptor-alpha subunit controlled by the Rac activator Dock2. Nat Immunol. 2007;8(10):1067–1075. [DOI] [PubMed] [Google Scholar]
- 30.Balic A, Harcus YM, Taylor MD, Brombacher F, Maizels RM. IL-4R signaling is required to induce IL-10 for the establishment of T(h)2 dominance. Int Immunol. 2006;18(10):1421–1431. [DOI] [PubMed] [Google Scholar]
- 31.Ahn JS, Agrawal B. IL-4 is more effective than IL-13 for in vitro differentiation of dendritic cells from peripheral blood mononuclear cells. Int Immunol. 2005;17(10):1337–1346. [DOI] [PubMed] [Google Scholar]
- 32.Hurdayal R, Nieuwenhuizen NE, Revaz-Breton M, et al. Deletion of IL-4 receptor alpha on dendritic cells renders BALB/c mice hypersusceptible to Leishmania major infection. PLoS Pathog. 2013;9(10):e1003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noval Rivas M, Burton OT, Oettgen HC, Chatila T. IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function. J Allergy Clin Immunol. 2016;138(3):801–811 e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith KA, Loser S, Varyani F, et al. Concerted IL-25R and IL-4Ralpha signaling drive innate type 2 effector immunity for optimal helminth expulsion. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motomura Y, Morita H, Moro K, et al. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40(5):758–771. [DOI] [PubMed] [Google Scholar]
- 36.Bal SM, Bernink JH, Nagasawa M, et al. IL-1beta, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol. 2016;17(6):636–645. [DOI] [PubMed] [Google Scholar]
- 37.Cardoso V, Chesne J, Ribeiro H, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549(7671):277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5(174):174ra126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, Toki S, Zhang J, et al. Prostaglandin I2 Signaling and Inhibition of Group 2 Innate Lymphoid Cell Responses. Am J Respir Crit Care Med. 2016;193(1):31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol. 2013;132(1):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Moltke J, O’Leary CE, Barrett NA, Kanaoka Y, Austen KF, Locksley RM. Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J Exp Med. 2017;214(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamani A, Wu D, Waggoner L, et al. The vascular endothelial specific IL-4 receptor alpha-ABL1 kinase signaling axis regulates the severity of IgE-mediated anaphylactic reactions. J Allergy Clin Immunol. 2018;142(4):1159–1172 e1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdel Aziz N, Nono JK, Mpotje T, Brombacher F. The Foxp3+ regulatory T-cell population requires IL-4Ralpha signaling to control inflammation during helminth infections. PLoS Biol. 2018;16(10):e2005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noval Rivas M, Burton OT, Wise P, et al. Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy. Immunity. 2015;42(3):512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burton OT, Noval Rivas M, Zhou JS, et al. Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity. 2014;41(1):141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdel-Gadir A, Schneider L, Casini A, et al. Oral immunotherapy with omalizumab reverses the Th2 cell-like programme of regulatory T cells and restores their function. Clin Exp Allergy. 2018;48(7):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelly VS, Coomes SM, Kannan Y, et al. Interleukin 4 promotes the development of ex-Foxp3 Th2 cells during immunity to intestinal helminths. J Exp Med. 2017;214(6):1809–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X, Di Rienzo A, Ober C. A population genetics study of single nucleotide polymorphisms in the interleukin 4 receptor alpha (IL4RA) gene. Genes Immun. 2001;2(3):128–134. [DOI] [PubMed] [Google Scholar]
- 49.Hershey GK, Friedrich MF, Esswein LA, Thomas ML, Chatila TA. The association of atopy with a gain-of-function mutation in the alpha subunit of the interleukin-4 receptor. N Engl J Med. 1997;337(24):1720–1725. [DOI] [PubMed] [Google Scholar]
- 50.Rosa-Rosa L, Zimmermann N, Bernstein JA, Rothenberg ME, Khurana Hershey GK. The R576 IL-4 receptor alpha allele correlates with asthma severity. J Allergy Clin Immunol. 1999;104(5):1008–1014. [DOI] [PubMed] [Google Scholar]
- 51.Ober C, Leavitt SA, Tsalenko A, et al. Variation in the interleukin 4-receptor alpha gene confers susceptibility to asthma and atopy in ethnically diverse populations. Am J Hum Genet. 2000;66(2):517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol. 2016;138(3):639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med. 2016;22(9):1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Del Rosso JQ. MONOCLONAL ANTIBODY THERAPIES for Atopic Dermatitis: Where Are We Now in the Spectrum of Disease Management? J Clin Aesthet Dermatol. 2019;12(2):39–41. [PMC free article] [PubMed] [Google Scholar]
- 55.Thibodeaux Q, Smith MP, Ly K, Beck K, Liao W, Bhutani T. A review of dupilumab in the treatment of atopic diseases. Hum Vaccin Immunother. 2019:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li-Hsien Wang YX, Andrew Murphy, Stevens Sean Inventor; Regeneron Pharmaceuticals Inc, assignee Humanized IL-4 and IL-4Rα animals. 2012. [Google Scholar]
- 57.Ana Kostic LK, Xia LiuBrendan, CLASSON J, Allen Radin Inventor; Regeneron Pharmaceuticals Inc, assignee Methods for treating eosinophilic esophagitis by administering an il-4r inhibitor. 2016. [Google Scholar]
- 58.Lai SY, Xu W, Gaffen SL, et al. The molecular role of the common gamma c subunit in signal transduction reveals functional asymmetry within multimeric cytokine receptor complexes. Proc Natl Acad Sci U S A. 1996;93(1):231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murata T, Taguchi J, Puri RK. Interleukin-13 receptor alpha’ but not alpha chain: a functional component of interleukin-4 receptors. Blood. 1998;91(10):3884–3891. [PubMed] [Google Scholar]
- 60.Zhang JL, Simeonowa I, Wang Y, Sebald W. The high-affinity interaction of human IL-4 and the receptor alpha chain is constituted by two independent binding clusters. J Mol Biol. 2002;315(3):399–407. [DOI] [PubMed] [Google Scholar]
- 61.Guttman-Yassky E, Bissonnette R, Ungar B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143(1):155–172. [DOI] [PubMed] [Google Scholar]
- 62.Sanchez-Borges M, Capriles-Hulett A, Ortega-Martell JA, Zubeldia IA. New and Potential Treatments for Atopic Dermatitis: Biologicals and Small Molecules. Curr Allergy Asthma Rep. 2019;19(3):18. [DOI] [PubMed] [Google Scholar]
- 63.Simpson EL, Akinlade B, Ardeleanu M. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med. 2017;376(11):1090–1091. [DOI] [PubMed] [Google Scholar]
- 64.Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. [DOI] [PubMed] [Google Scholar]
- 65.de Bruin-Weller M, Thaci D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol. 2018;178(5):1083–1101. [DOI] [PubMed] [Google Scholar]
- 66.Hamilton JD, Suarez-Farinas M, Dhingra N, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134(6):1293–1300. [DOI] [PubMed] [Google Scholar]
- 67.Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32(6):850–878. [DOI] [PubMed] [Google Scholar]
- 68.Hamilton JD, Ungar B, Guttman-Yassky E. Drug evaluation review: dupilumab in atopic dermatitis. Immunotherapy. 2015;7(10):1043–1058. [DOI] [PubMed] [Google Scholar]
- 69.Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faiz S, Giovannelli J, Podevin C, et al. Effectiveness and safety of dupilumab for the treatment of atopic dermatitis in a real-life French multicenter adult cohort. J Am Acad Dermatol. 2019. [DOI] [PubMed] [Google Scholar]
- 71.Ivert LU, Wahlgren CF, Ivert L, Lundqvist M, Bradley M. Eye Complications During Dupilumab Treatment for Severe Atopic Dermatitis. Acta Derm Venereol. 2019;99(4):375–378. [DOI] [PubMed] [Google Scholar]
- 72.Ou Z, Chen C, Chen A, Yang Y, Zhou W. Adverse events of Dupilumab in adults with moderate-to-severe atopic dermatitis: A meta-analysis. Int Immunopharmacol. 2018;54:303–310. [DOI] [PubMed] [Google Scholar]
- 73.Thomson J, Wernham AGH, Williams HC. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a critical appraisal. Br J Dermatol. 2018;178(4):897–902. [DOI] [PubMed] [Google Scholar]
- 74.Wollenberg A, Beck LA, Blauvelt A, et al. Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). The British journal of dermatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Busse WW. Biological treatments for severe asthma: A major advance in asthma care. Allergol Int. 2019;68(2):158–166. [DOI] [PubMed] [Google Scholar]
- 76.Castro M, Corren J, Pavord ID, et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. N Engl J Med. 2018;378(26):2486–2496. [DOI] [PubMed] [Google Scholar]
- 77.Rabe KF, Nair P, Brusselle G, et al. Efficacy and Safety of Dupilumab in Glucocorticoid-Dependent Severe Asthma. N Engl J Med. 2018;378(26):2475–2485. [DOI] [PubMed] [Google Scholar]
- 78.Pharmaceuticals R. Long-Term Safety Evaluation of Dupilumab in Patients With Asthma (LIBERTY ASTHMA TRAVERSE). In.
- 79.Pharmaceuticals R. Evaluation of Dupilumab in Children With Uncontrolled Asthma (VOYAGE). In:2019.
- 80.Chatila WPaT. Effect of IL-4RαR576 Polymorphism on Response to Dupilumab in Adolescents and Adults With Asthma (I-DAG). In:2019.
- 81.Menzella F, Montanari G, Patricelli G, et al. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bachert C, Zhang L, Gevaert P. Current and future treatment options for adult chronic rhinosinusitis: Focus on nasal polyposis. J Allergy Clin Immunol. 2015;136(6):1431–1440. [DOI] [PubMed] [Google Scholar]
- 83.Schneider JS. Subcutaneous Dupilumab and Mometasone Furoate Nasal Spray for Chronic Rhinosinusitis With Polyps. JAMA Otolaryngol Head Neck Surg. 2016;142(7):698–699. [DOI] [PubMed] [Google Scholar]
- 84.Jonstam K, Swanson BN, Mannent LP, et al. Dupilumab reduces local type 2 pro-inflammatory biomarkers in chronic rhinosinusitis with nasal polyposis. Allergy. 2019;74(4):743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bachert C, Hellings PW, Mullol J, et al. Dupilumab improves patient reported outcomes in patients with chronic rhinosinusitis with nasal polyps and comorbid asthma. J Allergy Clin Immunol Pract. 2019. [DOI] [PubMed] [Google Scholar]
- 86.Ackermann JA, Hofheinz K, Zaiss MM, Kronke G. The double-edged role of 12/15-lipoxygenase during inflammation and immunity. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862(4):371–381. [DOI] [PubMed] [Google Scholar]
- 87.Gundra UM, Girgis NM, Ruckerl D, et al. Alternatively activated macrophages derived from monocytes and tissue macrophages are phenotypically and functionally distinct. Blood. 2014;123(20):e110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kristjansson RP, Benonisdottir S, Davidsson OB, et al. A loss-of-function variant in ALOX15 protects against nasal polyps and chronic rhinosinusitis. Nat Genet. 2019;51(2):267–276. [DOI] [PubMed] [Google Scholar]
- 89.Ruffner MA, Kennedy K, Cianferoni A. Pathophysiology of eosinophilic esophagitis: recent advances and their clinical implications. Expert Rev Clin Immunol. 2018:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Durrani S, Rothenberg M. Recent advances in eosinophilic esophagitis. F1000Res 2017;6:1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davis BP, Stucke EM, Khorki ME, et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight. 2016;1(4):e86355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.REGENERON AND SANOFI ANNOUNCE POSITIVE PHASE 2 STUDY RESULTS FOR DUPILUMAB IN PATIENTS WITH ACTIVE MODERATE-TO-SEVERE EOSINOPHILIC ESOPHAGITIS [press release]. 2017.
- 93.Sastre J, Davila I. Dupilumab: A New Paradigm for the Treatment of Allergic Diseases. J Investig Allergol Clin Immunol. 2018;28(3):139–150. [DOI] [PubMed] [Google Scholar]
- 94.Albuhairi S, Rachid R. The Emerging Biologic Therapies on Food Allergy. Ann Allergy Asthma Immunol. 2019. [DOI] [PubMed] [Google Scholar]
- 95.Rial MJ, Barroso B, Sastre J. Dupilumab for treatment of food allergy. J Allergy Clin Immunol Pract. 2019;7(2):673–674. [DOI] [PubMed] [Google Scholar]
- 96.Pharmaceuticals R. Study in Pediatric Subjects With Peanut Allergy to Evaluate the Efficacy and Safety of Dupilumab as Adjunct to AR101 (Peanut Oral Immunotherapy). 2018.
- 97.Pharmaceuticals R. Study to Evaluate the Efficacy and Safety of Dupilumab Monotherapy in Pediatric Patients With Peanut Allergy. 2019.