To the Editor:
Numerous epidemiologic studies have reported associations between exposure to air pollutant and increased risk of allergic diseases, with a recent study estimating that 8–20% of asthma emergency room visits worldwide could be attributed to ozone exposure and 4–9% to exposure to particulate matter smaller than 2.5μm (PM2.5)1. Traffic pollution derived diesel particles represent a major source of PM2.5 in large urban centers and have been linked to increased susceptibility to asthma development and asthma exacerbations2. To determine the mechanisms behind these associations, we utilized a murine model of severe allergic airway disease where mice are co-exposed to diesel exhaust particles (DEP) and the common aeroallergen house dust mite (HDM) as previously described3. We demonstrated that exposure to DEP resulted in elevated BALF IL-17A levels and neutrophilia but none of the features associated with allergic airway inflammation3. However, when mice were exposed to both DEP and HDM, we observed increased HDM-induced Th2 responses and airway hyperresponsiveness (AHR) compared to mice exposed to HDM alone.3 The mechanism behind this DEP-induced increase in allergic Th2 responses remains poorly understood.
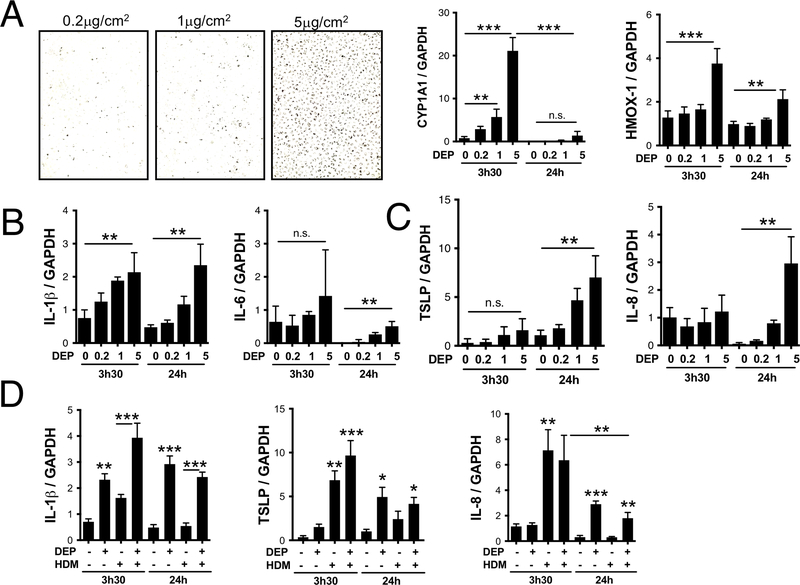
One likely mechanism could involve the production by stressed epithelial cells of innate cytokines like TSLP, IL-25 and IL-33, which promote and amplify Th2 responses via innate cells, skew dendritic cells toward a pro-Th2 phenotype, or directly act on Th2 cells in vivo4. Indeed, DEP has been reported to induce TSLP secretion by bronchial epithelial cells (HBEC)5. In vitro, monocyte-derived dendritic cells co-cultured with DEP-treated HBEC demonstrated a pro-Th2 phenotype characterized by elevated surface expression of OX40 Ligand and increased ability to induce IL-5 production by CD4+ T-cells5. Since in vivo DEP exposure does not induce IL-5 or eosinophilia but rather IL-17A and neutrophilia3, we compared the kinetics of TSLP mRNA generation in DEP-exposed HBEC with mRNA levels for cytokines known to promote Th17 differentiation (IL-1β and IL-6) and neutrophil recruitment (IL-8). Normal HBEC cells from the HBEC3 cell line (obtained from Dr John D. Minna, University of Texas Southwestern Medical Center) were cultured in 6 well plates until 80–90% confluence in 2ml of media (Keratinocyte-SFM + EGF and Bovine Pituitary Extract; Gibco). Cells were starved overnight in serum-free media before being stimulated with increasing doses of DEP (Figure 1A). We measured mRNA levels of CYP1A1, which is dose dependently induced by polycyclic aromatic hydrocarbons present on DEP (Figure 1A). When exposed to 5μg of DEP/cm2, HBEC undergo oxidative stress without any sign of cell death (data not shown), and mRNA for the anti-oxidant HMOX1 is induced within 3h of DEP exposure (Figure 1A). This early increase in oxidative stress is associated with dose dependent increases in IL-1β and, to a lesser extent, IL-6 mRNA levels, whereas IL-8 and TSLP are induced later (Figure 1B, 1C). This is in sharp contrast to stimulation with 25μg/ml of HDM, which strongly induces TSLP and IL-8 mRNA and to a lesser degree IL-1β within 3–4h (Figure 1D). Taken together, these data suggest that DEP-exposed epithelial cells will likely skew dendritic cells to promote Th17 differentiation in the draining lymph nodes upon migration, rather than promote Th2 differentiation.
Figure 1: Delayed TSLP induction by DEP stimulated bronchial epithelial cells.
HBECs were grown in 6 well plates to 90% confluence and starved over-night before stimulation with 0.2, 1 or 5μg/cm2 of DEP (A). Media was removed and replaced with TRIZOL 3h30 and 24h later. (A) CYP1A1 and HMOX-1 mRNA levels; (B) IL-1β and IL-6 mRNA levels and (C) TSLP and IL-8 mRNA levels were determined by real time quantitative PCR and normalized to GAPDH. (D) IL-1β, IL-8 and TSLP mRNA levels following exposure to 5μg/cm2 of DEP, 25μg/ml of HDM or both (data compiled from two separate experiments).
A recent study suggested that exposure to PM2.5 aggravates allergic airway inflammation through TSLP, based on a Western blot showing a dose dependent increase in TSLP lung levels following co-exposure to OVA and increasing doses of PM2.56. In order to determine if there is a causal link between TSLP and the synergistic increase in allergic airway inflammation observed in our model following DEP and HDM co-exposure, we exposed 6–8 week-old BALB/c mice lacking the receptor for TSLP and wild type control mice to 9 intratracheal exposure to either saline, DEP, HDM or HDM+DEP over a 3-week period (Figure 2A). A low dose of HDM (10μg of Dermatophagoides pteronyssinus extract from Greer Laboratories containing 2.2μg of protein, 0.1μg of Der p1 and 0.3 EU of endotoxin) was used to assess the impact of co-exposure to DEP (C-DEP was generated from a 4-cylinder Deutz diesel engine at the EPA and a detailed characterization has been published elsewhere7).
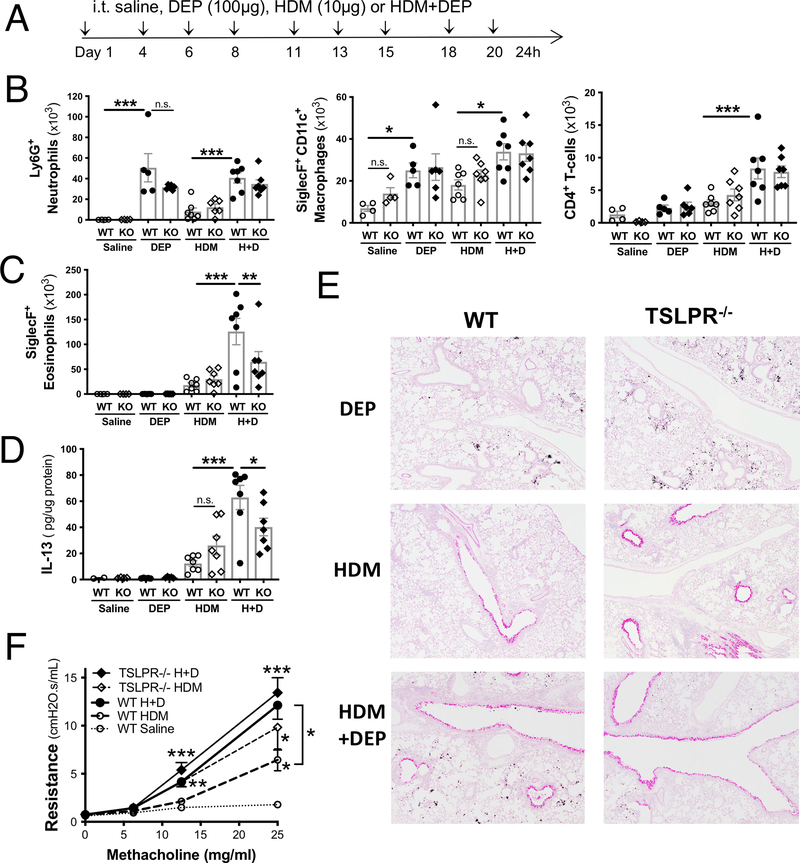
Figure 2: TLSP contributes to HDM+DEP induced eosinophilia but is not required for HDM+DEP induced AHR.
(A) Experimental protocol. (B) BALF levels of Gr-1+ neutrophils, SiglecF+CD11c+ macrophages, CD4+ T-cells, and SiglecF+ eosinophils (C) were assessed by flow cytometry (n=4–7 mice/group; 1 way-ANOVA, * p<0.05 ** p<0.01, *** p<0.001, n.s.= not significant). (D) IL13 levels were assessed in lung homogenates by ELISA and normalized to total lung protein levels. (E) Representative PAS stained lung sections. (F) Airway resistance was measured a day after the last challenge using FlexiVent (n=7–13 mice/group from two separate experiments; 2-way ANOVA, ** p<0.01, *** p<0.001).
Exposure to DEP alone induced an increase in the recruitment of alveolar macrophages and neutrophils to the BALF, but not eosinophils (Figure 2B, 2C). HDM+DEP co-exposure induced an increase in BALF eosinophil, neutrophils, macrophages and CD4+ T-cell levels compared to exposure with HDM alone (Figure 2B, 2C). In HDM+DEP exposed TSLP receptor deficient mice, BALF levels of macrophages, neutrophils, dendritic cells, and T cells were unchanged (Figure 2B), but BALF eosinophilia was significantly decreased compared to wild type control mice (Figure 2C). A similar decrease in lung tissue eosinophil levels was observed in HDM+DEP exposed TSLPR deficient mice (data not shown). Airway IL-13 levels were assessed in lung homogenates and normalized to total amount of protein (Figure 2D). The synergic increase in IL-13 lung levels observed following HDM+DEP co-exposure was significantly reduced in TSLP receptor deficient mice (Figure 2D). Taken together, these findings demonstrate that TSLP partially mediates type 2 inflammation in this model of pollution-induced severe allergic airway disease.
We next assessed mucus secretion using PAS stained lung sections and airway hyperresponsiveness (AHR) to increasing doses of methacholine using Scireq FlexiVent apparatus (Figure 2E, 2F). Exposure to DEP alone did not promote mucus production or airway resistance, whereas exposure to HDM did induce both and co-exposure to HDM+DEP further increased AHR (Figure 2E, 2F). The partial decrease in pulmonary IL-13 levels observed in HDM+DEP exposed TSLP receptor deficient mice did not lead to decreased airway resistance or a noticeable decrease in mucus production, as AHR and number of mucus positive cells were similar in wild type mice co-exposed to HDM+DEP (Figure 2E, 2F). Thus, TSLP is not responsible for the synergic increased in AHR observed following co-exposure to DEP and HDM.
Studies investigating the importance of TSLP in murine models of allergic airway disease have yielded conflicting results, including models that utilize airway exposure to HDM8, 9. Our interpretation of these apparently contradictory findings is that in murine models resulting in severe allergic Th2 inflammation, where mice are sensitized intraperitoneally in the presence of a Th2 skewing adjuvant (e.g. OVA/Alum model) or when mice are sensitized and challenged through the airways with large doses of HDM (25μg of protein in the study by Chen et al), TSLP is involved in sensitization, Th2 inflammation and AHR9. In contrast, when mice are exposed to suboptimal doses of HDM (2.2μg of protein in our case and 5–6μg in the study by Chu et al), TSLP has little to no impact on allergic airway disease8.
Thus, strength and limitations of our study include the use of full KO mice to assess the role of TSLP in the lungs, the use of a physiologically relevant three week model that is ideally suited to uncover a synergic contribution of DEP to allergic airway inflammation but is too short to assess the impact of TSLP on remodeling. The relative contribution of innate and adaptive lung cells to the observed modest contribution of TSLP to type 2 inflammation in this model of pollution-induced severe allergic airway disease remains to be investigated.
We demonstrate herein that in DEP-mediated increased allergic airway inflammation, the mixed Th2/Th17 responses driving disease severity are not dependent on TSLP, and may therefore rely on other epithelial-derived stress induced cytokines (IL-1α, IL-25, IL-33)4. Thus, while targeting TSLP in asthmatics has shown promising results10, our findings suggest that severe asthmatic patients with pollution-induced disease symptoms may not benefit from anti-TSLP therapy.
Acknowledgments
This work was supported by NIH grant 2U19AI70235-12. The HBEC3 cell line was provided by Dr Jeffrey Whitsett and originally obtained from Dr John D. Minna, University of Texas Southwestern Medical Center. TSLPR deficient mice were obtained from Steven Ziegler, Benaroya Research Institute, Seattle, WA. The DEP was kindly provided by Ian Gilmour (EPA, Research Triangle Park, NC 27711). We thank Angela Sadler for editorial assistance.
Funding information:
National Institutes of Health R01AI127392 and U19AI070235.
Footnotes
The authors declare no conflict of interest.
References:
- 1.Anenberg SC, Henze DK, Tinney V, Kinney PL, Raich W, Fann N, et al. Estimates of the Global Burden of Ambient PM2.5, Ozone, and NO2 on Asthma Incidence and Emergency Room Visits. Environ Health Perspect 2018; 126:107004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt EB, Myers JM, Ryan PH, Hershey GK. Air pollution and allergic diseases. Curr Opin Pediatr 2015; 27:724–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol 2013; 132:1194–204 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Grove KC, Provoost S, Brusselle GG, Joos GF, Maes T. Insights in particulate matter-induced allergic airway inflammation: Focus on the epithelium. Clin Exp Allergy 2018; 48:773–86. [DOI] [PubMed] [Google Scholar]
- 5.Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol 2010; 185:6636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Feng GZ, Du Q, Jin XX, Du XR. Fine particulate matter aggravates allergic airway inflammation through thymic stromal lymphopoietin activation in mice. Mol Med Rep 2017; 16:4201–7. [DOI] [PubMed] [Google Scholar]
- 7.Stevens T, Cho SH, Linak WP, Gilmour MI. Differential potentiation of allergic lung disease in mice exposed to chemically distinct diesel samples. Toxicol Sci 2009; 107:522–34. [DOI] [PubMed] [Google Scholar]
- 8.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, et al. IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 2013; 131:187–200 e1–8. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZG, Zhang TT, Li HT, Chen FH, Zou XL, Ji JZ, et al. Neutralization of TSLP inhibits airway remodeling in a murine model of allergic asthma induced by chronic exposure to house dust mite. PLoS One 2013; 8:e51268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in Adults with Uncontrolled Asthma. N Engl J Med 2017; 377:936–46. [DOI] [PubMed] [Google Scholar]