Abstract
Few studies have assessed the association of parental SES with brain measures in neonates, at a time when exposure to the postnatal environment is minimal. Social support may buffer the adverse consequences of SES, and has been associated with better cognitive – emotional development in children. We studied the association of prenatal SES and social support with neonatal brain structure, and toddler cognition and psychiatric symptoms. In a sample of 37 healthy neonates, we correlated a measure of SES and marital/partner status (an index of social support) with morphological features of the cerebral surface measured on high-resolution MRI scans, scanned between the 1st – 6th weeks of postnatal life. We then assessed how SES relates to cognitive and behavioral outcomes at age 24-months. We found that neonates born to mothers with lower SES had greater local volumes at the surface of the right occipital lobe, left temporal pole, and left inferior frontal and anterior cingulate regions. Partner status moderated the associations of SES on neonatal brain morphology. Lower SES was associated with poorer language scores and less severe ADHD and ODD symptoms. In summary, SES was associated with neonatal brain structure and language and behavioral outcomes at toddler age. Future studies with a greater sample size and longitudinal MRI scans will help to determine whether prenatal SES continues to relate to early brain development in the same or different brain regions.
Keywords: Neonate, Brain, Morphology, SES, language
Introduction
Lower socioeconomic status (SES), an index of economic and social resources, has numerous adverse developmental consequences (Brito et al., 2016). Lower SES is associated with poor physical and mental health (Brooks-Gunn and Duncan, 1997; Essex et al., 2006; Hoffman and Hatch, 2000; McLaughlin et al., 2010), as well as with lower cognitive abilities (Ozmert et al., 2005; Sarsour et al., 2011; Turkheimer et al., 2003) and poorer school achievement (Crosnoe et al., 2010; Marks, 2006). It has also been associated with altered brain development during childhood (Noble et al., 2006). For example, several studies of school-aged children have reported associations of lower SES with reduced gray matter volumes in subcortical structures, including the hippocampus and amygdala, and across the cortical surface (Hanson et al., 2011; Jednoróg et al., 2012; Merz et al., 2017). Cortical regions that support language processing and executive functioning, including the prefrontal and superior temporal areas, also significantly correlate with SES in school-age youth (Jednoróg et al., 2012; Noble et al., 2015b; Noble et al., 2012).
Very few published studies have examined the association of SES with brain measures in early infancy. Lower SES was associated with reduced gray matter volume of the frontal and parietal lobes in young children, 5 months to 4 years old (average age of 13 months) (Hanson et al., 2013). Lower SES was also associated with reduced functional activity of the frontal lobe using electroencephalogram in 6–9 month olds (Tomalski et al., 2013). Higher SES was associated with greater within-network connectivity in sensorimotor regions and lower outside-network connectivity in the default-mode network at 6 months of age, before correcting for multiple comparisons (Gao et al., 2015). These infant studies aid our understanding of the associations between SES on early brain development, although they vary widely in the age of the infants at the time of MRI scanning and the imaging modality employed, making conclusions difficult to draw about how SES relates to brain development in the fetus and in the earliest days of postnatal life. An important next step is to consider the independent association of SES and the neonatal brain, at a time when exposure to the postnatal environment has been minimal. The current study aims to fill this gap.
Identifying factors that may buffer the adverse association between lower SES and early brain development is also important. Marital or partner status (henceforth, simply “partner status”) is an index of the presence of family and social support, and has been associated with better cognitive and emotional development in children (Bacharach and Baumeister, 1998; Ricciuti and Scarr, 1990). Higher SES, having a partner, and having a better quality partner relationship associate positively with child cognition and physical and mental health (Duncan et al., 1994; Ricciuti and Scarr, 1990; Surkan and Poteat, 2011). The combination of single parenthood and lower SES was associated with problems with inhibitory control and cognitive flexibility in children from a sample of 60 families (Sarsour et al., 2011). Thus partner status may provide a buffer for the adverse associations of lower SES (Ghosh et al., 2010) and further investigation is warranted, given that family dynamics and social supports are potential targets for preventive interventions.
These prior studies suggest a gap in our understanding of how SES relates to prenatal and neonatal brain development, and they underscore the paucity of brain imaging studies that consider family and psychosocial support as a potential important moderator of those effects. We acquired anatomical MRI scans in healthy, term infants and obtained prenatal demographic and medical histories. These infants were followed through age twenty-four months. We test our primary hypothesis that lower SES is associated significantly with measures of brain structure in early postnatal life. We test our secondary hypothesis that partner status will moderate the association between SES and neonatal brain structure, such that the presence of partner support will attenuate the adverse association between SES and the neonatal brain structure. We also hypothesize that the brain regions significantly associated with SES will in turn be associated with later cognitive and behavioral capacities in these infants when they are toddlers.
Methods
Participants
Our cohort of 37 healthy infants (mean postmenstrual age [PMA] at scan = 42; SD = 1.9) was a subset of participants in a larger study of perinatal exposures on infant brain development. Pregnant women were recruited from 2005 to 2009 during the second to early third trimester of pregnancy from prenatal clinics at New York Presbyterian Hospital, Columbia University Medical Center. Inclusion criteria included a maternal age at conception of 18–45 years, no major prenatal or delivery complications, gestational age ≥ 37 weeks, birth weight >10th percentile relative to the national standards, no major congenital anomalies, and an uncomplicated neonatal nursery course as preterm birth, low birth weight, and nursery course putative to alter the developing brain (Duerden et al., 2016; Kwon et al., 2015; Scheinost et al., 2016b). Exclusion criteria were maternal history of a chronic medical disease, using drugs of abuse, smoking cigarettes, or drinking more than 1 ounce of alcohol per day during any trimester. Parents provided informed written consent for their infant to participate in the study including the MRI scan. Infants were imaged within the first 6 weeks of life. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute.
Procedures
Measures
Obstetrical and neonatal charts were reviewed by a co-investigator (neonatologist) to extract information on the pregnancy, labor, and delivery, and any other pertinent information. Postmenstrual age is defined as the time elapsed between the first day of the pregnant woman’s last normal menstrual period and the time of the MRI scan of their infant. Apgar is an assessment performed on the neonate following birth at 1- and 5-minutes following birth. The score includes breathing effort, heart rate, muscle tone, reflexes, and skin color. The total score ranges from 1 to 10. Gravida is the total number of confirmed pregnancies for each participant. Para is the number of births that each participant had after 20 weeks of gestation.
A previously validated psychosocial questionnaire was administered to each participant to obtain demographic information such as ethnicity, education, and family structure (Johnson et al., 1999). SES was obtained by utilizing the Hollingshead Index of Social Status. The Hollingshead Index provides an estimate of SES by using the highest educational and current occupational levels attained by the parent(s) (Hollingshead, 1975). Participants were asked to self-report the current highest level of education and occupation for themselves and if available another contributing adult (s) in the household. Weighted scores of education and occupation are derived. Occupations were coded according to the Hollingshead Four Factor occupational codes. Occupation scores range from 1–9, with scores of 1 indicating laborers and 9 indicating higher executives and major professionals. Educational attainment scores range from 1–7, with scores of 1 indicating completion of less than 7th grade and 7 indicating graduate training. Higher scores were indicative of higher SES. The Hollingshead measure is one of the most frequently used measures of socioeconomic position and has demonstrated good interrater reliability in previous research (Cirino et al., 2002). The Hollingshead in its original scoring system continue to be used as an estimate of SES across health research (Arentoft et al., 2015; Bava et al., 2018; Boylan et al., 2018; Bublitz et al., 2016; Daepp and Arcaya, 2017; Harris et al., 2014; Heptulla et al., 2016; Hur et al., 2015; Matthews et al., 2014; Mogi et al., 2019; Seyrek et al., 2017; Turkheimer et al., 2015; Turkheimer et al., 2003; Valenzuela et al., 2019). However, given more recent studies that define SES as education, income, or occupation alone (Brito et al., 2016; Merz et al., 2017; Noble et al., 2015a; Noble et al., 2015b; Noble et al., 2012; Noble et al., 2007; Noble et al., 2005; Noble et al., 2006), and the suggestion that occupation is one of the best estimates of SES as it reflects power, income, and education (https://macses.ucsf.edu/research/socialenviron/occupation.php), we also re-coded our occupation variable using an updated system (O*NET government codes [https://www.onetcenter.org/overview.html] based off the standard occupation classification [https://www.bls.gov/soc/]) (Choi et al., 2012).
Partner status was also obtained from the sociodemographic history form. For this study, a partner was defined as a spouse or if unmarried, an individual the mother was cohabitating with.
The Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III)(Bayley, 2005) was administered at 24 months of age by a master’s level psychologist. This measure assesses developmental functioning in early childhood from ages 1 to 42 months. We included the cognitive scale that measures visual processing, attention, and habituation and the language scale that measures receptive communication and expressive communication skills. The cognitive and language scales were used for analyses because cognitive function correlates with SES in school-age children (Ozmert et al., 2005; Sarsour et al., 2011; Turkheimer et al., 2003).
The Child Behavior Checklist (CBCL)(Achenbach, 2009) was administered to the parents when their child was 24 months of age. This measure assesses the emotional status of individuals ranging from ages 24 months to 21 years. The DSM syndrome scales, affective, anxiety, pervasive developmental disorder (PDD), attention - deficit hyperactivity disorder (ADHD), and oppositional defiant disorder (ODD) were used for analyses because of the correlations of both externalizing and internalizing symptoms with lower SES in school-age children found in prior studies (Essex et al., 2006; McLaughlin et al., 2010). Higher t-scores on the scales reflect greater symptom report.
MRI Scanning
The infants were fed, swaddled, and acclimated to the scanner environment and noise by listening to a tape recording of the scanner sounds played before each pulse sequence. The infants were given time to fall into deep sleep, without the use of sedatives, on the scanner bed before the start of each sequence. Foam and wax earplugs along with ear shields (Natus Medical Inc., San Carlos, CA) were applied to dampen scanner noise. MRI safe ECG and pulse oximetry leads were placed and heart rate and oxygen saturation were continually monitored during the scan (InVivo Research, Orlando, FL). As part of the standard protocol, scans were stopped at any signs of infant discomfort or changes in vital signs. This was not necessary for any of the infants in the current sample.
Images were obtained using a 3 Tesla GE Signa MRI scanner (Milwaukee, Wisconsin) and an 8-channel head coil. A 3-plane localizer was used to position the T2-weighted axial images parallel to the anterior–posterior commissure line. The T2-weighted images were acquired using a 2D, multiple-shot, fast spin echo pulse sequence that employed PROPELLER (Periodically Rotated Overlapping Parallel Lines with Enhanced Reconstruction) to reduce motion artifacts in reconstructed MR images (Pipe, 1999). The pulse sequence parameters were: repetition time (TR)=10,000 ms; echo time (TE)=130 ms; echo train length (ETL)=32; matrix size=192×192; field of view (FOV)=190×190 mm; phase field of view=100%; slice thickness=1.0mm; slice spacing=0mm; readout bandwidth=83.33 KHz; number of excitations (NEX)=1×2 (i.e., two images are acquired and averaged off-line, allowing us to use one of the acquisitions if the infant moved). The spatial resolution of the T2-weighted images was 1mm3.
Image Processing
The anatomical T2-weighted images for each infant were processed using a combination of automated and manual editing procedures that have been previously validated (Bansal et al., 2008a; Bansal et al., 2005; Bansal et al., 2008b; Peterson et al., 2003; Peterson et al., 2000; Sobel et al., 2010). Briefly, morphometric analyses were performed by operators blinded to participant characteristics. Large-scale variations in image intensity were removed (Sled et al., 1998) and images were reformatted to a standard orientation using midline landmarks (anterior and posterior commissure) to correct for head rotation and tilt. We isolated the brain from nonbrain tissue using an isointensity contour function with manual edits that were confirmed by a second operator (Peterson et al., 2000; Peterson et al., 2009). Connecting dura was removed manually on each slice in the sagittal view and confirmed in the orthogonal views. The brain was divided into hemispheres using a curvilinear plane positioned through standard midline landmarks. The cerebellum was removed where the peduncles join the brainstem, the brainstem was transected at the pontomedullary junction, and the brain was split into two hemispheres. The operator interrater reliability was assessed on 10 scans and intraclass correlation coefficients were greater than 0.95.
Template Selection
We applied a rigorous, two-step procedure to select the brain of a single infant from the sample as the template to ensure that it was morphologically representative of the brains in our cohort (Bansal et al., 2005; Peterson, 2010; Sobel et al., 2010; Spann et al., 2014; Spann et al., 2015). We selected a single representative infant brain as the template, rather than the brain generated by averaging the brains of all infants in our cohort, because a single brain has well-defined tissue interfaces that reduce errors when determining point correspondences across its surface with the surfaces of the other brains in the sample (Bansal et al., 2005; Goh et al., 2011). First, a preliminary template brain was selected that was closest in PMA and sex to the mean of the entire sample. The remaining participants were coregistered to the preliminary template brain and the Euclidian distances between the corresponding points on the surface of this preliminary template and each participant brain were computed. Then each participant brain was compared to the average distance across the entire surface for the sample. The brain of the infant having a surface contour closest to the average contour defined by the average distance at each point was selected as the final template. The procedure has been shown to be reliable regardless of the specific template brain selected for morphological analysis (Spann et al., 2015).
Deformation-Based Measures of Brain Morphology
We calculated distances from the surfaces of each neonatal brain from the corresponding points on the surface of a template brain using previously validated methods (Bansal et al., 2005) that permit fine-grained analyses of localized morphological features across the cerebral surface (Peterson, 2010). First we applied to each brain a similarity transformation consisting of seven parameters (three translations along the X, Y, and Z axes, three different rotations about the three axes, and one global scaling that scales the entire brain by the same amount along the three axes) to coregister each brain to the template while maximizing mutual information between the brains (Wells et al., 1996). Second, we nonlinearly transformed each brain to the template using a high-dimensional, non-rigid warping algorithm based on fluid dynamics (Christensen et al., 1996) so that the two brains matched perfectly in size and shape. The nonlinear deformation was then reversed, thereby establishing a point-to-point correspondence across the surfaces of the two brains. We then measured the Euclidean distances between corresponding points across the two surfaces, encoding the distances as positive for outward and negative for inward deformations relative to the template.
Statistical Analyses
We applied multiple linear regression to brain measures at each point across the cerebral surface to test the hypothesis that lower SES altered neonatal brain structure. For surface analyses, the dependent measure was the signed Euclidean distances at each point on the surface of the template for each infant brain. The independent variable was SES, and covariates were PMA at the time of scan and sex. As exploratory analyses, we repeated the primary hypothesis model, relating SES as measured by occupation (O*NET coding) to neonatal brain structure (see Measures section for justification). Secondary analyses were performed to evaluate the interaction of SES and partner status on neonatal brain maturation. The independent variables were SES, partner status, and the interaction term of SES-by-partner status, and covariates were PMA and sex, and the dependent variable was the signed Euclidean distances. We also used linear regression to assess the associations of developmental outcomes with distance values of regions of brain morphology that were significantly associated with SES. The dependent measure was the signed Euclidean distance from the surface of the template brain. The independent variables, entered separately, were BSID-III cognitive and language standard scores and CBCL scales at 24 months of age. Covariates were also PMA and sex.
P-values were adjusted for the number of statistical comparisons across the brain surface using False Discovery Rate (Logan and Rowe, 2004), color-coded, and displayed on the template brain, with warm colors (red and yellow) denoting outward deviations or protrusions and cool colors (purple and blue) denoting inward deviations or indentations associated with the independent variables. For the purposes of simplicity, the regional findings for surface distances relative to the template brain are interpreted and discussed as effects of local volumes across the cerebral surface.
Atlas-Based Visualization of Findings
To aid in localization of findings, a digital brain atlas of an infant with a parcellation scheme (Oishi et al., 2011) was registered to the template using the same procedures outlined for surface analysis. Then the major sulci that were represented in the atlas and that are usually present in term newborn brains (the Sylvian and interhemispheric fissures; central, pre-central, post-central, and superior temporal sulci) were digitized and represented on the 3-D surface rendering of the template brain (Battin et al., 1998; Duvernoy, 1991; Kennedy et al., 1998; Ono, 1990; Sowell et al., 2002; van der Knaap et al., 1996).
Results
Sample Description
Maternal and neonatal demographic information, including gestational age at birth, ethnicity, gender, gravida, and para are presented in Tables 1 and 2. Mean years of education for the mother’s was high school. Approximately 41% of the mothers’ had a partner. Age at time of pregnancy was 26.2 (SD = 6.4).
Table 1.
Maternal Demographic Information
| Variable | Mean (SD) |
|---|---|
| Age at pregnancy, years | 26.2 (6.4) |
| Spouse/Partner, n (%) | 15 (40.5%) |
| Hollingshead – SES | 36.7 (8.0) |
| Years of Education | 12.7 (2.8) |
| Gravida | 3.1 (1.9) |
| Para | 1.1 (1.1) |
| Type of Delivery, n (%) | |
| Vaginal Delivery | 21 (56.8%) |
| Assisted Vaginal Delivery | 3 (8.1%) |
| Cesarean Section | 12 (32.4%) |
| Race, n (%) | |
| White | 1 (2.7%) |
| Hispanic | 33 (89.2%) |
| African American | 3 (8.1%) |
Abbreviations: n, number; SD, standard deviation
Table 2.
Neonatal Demographic Information
| Variable | Mean (SD) |
|---|---|
| Postmenstrual age at scan, wk | 42.0 (1.9) |
| Gestational age at birth, wk | 39.2 (1.2) |
| Birth weight, g | 3320.4 (471.8) |
| Birth length, cm | 50.6 (2.6) |
| Head Circumference, cm | 34.5 (1.3) |
| 5 minute Apgar Score | 9 (0.2) |
| Male, n (%) | 24 (64.9%) |
Abbreviations: cm, centimeters; g, grams; wk, weeks; SD, standard deviation.
The majority of infants were born via normal vaginal delivery at 39.2 (SD = 1.2) weeks gestation with Apgar scores of 9 (SD = 0.2) at 5 minutes. Birth weight, length, and head circumference fell within normal limits. The majority of infants were male and of Hispanic ethnicity. Infants participated in MRI scans at mean postmenstrual age of 42.0 (SD = 1.9) weeks.
SES was not significantly correlated with or different from any maternal or neonatal factors, with the exception of race/ethnicity. There was no significant difference in SES among the mothers who had a partner compared to mothers’ who did not have a partner (meanpartner = 35.9, meanno partner = 33.8, F (1, 28) = 0.73, p = 0.40).
Of the 37 participants, 17 (45.9%) had behavioral follow-up data at 24-months of age. The demographic information for the toddlers are presented in Supplemental Table 1.
Primary Hypothesis Testing
SES.
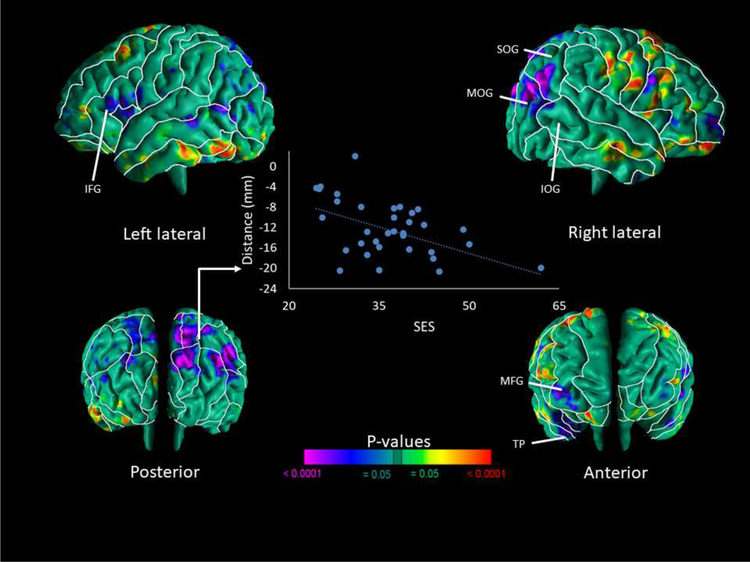
We detected significant inverse correlations of local brain volumes in the infants with SES in the superior and middle occipital gyri primarily of the right hemisphere, parieto-occipital region of both hemispheres, middle frontal and temporal pole regions of the right hemisphere, and the inferior frontal and anterior cingulate regions of the left hemisphere (Fig. 1). We also detected significant positive correlations with SES in the frontoparietal region of the right hemisphere and the inferior temporal lobe of the left hemisphere. Since SES significantly differed across the ethnic groups, we performed a post-hoc partial correlation. SES controlling for ethnicity was correlated with significant brain morphology (distance values) from the main effect analysis. The main effect findings remained unchanged. In supplemental figure 1, when SES is measured by the occupation along (O*NET coding) instead of the Hollingshead, the findings are comparable.
Figure 1. Correlation of Cerebral Surface Measures with SES.
Maps are shown for the correlations of surface measures of the neonatal brain with SES. Results are FDR-corrected for multiple comparisons and shown in red and yellow are local protrusions and in blue and purple are local indentations of the cerebral surface, which are regarded as smaller or larger, respectively local volume of the cerebral surface. We found significant inverse correlations of local brain volumes in the infants with SES in the superior and middle occipital gyri primarily of the right hemisphere, parieto-occipital region of both hemispheres, middle frontal and temporal pole regions of the right hemisphere, and inferior frontal and anterior cingulate regions of the left hemisphere. We also detected significant positive correlations of local brain volumes in the infants with SES in the frontoparietal region of the right hemisphere and the inferior temporal lobe of the left hemisphere. The scatterplot of the inverse correlation with SES and surface measures of the SOG. Surface distances (in mm from the corresponding point on the surface template brain) are plotted on the y-axis. Blue scatter points represent SES values. Abbreviations: IFG – Inferior Frontal Gyrus; IOG – Inferior Occipital Gyrus; MFG – Medial Frontal Gyrus; MOG – Middle Occipital Gyrus; SOG – Superior Occipital Gyrus; TP – Temporal Pole.
Secondary Hypothesis Testing
Partner Status.
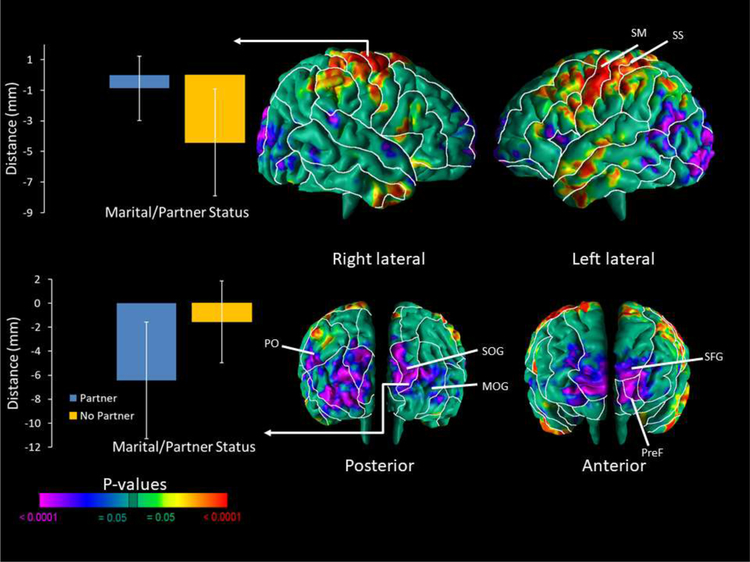
Infants whose mother had a partner compared to those who did not have a partner had smaller local volumes in the prefrontal and occipital (diffuse) regions of both hemispheres and the angular gyrus of the left hemisphere (Fig. 2). There were greater local volumes in the fronto-parietal and inferior temporal regions of both hemispheres.
Figure 2. Correlations of Cerebral Surface Measures with Partner Status.
Maps are shown for partner status correlations with morphological measures of the cerebral surface. The p-values are adjusted for multiple comparisons with FDR. We found significant inverse correlations of local brain volumes in the neonates based on maternal partner status in the prefrontal region and diffuse across the occipital lobe of both hemispheres, and the angular gyrus of the left hemisphere. There were positive correlations of local volume in the fronto-parietal and inferior temporal regions of both hemispheres. A sample of the average local volumes (or, more accurately, distances in mm from the most significant corresponding point on the surface of the template brain in the region denoted) are displayed in bar charts for the SM and SOG of the right hemisphere. Abbreviations: PreF – Prefrontal; MOG – Middle Occipital Gyrus; PO – Parieto-occipital; SM – Somatomotor; SS – Somatosensory; SFG – Superior Frontal Gyrus; SOG – Superior Occipital Gyrus.
SES-by-Partner Status Interaction.
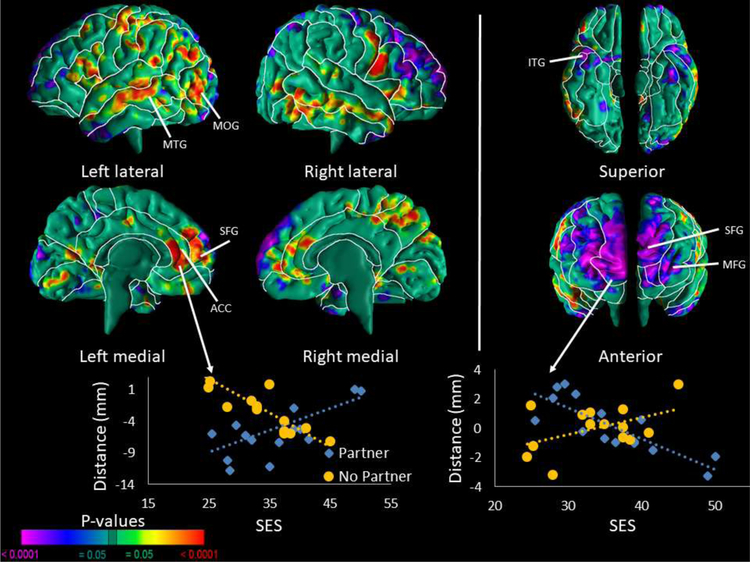
Infants whose mother had lower SES and who also had a partner compared to those who did not have a partner had smaller local volumes in the middle and superior frontal, temporal pole, and inferior temporal regions of both hemispheres, and the parieto-occipital region of the right hemisphere (Fig. 3). They also had greater local volumes in the middle temporal and occipital, inferior frontal, and medial superior frontal regions of both hemispheres, and the anterior cingulate of the left hemisphere. This similar pattern was true for pregnant women with higher SES who did not have a partner compared to those who had a partner resulting in a cross-over effect.
Figure 3. Interaction of SES with Partner Status on Cerebral Surface Measures.
Correlations of surface measures with SES in infants with mothers who have a partner compared to those who do not have a partner. The p-values are adjusted for multiple comparisons with FDR. We found significant inverse correlations of local brain volumes in the neonates for the interaction of SES with partner status in the middle and superior frontal, temporal pole, and inferior temporal regions of both hemispheres, and the parieto-occipital region of the right hemisphere. The correlation suggests that neonates whose mother had lower SES and a partner compared to those whose mother did not have a partner had smaller local volumes in the regions note above. We also detected positive correlations of local volume in the middle temporal and occipital, inferior frontal, and medial superior frontal regions of both hemispheres, and anterior cingulate region of the left hemisphere. Since the right parieto-occipital and temporal pole, and left inferior frontal regions were significant in the main effect model with SES, partner status moderates SES in these brain regions.
The scatterplot for this interaction are shown for the ACC of the left hemisphere and the SFG of the right hemisphere. Surface distances (in mm from the corresponding point on the surface template brain) are plotted on the y-axis. Blue scatter points represent infants whose mother had a partner and yellow scatter points represent infants whose mother did not have a partner; surface distances are in mm from the corresponding point on the surface of the template brain. Abbreviations: ACC – Anterior Cingulate; ITG – Inferior Temporal Gyrus; MTG – Middle Temporal Gyrus; MOG – Middle Occipital Gyrus; SFG – Superior Frontal Gyrus; SOG – Superior Occipital Gyrus.
SES and toddler cognitive and behavioral outcome.
SES was correlated positively with the BSID-III language, but not the cognitive score (Table 3). SES also correlated positively with the CBCL ADHD and ODD scores, but not the affective, anxiety, or PDD scores. Since the BSID-III language and CBCL ADHD and ODD scales correlated positively with SES, we performed additional analyses to determine whether the scales correlated with brain morphology that was significantly associated with SES.
Table 3.
Correlations between prenatal SES and cognitive and behavioral outcomes
| Prenatal SES | ||
|---|---|---|
| Variable | r | p-value |
| BSID-III (Standard Scores) | ||
| Cognitive | 0.002 | 0.99 |
| Language | 0.58 | 0.02* |
| CBCL – DSM Scales (T-scores) | ||
| Affective Problems | 0.33 | 0.24 |
| Anxiety Problems | 0.34 | 0.22 |
| Pervasive Developmental Problems | 0.12 | 0.67 |
| ADHD Problems | 0.59 | 0.02* |
| Oppositional Defiant Problems | 0.64 | 0.01* |
Abbreviations: BSID-III, Bayley Scales of Infant Development – Third Edition; CBCL, Child Behavior Checklist
p-value significant at ≤ 0.05.
No outcome variables correlated with brain regions that were significantly associated with SES (not shown). The local volumes of the right parieto-occipital region had an inverse correlation that approached significance with the CBCL ODD scale (r = −0.44, p = 0.09).
Discussion
Our goal in this study was to identify morphological features of the neonatal brain associated with lower SES, how partner status, as a proxy for social support may moderates those correlations, and how those associations relate to cognitive and behavioral outcomes at 24-months of age. Local volumes within the superior and middle occipital gyrus and parieto-occipital region bilaterally, right middle frontal and temporal pole regions, and left inferior frontal region and anterior cingulate gyrus were associated with SES. The interaction of SES with partner status was significant in additional regions (primarily superior and middle frontal and inferior and middle temporal gyri) and in regions (parieto-occipital, inferior frontal, and temporal pole) overlapping those of the main findings related to SES. For the independent variables, SES and partner status, the main effects are interpretable in locations where the interaction term is not significant. SES did not correlate significantly with overall cognitive scores on the BSID-III or internalizing behavior symptoms on the CBCL at age 24 months. However, infants with lower SES compared with higher SES had lower language scores and less severe ADHD and ODD symptoms.
In contrast to studies with older infants and children, we observed that lower SES was associated with larger local brain volumes in infants, albeit in similar brain regions as reported previously (Hanson et al., 2013; Noble et al., 2006). Brain maturation in early life involves both progressive and regressive processes. Soon after birth the brain grows rapidly as a consequence of both glial cell multiplication and dendritic arborization that will support subsequent synaptogenesis (Gilmore et al., 2007; Huttenlocher et al., 1982a), whereas in later infancy through the school-age years the same brain regions may disproportionately slow in their growth due to apoptosis or synaptic pruning (Huttenlocher et al., 1982a; Huttenlocher et al., 1982b). Thus, SES may relate to the direction of the volumetric findings by altering maturational processes that determine these region-specific growth trajectories (Merz et al., 2017). Consistent with this possibility, several researchers have reported that early life adversity is associated with accelerated brain maturation during infancy and childhood (Gee et al., 2013; Posner et al., 2016). Because our study was cross-sectional rather than longitudinal, however, we cannot definitively attribute our findings to accelerated maturation of the cerebral surface. Nevertheless, our interpretations do provide a framework and set of hypotheses for future studies to consider and test.
Prior studies vary greatly in their imaging measures, time points of assessment, and measures of SES, making their findings difficult to compare directly with one another and with ours. Nevertheless, the brain regions associated with lower SES that have been identified in prior studies are generally consistent with our findings. As reported in prior studies, we found associations of SES with local volumes in parietal and occipital regions (Hanson et al., 2013; Noble et al., 2006). Although we did not detect significant associations in frontal regions (Hanson et al., 2013; Noble et al., 2015b; Tomalski et al., 2013), we did detect associations in a smaller region within the anterior portion of the superior temporal lobe. In addition, lower SES was associated with larger local volumes in the anterior cingulate gyrus, a brain region involved in emotion regulation and attention processing (Bush et al., 2000).
The moderating associations of partner status on SES are in brain regions that both overlap with the main associations of SES and that are distinct from them. These brain regions support several functional capacities, including emotion regulation, attention, sensory processing, and language. Marital or partner status, and the number of family members, have both been found to reduce the detrimental role of lower SES on cognitive, physical, and mental health outcomes in children (Ghosh et al., 2010; Sullivan et al., 2012). Similarly, prior studies have reported that a two-parent household and better parent-child relationships can help reduce the cognitive and mental health challenges associated with early life adversity, including premature birth and institutionalization following parental loss (Ment et al., 2003; Vantieghem et al., 2017). Nevertheless, our findings add another layer to this previous literature, indicating that the presence of a partner is complex, multifaceted, and beyond what the current study can disentangle. To parse this apart, future studies would need to investigate the individual facets that partner status represents (e.g., emotional, monetary, and social support). Overall, these results underscore the value of assessing the moderating associations of social support in future studies.
A wide range of cognitive and behavioral outcomes have been associated with lower SES (Brooks-Gunn and Duncan, 1997). We found that lower SES was associated with poorer language outcome at 24-months of age, consistent with prior findings of deficits in language-related functions including reading, phonological processing, and intellectual and executive capacities that require language-based reasoning (Markant et al., 2016; Sarsour et al., 2011). While the language outcome was not significantly associated with the brain regions from the main effect analysis of SES, SES demonstrated an association with inferior frontal and temporal regions, which support language production. Prior studies have reported that the superior temporal lobe, an important language region, modulated SES-related differences in reading and phonological language performance (Noble et al., 2006). In contrast to prior research, however, we found that lower compared to higher SES was associated with less severe ADHD and ODD symptoms. Prior studies have found that older children in lower SES environments exhibit more severe internalizing and externalizing symptoms (Essex et al., 2006; McLaughlin et al., 2010; Merz et al., 2017). Our findings may differ from theirs because of the unique community sample we have available, which consisted predominantly of Hispanic families. Minority populations with higher SES can experience greater stress, less family support, less time with their child if the mother is working, and challenges with acculturation (Cardoso et al., 2016; Foster et al., 2000; Gorman-Smith et al., 2000; Jones et al., 2008). These factors may lead to more externalizing behaviors in the child.
Our study has several limitations. The study sample is representative of the Washington Heights district of New York City, a primarily Latino community, but is not representative of the national demographic. The findings therefore may not generalize to the national population. Our sample of infants with MRI data was small and only provided sufficient power to detect a large effect of our primary hypothesis. In addition, several infants were unable to complete follow-up cognitive and behavioral assessments at 24 months of age, reducing power to detect significance when testing our secondary hypothesis. Scheduling of MRI’s post-conception relates to scanner and maternal availability. The infants participated in MRI scans generally within the first two weeks after birth. Within that timeframe, they had the opportunity to interact with their mothers, health professionals, and other family members. Thus, postnatal environmental factors could have contributed to infant brain development during that time, albeit minimally compared with the older infants of prior studies. Similarly, recruitment of mothers spanned the second to third trimesters of pregnancy. As such, maternal prenatal assessments, which included psychosocial data collection, may have varied somewhat throughout the pregnancy.
Our results add to the rapidly growing literature investigating the associations of prenatal exposures with the neonatal brain (Qiu et al., 2017; Scheinost et al., 2016a; Scheinost et al., 2017). Our findings are novel as they demonstrate that prenatal exposure to lower SES was associated with larger local volumes at the neonatal cerebral surface, particularly in the occipital lobes, and it was associated with poorer language and fewer externalizing symptoms subsequently as toddlers. Our study also extends findings from prior studies of SES and the developing brain by assessing the potential moderating effects of social support, as measured by partner status. To our knowledge, no other imaging studies of the brain correlates of SES have included social support factors as a potential moderator. Our ability to scan infants shortly after birth provides a window into the contributions of prenatal and very early postnatal environment on the developing brain. Future longitudinal imaging and corresponding assessments of the social environment and behavior will help to identify the transient and enduring associations between SES and brain development in young children, as well as their functional implications for subsequent cognitive, emotional, and behavioral development (Gao et al., 2015).
Supplementary Material
Supplemental Figure 1. Comparison of the Correlation of Cerebral Surface Measures with SES coded in two different ways, via Hollingshead and Occupation alone.
Maps of SES coded in two different ways related to morphological measures of the cerebral surface are presented. (A) The main findings of a correlation between SES as measured by the Hollingshead and the infants’ local volumes of the cerebral surface are the same as presented in Figure 1. (B) The primary hypothesis model (A and Figure 1) was repeated with SES measured by occupation using the O*NET database coding system. The results are similar, with significant inverse correlations of local brain volumes in the superior and middle occipital gyri primarily of the right hemisphere, middle and inferior frontal, and anterior cingulate regions of the left hemisphere.
Acknowledgements:
This research was supported by the NIDA R01DA017820 (BSP and TSR), NIMH P50MH090966 (BSP), and NIMH T32MH016434 (BSP and MNS), National Center for Advancing Translational Sciences Grants KL2RR024157 and KL2TR000081 (MNS), National Institute of Child Health and Development Grant HD092589–02 (MNS), and MJS Foundation (Whitaker Scholar Developmental Neuropsychiatry program; MNS). Special thanks to Juan Sanchez-Peña, Siddhant Sawardekar, I-Chin Chiang, Deborah Jaspen, Beth Jewett, Dana Serino, Samantha Garavelli, Kirwan Walsh, David Semanek, Kathleen Durkin, Erica Lambeth, and Nelson Chen for their invaluable assistance.
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interests: The authors have no conflicts of interest to disclose.
References
- Achenbach TM, 2009. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory, and Applications University of Vermont Research Center for Children, Youth, & Families, Burlington, VT. [Google Scholar]
- Arentoft A, Byrd D, Monzones J, Coulehan K, Fuentes A, Rosario A, Miranda C, Morgello S, Rivera Mindt M, 2015. Socioeconomic Status and Neuropsychological Functioning: Associations in an Ethnically Diverse HIV+ Cohort. Clin Neuropsychol 29, 232–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacharach VR, Baumeister AA, 1998. Effects of maternal intelligence, marital status, income, and home environment on cognitive development of low birthweight infants. J Pediatr Psychol 23, 197–205. [DOI] [PubMed] [Google Scholar]
- Bansal R, Gerber AJ, Peterson BS, 2008a. Brain morphometry using anatomical magnetic resonance imaging. J Am Acad Child Adolesc Psychiatry 47, 619–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R, Staib LH, Whiteman R, Wang YM, Peterson BS, 2005. ROC-based assessments of 3D cortical surface-matching algorithms. Neuroimage 24, 150–162. [DOI] [PubMed] [Google Scholar]
- Bansal R, Staib LH, Xu D, Laine AF, Royal J, Peterson BS, 2008b. Using perturbation theory to compute the morphological similarity of diffusion tensors. IEEE Trans Med Imaging 27, 589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battin MR, Maalouf EF, Counsell SJ, Herlihy AH, Rutherford MA, Azzopardi D, Edwards AD, 1998. Magnetic resonance imaging of the brain in very preterm infants: visualization of the germinal matrix, early myelination, and cortical folding. Pediatrics 101, 957–962. [DOI] [PubMed] [Google Scholar]
- Bava L, Johns A, Kayser K, Freyer DR, 2018. Cognitive outcomes among Latino survivors of childhood acute lymphoblastic leukemia and lymphoma: A cross-sectional cohort study using culturally competent, performance-based assessment. Pediatr Blood Cancer 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N, 2005. Bayley Scales of Infant and Toddler Development-Third Edition: Administration Manual Harcourt Assessment, San Antonio, TX. [Google Scholar]
- Boylan JM, Cundiff JM, Jakubowski KP, Pardini DA, Matthews KA, 2018. Pathways Linking Childhood SES and Adult Health Behaviors and Psychological Resources in Black and White Men. Ann Behav Med 52, 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, Fifer WP, Myers MM, Elliott AJ, Noble KG, 2016. Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Dev Cogn Neurosci 19, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan GJ, 1997. The effects of poverty on children. Future Child 7, 55–71. [PubMed] [Google Scholar]
- Bublitz MH, Vergara-Lopez C, O’Reilly Treter M, Stroud LR, 2016. Association of Lower Socioeconomic Position in Pregnancy with Lower Diurnal Cortisol Production and Lower Birthweight in Male Infants. Clin Ther 38, 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI, 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4, 215–222. [DOI] [PubMed] [Google Scholar]
- Cardoso JB, Goldbach JT, Cervantes RC, Swank P, 2016. Stress and Multiple Substance Use Behaviors Among Hispanic Adolescents. Prev Sci 17, 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Hu H, Tak S, Mukherjee B, Park SK, 2012. Occupational noise exposure assessment using O*NET and its application to a study of hearing loss in the US general population. Occup Environ Med 69, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen GE, Rabbitt RD, Miller MI, 1996. Deformable templates using large deformation kinematics. IEEE Trans Image Process 5, 1435–1447. [DOI] [PubMed] [Google Scholar]
- Cirino PT, Chin CE, Sevcik RA, Wolf M, Lovett M, Morris RD, 2002. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment 9, 145–155. [DOI] [PubMed] [Google Scholar]
- Crosnoe R, Leventhal T, Wirth RJ, Pierce KM, Pianta RC, Network, N.E.C.C.R., 2010. Family socioeconomic status and consistent environmental stimulation in early childhood. Child Dev 81, 972–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daepp MIG, Arcaya MC, 2017. The effect of health on socioeconomic status: Using instrumental variables to revisit a successful randomized controlled trial. Econ Hum Biol 27, 305–314. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Guo T, Dodbiba L, Chakravarty MM, Chau V, Poskitt KJ, Synnes A, Grunau RE, Miller SP, 2016. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann Neurol 79, 548–559. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Brooks-Gunn J, Klebanov PK, 1994. Economic deprivation and early childhood development. Child Dev 65, 296–318. [PubMed] [Google Scholar]
- Duvernoy HM, Cabanis EA, & Vannson JL, 1991. The human brain: surface, three-dimensional sectional anatomy and MRI Springer-Verlag Wien, New York. [Google Scholar]
- Essex MJ, Kraemer HC, Armstrong JM, Boyce WT, Goldsmith HH, Klein MH, Woodward H, Kupfer DJ, 2006. Exploring risk factors for the emergence of children’s mental health problems. Arch Gen Psychiatry 63, 1246–1256. [DOI] [PubMed] [Google Scholar]
- Foster HW, Wu L, Bracken MB, Semenya K, Thomas J, 2000. Intergenerational effects of high socioeconomic status on low birthweight and preterm birth in African Americans. J Natl Med Assoc 92, 213–221. [PMC free article] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W, 2015. Functional Network Development During the First Year: Relative Sequence and Socioeconomic Correlations. Cereb Cortex 25, 2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N, 2013. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A 110, 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JK, Wilhelm MH, Dunkel-Schetter C, Lombardi CA, Ritz BR, 2010. Paternal support and preterm birth, and the moderation of effects of chronic stress: a study in Los Angeles county mothers. Arch Womens Ment Health 13, 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YS, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, Gerig G, 2007. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci 27, 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh S, Bansal R, Xu D, Hao X, Liu J, Peterson BS, 2011. Neuroanatomical correlates of intellectual ability across the life span. Dev Cogn Neurosci 1, 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman-Smith D, Tolan PH, Henry DB, Florsheim P, 2000. Patterns of family functioning and adolescent outcomes among urban African American and Mexican American families. J Fam Psychol 14, 436–457. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD, 2011. Association between income and the hippocampus. PLoS One 6, e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, Pollak SD, 2013. Family poverty affects the rate of human infant brain growth. PLoS One 8, e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MN, Lundien MC, Finnie DM, Williams AR, Beebe TJ, Sloan JA, Yawn BP, Juhn YJ, 2014. Application of a novel socioeconomic measure using individual housing data in asthma research: an exploratory study. NPJ Prim Care Respir Med 24, 14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptulla R, Hashim R, Johnson DN, Ilkowitz JT, DiNapoli G, Renukuntla V, Sivitz J, 2016. Evaluating emergency preparedness and impact of a hurricane sandy in pediatric patients with diabetes. Disaster Mil Med 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S, Hatch MC, 2000. Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol 19, 535–543. [PubMed] [Google Scholar]
- Hollingshead AB, 1975. Four factor index of social status Yale University, New Haven, CT, pp. 1–22. [Google Scholar]
- Hur JW, Choi SH, Yun JY, Chon MW, Kwon JS, 2015. Parental socioeconomic status and prognosis in individuals with ultra-high risk for psychosis: A 2-year follow-up study. Schizophr Res 168, 56–61. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, De Courten C, Garey LJ, Van der Loos H, 1982a. Synaptic development in human cerebral cortex. Int J Neurol 16–17, 144–154. [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H, 1982b. Synaptogenesis in human visual cortex--evidence for synapse elimination during normal development. Neurosci Lett 33, 247–252. [DOI] [PubMed] [Google Scholar]
- Jednoróg K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, Dehaene-Lambertz G, Ramus F, 2012. The influence of socioeconomic status on children’s brain structure. PLoS One 7, e42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HL, Nusbaum BJ, Bejarano A, Rosen TS, 1999. An ecological approach to development in children with prenatal drug exposure. Am J Orthopsychiatry 69, 448–456. [DOI] [PubMed] [Google Scholar]
- Jones DJ, Forehand R, Rakow A, Colletti CJ, McKee L, Zalot A, 2008. The specificity of maternal parenting behavior and child adjustment difficulties: a study of inner-city African American families. J Fam Psychol 22, 181–192. [DOI] [PubMed] [Google Scholar]
- Kennedy DN, Lange N, Makris N, Bates J, Meyer J, Caviness VS Jr., 1998. Gyri of the human neocortex: an MRI-based analysis of volume and variance. Cereb Cortex 8, 372–384. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Scheinost D, Lacadie C, Sze G, Schneider KC, Dai F, Constable RT, Ment LR, 2015. Adaptive mechanisms of developing brain: Cerebral lateralization in the prematurely-born. Neuroimage 108, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BR, Rowe DB, 2004. An evaluation of thresholding techniques in fMRI analysis. Neuroimage 22, 95–108. [DOI] [PubMed] [Google Scholar]
- Markant J, Ackerman LK, Nussenbaum K, Amso D, 2016. Selective attention neutralizes the adverse effects of low socioeconomic status on memory in 9-month-old infants. Dev Cogn Neurosci 18, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks GB, 2006. Environmental factors and gene-environment interactions in the aetiology of asthma. Clin Exp Pharmacol Physiol 33, 285–289. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Hall M, Dahl RE, 2014. Sleep in healthy black and white adolescents. Pediatrics 133, e1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Kubzansky LD, Dunn EC, Waldinger R, Vaillant G, Koenen KC, 2010. Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depress Anxiety 27, 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Vohr B, Allan W, Katz KH, Schneider KC, Westerveld M, Duncan CC, Makuch RW, 2003. Change in cognitive function over time in very low-birth-weight infants. JAMA 289, 705–711. [DOI] [PubMed] [Google Scholar]
- Merz EC, Tottenham N, Noble KG, 2017. Socioeconomic Status, Amygdala Volume, and Internalizing Symptoms in Children and Adolescents. J Clin Child Adolesc Psychol, 1–12. [DOI] [PMC free article] [PubMed]
- Mogi T, Tsunoda T, Yoshino A, 2019. Altered upright face recognition and presence of face inversion effect in temporal lobe epilepsy: An event-related potential study. Psychiatry Clin Neurosci [DOI] [PubMed]
- Noble KG, Engelhardt LE, Brito NH, Mack LJ, Nail EJ, Angal J, Barr R, Fifer WP, Elliott AJ, Network P, 2015a. Socioeconomic disparities in neurocognitive development in the first two years of life. Dev Psychobiol 57, 535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Van Zijl P, Mostofsky S, Kaufmann WE, Kenet T, Dale AM, Jernigan TL, Sowell ER, 2015b. Family income, parental education and brain structure in children and adolescents. Nat Neurosci 18, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER, 2012. Neural correlates of socioeconomic status in the developing human brain. Dev Sci 15, 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ, 2007. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev Sci 10, 464–480. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ, 2005. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev Sci 8, 74–87. [DOI] [PubMed] [Google Scholar]
- Noble KG, Wolmetz ME, Ochs LG, Farah MJ, McCandliss BD, 2006. Brain-behavior relationships in reading acquisition are modulated by socioeconomic factors. Dev Sci 9, 642–654. [DOI] [PubMed] [Google Scholar]
- Oishi K, Mori S, Donohue PK, Ernst T, Anderson L, Buchthal S, Faria A, Jiang H, Li X, Miller MI, van Zijl PC, Chang L, 2011. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage 56, 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Kubik S, & Abernathey CD , 1990. Atlas of the cerebral sulci Georg Thieme Verlag, Stuttgart. [Google Scholar]
- Ozmert EN, Yurdakök K, Soysal S, Kulak-Kayikçi ME, Belgin E, Ozmert E, Laleli Y, Saraçbasi O, 2005. Relationship between physical, environmental and sociodemographic factors and school performance in primary schoolchildren. J Trop Pediatr 51, 25–32. [DOI] [PubMed] [Google Scholar]
- Peterson BS, 2010. Form determines function: new methods for identifying the neuroanatomical loci of circuit-based disturbances in childhood disorders. J Am Acad Child Adolesc Psychiatry 49, 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, Gore JC, Duncan CC, Makuch R, Ment LR, 2003. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111, 939–948. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR, 2000. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284, 1939–1947. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramaratne P, Weissman MM, 2009. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci U S A 106, 6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipe JG, 1999. Motion correction with PROPELLER MRI: application to head motion and free-breathing cardiac imaging. Magn Reson Med 42, 963–969. [DOI] [PubMed] [Google Scholar]
- Posner J, Cha J, Roy AK, Peterson BS, Bansal R, Gustafsson HC, Raffanello E, Gingrich J, Monk C, 2016. Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry 6, e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Shen M, Buss C, Chong YS, Kwek K, Saw SM, Gluckman PD, Wadhwa PD, Entringer S, Styner M, Karnani N, Heim CM, O’Donnell KJ, Holbrook JD, Fortier MV, Meaney MJ, group, t.G.s., 2017. Effects of Antenatal Maternal Depressive Symptoms and Socio-Economic Status on Neonatal Brain Development are Modulated by Genetic Risk. Cereb Cortex 27, 3080–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciuti AE, Scarr S, 1990. Interaction of early biological and family risk factors in predicting cognitive development. Journal of Applied Developmental Psychology 11, 1–12. [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, Boyce WT, 2011. Family socioeconomic status and child executive functions: the roles of language, home environment, and single parenthood. J Int Neuropsychol Soc 17, 120–132. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Lacadie C, Sze G, Sinha R, Constable RT, Ment LR, 2016a. Prenatal stress alters amygdala functional connectivity in preterm neonates. Neuroimage-Clinical 12, 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Kwon SH, Shen X, Lacadie C, Schneider KC, Dai F, Ment LR, Constable RT, 2016b. Preterm birth alters neonatal, functional rich club organization. Brain Structure & Function 221, 3211–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D, Sinha R, Cross SN, Kwon SH, Sze G, Constable RT, Ment LR, 2017. Does prenatal stress alter the developing connectome? Pediatr Res 81, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyrek S, Cop E, Sinir H, Ugurlu M, Şenel S, 2017. Factors associated with Internet addiction: Cross-sectional study of Turkish adolescents. Pediatr Int 59, 218–222. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC, 1998. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17, 87–97. [DOI] [PubMed] [Google Scholar]
- Sobel LJ, Bansal R, Maia TV, Sanchez J, Mazzone L, Durkin K, Liu J, Hao X, Ivanov I, Miller A, Greenhill LL, Peterson BS, 2010. Basal ganglia surface morphology and the effects of stimulant medications in youth with attention deficit hyperactivity disorder. Am J Psychiatry 167, 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Peterson BS, Mattson SN, Welcome SE, Henkenius AL, Riley EP, Jernigan TL, Toga AW, 2002. Mapping cortical gray matter asymmetry patterns in adolescents with heavy prenatal alcohol exposure. Neuroimage 17, 1807–1819. [DOI] [PubMed] [Google Scholar]
- Spann MN, Bansal R, Rosen TS, Peterson BS, 2014. Morphological features of the neonatal brain support development of subsequent cognitive, language, and motor abilities. Hum Brain Mapp 35, 4459–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann MN, Serino D, Bansal R, Hao X, Nati G, Toth Z, Walsh K, Chiang IC, Sanchez-Peña J, Liu J, Kangarlu A, Liu F, Duan Y, Shova S, Fried J, Tau GZ, Rosen TS, Peterson BS, 2015. Morphological features of the neonatal brain following exposure to regional anesthesia during labor and delivery. Magn Reson Imaging 33, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K, Raley RK, Hummer RA, Schiefelbein E, 2012. The potential contribution of marital-cohabitation status to racial, ethnic, and nativity differentials in birth outcomes in Texas. Matern Child Health J 16, 775–784. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Poteat T, 2011. Relevance of the quality of partner relationships and maternal health to early child wellness. J Dev Behav Pediatr 32, 292–300. [DOI] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff-Smith A, Johnson MH, Kushnerenko E, 2013. Socioeconomic status and functional brain development - associations in early infancy. Dev Sci 16, 676–687. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Beam CE, Davis DW, 2015. The Scarr-Rowe Interaction in Complete Seven-Year WISC Data from the Louisville Twin Study: Preliminary Report. Behav Genet 45, 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II, 2003. Socioeconomic status modifies heritability of IQ in young children. Psychol Sci 14, 623–628. [DOI] [PubMed] [Google Scholar]
- Valenzuela D, Singer J, Lee T, Hu A, 2019. The Impact of Socioeconomic Status on Voice Outcomes in Patients With Spasmodic Dysphonia Treated With Botulinum Toxin Injections. Ann Otol Rhinol Laryngol 128, 316–322. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, van Wezel-Meijler G, Barth PG, Barkhof F, Ader HJ, Valk J, 1996. Normal gyration and sulcation in preterm and term neonates: appearance on MR images. Radiology 200, 389–396. [DOI] [PubMed] [Google Scholar]
- Vantieghem MR, Gabard-Durnam L, Goff B, Flannery J, Humphreys KL, Telzer EH, Caldera C, Louie JY, Shapiro M, Bolger N, Tottenham N, 2017. Positive valence bias and parent-child relationship security moderate the association between early institutional caregiving and internalizing symptoms. Dev Psychopathol 29, 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells WM 3rd, Viola P, Atsumi H, Nakajima S, Kikinis R, 1996. Multi-modal volume registration by maximization of mutual information. Med Image Anal 1, 35–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Comparison of the Correlation of Cerebral Surface Measures with SES coded in two different ways, via Hollingshead and Occupation alone.
Maps of SES coded in two different ways related to morphological measures of the cerebral surface are presented. (A) The main findings of a correlation between SES as measured by the Hollingshead and the infants’ local volumes of the cerebral surface are the same as presented in Figure 1. (B) The primary hypothesis model (A and Figure 1) was repeated with SES measured by occupation using the O*NET database coding system. The results are similar, with significant inverse correlations of local brain volumes in the superior and middle occipital gyri primarily of the right hemisphere, middle and inferior frontal, and anterior cingulate regions of the left hemisphere.