Abstract
Background:
Landmark studies have demonstrated the safety and efficacy of implantable cardioverter-defibrillators (ICDs) in selected stable ambulatory patients with heart failure (HF) with a reduced ejection fraction receiving optimal medical therapy. It is not known whether a recent hospitalization for HF prior to ICD placement is associated with subsequent outcomes.
Methods:
A post-hoc analysis was performed of Medicare beneficiaries enrolled in the National Cardiovascular Data Registry’s (NCDR®) ICD Registry™ with a known diagnosis of HF and an EF ≤35% underdoing a new ICD placement for primary prevention. Patients were grouped based on timing of ICD placement from last hospitalization for HF. The association between timing of ICD placement and outcomes was assessed using multivariable logistic regression models.
Results:
The final analytical cohort included 81,180 patients undergoing initial ICD placement for primary prevention who were currently hospitalized for HF (N = 11,563, 14%), hospitalized for HF within 3 months (N = 6,252, 8%), or hospitalized for HF more than 3 months prior or had no previous hospitalizations for HF (N = 63,365, 78%). Patients currently or recently hospitalized for HF had a higher unadjusted composite periprocedural complication rate (2.60% vs. 1.71% vs. 1.25%, p value <0.001). After adjusting for potential confounders, patients currently hospitalized for HF were at higher risk for death (Odds Ratio [OR] 2.25, 95% Confidence Interval [CI] 2.02-2.52; p-value <0.001) and all-cause re-admission (OR 1.89, 95% CI 1.79-1.99; p-value <0.001) at 90 days.
Conclusion:
Older patients currently or recently hospitalized for HF undergoing initial ICD placement for primary prevention experienced a higher rate of periprocedural complications and were at increased risk of death compared to those receiving an ICD without recent HF hospitalization. Additional prospective, real-world, pragmatic, comparative effectiveness studies should be conducted to define the optimal timing of ICD placement.
Keywords: heart failure, hospitalization, implantable cardioverter-defibrillator, outcomes
INTRODUCTION
Although the prognosis of ambulatory patients with heart failure with a reduced ejection fraction (HFrEF) has been revolutionized by the development of guideline-directed medical therapies, there are more than one million admissions for HF annually in the United States.1–6 Hospitalization is a particularly important inflection point in the natural history of HF; early post-discharge readmission and mortality rates may be as high as 30% and 15%, respectively.7 However, despite the attendant morbidity and mortality of hospitalizations for HF, landmark trials for evidence-based medications and devices enrolled stable outpatients on optimal medical therapy (OMT),8–10 and it is uncertain whether the time from last hospitalization for HF to implantable-cardioverter defibrillator (ICD) placement is associated with outcomes.11–13
The best time for implementing HF therapies is particularly relevant to ICD placement which is known to pose additional initial risk to patients when implanted during an index admission for HF.14 The National Cardiovascular Data Registry’s (NCDR®) ICD Registry™ provides a unique opportunity to systematically analyze in-hospital adverse events and post-discharge outcomes based on timing of ICD placement from last hospitalization for HF. Specifically, the objectives of this analysis were to systematically describe the clinical characteristics, periprocedural complications, and post-procedural readmissions and mortality based on the timing of ICD placement for primary prevention of sudden cardiac death (SCD) from last hospitalization for HF.
METHODS
Data Sources
Patient and device implantation data were obtained from the NCDR® ICD Registry™. The data cannot be made available to other researchers by the authors for purposes of reproducing the results or replicating the procedure, because by contract with participating sites, analyses of NCDR data are to be performed by contracted data analytic centers. Demographics, clinical characteristics, and procedural data were collected using standardized definitions.15, 16 Data on periprocedural complications are recorded in the NCDR® ICD Registry™, while 30-day and 90-day complications and reoperations were obtained by linking the NCDR® ICD Registry™ with the Medicare Inpatient Institutional Claims, Outpatient Institutional Claims, and Master Summary Beneficiary Files. These databases contain claims for inpatient admissions, outpatient procedures, and enrollment and vital status for Medicare fee-for-service (FSS) patients. The study protocol was approved by the Institutional Review Board (IRB) and/or ethics committee at each participating site. All patients provided written informed consent.
Study Population
All patients enrolled in the NCDR® ICD Registry™ with a diagnosis of HF and an EF ≤35% undergoing a new ICD implant for primary prevention between January 1, 2010 through December 31, 2014 were considered for inclusion. Relevant exclusion criteria included patients currently hospitalized for a non-elective reason other than HF, acute myocardial infarction within 40 days or revascularization within 90 days, prior ventricular tachycardia/ventricular fibrillation arrest, arrhythmogenic syndromes with an increased risk of sudden cardiac death (e.g. Brugada, catecholaminergic polymorphic ventricular tachycardia, long QT syndrome, etc.), epicardial lead placement, lead only procedures, and device replacements.
The NCDR® ICD Registry™ was linked to Medicare data by social security number, date of birth, and gender. Only patients matching a beneficiary on all three criteria were included. Our cohort was further restricted to patients enrolled in Medicare FFS for at least three months prior and three months following their procedure in order to permit the ascertainment both of antecedent hospitalizations and the outcomes of interest.
Outcomes
Composite and cause-specific event rates were calculated for complications occurring during the periprocedural and 30- and 90- day periods. Periprocedural complications were ascertained based on the NCDR® ICD Registry™ case report form completed at the point-of-care by the treating physician and/or ancillary support staff. To identify 30-day and 90-day device-related complications, reoperations were first identified using codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and Current Procedural Terminology (CPT), respectively, for inpatient and outpatient procedures.17 The subset of reoperations due to device-related complications were then determined based on whether the primary diagnosis was the result of mechanical complication of the device, infection (i.e. device infection, endocarditis, or systemic infection), ICD pocket-related complication, or other complication related to perforation, inflammation, and venous obstruction or thromboembolism.17 In addition, device-related complications not requiring reoperation were identified as hospitalizations (i.e. inpatient admissions, observation stays, and emergency department visits) with a primary diagnosis consistent with a device-related complication (i.e. ICD-9-CM codes) in the absence of a procedure code indicating device reoperation. Device-related complications not resulting in an acute care episode (i.e. inpatient admissions, observation stays, and emergency department visit) or reoperation in an inpatient or ambulatory setting were not included.
Statistical Analysis
Study participants were grouped based on timing of ICD placement from last hospitalization for HF (i.e. currently hospitalized for HF vs. hospitalized for HF ≤3 months vs. hospitalized for HF >3 months prior or no previous admission for HF) identified using admissions from inpatient institutional claims. Three months was selected as the cutoff between a recent and more remote hospitalization for HF as this is the timeframe that is generally recommended in the guidelines for reassessing clinical status and/or EF following implementation of OMT and/or coronary revascularization (i.e. percutaneous coronary intervention or coronary artery bypass grafting). Baseline clinical characteristics were expressed as percentages for categorical variables and means and standard deviations for continuous variables. The unadjusted periprocedural, 30-day, and 90-day complication event rates were calculated as an incidence rate (i.e. # of events / # of procedures) with 95% confidence interval (CI). In addition, as a sensitivity analysis aggregate periprocedural, 30-day, and 90-day complication rates were determined by device type (i.e. single chamber ICD vs. dual chamber ICD vs. cardiac resynchronization therapy-defibrillator [CRT-D]). Comparisons of unadjusted composite and cause-specific event rates were adjusted for multiple comparisons using the Holm procedure.
Multivariable logistic regression employing generalized estimating equations using an exchangeable working correlation structure was utilized to assess the association between timing of ICD placement from last hospitalization for HF and in-hospital (i.e. any adverse event and mortality), 30-day (i.e. all-cause mortality, all-cause readmissions, and cardiovascular [CV] readmissions), and 90-day (i.e. all-cause mortality, all-cause readmissions, and CV readmissions) outcomes. For each model, odds ratios (OR) with 95% confidence intervals (CI) were calculated. Models were adjusted for potential confounders including age, sex, EF, New York Heart Association (NYHA) functional class, QRS duration, systolic blood pressure, serum sodium, serum creatinine, blood urea nitrogen (BUN), hemoglobin, cardiac (i.e. hypertension, cerebrovascular disease, previous percutaneous coronary intervention, prior coronary artery bypass graft, syncope, and previous cardiac arrest) and non-cardiac comorbidities (i.e. diabetes mellitus [DM], chronic obstructive pulmonary disease [COPD], and end-stage renal disease requiring dialysis), and device type (i.e. single chamber ICD vs. dual chamber ICD vs. CRT-D). Covariates included in each model were selected using a stepwise selection approach. The probability of entry into the model was set at p-value ≤ 0.50 and probability of removal p-value ≥ 0.10. Prior to modeling, missing continuous and categorical variables were imputed using fully conditional specification methods.18 Dichotomous variables with missing values were assumed to be absence of the disease. Missingness rates ranged from 0% - 2.1% with the large majority of variables missing < 1%. Additional analyses were performed to assess for an interaction between device type (i.e. single chamber ICD vs. dual chamber ICD vs. CRT-D) and timing of ICD placement from last hospitalization for HF and outcomes.
All analyses were two-sided and a p-value <0.05 was set as the threshold for statistical significance. Statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Funding Source and Manuscript Preparation
The American College of Cardiology (Washington, D.C.) provides ongoing financial and material support for the NCDR® ICD Registry™. Database management and statistical analysis were performed by the Center for Outcomes Research and Evaluation, Yale University School of Medicine (New Haven, CT). The authors take responsibility for the manuscript’s integrity and had authority over its preparation and the decision to publish.
RESULTS
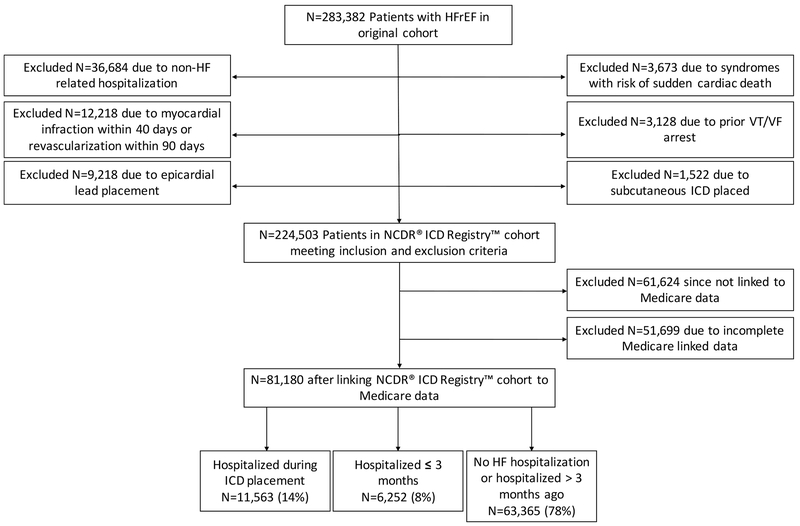
A total of 283,382 HFrEF patients undergoing initial ICD placement for primary prevention of SCD were identified (Figure 1). The primary reasons for exclusion were currently hospitalized for a non-elective reason other than HF (N = 36,684), acute myocardial infarction within 40 days or revascularization within 90 days (N = 12,218), and epicardial lead placement (N = 9,219). After linking the NCDR® ICD Registry™ cohort to Medicare data, the final analytical cohort included 81,180 patients. Patients were divided into the following groups based on timing of ICD placement from last hospitalization for HF: currently hospitalized for HF (N = 11,563, 14%), hospitalized for HF within 3 months (N = 6,252, 8%), or hospitalized for HF more than 3 months prior or no previous admission for HF (N = 63,365, 78%).
Figure 1.
A consort diagram showing the derivation of the final analytical cohort.
Patient Characteristics at Implantation
Patients currently or recently hospitalized for HF tended to be non-white (25% versus [vs.] 22% vs. 15%) compared to patients without a recent admission for HF (Table 1). These patients also tended to have a lower EF (23±7 vs. 24±7 vs. 26±6), were more likely to report NYHA functional class III/IV symptoms (85% vs. 78% vs. 62%), and had a higher burden of cardiac (i.e. atrial fibrillation) and non-cardiac (i.e. DM and COPD) comorbidities. Patients currently or recently admitted for HF also had a lower hemoglobin (12.1±1.9 g/dL vs. 12.5±1.8 vs. 13.2±1.7 g/dL) and worse renal function as measured by BUN (32±16 vs. 31±15 vs. 25±12) compared to patients without a recent hospitalization for HF. Among patients currently or recently hospitalized for HF, the rate of prescription of angiotensin converting-enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) was lower (72% vs. 75% vs. 81%) and they were more likely to be receiving loop diuretics (81% vs. 86% vs. 68%) and digoxin (24% vs. 22% vs. 17%). Finally, patients receiving a primary prevention ICD during or within 3 months of an index admission for HF were more likely to receive a CRT-D device (59% vs. 60% vs. 53%) as opposed to a single chamber or dual chamber ICD.
Table 1.
Clinical characteristics based on timing of ICD placement from last hospitalization for HF.
| Overall (N = 81,180) | Patients Admitted for HF (N = 11563) | HF Admission ≤3 months† (N = 6,252) | HF Admission >3 months† (N = 63,365) | |
|---|---|---|---|---|
| Demographics* | ||||
| Age - Mean (SD) | 73 (8) | 73 (9) | 74 (8) | 73 (8) |
| Sex: Female | 28 | 29 | 32 | 27 |
| Race | ||||
| White non-Hispanic | 83 | 75 | 78 | 85 |
| Black non-Hispanic | 11 | 16 | 15 | 9 |
| Hispanic | 4 | 6 | 5 | 4 |
| Other | 2 | 2 | 2 | 2 |
| Clinical Characteristics | ||||
| NICM | 38 | 40 | 39 | 38 |
| LVEF, Mean (SD) | 26 (6) | 23 (7) | 24 (7) | 26 (6) |
| NYHA Class | ||||
| I | 2 | 1 | 1 | 3 |
| II | 31 | 15 | 21 | 36 |
| III | 63 | 74 | 73 | 60 |
| IV | 3 | 11 | 5 | 2 |
| Atrial fibrillation/flutter | 40 | 48 | 44 | 38 |
| Cerebrovascular Disease | 17 | 20 | 19 | 17 |
| Currently on Dialysis | 3 | 4 | 4 | 3 |
| Diabetes | 42 | 49 | 49 | 41 |
| Chronic Lung Disease | 25 | 34 | 31 | 22 |
| QRS Duration, Median (25th-75th) | 127 (103-154) | 130 (104-154) | 130 (104-156) | 126 (102-154) |
| Left Bundle Branch Block | 35 | 34 | 36 | 35 |
| BUN, Mean (SD) | 26 (14) | 32 (16) | 31 (15) | 25 (12) |
| Sodium, Mean (SD) | 139 (3) | 138 (4) | 138 (4) | 139 (3) |
| Hemoglobin, Mean (SD) | 13.0 (1.8) | 12.1 (1.9) | 12.5 (1.8) | 13.2 (1.7) |
| Procedure | ||||
| Implanted Device Type | ||||
| Single Chamber | 19 | 16 | 17 | 19 |
| Dual Chamber | 27 | 25 | 22 | 27 |
| CRT-D | 55 | 59 | 60 | 53 |
| Discharge Medications | ||||
| ACEI | 58 | 55 | 55 | 59 |
| ARB | 21 | 17 | 20 | 22 |
| Beta-Blockers | 91 | 89 | 90 | 91 |
| Hydralazine | 5 | 10 | 9 | 4 |
| Long Acting Nitroglycerin | 13 | 17 | 17 | 12 |
| Digoxin | 18 | 24 | 22 | 17 |
| Diuretics | 71 | 81 | 86 | 68 |
| Aspirin | 71 | 70 | 70 | 72 |
| Statins | 70 | 66 | 67 | 71 |
| Non-Statin | 11 | 8 | 10 | 12 |
| Antiarrhythmic Agents | 13 | 18 | 16 | 11 |
| Channel Blockers | 9 | 8 | 9 | 9 |
| Warfarin | 30 | 34 | 32 | 29 |
| Thienopyridines | 24 | 22 | 24 | 24 |
all numbers are calculated using complete cases within each variable
3 months defined as 90 days
Abbreviations: ICD = implantable cardioverter-defibrillation; HF = heart failure; SD = standard deviation; NICM = nonischemic cardiomyopathy; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; CRT-D = cardiac resynchronization therapy-defibrillator; ACEI = angiotensin converting-enzyme inhibitor; angiotensin receptor blocker.
Periprocedural and Post-Procedural Complication Rates
The composite periprocedural complication rate was 1.47% (95% CI 1.39%-1.56%) (Table 2). Among patients currently hospitalized for HF, hospitalized for HF within 3 months, or hospitalized for HF more than 3 months prior or no previous admission for HF, respectively, the composite periprocedural complication rate was 2.60% (2.32%-2.91%), 1.71% (1.40%-2.06%), and 1.25% (1.16%-1.33%) (p-value <0.001). This finding was driven by a higher rate of stroke/transient ischemic attack (TIA), myocardial infarction (MI), cardiac arrest, and in-hospital death as shown in Table 2. With the notable exception of ICD pocket hematoma, patients currently or recently hospitalized for HF experienced similar rates of other complications including cardiac perforation, cardiac venous dissection, pericardial tamponade, pneumothorax, and hemothorax (p-value ≥0.05 for all comparisons).
Table 2.
Complication rates based on timing of ICD placement from last HF hospitalization.
| Overall | Patients Admitted for HF | HF Admission ≤3 months | HF Admission >3 months | Adjusted p-value‡ | |||||
|---|---|---|---|---|---|---|---|---|---|
| # | Rate (95% CI) | # | Rate (95% CI) | # | Rate (95% CI) | # | Rate (95% CI) | ||
| Periprocedural | |||||||||
| Any | 1,197 | 1.47 (1.39-1.56) | 301 | 2.60 (2.32-2.91) | 107 | 1.71 (1.40-2.06) | 789 | 1.25 (1.16-1.33) | <0.001 |
| In Hospital Death | 161 | 0.20 (0.17-0.23) | 101 | 0.87 (0.71-1.06) | 20 | 0.32 (0.20-0.49) | 40 | 0.06 (0.05-0.09) | <0.001 |
| Cardiac Arrest | 142 | 0.17 (0.15-0.21) | 60 | 0.52 (0.40-0.67) | 21 | 0.34 (0.21-0.51) | 61 | 0.10 (0.07-0.12) | <0.001 |
| Myocardial Infarction | 21 | 0.03 (0.02-0.04) | 9 | 0.08 (0.04-0.15) | 0 | - | 12 | 0.02 (0.01-0.03) | 0.0110 |
| Cardiac Perforation | 94 | 0.12 (0.09-0.14) | 15 | 0.13 (0.07-0.21) | 7 | 0.11 (0.05-0.23) | 72 | 0.11 (0.09-0.14) | 0.8920 |
| Cardiac Venous Dissection | 160 | 0.20 (0.17-0.23) | 15 | 0.13 (0.07-0.21) | 11 | 0.18 (0.09-0.31) | 134 | 0.21 (0.18-0.25) | 1.000 |
| Pericardial Tamponade | 111 | 0.14 (0.11-0.16) | 17 | 0.15 (0.09-0.24) | 7 | 0.11 (0.05-0.23) | 87 | 0.14 (0.11-0.17) | 1.000 |
| Stroke/TIA | 43 | 0.05 (0.04-0.07) | 14 | 0.12 (0.07-0.20) | 5 | 0.08 (0.03-0.19) | 24 | 0.04 (0.02-0.06) | 0.018 |
| Hematoma | 257 | 0.32 (0.28-0.36) | 77 | 0.67 (0.53-0.83) | 22 | 0.35 (0.22-0.53) | 158 | 0.25 (0.21-0.29) | <0.001 |
| Infection Requiring Antibiotics | 45 | 0.06 (0.04-0.07) | 17 | 0.15 (0.09-0.24) | 7 | 0.11 (0.05-0.23) | 21 | 0.03 (0.02-0.05) | <0.001 |
| Pneumothorax | 318 | 0.39 (0.35-0.44) | 33 | 0.29 (0.20-0.40) | 27 | 0.43 (0.28-0.63) | 258 | 0.41 (0.36-0.46) | 1.000 |
| Hemothorax | 21 | 0.03 (0.02-0.04) | 7 | 0.06 (0.02-0.12) | 1 | 0.02 (0.00-0.09) | 13 | 0.02 (0.01-0.04) | 0.596 |
| Urgent Surgery | 23 | 0.03 (0.02-0.04) | 5 | 0.04 (0.01-0.10) | 1 | 0.02 (0.00-0.09) | 17 | 0.03 (0.02-0.04) | 1.000 |
| 30-Day Complications | |||||||||
| N* | 76,263 | 10,792 | 6,003 | 59,468 | |||||
| Any complication | 1,610 | 2.11 (2.01-2.22) | 565 | 5.24 (4.82-5.67) | 198 | 3.30 (2.86-3.78) | 847 | 1.42 (1.33-1.52) | <0.001 |
| Any complication requiring reoperation | 209 | 0.27 (0.24-0.31) | 38 | 0.35 (0.25-0.48) | 19 | 0.32 (0.19-0.49) | 152 | 0.26 (0.22-0.30) | 1.000 |
| Hemothorax, Pneumothorax, or Pleural Effusion | 39 | 0.05 (0.04-0.07) | 12 | 0.11 (0.06-0.19) | 5 | 0.08 (0.03-0.19) | 22 | 0.04 (0.02-0.06) | 0.057 |
| Pocket-related complication | 134 | 0.18 (0.15-0.21) | 27 | 0.25 (0.16-0.36) | 13 | 0.22 (0.12-0.37) | 94 | 0.16 (0.13-0.19) | 0.885 |
| Cardiac Tamponade or Pericardial Effusion | 48 | 0.06 (0.05-0.08) | 2 | 0.02 (0.00-0.07) | 2 | 0.03 (0.00-0.12) | 44 | 0.07 (0.05-0.10) | 0.817 |
| Any complication without reoperation | 1,407 | 1.84 (1.75-1.94) | 529 | 4.90 (4.50-5.33) | 179 | 2.98 (2.57-3.44) | 699 | 1.18 (1.09-1.27) | <0.001 |
| Death | 695 | 0.91 (0.85-0.98) | 324 | 3.00 (2.69-3.34) | 95 | 1.58 (1.28-1.93) | 276 | 0.46 (0.41-0.52) | <0.001 |
| Hemothorax, Pneumothorax, or Pleural Effusion | 374 | 0.49 (0.44-0.54) | 124 | 1.15 (0.96-1.37) | 58 | 0.97 (0.73-1.25) | 192 | 0.32 (0.28-0.37) | <0.001 |
| Pocket-related complication | 279 | 0.37 (0.32-0.41) | 79 | 0.73 (0.58-0.91) | 22 | 0.37 (0.23-0.55) | 178 | 0.30 (0.26-0.35) | <0.001 |
| Cardiac Tamponade or Pericardial Effusion | 150 | 0.20 (0.17-0.23) | 25 | 0.23 (0.15-0.34) | 17 | 0.28 (0.17-0.45) | 108 | 0.18 (0.15-0.22) | 1.000 |
| 90-Day Complications | |||||||||
| N† | 72,608 | 10,306 | 5,728 | 56,574 | |||||
| Any complication | 3,764 | 5.18 (5.02-5.35) | 799 | 7.75 (7.24-8.29) | 358 | 6.25 (5.64-6.91) | 2,607 | 4.61 (4.44-4.78) | <0.001 |
| Any complication requiring reoperation | 1,926 | 2.65 (2.54-2.77) | 258 | 2.50 (2.21-2.82) | 143 | 2.50 (2.11-2.93) | 1,525 | 2.70 (2.56-2.83) | 1.000 |
| Mechanical complication | 1,537 | 2.12 (2.01-2.22) | 186 | 1.80 (1.56-2.08) | 117 | 2.04 (1.69-2.44) | 1,234 | 2.18 (2.06-2.30) | 0.607 |
| Device infection | 444 | 0.61 (0.56-0.67) | 77 | 0.75 (0.59-0.93) | 32 | 0.56 (0.38-0.79) | 335 | 0.59 (0.53-0.66) | 1.000 |
| Systemic infection | 176 | 0.24 (0.21-0.28) | 43 | 0.42 (0.30-0.56) | 13 | 0.23 (0.12-0.39) | 120 | 0.21 (0.18-0.25) | 0.010 |
| Endocarditis | 41 | 0.06 (0.04-0.08) | 13 | 0.13 (0.07-0.22) | 5 | 0.09 (0.03-0.20) | 23 | 0.04 (0.03-0.06) | 0.034 |
| Other infection | 65 | 0.09 (0.07-0.11) | 10 | 0.10 (0.05-0.18) | 8 | 0.14 (0.06-0.28) | 47 | 0.08 (0.06-0.11) | 1.000 |
| Any complication without reoperation | 2,025 | 2.79 (2.67-2.91) | 579 | 5.62 (5.18-6.08) | 232 | 4.05 (3.55-4.59) | 1,214 | 2.15 (2.03-2.27) | <0.001 |
| Mechanical complication | 622 | 0.86 (0.79-0.93) | 141 | 1.37 (1.15-1.61) | 61 | 1.06 (0.82-1.37) | 420 | 0.74 (0.67-0.82) | <0.001 |
| Device infection | 219 | 0.30 (0.26-0.34) | 49 | 0.48 (0.35-0.63) | 21 | 0.37 (0.23-0.56) | 149 | 0.26 (0.22-0.31) | 0.017 |
| Systemic infection | 1,186 | 1.63 (1.54-1.73) | 392 | 3.80 (3.44-4.19) | 156 | 2.72 (2.32-3.18) | 638 | 1.13 (1.04-1.22) | <0.001 |
| Endocarditis | 55 | 0.08 (0.06-0.10) | 18 | 0.17 (0.10-0.28) | 9 | 0.16 (0.07-0.30) | 28 | 0.05 (0.03-0.07) | 0.000 |
| Other infection | 268 | 0.37 (0.33-0.42) | 77 | 0.75 (0.59-0.93) | 29 | 0.51 (0.34-0.73) | 162 | 0.29 (0.24-0.33) | <0.001 |
Patients with at least 1 month of FFS follow up
Patients with at least 3 months of FFS follow up
Adjusted using the Holm procedure for multiple comparisons
Abbreviations: ICD = implantable cardioverter-defibrillation; HF = heart failure; N = number.
The composite 30-day complication rate was 2.11% (2.01%-2.22%). Among patients currently hospitalized for HF, hospitalized for HF within 3 months, or hospitalized for HF more than 3 months prior or no previous admission for HF, respectively, the composite 30-day complication rate was 5.24% (4.82%-5.67%), 3.30% (2.86%-3.78%), and 1.42% (1.33%-1.52%) (p-value <0.001). The higher 30-day complication rate observed among patients currently or recently hospitalized for HF was driven principally by a higher mortality rate and to a lesser extent ICD pocket-related complications and pneumothorax/hemothorax/pleural effusion not requiring reoperation as shown in Table 2. However, there was no difference in complications requiring reoperation including pocket-related issues, pneumothorax/hemothorax/pleural effusion, and pericardial effusion/tamponade (p-value ≥0.05 for all comparisons).
Similarly, the composite 90-day complication rate was 5.18% (5.02%-5.35%). Among patients currently hospitalized for HF, hospitalized for HF within 3 months, or hospitalized for HF more than 3 months prior or no previous admission for HF, respectively, the composite 90-day complication rate was 7.75% (7.24%-8.29%), 6.25% (5.64%-6.91%), and 4.61% (4.44%-4.79%) (p-value <0.001). In addition, there was no difference in 90-day complications requiring reoperation. However, compared to patients with no recent hospitalization for HF, patients currently or recently hospitalized for HF experienced higher 90-day rates of infections including device infections not requiring reoperation and endocarditis and other systemic infections irrespective of operative status as shown in Table 2.
A sensitivity analysis was performed to look at the periprocedural, 30-day, and 90-day complication rate by device type (i.e. single chamber ICD vs. dual chamber ICD vs. CRT-D). In general, the trends in complication rates were similar in all three groups (Supplemental Table 1). Regardless of device type, patients currently hospitalized for HF or hospitalized for HF within 3 months were at higher risk of periprocedural, 30-day, and 90-day complications compared to patients hospitalized for HF more than 3 months prior or with no previous admission for HF. The higher 30-day and 90-day overall complication rates was driven by complications not requiring reoperation.
Timing from Last Hospitalization for Heart Failure and Outcomes
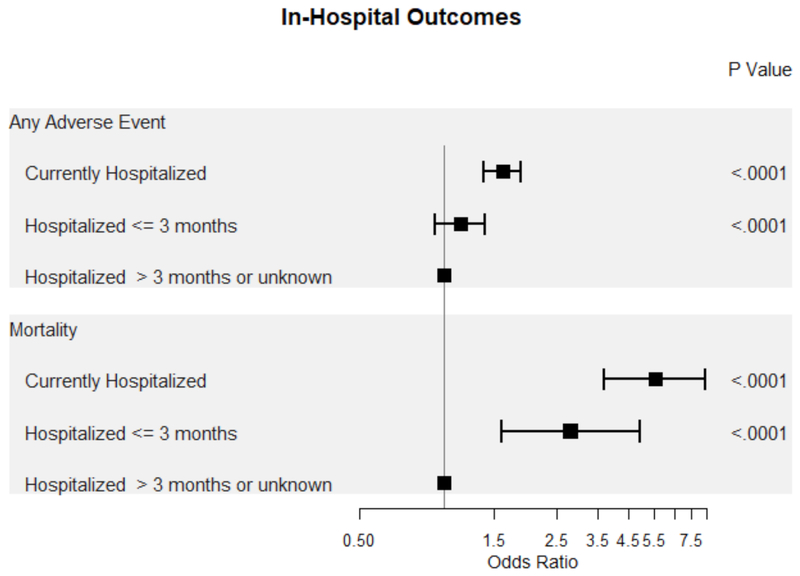
After multivariable adjustment, patients currently hospitalized for HF had higher odds of any adverse event (OR 1.61, 95% CI 1.38–1.87, p-value <0.001) and in-hospital mortality (OR 5.56, 95% CI 3.68–8.39, p-value <0.001) compared to patients without a hospitalization for HF within the past 3 months (Table 3, Figure 2). In contrast, patients hospitalized for HF within the last 3 months had higher odds of in-hospital mortality (OR 2.79, 95% CI 1.59-4.90, p-value <0.001) but not any adverse event (p-value = 0.195).
Table 3.
Multivariable adjusted odds ratios (95% confidence interval) between timing of ICD placement from last HF hospitalization and outcomes.
| Group | p-value | |||||
|---|---|---|---|---|---|---|
| Outcome | Currently Hospitalized (1) | Hospitalized ≤3 months (2) | Hospitalized >3 months (3) | overall | (1) vs. (3) | (2) vs. (3) |
| In Hospital | ||||||
| Any Adverse Event | 1.61 (1.38-1.87) | 1.14 (0.93-1.4) | 1 | <0.001 | <0.001 | 0.195 |
| Mortality | 5.56 (3.68-8.39) | 2.79 (1.59-4.9) | 1 | <0.001 | <0.001 | <0.001 |
| 30-Day Outcomes | ||||||
| All-Cause Mortality | 2.62 (2.17-3.18) | 1.92 (1.50-2.45) | 1 | <0.001 | <0.001 | <0.001 |
| All-Cause Readmissions | 1.99 (1.87-2.13) | 1.57 (1.45-1.69) | 1 | <0.001 | <0.001 | <0.001 |
| CV Readmissions | 2.39 (2.16-2.64) | 2.02 (1.79-2.28) | 1 | <0.001 | <0.001 | <0.001 |
| 90-Day Outcomes | ||||||
| All- Cause Mortality | 2.25 (2.02-2.52) | 1.56 (1.35-1.81) | 1 | <0.001 | <0.001 | <0.001 |
| All-Cause Readmissions | 1.89 (1.79-1.99) | 1.66 (1.56-1.77) | 1 | <0.001 | <0.001 | <0.001 |
| CV Readmissions | 2.26 (2.11-2.43) | 2.15 (1.98-2.34) | 1 | <0.001 | <0.001 | <0.001 |
Abbreviations: ICD = implantable cardioverter-defibrillation; HF = heart failure; CV = cardiovascular.
Figure 2.
Forrest plot of in-hospital outcomes based on timing of ICD placement from last hospitalization for HF.
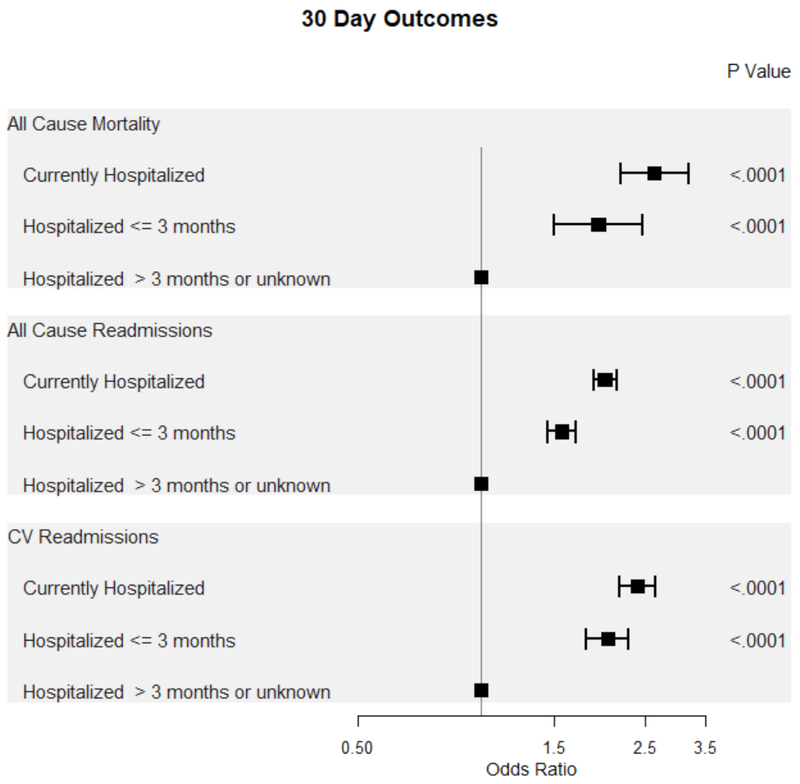
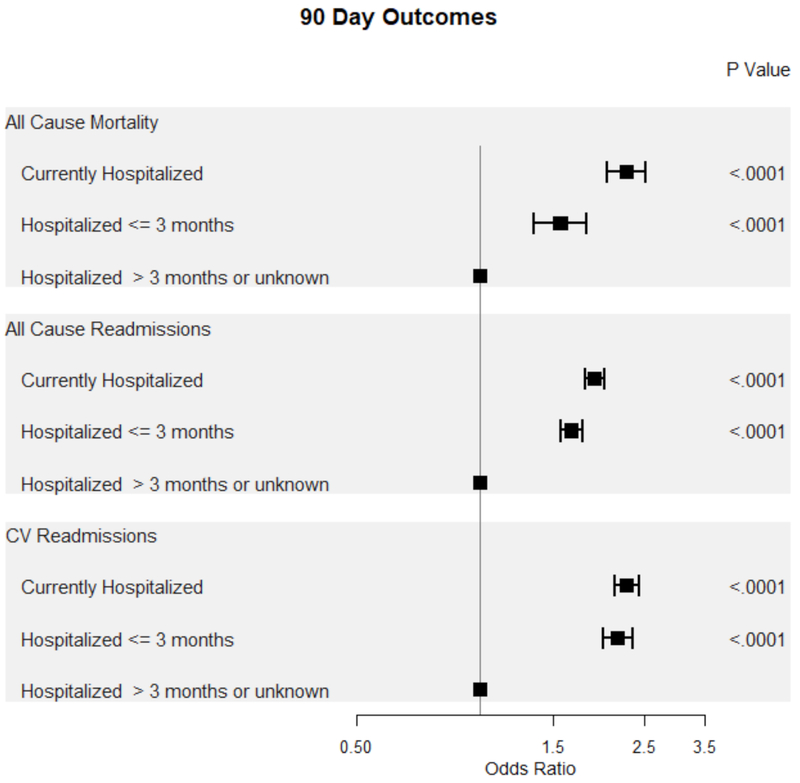
Patients currently or recently hospitalized for HF were at increased odds of all-cause mortality, all-cause admissions, and cardiovascular (CV) admissions within both 30 and 90 days compared to patients without a recent admission for HF (Figure 3, Figure 4). Notably, the odds of all-cause and CV-related morbidity and mortality was highest among patients undergoing ICD placement for primary prevention during a hospitalization for HF.
Figure 3.
Forest plot of 30-day outcomes based on timing of ICD placement from last hospitalization for HF.
Figure 4.
Forest plot of 90-day outcomes based on timing of ICD placement from last hospitalization for HF.
In addition, a sensitivity analysis was performed to look at in-hospital, 30-day, and 90-day outcomes based on timing from last hospitalization for HF stratified by device type (i.e. single chamber ICD vs. dual chamber ICD vs. CRT-D). The results were similar when stratified by device type for in-hospital and 30-day outcomes (Supplemental Table 2). In contrast, patients undergoing placement of a CRT-D device and single chamber ICD for primary prevention during index hospitalization were, respectively, at higher risk of all-cause 90-day admissions and CV 90-day admissions compared to patients admitted for HF within the past 3 months (Supplemental Table 3, Supplemental Table 4).
DISCUSSION
The objectives of this post-hoc analysis of the NCDR® ICD Registry™ were to systematically describe the clinical characteristics, periprocedural complications, and post-procedural outcomes based on the timing of ICD placement for primary prevention from last hospitalization for HF. Approximately 20% of patients had an ICD placed during a hospitalization for HF or within 3 months of admission for a primary diagnosis of HF. Patients currently or recently hospitalized for HF tended to have a lower EF, were more symptomatic, had a higher burden of cardiac and non-cardiac comorbidities, and were less likely to be treated with an ACEI/ARB at baseline. Patients currently or recently hospitalized for HF had higher composite periprocedural, 30-day, and 90-day complication rates driven by a higher rate of stroke/TIA, MI, cardiac arrest, death, and minor access site and pocket-related complications not requiring reoperation. Finally, patients currently or recently hospitalized for HF were at higher risk of in-hospital mortality and post-procedural readmission or death independent of traditional risk factors.
Data from the Get With the Guidelines-HF registry suggest that among patients hospitalized for a primary diagnosis of HFrEF, fewer than 25% had an ICD in situ.19 It has also been well-documented in clinical trials of acute HF that ICDs may be underutilized and substantial geographic disparities exist.20–24 Although we found that only approximately 20% of patients underwent initial ICD placement for primary prevention within 3 months of a hospitalization for HF and implanting a primary prevention ICD is associated with worse outcomes, hospitalization represents an opportunity to review background guideline-directed medical and device-based therapies for HF and refer patients for consideration of ICD placement in the ambulatory setting. A recent meta-analysis of landmark clinical trials of pharmacotherapy in HF found that among HFrEF patients without an ICD, the incidence of SCD has declined from 2.4% to 1.0% at 90 days as a result of the cumulative benefit of evidence-based medications.25, 26 In contrast, it is known that following an index hospitalization for HFrEF the incidence of SCD may be as high as 2% within 30 days and more than 25% of deaths may be due to fatal arrhythmias.27–29 Observational studies of HF patients receiving ICD during an index HF hospitalization have shown significantly better survival compared to otherwise eligible patients that have not received an ICD during the HF hospitalization.11–13 However, as the present study only compared outcomes among patients receiving an ICD, the magnitude of benefit, risks, and risk/benefit ratio related to timing of ICD implant cannot be determined.
The data presented in this analysis of the NCDR® ICD Registry™ suggest that patients currently or recently hospitalized for HF had higher aggregate periprocedural, 30-day, and 90-day complication rates compared to patients without a recent hospitalization for HF. In addition, the differences in the periprocedural, 30-day, and 90-day complication rates were driven by a higher rate of stroke/TIA, MI, cardiac arrest, and death with a few notable exceptions. However, as all of these risks have been previously shown to be increased in HFrEF patients with a current or recent hospitalization, irrespective of device placement, they may be entirely unrelated. Potentially more concerning is the higher incidence of device infections and endocarditis seen at 90 days, which may be explained by multiple factors including, but not limited to, acuity of illness, prolonged hospitalization/deconditioning, exposure to nosocomial pathogens, and indwelling intravenous lines and/or urinary catheters. Given the attendant morbidity and mortality associated with cardiovascular implantable electronic device (CIED) infections, strategies to mitigate this risk including patient selection, periprocedural antibiotics, and sterile wound care and antiseptic bandages should be reinforced. In contrast, it should be noted that this study found similar rates of periprocedural complications directly related to ICD placement including cardiac perforation, cardiac venous dissection, pericardial tamponade, pneumothorax, and hemothorax irrespective of timing from last hospitalization for HF.
Importantly, after adjusting for potential confounders, patients currently or recently hospitalized for HF undergoing ICD placement for primary prevention were also at higher risk for all-cause death and readmission compared to patients without a recent hospitalization for HF. While this is not entirely unexpected, it raises the question of whether a strategy of early (i.e. during hospitalization or soon after discharge) vs. delayed (i.e. 3 or more months) ICD placement is indicated. Although ICDs may be underutilized in patients hospitalized for HF and this patient population is at relatively higher risk for SCD, providers and patients must assess the competing risks vs. benefits in making treatment decisions. Patients admitted for a primary diagnosis of HF experience an exceptionally poor short-term prognosis and more than 40% of deaths occurring in the post-discharge vulnerable phase may be due to progressive HF and/or cardiogenic shock.27 In addition, an unknown proportion of the patients experiencing SCD early post-discharge may be due to nonshockable rhythms (i.e. asystole or pulseless electrical activity).30 It is plausible that due to the overall dismal prognosis and competing risk of deaths, patients hospitalized for HF may behave similarly to the post-MI population in which early ICD placement has not been shown to beneficial.31, 32 Thus, there is a strong theoretical rationale for a strategy trial to determine the optimal timing of ICD placement for primary prevention from last hospitalization for HF.
Several limitations of this study should be mentioned. First, this study was conceived post-hoc and timing of ICD placement was not randomized. As a result, the relationships could reflect unmeasured or residual confounding. Second, there were not comparisons made to a comparable cohort of patients eligible for ICD placement that did not receive this therapy. Third, Medicare claims data were used to identify 30- and 90-day complications and claims data may be inaccurate and lack the complexity and granularity compared with patient data extracted directly from clinical records. However, Medicare Claims Data are the only available data source that has nationwide coverage, permitting follow-up of a large cohort of patients. Fourth, this study included only Medicare FSS patients age 65 years or older and may not be generalizable to a younger HF population. Fifth, this study likely underestimates the overall burden of morbidity as events not leading to an acute care episode or reoperation (i.e. inappropriate shocks) were not included. Finally, the rate of new device implants for whom these data are most relevant (HFrEF currently or recently hospitalized for a primary diagnosis of HF) is declining.
In conclusion, approximately 20% of patients undergoing ICD placement for primary prevention were currently or recently hospitalized for HF. The aggregate periprocedural, 30-day, and 90-day complication rates were higher among this subset of patients and driven by a higher rate of stroke/TIA, MI, cardiac arrest, and death. Additional research is required to clarify the signal of increased pocket infection, endocarditis, and other systemic infections as well as to identify approaches to mitigating the overall risk of infection in patients hospitalized for HF. Finally, given the highlighted safety concerns and the overall poor short-term prognosis and high competing risk of death due to progressive pump failure in patients hospitalized for HF, future prospective, real-world, pragmatic, comparative effectiveness studies should be conducted to define the optimal timing of ICD placement for primary prevention from last hospitalization for HF.
Supplementary Material
CLINICAL PERSPECTIVE.
1.). What is new?
Landmark clinical trials have demonstrated the safety and efficacy of implantable cardioverter-defibrillators (ICDs) in selected stable ambulatory patients with heart failure with a reduced ejection (HFrEF) fraction.
However, the optimal timing of ICD placement for primary prevention following an index hospitalization for HF remains unknown.
2.). What are the clinical implications?
This study found that patients currently or recently admitted for a diagnosis of HF who received a primary prevention ICD experienced a higher adjusted rate of periprocedural complications and were at increased risk of readmission or death independent of traditional risk factors.
These data highlight the need for additional prospective, real-world, pragmatic, comparative effectiveness studies to assess the relative risks and benefits of an early vs. delayed strategy for ICD placement following hospitalization for HF.
Acknowledgments
Funding Source: The American College of Cardiology (Washington, D.C.) provides ongoing financial and material support for the NCDR® ICD Registry™.
Disclosures: APA is supported by a NHLBI T32 postdoctoral training grant (5T32HL069749-14). MF is supported by a NHLBI T32 postdoctoral training grant (5T32HL007101-42) and an American Heart Association grant (17MCPRP33460225) and reports consulting for Coridea, AxonTherapies, and Galvani. GCF reports consulting for Abbott (modest), Amgen (modest), Janssen (modest), Novartis (significant), and Medtronic (modest). FAM reports a contract with the American College of Cardiology (through the University of Colorado) for his role as Chief Science Officer, NCDR. All other authors declare no relevant financial disclosures.
References
- 1.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN and Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 4.Writing Committee M, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW and Westlake C 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–293. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr. , Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ and Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology F and American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC, Investigators O-H and Coordinators. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. [DOI] [PubMed] [Google Scholar]
- 8.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp-Channing N, Davidson-Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH and Sudden Cardiac Death in Heart Failure Trial I. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. [DOI] [PubMed] [Google Scholar]
- 9.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML and Multicenter Automatic Defibrillator Implantation Trial III. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. [DOI] [PubMed] [Google Scholar]
- 10.Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SC, Joglar JA, Kay GN, Matlock DD, Myerburg RJ and Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017. pii: CIR.0000000000000548. doi: 10.1161/CIR.0000000000000548. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Chen CY, Stevenson LW, Stewart GC, Bhatt DL, Desai M, Seeger JD, Williams L, Jalbert JJ and Setoguchi S. Real world effectiveness of primary implantable cardioverter defibrillators implanted during hospital admissions for exacerbation of heart failure or other acute co-morbidities: cohort study of older patients with heart failure. BMJ. 2015;351:h3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CY, Stevenson LW, Stewart GC, Seeger JD, Williams L, Jalbert JJ and Setoguchi S. Impact of baseline heart failure burden on post-implantable cardioverter-defibrillator mortality among medicare beneficiaries. J Am Coll Cardiol. 2013;61:2142–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setoguchi S, Nohria A, Rassen JA, Stevenson LW and Schneeweiss S. Maximum potential benefit of implantable defibrillators in preventing sudden death after hospital admission because of heart failure. CMAJ. 2009;180:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodson JA, Reynolds MR, Bao H, Al-Khatib SM, Peterson ED, Kremers MS, Mirro MJ, Curtis JP and Ncdr. Developing a risk model for in-hospital adverse events following implantable cardioverter-defibrillator implantation: a report from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2014;63:788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammill SC, Kremers MS, Kadish AH, Stevenson LW, Heidenreich PA, Lindsay BD, Mirro MJ, Radford MJ, McKay C, Wang Y, Lang CM, Pontzer K, Rumsfeld J, Phurrough SE, Curtis JP and Brindis RG. Review of the ICD Registry’s third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6:1397–1401. [DOI] [PubMed] [Google Scholar]
- 16.Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, Beachy J, Blum Meisnere L, Conyers del M, Reynolds MR, Heidenreich PA, Al-Khatib SM, Pina IL, Blake K, Norine Walsh M, Wilkoff BL, Shalaby A, Masoudi FA and Rumsfeld J. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–65. [DOI] [PubMed] [Google Scholar]
- 17.Ranasinghe I, Parzynski CS, Freeman JV, Dreyer RP, Ross JS, Akar JG, Krumholz HM and Curtis JP. Long-Term Risk for Device-Related Complications and Reoperations After Implantable Cardioverter-Defibrillator Implantation: An Observational Cohort Study. Ann Intern Med. 2016. doi: 10.7326/M15-2732. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC, Get With the Guidelines Scientific Advisory C and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 20.Gheorghiade M, Konstam MA, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C and Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan I. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. [DOI] [PubMed] [Google Scholar]
- 21.Konstam MA, Gheorghiade M, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C and Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan I. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 22.Gheorghiade M, Bohm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP, Investigators A and Coordinators. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. [DOI] [PubMed] [Google Scholar]
- 23.Blair JE, Zannad F, Konstam MA, Cook T, Traver B, Burnett JC Jr., Grinfeld L, Krasa H, Maggioni AP, Orlandi C, Swedberg K, Udelson JE, Zimmer C, Gheorghiade M and Investigators E. Continental differences in clinical characteristics, management, and outcomes in patients hospitalized with worsening heart failure results from the EVEREST (Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan) program. J Am Coll Cardiol. 2008;52:1640–1648. [DOI] [PubMed] [Google Scholar]
- 24.Greene SJ, Fonarow GC, Solomon SD, Subacius H, Maggioni AP, Bohm M, Lewis EF, Zannad F, Gheorghiade M, Investigators A and Coordinators. Global variation in clinical profile, management, and post-discharge outcomes among patients hospitalized for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail. 2015;17:591–600. [DOI] [PubMed] [Google Scholar]
- 25.Shen L, Jhund PS and McMurray JJV. Declining Risk of Sudden Death in Heart Failure. N Engl J Med. 2017;377:1794–1795. [DOI] [PubMed] [Google Scholar]
- 26.Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, Dargie HJ, Granger CB, Kjekshus J, Kober L, Latini R, Maggioni AP, Packer M, Pitt B, Solomon SD, Swedberg K, Tavazzi L, Wikstrand J, Zannad F, Zile MR and McMurray JJV. Declining Risk of Sudden Death in Heart Failure. N Engl J Med. 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor CM, Miller AB, Blair JE, Konstam MA, Wedge P, Bahit MC, Carson P, Haass M, Hauptman PJ, Metra M, Oren RM, Patten R, Pina I, Roth S, Sackner-Bernstein JD, Traver B, Cook T, Gheorghiade M and Efficacy of Vasopressin Antagonism in heart Failure Outcome Study with Tolvaptan i. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J. 2010;159:841–849 e841. [DOI] [PubMed] [Google Scholar]
- 28.Pokorney SD, Al-Khatib SM, Sun JL, Schulte P, O’Connor CM, Teerlink JR, Armstrong PW, Ezekowitz JA, Starling RC, Voors AA, Velazquez EJ, Hernandez AF and Mentz RJ. Sudden cardiac death after acute heart failure hospital admission: insights from ASCEND-HF. Eur J Heart Fail. 2018;20:525–532. [DOI] [PubMed] [Google Scholar]
- 29.Felker GM, Teerlink JR, Butler J, Hernandez AF, Miller AB, Cotter G, Davison BA, Filippatos G, Greenberg BH, Ponikowski P, Voors AA, Hua TA, Severin TM, Unemori E and Metra M. Effect of serelaxin on mode of death in acute heart failure: results from the RELAX-AHF study. J Am Coll Cardiol. 2014;64:1591–1598. [DOI] [PubMed] [Google Scholar]
- 30.Ambrosy AP, Fudim M and Chioncel O. Sudden cardiac death following admission for acute heart failure: adding insult to injury. Eur J Heart Fail. 2018;20:533–535. [DOI] [PubMed] [Google Scholar]
- 31.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, Fain E, Gent M, Connolly SJ and Investigators D. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–2488. [DOI] [PubMed] [Google Scholar]
- 32.Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, Kornacewicz-Jach Z, Sredniawa B, Lupkovics G, Hofgartner F, Lubinski A, Rosenqvist M, Habets A, Wegscheider K, Senges J and Investigators I. Defibrillator implantation early after myocardial infarction. N Engl J Med. 2009;361:1427–1436. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.