Abstract
Background/Objectives
γ-Tocopherol has unique properties that protect against nitrogen oxide-mediated cellular damage. To elucidate the potential role of γ-tocopherol in the aging process, we examined the associations of serum γ-tocopherol levels with all-cause and cause-specific mortality.
Subjects/Methods
Among participants in the biorepository subcohort of the Multiethnic Cohort Study, pre-cancer diagnostic serum γ-tocopherol levels were measured in a subset of 3904 men and 4461 women. Of these, 22.7% of men and 13.5% of women died during a mean follow-up time of 9.6±2.6 years. Hazard ratios (HRs) and 95% confidence intervals (95%CIs) for mortality associated with γ-tocopherol were estimated by Cox proportional hazards regression.
Results
Positive associations of serum γ-tocopherol with all-cause, cancer and cardiovascular disease mortality (CVD) (Ptrend<0.05) were detected after adjusting for age, race-ethnicity and serum cholesterol levels. The respective HRs (95%CIs) for the highest versus the lowest sex-specific γ-tocopherol quartile were 1.43 (1.17–1.74), 1.79 (1.22–2.64), and 1.52 (1.10–2.11) for men and 1.58 (1.25–2.00), 1.59 (1.05–2.41), and 1.59 (1.07–2.37) for women. Associations remained significant for all-cause mortality among women after further adjusting for smoking variables and history of cancer, CVD, diabetes, and hypertension at cohort entry (highest vs. lowest γ-tocopherol quartile: HR=1.38; 95%CI=1.08–1.75; Ptrend= 0.005). Overall, associations with all-cause mortality were consistent across race/ethnicity and were significant in three of ten sex-specific racial/ethnic groups in the fully adjusted models with no interactions between ethnicity and γ-tocopherol.
Conclusions
The positive association between γ-tocopherol and mortality suggests a potential physiological role for γ-tocopherol in response to pathological conditions.
INTRODUCTION
The tocopherols are important agents that prevent cellular oxidative damage and are thought to protect against the development of cancer and other aging-related diseases [1, 2]. Although α-tocopherol has the highest level of vitamin E bioactivity of all the tocopherols [3] and, consequently, has been the target of the most research over the years, animal studies have shown that other tocopherols also play a significant role in inhibiting tumor formation independent of vitamin E bioactivity [2]. In particular, γ-tocopherol has been identified as a key carcinogenesis modifier and cellular antioxidant that limits damage to cells resulting from the enzymatic production of nitric oxide (NO), a mediator of inflammation through its oxidation products [4–6]. Paradoxically, γ-tocopherol at high concentrations can also enhance cellular NO synthesis resulting in an enhanced cellular immune response and potentially increased oxidative damage [4].
The Institute of Medicine (IOM) has identified a concentration of 5.16 μg/mL (12 μM) α-tocopherol in blood as sufficient to meet vitamin E requirements in humans with respect to acute consequences of vitamin E deficiency. The IOM further concludes that only α-tocopherol is required for vitamin E function and that there is inadequate evidence to recommend a requirement for γ-tocopherol [7]. However, the IOM report points out the need for more research to determine the levels and types of tocopherols that are important for optimal nutrition and their mechanisms in the prevention of aging related chronic diseases. In particular, serum levels of γ-tocopherol appear to be independent of dietary intake and physiologically regulated [8], possibly as a consequence of chronic inflammation. This was suggested by the positive associations of γ-tocopherol with circulating C-reactive protein (CRP) and urinary isoprostane (both indicators of chronic inflammation) [9] as well as by animal and cell studies showing γ-tocopherol levels increase in response to inflammation [10, 11]. Previous work also found that γ-tocopherol was positively associated with various disease states and risk factors that are associated with inflammation, such as diabetes [12], Alzheimer’s Disease [13], smoking [14], obesity [15] and poor diet [16]. Further, infection is often a source of chronic inflammation and it has been reported that elevated γ-tocopherol levels are associated with higher clearance of anal human papillomavirus infection [17].
Epidemiological evidence regarding associations of γ-tocopherol with aging and the etiology of aging related diseases is limited and results are equivocal. While some studies reported inverse associations of γ-tocopherol with certain types of cancers [18–20], null [21–23] or positive associations have been found in others [20, 24]. Further, a nested case-control study with 39,242 Japanese subjects found that serum γ-tocopherol levels were inversely associated with ischemic stroke mortality in men but positively associated with hemorrhagic stroke mortality in women [25]. However, to our knowledge, no study to-date has assessed the relation between γ-tocopherol and overall risk of death and its consistency across racial/ethnic groups. Because of its unique paradoxical relationship and the broad array of evidence from basic science studies as well as epidemiologic associations, we hypothesize that elevated γ-tocopherol levels generally reflect increased risk of premature mortality, which may in turn serve as an indicator of adverse physiologic conditions such as chronic inflammation and heightened systemic and/or tissue-specific oxidation. Support for this concept was further suggested by a recent cross-sectional study using a nationally representative sample showing that adults with γ-tocopherol levels in the highest quartile (75–100th percentile) had much shorter telomere length (a biomarker for biological aging) than those at the lowest quartile (0–25th percentile), accounting for approximately three years of additional biological aging [26]. Thus, the current study examined the associations of serum levels of γ-tocopherol with all-cause mortality, cancer mortality, and cardiovascular disease (CVD) mortality among men and women with white, African American, Native Hawaiian, Japanese American, and Latino ancestry who participated in the biorepository subcohort of the Multiethnic Cohort (MEC) Study.
PARTICIPANTS AND METHODS
Study Population
The MEC is a prospective cohort study assembled in Hawaii and Los Angeles during 1993–1996 funded by the National Cancer Institute to investigate the association of dietary, lifestyle, and genetic factors with cancer incidence. Details on recruitment and baseline demographic information have been described previously [27]. Briefly, subjects from five ethnic groups (white, African American, Native Hawaiian, Japanese American, and Latino) were identified through drivers’ license files, voter registration lists in Hawaii and Medicare files in California and recruited by mailing a self-administered, 26-page questionnaire that included questions on anthropometric measures, medical history, family history of cancer, lifestyle factors, and a food frequency questionnaire of over 180 items and dietary supplements. The validity of the dietary questionnaire was confirmed in a calibration study using three 24-hour dietary recalls [28]. A total of 215,251 men and women aged 45 to 75 years were included at baseline representing the general target population as verified by a comparison of the cohort distributions with corresponding census data for the two geographical areas [27]. The study protocol was approved by the Institutional Review Boards of the University of Hawaii and the University of Southern California and informed consent was obtained from all subjects.
The MEC biorepository was established primarily during 2001–2006 by asking surviving cohort members residing in the catchment area of the University of Hawaii and University of Southern California to provide blood and urine specimens [29]. A total of 67,594 cohort members (approximately 32% of original MEC members) contributed to the biorepository and of all blood samples collected, 18% were analyzed for biomarkers. Participants were free of cancer at the time when blood and urine were collected. When comparing the characteristics of individuals who provided specimens with those who did not, participants were younger by an average of three years, had more education, and were less likely to be current smokers. However, there were no substantial differences between the two groups in several demographic characteristics and cancer risk factors including body mass index (BMI), dietary fat and vegetable intake, physical activity, and family history of cancer suggesting that the biorepository participants are broadly representative of all cohort members. Mortality from cancer and other causes was available through linkages with death certificate files in both states. For cohort members who moved to other states, information on deaths was obtained from the National Death Index [27, 30].
Serum Assays
A panel of biomarkers were assayed in the MEC biorepository subcohort that included controls from nested cancer case-control studies. Lipid-soluble micronutrients were assessed in 8,664 individuals from this subcohort. After excluding participants with invalid biomarker measurements, the final analysis cohort consisted of 8,365 participants (3904 men and 4461 women). All serum samples were collected after an overnight fast, stored at −80°C immediately after blood collection, and had not been previously thawed before extraction to minimize the outcome variations due to storage conditions such as temperature and storage duration from collection to processing. Serum concentrations of tocopherols were analyzed using high-pressure liquid chromatography with photodiode-array detection according to our earlier protocol [31, 32]. The β- and γ-tocopherols were measured together. Given that β-tocopherol comprises only a tiny fraction of total tocopherols, the combined values represent predominantly γ-tocopherol. Based on nine triplet pooled samples prepared for quality control, the respective within- and between-batch coefficients of variation were 2.2±2.0% and 5.9±4.7% for β- and γ-tocopherols combined.
Statistical Analyses
Serum γ-tocopherol levels were categorized into quartiles for men and women, separately. Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95%CIs) for associations of γ-tocopherol with all-cause, cancer, and CVD-specific mortality among men and women. Deaths due to injuries (0.3% of all deaths) were excluded from all-cause mortality. Survival time was defined from the date of blood draw until death or the end of follow-up on December 31, 2013. For cancer and CVD-specific models, participants who had died from other causes were censored. Two models were estimated: Model 1 (minimally adjusted model) only included age at blood draw, race/ethnicity (as a strata variable: white, African American, Native Hawaiian, Japanese American, and Latino) and serum cholesterol levels while Model 2 (fully adjusted model) further included smoking variables and history of cancer, CVD (heat attack, angina, stroke), diabetes, and hypertension at cohort entry (before blood draw). The adjustment for serum cholesterol levels in both models was because previous research found that tocopherols and cholesterol levels were highly correlated [19]. The adjustment for smoking was based on a comprehensive smoking model that was developed for a lung cancer study in the MEC [33]. The smoking model explicitly included average number of cigarettes; average number of cigarettes squared; indicator variables for former and current smokers; number of years smoked (time-dependent); and number of years since quitting (time-dependent). We also considered BMI, alcohol use and season of blood draw as covariates but did not include them in the final models because they did not alter the risk estimates. Sensitivity analyses were performed after excluding participants who died within one year after blood draw. Research evidence showed a direct relationship between CRP and mortality [34] and γ-tocopherol was positively associated with CRP [9]. Thus, we added CRP (log-transformed values) in Model 1 to determine if γ-tocopherol contributed any information beyond CRP to predict mortality (Model 3).
In order to determine whether the associations between γ-tocopherol and mortality, if present, were consistent across racial/ethnic groups in MEC, analyses were repeated separately by sex-specific racial/ethnic groups. Due to the relatively small number of participants in some racial/ethnic groups, sex-specific tertiles of γ-tocopherol (for the overall study population of men and women, respectively) were used in these analyses. Interaction terms between serum γ-tocopherol levels and race/ethnicity in relation to mortality were estimated. As supplementation with large doses of α-tocopherol may significantly depress γ-tocopherol concentrations in the serum [9], we performed sensitivity analyses by excluding participants taking vitamin E supplements (mainly containing α-tocopherol) at cohort entry and participants whose serum α-tocopherol concentrations were ≥18 ug/mL. Further, we also assessed the association of serum α-tocopherol levels (categorized into sex-specific quartiles) with all-cause mortality using Cox proportional hazards regression to determine whether the association for α-tocopherol was similar to that for γ-tocopherol. The proportionality assumption was tested by Schoenfeld residuals and was found to be valid. SAS statistical software version 9.4 (SAS Institute, Inc., Cary, North Carolina) was used to conduct all analyses. All tests were two-sided, and P < 0.05 was used as the critical value for statistical significance.
RESULTS
This analysis included 8365 participants (3,904 men and 4,461 women); of these, 1,489 (886 men [22.7%] and 603 women [13.5%]) had died during a mean follow-up time of 9.6±2.6 years. The racial/ethnic distribution for the study population was 8% white, 28% African American, 26% Native Hawaiian, 30% Japanese American, and 8% Latino, which differed from the original parent MEC biorepository subcohort due to the selection process of matching controls to case characteristics. The mean age at blood draw of participants in the current study was 68.3 ± 8.5 years, which was similar to the MEC biorepository cohort. Major disease-related causes of death were cancer (30.5%), major CVD (35.5%), respiratory/pulmonary diseases (4.9%), diabetes (2.1%), and infectious disease (1.0%). These rates were comparable to those observed in the original MEC (data not shown). Differences in age, race/ethnicity, BMI, smoking related variables, alcohol use, hypercholesterolemia and history of cancer, CVD, diabetes or hypertension were observed between participants who died and participants who remained alive during the follow-up. γ-Tocopherol levels were positively associated with smoking, adiposity, hypercholesterolemia, and history of cancer, CVD, diabetes, or hypertension and inversely associated with moderate/vigorous physical activity and α-tocopherol concentrations (Table 1).
Table 1.
Characteristics of Study Participants by γ-Tocopherol Quartiles and by All-Cause Mortality
| by γ-Tocopherol Quartiles (Q1-Q4, μg/mL) |
Participants |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 (0 −1.18) | Q2 (1.18–1.65) | Q3 (1.65–2.34) | Q4 (2.34–13.97) | Ptrenda | All (N=8365) | Alive (N=6876) | Dead (N=1489) | Pb | |
| Age at blood draw (y) | 69.7 ± 8.5 | 68.9 ± 8.6 | 67.9 ± 8.6 | 67.1 ± 8.3 | <0.0001 | 68.3 ± 8.5 | 67.1 ± 8.1 | 74.4 ± 7.4 | <0.0001 |
| Sex, n (%) | |||||||||
| Men | 968 (46.3) | 1015 (48.5) | 1012 (48.4) | 909 (43.5) | 0.075 | 3904 (46.7) | 3018 (43.9) | 886 (59.5) | <0.0001 |
| Women, | 1123 (53.7) | 1077 (51.5) | 1077 (51.6) | 1183 (56.5) | 0.075 | 4461 (53.3) | 3858 (56.1) | 603 (40.5) | <0.0001 |
| Race/ethnicity, n (%) | |||||||||
| African American | 469 (22.4) | 522 (25.0) | 580 (27.7) | 727 (34.8) | <0.0001 | 2297 (27.5) | 1831 (26.6) | 466 (31.3) | 0.0003 |
| Latino | 91 (4.4) | 143 (6.8) | 187 (8.9) | 276 (13.2) | <0.0001 | 697 (8.3) | 488 (7.1) | 209 (14.1) | <0.0001 |
| Native Hawaiian | 426 (20.4) | 494 (23.6) | 631 (30.2) | 584 (27.9) | <0.0001 | 2134 (25.5) | 1870 (27.2) | 264 (17.7) | <0.0001 |
| Japanese American | 935 (44.7) | 729 (34.8) | 533 (25.5) | 348 (16.6) | <0.0001 | 2545 (30.4) | 2219 (32.3) | 326 (21.9) | <0.0001 |
| White | 170 (8.1) | 204 (9.8) | 161 (7.7) | 157 (7.5) | 0.14 | 692 (8.3) | 468 (6.8) | 224 (15.0) | <0.0001 |
| Body mass index (kg/m2) | 25.3 ± 4.2 | 26.1 ± 4.5 | 27.1 ± 5.2 | 28.4 ± 5.6 | <0.0001 | 26.7 ± 5.0 | 26.6 ± 4.9 | 27.1 ± 5.4 | 0.001 |
| Smoking statusc, n (%) | |||||||||
| Never | 1048 (50.2) | 1038 (49.9) | 938 (45.3) | 852 (41.2) | <0.0001 | 3875 (46.3) | 3332 (48.5) | 543 (36.5) | <0.0001 |
| Former | 846 (40.6) | 832 (40.0) | 849 (41.0) | 862 (41.6) | 0.53 | 3388 (40.5) | 2689 (39.1) | 699 (46.9) | <0.0001 |
| Current | 192 (9.2) | 210 (10.1) | 284 (13.7) | 356 (17.2) | <0.0001 | 1042 (12.5) | 806 (11.7) | 236 (15.9) | <0.0001 |
| Years of smokingc | 9.9 ± 13.2 | 10.2 ± 13.2 | 11.3 ± 13.7 | 12.6 ± 14.1 | <0.0001 | 11.0 ± 13.6 | 10.1 ± 13.0 | 15.3 ± 15.5 | <0.0001 |
| Number of cigarettes per dayc | 7.2 ± 9.3 | 7.4 ± 9.4 | 7.9 ± 9.3 | 8.2 ± 9.2 | <0.0001 | 7.7 ± 9.3 | 7.3 ± 9.1 | 9.6 ± 9.9 | <0.0001 |
| Alcohol use (g/day) | 6.3 ± 15.7 | 8.3 ± 22.4 | 9.7 ± 28.9 | 8.5 ± 22.3 | 0.006 | 8.5 ± 22.8 | 7.6 ± 21.4 | 10.8 ± 28.2 | <0.0001 |
| Moderate/vigorous activity (hour/day) | 1.35 ± 1.38 | 1.36 ± 1.39 | 1.27 ± 1.34 | 1.24 ± 1.45 | 0.003 | 1.30 ± 1.39 | 1.32 ± 1.37 | 1.24 ± 1.46 | 0.07 |
| History of diabetes, n (%) | 59 (2.8) | 68 (3.2) | 86 (4.1) | 126 (6.3) | <0.0001 | 339 (4.1) | 180 (2.6) | 159 (10.7) | <0.0001 |
| History of hypertension, n (%) | 767 (36.7) | 757 (36.2) | 797 (38.1) | 868 (41.5) | 0.0005 | 3189 (38.1) | 2457 (35.7) | 732 (49.2) | <0.0001 |
| History of cancer, n (%) | 87 (4.2) | 104 (5.0) | 99 (4.7) | 122 (5.8) | 0.02 | 412 (4.9) | 260 (3.8) | 152 (10.2) | <0.0001 |
| History of cardiovascular disease, n (%) | 149 (24.4) | 145 (23.7) | 130 (21.3) | 187 (30.6) | 0.06 | 611 (7.3) | 382 (5.6) | 229 (15.4) | <0.0001 |
| Hypercholesterolemiad, n (%) | 774 (20.6) | 938 (25.0) | 941 (25.0) | 1107 (29.4) | <0.0001 | 3758 (44.9) | 3220 (46.8) | 538 (36.1) | <0.0001 |
| α-Tocopherol (μg/mL) | 11.23 ± 4.62 | 10.73 ± 5.26 | 8.81 ± 4.65 | 8.53 ± 3.97 | <0.0001 | 9.82 ± 4.79 | 9.87 ± 4.76 | 9.61 ± 4.96 | 0.07 |
Note: Data are given as mean ± standard deviation unless otherwise specified
P for trend across γ-tocopherol quartiles.
P for difference between participants who were alive and participants who died during follow-up.
60 participants had missing value for smoking status; 196 participants had missing values for other smoking related variables.
Hypercholesterolemia: cholesterol ≥200 mg/dL
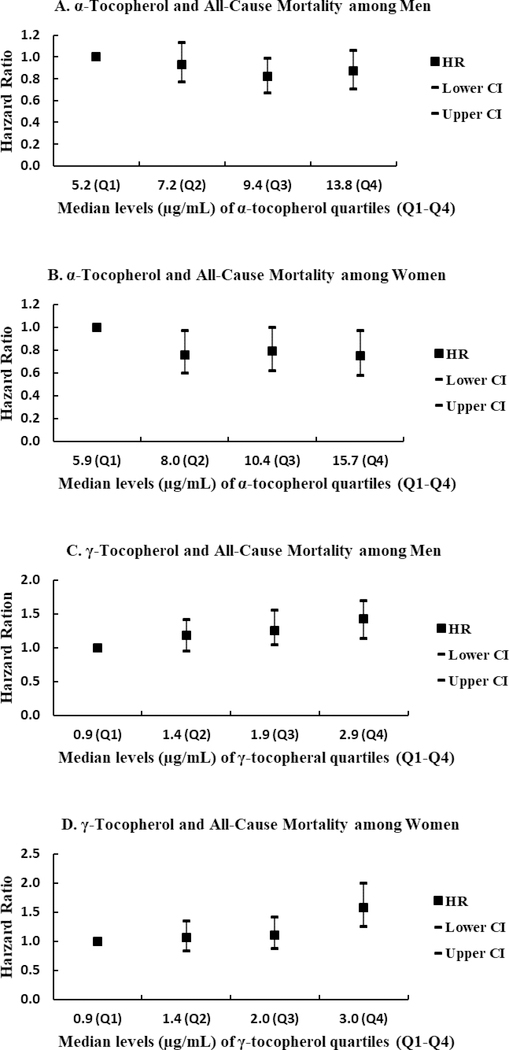
Serum γ-tocopherol levels were positively associated with all-cause mortality in both men and women after adjusting for age at blood draw, race/ethnicity and serum cholesterol levels (men: Ptrend=0.0005; women: Ptrend<0.0001). The mortality risk was approximately 40% higher among men and 60% higher among women for the highest versus the lowest quartile of γ-tocopherol (men: HR=1.43, 95%CI=1.17–1.74; women: HR=1.58, 95%CI=1.25–2.00). The association remained significant for women when the model was additionally adjusted for history of cancer, CVD, diabetes, and hypertension and smoking variables at cohort entry (the highest versus the lowest γ-tocopherol quartile for women: HR=1.38, 95%CI=1.08–1.75; Ptrend= 0.005). Although attenuated, the relation between γ-tocopherol and all-cause mortality remained significant when CRP was included in Model 1 (the highest versus the lowest quartile for men: HR=1.34, 95%CI=1.09–1.63; Ptrend= 0.006; for women: HR=1.45, 95%CI=1.14–1.83; Ptrend= 0.001) (Table 2). Further, γ-tocopherol and α-tocopherol appeared to have opposite associations with mortality risk; while positive associations were observed for γ-tocopherol with all-cause mortality among both men and women, higher serum α-tocopherol levels were more likely to be associated with lower risk of mortality (all-cause) particularly for women in the age, ethnicity, and cholesterol adjusted models (Figure 1).
Table 2.
HR and 95% CI for All-Cause Mortality by γ-Tocopherol Quartiles
| γ-Tocopherol Quartiles (μg/mL) | Median (μg/mL) | Participants (N) | Deaths (N) | HR (95% CI)a | Ptrenda |
|---|---|---|---|---|---|
| Men | |||||
| Model 1b | |||||
| Q1 (0.27–1.17) | 0.96 | 973 | 198 | 1.00 | |
| Q2 (1.17–1.63) | 1.39 | 974 | 211 | 1.18 (0.97–1.43) | |
| Q3 (1.63–2.28) | 1.92 | 968 | 223 | 1.25 (1.02–1.52) | |
| Q4 (2.28–11.02) | 2.86 | 972 | 237 | 1.43 (1.17–1.74) | 0.0005 |
| Model 2c | |||||
| Q1 (0.27–1.17) | 0.96 | 971 | 192 | 1.00 | |
| Q2 (1.17–1.63) | 1.39 | 968 | 209 | 1.12 (0.92–1.38) | |
| Q3 (1.63–2.28) | 1.92 | 960 | 220 | 1.23 (1.00–1.50) | |
| Q4 (2.28–11.02) | 2.86 | 963 | 235 | 1.15 (0.93–1.41) | 0.23 |
| Model 3d | |||||
| Q1 (0.27–1.17) | 0.96 | 973 | 198 | 1.00 | |
| Q2 (1.17–1.63) | 1.39 | 974 | 211 | 1.16 (0.96–1.42) | |
| Q3 (1.63–2.28) | 1.92 | 968 | 223 | 1.23 (1.01–1.49) | |
| Q4 (2.28–11.02) | 2.86 | 972 | 237 | 1.34 (1.09–1.63) | 0.006 |
| Women | |||||
| Model 1b | |||||
| Q1 (0–1.17) | 0.97 | 1113 | 136 | 1.00 | |
| Q2 (1.17–1.66) | 1.40 | 1112 | 139 | 1.06 (0.84–1.35) | |
| Q3 (1.66–2.39) | 1.98 | 1114 | 138 | 1.11 (0.87–1.41) | |
| Q4 (2.39–13.97) | 2.98 | 1113 | 181 | 1.58 (1.25–2.00) | <0.0001 |
| Model 2c | |||||
| Q1 (0–1.17) | 0.97 | 1110 | 136 | 1.00 | |
| Q2 (1.17–1.66) | 1.40 | 1106 | 139 | 1.03 (0.81–1.32) | |
| Q3 (1.66–2.39) | 1.98 | 1101 | 137 | 1.04 (0.82–1.34) | |
| Q4 (2.39–13.97) | 2.98 | 1110 | 179 | 1.38 (1.08–1.75) | 0.005 |
| Model 3d | |||||
| Q1 (0–1.17) | 0.97 | 1113 | 136 | 1.00 | |
| Q2 (1.17–1.66) | 1.40 | 1112 | 139 | 1.07 (0.85–1.37) | |
| Q3 (1.66–2.39) | 1.98 | 1114 | 138 | 1.07 (0.84–1.36) | |
| Q4 (2.39–13.97) | 2.98 | 1113 | 181 | 1.45 (1.14–1.83) | 0.001 |
HR, 95% CI and P values were estimated by Cox proportional hazards models.
Model 1: Adjusted by Cox regression for age at blood draw, race/ethnicity (as a strata variable) and serum cholesterol levels.
Model 2: Adjusted by Cox regression for variables in Model 1, and further adjusted for history of cancer, cardiovascular diseases, diabetes, and hypertension and smoking related variables.
Model 3: Adjusted by Cox regression for variables in Model 1, and further adjusted for C-reactive protein (log-transformed).
Figure 1.
Associations of serum α-tocopherol and γ-tocopherol levels with all-cause mortality among men and women. Tocopherol levels were categorized into sex-specific quartiles (Q1-Q4) and the lowest quartile (Q1) was used as the reference quartile. Hazard ratios (HRs) and 95% confidence intervals (CIs) were plotted against the median level (μg/mL) of each α-tocopherol or γ-tocopherol quartile. HRs and 95%CIs were estimated by Cox proportional hazards regression adjusting for age at blood draw, race/ethnicity (as a strata variable) and serum cholesterol levels.
Serum γ-tocopherol levels were also positively associated with cancer mortality (Table 3) among men (Ptrend=0.005) and women (Ptrend= 0.006) after adjusting for age at blood draw, ethnicity, and serum cholesterol. Mortality risk was approximately 80% higher among men (HR=1.79, 95%CI=1.22–2.64) and 60% higher among women (HR=1.59, 95%CI=1.05–2.41) for the highest versus the lowest quartile of γ-tocopherol. Although the association was attenuated after further adjusting for history of major diseases (cancer, CVD, diabetes, hypertension) and smoking variables, mortality risk was 80% higher for the second highest compared to the lowest quartile among men (HR=1.83, 95%CI=1.26–2.67). Similarly, positive associations were observed between γ-tocopherol and CVD mortality risk among men (Ptrend=0.01) and women (Ptrend=0.01) in the age, ethnicity and cholesterol adjusted models with an approximately 50 – 60% higher risk for the highest relative to the lowest γ-tocopherol quartile (men: HR=1.52, 95%CI=1.10–2.11; women: HR=1.59, 95%CI=1.07–2.37). Likewise, the associations for γ-tocopherol with CVD mortality risk were attenuated for both men and women in the fully adjusted models (Table 3). In addition, results did not change materially from the overall models after excluding participants who died within one year after blood draw (Supplementary Table 1) as well as by excluding participants taking vitamin E supplements at cohort entry and participants having serum α-tocopherol concentrations ≥18 ug/mL (Supplementary Table 2).
Table 3.
HR and 95% CI for Cancer and Cardiovascular Disease (CVD) Mortality by γ-Tocopherol Quartiles
| γ-Tocopherol Quartiles (μg/mL) | Participants (N) | Cancer Mortality |
CVD Mortality |
||||
|---|---|---|---|---|---|---|---|
| Death (N) | HR (95% CI)a | Ptrenda | Death (N) | HR (95% CI)a | Ptrenda | ||
| Men | Men | ||||||
| Model 1b | |||||||
| Q1 (0.27–1.18) | 976 | 45 | 1.00 | 71 | 1.00 | ||
| Q2 (1.18–1.63) | 976 | 64 | 1.49 (1.01–2.18) | 73 | 1.16 (0.84–1.62) | ||
| Q3 (1.63–2.28) | 976 | 84 | 2.00 (1.39–2.90) | 82 | 1.24 (0.89–1.71) | ||
| Q4 (2.28–11.02) | 976 | 76 | 1.79 (1.22–2.64) | 0.005 | 92 | 1.52 (1.10–2.11) | 0.01 |
| Model 2c | |||||||
| Q1 (0.27–1.18) | 974 | 44 | 1.00 | 71 | 1.00 | ||
| Q2 (1.18–1.62) | 970 | 63 | 1.36 (0.92–2.01) | 73 | 1.16 (0.83–1.63) | ||
| Q3 (1.62–2.27) | 968 | 83 | 1.83 (1.26–2.67) | 81 | 1.28 (0.91–1.78) | ||
| Q4 (2.27–11.02) | 967 | 75 | 1.46 (0.98–2.17) | 0.10 | 91 | 1.21 (0.86–1.70) | 0.33 |
| Women | Women | ||||||
| Model 1b | |||||||
| Q1 (0–1.17) | 1115 | 44 | 1.00 | 46 | 1.00 | ||
| Q2 (1.17–1.66) | 1116 | 38 | 0.86 (0.55–1.33) | 47 | 1.06 (0.70–1.59) | ||
| Q3 (1.65–2.39) | 1116 | 40 | 0.97 (0.63–1.51) | 54 | 1.22 (0.82–1.82) | ||
| Q4 (2.39–13.97) | 1114 | 63 | 1.59 (1.05–2.41) | 0.0057 | 63 | 1.59 (1.07–2.37) | 0.01 |
| Model 2c | |||||||
| Q1 (0–1.17) | 1112 | 44 | 1.00 | 46 | 1.00 | ||
| Q2 (1.17–1.66) | 1110 | 38 | 0.80 (0.51–1.26) | 47 | 1.11 (0.73–1.68) | ||
| Q3 (1.65–2.39) | 1103 | 39 | 0.94 (0.60–1.47) | 54 | 1.18 (0.78–1.77) | ||
| Q4 (2.39–13.97) | 1101 | 63 | 1.50 (0.98–2.28) | 0.01 | 62 | 1.40 (0.93–2.11) | 0.10 |
HR, 95% CI and P values were estimated by Cox proportional hazards models.
Model 1: Adjusted by Cox regression for age at blood draw, race/ethnicity (as a strata variable) and serum cholesterol levels.
Model 2: Adjusted by Cox regression for variables in Model 1, and further adjusted for history of cancer, cardiovascular diseases, diabetes, and hypertension at cohort entry and smoking related variables.
After analyses were repeated separately by sex-specific racial/ethnic groups, positive associations for γ-tocopherol with all-cause mortality were observed in six out of ten sex-specific racial/ethnic groups (white, Japanese, and African American men and white, African American and Native Hawaiian women) in the age and cholesterol adjusted models (Ptrend<0.05) and none of the HRs in the other ethnic groups indicated significant association (data not shown). The associations remained significant for white men (Ptrend=0.01), white women (Ptrend=0.03) and Native Hawaiian women (Ptrend=0.01) in the fully adjusted models (Supplementary Table 3). Overall, the associations of γ-tocopherol with all-cause mortality were consistent across racial/ethnic groups in the study. No interactions between ethnicity and γ-tocopherol in relation to mortality were observed.
DISCUSSION
In the current study, serum γ-tocopherol levels were positively associated with all-cause mortality, as well as cancer and CVD-specific mortality among men and women adjusting for age, ethnicity and serum cholesterol levels after close to ten years of follow-up. The associations remained significant for all-cause mortality among women after further adjustment for history of major diseases such as cancer, CVD, diabetes, and hypertension and smoking variables at cohort entry. Sub-group analyses of the associations between γ-tocopherol and all-cause mortality were significant for six out of ten sex-specific racial/ethnic groups in the minimally adjusted models and remained significant for three of these groups in the fully adjusted models without interactions between ethnicity and γ-tocopherol.
A limited number of studies so far have examined the association between γ-tocopherol and mortality and the results are conflicting. Nagao et al. reported that serum γ-tocopherol was inversely associated with ischemic stroke mortality among men but positively associated with hemorrhagic stroke mortality among women from a large sample of Japanese participants (N=39,242) followed for 13 years [25]. Although Cooney et al. found no association of serum γ-tocopherol levels with all-cause or cancer specific mortality among colorectal cancer patients, they observed a strong association of CRP with both suggesting that inflammation may be a significant contributing factor to poor survival of cancer patients [34]. Although attenuated, the association between γ-tocopherol and all-cause mortality in our study remained significant when CRP was included in the minimally adjusted model. This may suggest that γ-tocopherol makes its own contribution beyond CRP to the prediction of mortality. As γ-tocopherol rises in response to inflammation [5, 35] despite its anti-inflammatory and protective effect against nitrogen oxide radicals, it may be elevated in individuals with serious disease who are at high risk of death as well as simultaneously function to reduce risk of death [10, 36].
Many biological effects of γ-tocopherol have been observed in vitro with potentially important biological and health consequences, including effects on sphingolipid metabolism, inflammation, nuclear peroxisome proliferator-activated receptor, neoplastic transformation, tumor cell proliferation, apoptosis, natriuresis, and immune function [37]. The results presented here are consistent with a hypothesis that elevated γ-tocopherol levels are predictors of all-cause, cancer and CVD mortality in the age, ethnicity, and cholesterol adjusted models, yet when further adjusted for the major risk factors for premature death such as smoking and history of major chronic diseases including cancer, CVD, diabetes, and hypertension the association is reduced significantly except for all-cause mortality for women. Although the association for all-cause mortality was not significant for men in the fully adjusted model, it nevertheless showed the same direction as that observed for women. Similarly, while significant associations between γ-tocopherol and all-cause mortality were observed for three out of ten sex-specific racial/ethnic groups in the fully adjusted models, the associations appeared consistent across race/ethnicity in the study. Our findings that serum γ-tocopherol levels were positively associated with mortality risk, whereas an inverse association with mortality was observed for α-tocopherol further suggest the potentially different physiologic properties and functions between α- and γ-tocopherols. In animals and cultured fibroblasts, γ-tocopherol levels rise in response to inflammatory signals, whereas in contrast α-tocopherol appears to be a primary reactant against cellular oxygen-derived oxidants and a net rise in levels is not observed [5, 35].
To the best of our knowledge, this is the first study to examine the association of serum γ-tocopherol levels and all-cause mortality in a population of five racial/ethnic groups. The large sample size and relatively long follow-up time enabled us to detect significant associations between γ-tocopherol and the risk of mortality. However, our study had limitations. The use of only one serum γ-tocopherol measurement may have misclassified exposure status for some individuals and, thereby, attenuated risk estimates. This is supported by a nested case control study of 88 colorectal cancer cases [38], where Kabat et al. reported higher significance for protective associations of pro-vitamin A carotenoids when repeated measurements of serum antioxidant levels were used. Interestingly a non-significantly increased risk of approximately 50% was observed for elevated γ –tocopherol with colon cancer incidence [38]. In the current study, adjustment for season of blood draw to account for potential seasonal variations in γ-tocopherol levels did not significantly change the results; however, repeated measurements might have strengthened the association observed. In addition, we did not have updated information on some of the relevant variables such as smoking and supplement intake, for example supplemental vitamin E intake at the time of blood draw for all the study participants. Due to the selection for biomarker analysis, the prevalence of certain chronic diseases (e.g., diabetes) was low. Also chronic disease history was assessed at cohort entry and not thereafter. Lastly, there were discrepancies in racial/ethnic distributions between the current sample of participants and the original MEC population due to differential participation in the biorepository and selection into case-control studies.
In conclusion, the novel results from the current study suggest that serum γ-tocopherol levels were significantly associated with all-cause as well as cancer and CVD-specific mortality among both men and women in the age, ethnicity and cholesterol adjusted models. The association remained significant for all-cause mortality for women after further adjustment for major risk factors of premature death such as smoking and history of major chronic diseases including cancer, CVD, diabetes, and hypertension. The observed positive association between γ-tocopherol and mortality risk suggests a potential role for γ-tocopherol as a biomarker in response to pathological conditions and, therefore, more research should be conducted to assess its utility as a generalized marker for mortality risk in populations.
Supplementary Material
Acknowledgments
FUNDING
The Multiethnic Cohort has been supported by NCI grants R37 CA54281 and U01 CA164973. The Analytical Biochemistry Shared Resource of the University of Hawaii Cancer Center is supported, in part, by grant P30 CA71789.
Footnotes
CONFLICT OF INTEREST
Authors declaim no conflict of interest.
REFERENCES
- 1.Pazdro R, Burgess JR. The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev 2010;131(4):276–286. [DOI] [PubMed] [Google Scholar]
- 2.Ju J, Picinich SC, Yang Z, Zhao Y, Suh N, Kong AN, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis 2010;31(4):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traber M Vitamin E In: Bowman BA, Russell MR (eds). Present Knowledge in Nutrition. (ILSI Press, Washington, DC, 2006). pp. 211–219. [Google Scholar]
- 4.Tanaka Y and Cooney RV. Chemical and biological properties of to copherols and their relation to cancer incidence and progression In: Preedy VR, Watson RR (eds). The Encyclopedia of Vitamin E. (Cromwell Press,Trowbridge, UK, 2007). pp. 853–863. [Google Scholar]
- 5.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med 2002;33(11):1534–1542. [DOI] [PubMed] [Google Scholar]
- 6.Sjoholm A, Berggren PO, Cooney RV. gamma-tocopherol partially protects insulin-secreting cells against functional inhibition by nitric oxide. Biochem Biophys Res Commun 2000;277(2):334–340. [DOI] [PubMed] [Google Scholar]
- 7.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. (National Academy Press; Washington, DC, 2000). [PubMed] [Google Scholar]
- 8.Li Y, Sen A, Ren J, Askew LM, Sidahmed E, Brenner DE, et al. Effects of vitamin E from supplements and diet on colonic alpha- and gamma-tocopherol concentrations in persons at increased colon cancer risk. Nutr Cancer 2015;67(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooney RV, Franke AA, Wilkens LR, Gill J, Kolonel LN. Elevated plasma gamma-tocopherol and decreased alpha-tocopherol in men are associated with inflammatory markers and decreased plasma 25-OH vitamin D. Nutr Cancer 2008;60 Suppl 1:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J 2003;17(8):816–822. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Wood LA, Cooney RV. Enhancement of intracellular gamma-tocopherol levels in cytokine-stimulated C3H 10T1/2 fibroblasts: relation to NO synthesis, isoprostane formation, and tocopherol oxidation. BMC Chem Biol 2007;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel CJ, Bhattacharya J, Butte AJ. An Environment-Wide Association Study (EWAS) on type 2 diabetes mellitus. PloS one 2010; 5(5):e10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullan K, Williams MA, Cardwell CR, McGuinness B, Passmore P, Silvestri G, Woodside JV, McKay GJ. Serum concentrations of vitamin E and carotenoids are altered in Alzheimer’s disease: A case-control study. Alzheimer’s & dementia 2017; 3(3):432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, et al. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr 2003;77(1):160–166. [DOI] [PubMed] [Google Scholar]
- 15.Chai W, Conroy SM, Maskarinec G, Franke AA, Pagano IS, Cooney RV. Associations between obesity and serum lipid-soluble micronutrients among premenopausal women. Nutr Res 2010; 30(4):227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morimoto Y, Beckford F, Cooney RV, Franke AA, Maskarinec G. Adherence to cancer prevention recommendations and antioxidant and inflammatory status in premenopausal women. Br J Nutr 2015;114(1):134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shvetsov YB, Hernandez BY, Wilkens LR, Thompson PJ, Franke AA, Zhu X, et al. Plasma micronutrients and the acquisition and clearance of anal human papillomavirus infection: the Hawaii HPV cohort study. Cancer Res 2010;70(23):9787–9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helzlsouer KJ, Huang HY, Alberg AJ, Hoffman S, Burke A, Norkus EP, et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst 2000;92(24):2018–2023. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein SJ, Wright ME, Pietinen P, King I, Tan C, Taylor PR, et al. Serum alpha-tocopherol and gamma-tocopherol in relation to prostate cancer risk in a prospective study. J Natl Cancer Inst 2005;97(5):396–399. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein SJ, Peters U, Ahn J, Friesen MD, Riboli E, Hayes RB, et al. Serum alpha-tocopherol and gamma-tocopherol concentrations and prostate cancer risk in the PLCO Screening Trial: a nested case-control study. PLoS One 2012;7(7):e40204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui R, Liu ZQ, Xu Q. Blood alpha-tocopherol, gamma-tocopherol levels and risk of prostate cancer: a meta-analysis of prospective studies. PLoS One 2014;9(3):e93044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill JK, Franke AA, Steven Morris J, Cooney RV, Wilkens LR, Le Marchand L, et al. Association of selenium, tocopherols, carotenoids, retinol, and 15-isoprostane F(2t) in serum or urine with prostate cancer risk: the multiethnic cohort. Cancer Causes Control 2009;20(7):1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto Y, Ollberding NJ, Cooney RV, Wilkens LR, Franke AA, Le Marchand L, et al. Prediagnostic serum tocopherol levels and the risk of non-hodgkin lymphoma: the multiethnic cohort. Cancer Epidemiol Biomarkers Prev 2013;22(11):2075–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epplein M, Shvetsov YB, Wilkens LR, Franke AA, Cooney RV, Le Marchand L, et al. Plasma carotenoids, retinol, and tocopherols and postmenopausal breast cancer risk in the Multiethnic Cohort Study: a nested case-control study. Breast Cancer Res 2009;11(4):R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagao M, Moriyama Y, Yamagishi K, Iso H, Tamakoshi A, Group JS. Relation of serum alpha- and gamma-tocopherol levels to cardiovascular disease-related mortality among Japanese men and women. J Epidemiol 2012;22(5):402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tucker LA. Alpha- and Gamma-Tocopherol and Telomere Length in 5768 US Men and Women: A NHANES Study. Nutrients 2017;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151(4):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol 2000; 151(4):358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SY, Wilkens LR, Henning SM, Le Marchand L, Gao K, Goodman MT, et al. Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer Causes Control 2009;20(2):211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SY, Wilkens LR, Maskarinec G, Haiman CA, Kolonel LN, Marchand LL. Weight change in older adults and mortality: the Multiethnic Cohort Study. Int J Obes (Lond) 2018;42(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franke AA, Custer LJ, Cooney RV. Synthetic carotenoids as internal standards for plasma micronutrient analyses by high-performance liquid chromatography. J Chromatogr 1993;614(1):43–57. [DOI] [PubMed] [Google Scholar]
- 32.Epplein M, Franke AA, Cooney RV, Morris JS, Wilkens LR, Goodman MT, et al. Association of plasma micronutrient levels and urinary isoprostane with risk of lung cancer: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev 2009;18(7):1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med 2006;354(4):333–342. [DOI] [PubMed] [Google Scholar]
- 34.Cooney RV, Chai W, Franke AA, Wilkens LR, Kolonel LN, Le Marchand L. C-reactive protein, lipid-soluble micronutrients, and survival in colorectal cancer patients. Cancer Epidemiol Biomarkers Prev 2013;22(7):1278–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnett TS, Tanaka Y, Harwood PJ, Cooney RV. Mechanisms of phytochemical inhibition of carcinogenesis: elucidating the role of γ-tocpherol in nutrition In: Shibamoto IT, Terao J, Osawa T (eds). Functional Foods for Disease Prevention. (American Chemical Society, Washington, D.C, 1998). pp. 45–58. [Google Scholar]
- 36.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. Gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A 2000;97(21):11494–11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das Gupta S, Suh N. Tocopherols in cancer: An update. Mol Nutr Food Res 2016;60(6):1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabat GC, Kim MY, Sarto GE, Shikany JM, Rohan TE. Repeated measurements of serum carotenoid, retinol and tocopherol levels in relation to colorectal cancer risk in the Women’s Health Initiative. Eur J Clin Nutr 2012; 66:549–554. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.