Abstract
Particulate matter (PM) is a type of air pollutant that poses a risk to human health. In the ocular system, PM causes or aggravates dry eye syndrome (DES) by damaging the corneal and conjunctival epithelia. Liriope platyphylla has been used traditionally as an expectorant, antitussive agent, and tonic in Korea. However, the effects of Liriope platyphylla extract (LPE) on PM-induced ocular damage have not been elucidated. In this study, we evaluated the in vivo protective effect of LPE against PM-induced DES in rats. Topical administration of LPE attenuated the PM-induced decrease in tear volume and reduced corneal epithelial irregularity and damage. LPE also protected against PM-induced disruption of the corneal mucin-4 layer and reduction in the conjunctival goblet cell density. These findings suggest that LPE has protective effects against PM-induced DES.
1. Introduction
Particulate matter (PM), a type of environmental air pollution, is a mixture of solid and liquid particles such as sulfite, nitrogen oxide, carbon oxide, heavy metals, benzopyrene, polycyclic aromatic hydrocarbons (PAHs), nitro-substituted PAHs (nitro-PAHs), polychlorinated biphenyl (PCB) congeners, and chlorinated pesticides [1]. PM can penetrate human organs, and it poses a health risk. In the skin, PM exposure increases reactive oxygen species (ROS), epidermal thickening, and dermal inflammation with neutrophil infiltration [2]. Extended PM exposure significantly contributes to the burden of cardiovascular events [3]. Additionally, PM exposure contributes to increased risk and mortality burden of lung cancer [4, 5] and chronic kidney disease [6].
The eyes are another organ damaged by PM exposure. Continued and direct PM exposure may cause various eye symptoms or diseases [7], including allergic conjunctivitis during the nonpollen season [8]; recent studies have found a correlation between dry eye syndrome (DES) and PM exposure [9, 10]. DES affects tears and the ocular surface, resulting in discomfort, tear film instability, and visual disturbances that decrease the quality of life for many patients [11, 12].
DES is a common eye disease, and the number of cases has been increasing around the world. In the United States, 6.8% of adults were diagnosed with DES (∼16.4 million people) in 2017 [13]. Young adults (18 to 45 years) have a 2.7% prevalence, while the rates among adults over 40 years can be as high as 75% [14].
DES can be caused by various factors such as aging, smoking, health condition, and medications, but it can also be caused by PM exposure. Continuous PM exposure reduces tear film stability, influences the tear osmolality [9], delays corneal epithelium wound healing by inhibiting cell migration [15], and increases oxidation stress on the ocular surface [16]. Furthermore, PAH mixtures increase eye irritation and photosensitivity [17], and high concentrations of heavy metals are associated with DES induction [17, 18]. Therefore, PM exposure can induce multifactorial damage to the eyes via the inflammation response and oxidation stress, which increases the possibility of causing or worsening DES.
Liriope platyphylla (LP) is a perennial herbaceous evergreen belonging to the lily family. It is cultivated for medicinal or ornamental purposes in Korea, China, Japan, Europe, etc. LP has been used traditionally as an expectorant, antitussive agent, and tonic in Korea [19]. The Liriope platyphylla extract (LPE) contains bioactive compounds such as spicatoside A, pennogenin, homo-isoflavonoid, and sitosterol [20, 21].
LP has recently shown clinical effects on diabetes, neurodegenerative disorders, and obesity [22–24]. The root of LP affects hepatitis B viral gene expression and viral DNA replication by nuclear factor (NF-κB) inhibition [25]. Mixtures containing LP have an ability to increase the number of goblet cells and thicken the mucosal layer and muscle fibers [26]. Additionally, LP has shown anti-inflammatory, antioxidant, and wound healing abilities [27, 28]. However, LPE effects have not been well defined in the DES context. Therefore, this study aimed to evaluate the ocular protective effects of LPE on PM-induced DES.
2. Materials and Methods
2.1. Preparation of LPE
LP (5 kg) was ground, dried, and boiled with 40 L distilled water at 100°C for 3 h. Thereafter, aqueous LPE was concentrated by freeze-drying into powdered form. The concentrated LPE powder was deposited in the herbarium of the Korea Institute of Oriental Medicine (Daejeon, Republic of Korea).
2.2. Animals and Experiment Design
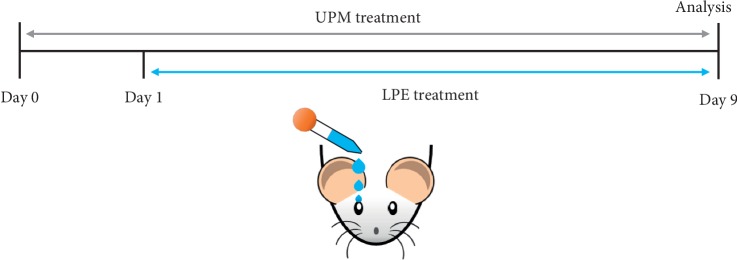
The animal experiments were conducted in accordance with the Institutional Animal Care and Use Committee approved protocol (IACUC approval ID: 17-060). Six-week-old female Sprague Dawley (SD) rats were purchased from Orient Bio (Seongnam, Korea). Rats were maintained in specific pathogen-free facilities with temperature 22–24°C, relative humidity 50–60%, and 12 h light/12 h dark cycles by the Korea Institute of Oriental Medicine. Urban particulate matter (UPM) was purchased from Sigma-Aldrich (St. Louis, MO, USA; NIST SRM 1648a) and mixed with saline at 20 mg/mL. SD rats were acclimated for 1 week and separated into five groups: (1) Control (CTL); (2) UPM Vehicle group (Veh); (3) UPM with 1 mg/mL LPE; (4) UPM with 5 mg/mL LPE; and (5) UPM with 10 mg/mL LPE). The schematic illustration of the animal experiment is shown in Figure 1. On day 0 through 9, the UPM solution was administered three times via an eyedropper to induce DES. On days 1 through 9, the LPE solution was administered three times via eyedropper after each UPM treatment. On the final day, the UPM and LPE treatment was done only once.
Figure 1.
Schematic illustration of animal experiment design. UPM, urban particulate matter; LPE, Liriope platyphylla extract.
2.3. Tear Volume
Tear volume was measured using the phenol red thread (Tianjin Jingming New Technological Development, Tianjin, China). SD rats were anesthetized by intraperitoneal injection of 40 mg/kg pentobarbital (Entobar, Hanlim pharm. Co., LTD, Seoul, Korea). Threads were placed into the third point from the lateral canthus of the lower eyelid for 1 min. The length of the red part was measured using a microscope (SZ61, Olympus, Tokyo, Japan) and presented as the tear volume in millimeters.
2.4. Corneal Irregularity Score
The corneal irregularity score was analyzed by the reflection of a ring-shaped slit light on the eye surface using a stereoscopic microscope (SZ61, Olympus, Tokyo, Japan). The corneal irregularity was defined using the following scale: 0, no distortion; 1, distortion in one quadrant; 2, distortion in two quadrants; 3, distortion in three quadrants; 4, distortion in all four quadrants; and 5, severe distortion in which no ring was visible.
2.5. Terminal Deoxynucleotidyl Transferases dUTP Nick-End Labeling (TUNEL) Assay
Terminal deoxynucleotidyl transferases dUTP nick-end labeling (TUNEL) was applied to identify corneal cell apoptosis. At necropsy process, the eyes were removed from rats and fixed using 10% formalin solution for 24 h at room temperature. Fixed tissues were dehydrated in ethanol, cleared in xylene, and embedded in paraffin. The paraffin-embedded eye tissues were sectioned using a microtome (Leica, Wetzlar, Germany). Prepared paraffin sections were deparaffinized and stained using the DeadEnd apoptosis detection system (Promega, Madison, WI, USA) according to the manufacturer's protocols. Stained corneal sections were observed using a fluorescence microscope (Olympus). Images of corneal sections were quantified by counting the TUNEL-positive cells.
2.6. Immunohistochemistry of Mucin 4 (MUC4)
Corneal paraffin sections were deparaffinized and blocked using the CAS-Block™ Histochemical Reagent (Thermo, Waltham, MA, USA). Sections were washed with PBS and incubated overnight at 4°C with Mucin 4 (MUC4) (Thermo). After incubation, the sections were washed using PBS, marked with an LSAB kit (DAKO, Santa Clara, CA, USA) and specified with a DAB substrate kit (DAKO). The nuclei were counterstained using Hematoxylin qs (VECTOR LABORATORIES, INC., Burlingame, CA, USA). MUC4 levels were analyzed by examining the immunoreactive intensity per unit area (mm2) using ImageJ software from NIH (National Institutes of Health, Bethesda, MD, USA).
2.7. Periodic Acid-Schiff (PAS) Staining
To confirm the number of goblet cells, paraffin sections of the conjunctiva tissue were stained using the Periodic acid-Schiff (PAS) staining system (Merck, Kenilworth, NJ, USA). After staining, the samples were observed using a DP80 digital camera (Olympus), and the resulting goblet cell images were analyzed using ImageJ software.
2.8. Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test between groups using the Prism 7.0 Software (GraphPad, San Diego, CA, USA). The data from the fluorescein staining score and irregularity score were analyzed using the Kruskal–Wallis nonparametric ANOVA with Dunn's multiple comparisons test; p values <0.05 indicated statistical significance.
3. Results
3.1. LPE Recovers Tear Secretion
To assess the degree of UPM-induced DES in SD rats, we measured tear volume with and without LPE (Figure 2). The UPM-treated group (9.0 ± 0.65 mm) showed considerably reduced tear volume compared to that of the control group (5.1 ± 0.32 mm). The tear volume was significantly recovered in the 5 and 10 mg/mL LPE-treated groups, respectively, and was similar to that of the control group (8.4 ± 0.88 and 8.1 ± 0.47 mm, respectively).
Figure 2.
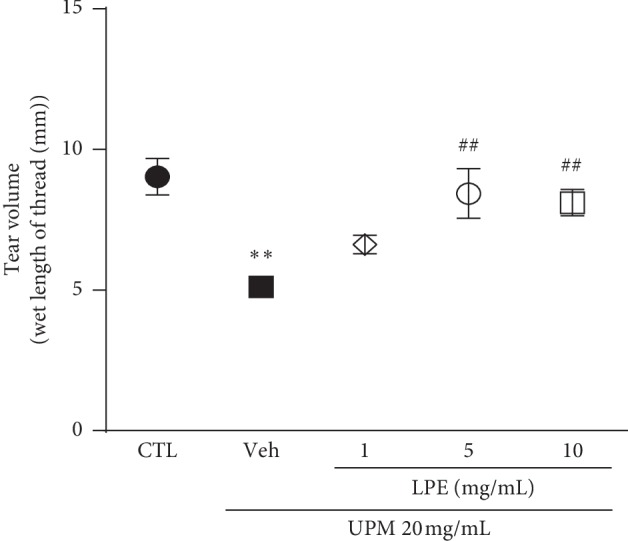
Tear volume analysis of urban particulate matter (UPM)-induced dry eye syndrome (DES) with and without Liriope platyphylla extract (LPE) treatment (1, 5, and 10 mg/mL). CTL, Control; Veh, Vehicle. Data shown are mean ± standard error. ∗∗p < 0.01 vs. Control; ##p < 0.01 vs. Vehicle.
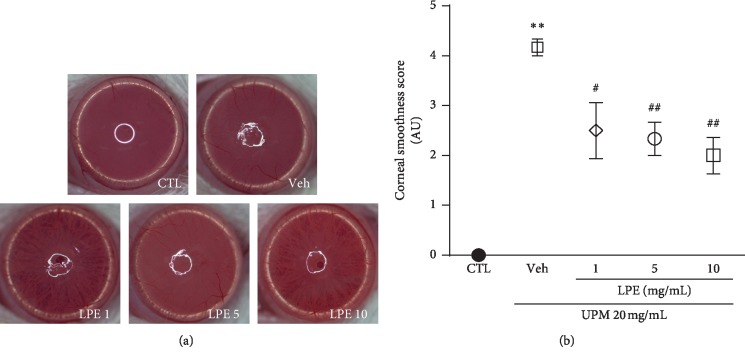
3.2. LPE Effects on Corneal Smoothness
The corneal irregularity score is an index of the corneal surface smoothness and damage (Figure 3). The corneal irregularity score of the UPM-treated group (4.2 ± 0.17 AU) increased compared to the control group. The corneal irregularity scores in the 1, 5, and 10 mg/mL LPE-treated groups were lower than those in the Veh group (2.5 ± 0.56, 2.3 ± 0.33, and 2.0 ± 0.37 AU, respectively).
Figure 3.
The effect of Liriope platyphylla extract (LPE) on corneal smoothness in urban particulate matter (UPM)-induced dry eye syndrome (DES). (a) Representative corneal images of Control (CTL), Vehicle (Veh), and LPE (1, 5, and 10 mg/mL) groups. (b) Graph of corneal irregularity scores. Data shown are mean ± standard error. ∗∗p < 0.01 vs. CTL; #p < 0.05 or ##p < 0.01 vs. Veh.
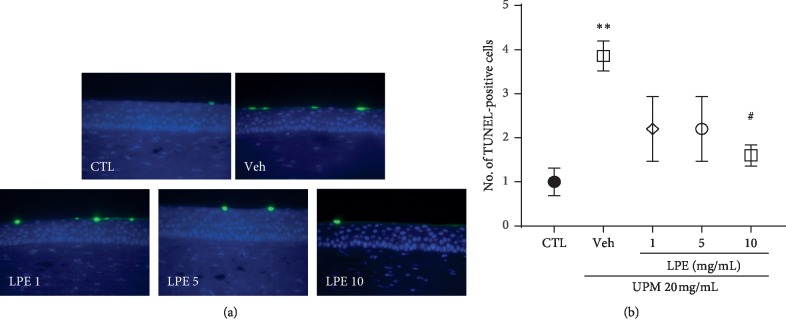
3.3. LPE Protects the Corneal Surface
As shown in Figure 4(a), the UPM-treated group showed a stronger apoptotic signal on the ocular surface than the CTL group. The 10 mg/mL LPE group showed a weaker apoptotic signal than the UPM-treated group. For a detailed assay, the number of TUNEL-positive cells was counted in areas of the same size (Figure 4(b)). The UPM-treated group (3.9 ± 0.34) showed more TUNEL-positive cells than the CTL group (1.0 ± 0.32). The 10 mg/mL LPE group (1.6 ± 0.24) showed significantly lesser TUNEL-positive cells than the UPM-treated group.
Figure 4.
Terminal deoxynucleotidyl transferases dUTP nick-end labeling (TUNEL) assay. (a) Representative images of TUNEL-positive cells within the Control (CTL), Vehicle (Veh), and Liriope platyphylla extract (LPE) (1, 5, and 10 mg/mL) groups. (b) Graph of the number of TUNEL-positive cells. Data shown are mean ± standard error. ∗∗p < 0.01 vs. CTL; #p < 0.05 vs. Veh.
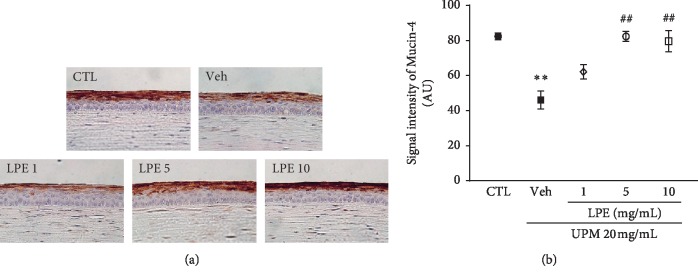
3.4. LPE Recovers the Level of MUC4
Figure 5(a) shows images of MUC4-stained corneal sections. The Veh group had lower MUC4 levels than the CTL group. The LPE-treated group presented a gradual, dose-dependent increase in the MUC4 layer. We quantified the signal intensity of the MUC4 layer for a detailed assay (Figure 5(b)). The signal intensity of MUC4 in the UPM-treated group (46.0 ± 5.20 AU) was decreased compared to that of the CTL group (82.4 ± 1.88 AU). The 1 mg/mL LPE-treated group showed a slightly increased MUC4 signal compared to that of the Veh group. The 5 and 10 mg/mL LPE-treated groups (82.3 ± 2.90 and 79.5 ± 6.04 AU, respectively) had enhanced MUC4 intensity, similar to the CTL group.
Figure 5.
The effect of Liriope platyphylla extract (LPE) on a mucin protein (MUC4) in urban particulate matter (UPM)-induced dry eye syndrome (DES). (a) Representative images of MUC4-stained corneal sections of the Control (CTL), Vehicle (Veh), and LPE (1, 5, and, 10 mg/mL) groups. (b) Graph of MUC4 signal intensity. Data shown are mean ± standard error. ∗∗p < 0.01 vs. CTL; ##p < 0.01 vs. Veh.
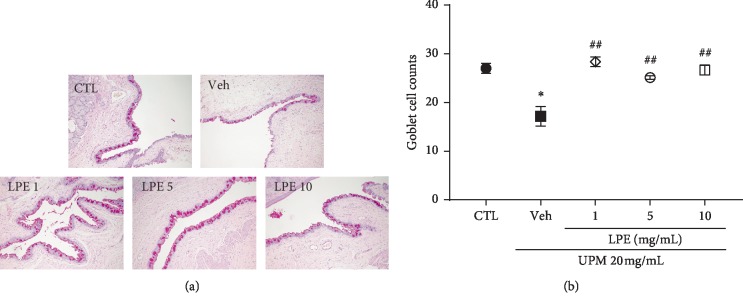
3.5. LPE Restores Conjunctival Goblet Cells
As shown in Figure 6, the UPM-treated group (17.2 ± 2.00) showed fewer goblet cells than the CTL group (27.0 ± 1.00). The number of goblet cells recovered in the 1, 5, and 10 mg/mL LPE-treated groups was 28.4 ± 1.00, 25.1 ± 0.40, and 26.7 ± 1.03, respectively.
Figure 6.
Effect of Liriope platyphylla extract (LPE) on conjunctival goblet cells in urban particulate matter (UPM)-induced dry eye syndrome (DES). (a) Representative images of goblet cells stained with periodic acid-Schiff (PAS). (b) Graph of goblet cell counts. Data shown are mean ± standard error. ∗p < 0.05 vs. Control (CTL); ##p < 0.01 vs. Vehicle (Veh).
4. Discussion
Accelerated industrialization and urbanization have been generating increased levels of environmental pollutants such as PM [29]. Although several studies have addressed the effects of PM on the development of human cardiovascular [3] and respiratory diseases [30], our current study focused on eye disease because the eyes are in direct contact with PM. Several recent studies have reported that PM causes or aggravates DES [3, 31]. We applied UPM from NIST as a standard reference PM to induce DES because the UPM constituents (such as polycyclic aromatic hydrocarbons (PAHs), nitro-substituted PAHs (nitro-PAHs), polychlorinated biphenyl (PCB) congeners, and chlorinated pesticides) are similar to the atmospheric PM within industrialized urban areas.
DES is driven by low lacrimal flow and high evaporation that leads to reduced tear volume [32]. The exposure to PM also induces significant toxicity and damage to the ocular surface, including the cornea and conjunctiva [16]. Damaged corneal and conjunctival epithelia lead to barrier dysfunction, which is ascribed to dry eye syndrome [33]. Epithelial damage is mainly caused by cell death, including apoptosis. It has been reported that PM may directly cause corneal epithelial apoptosis [16]. In this study, we observed that PM decreased tear volume and LPE recovered the decreased tear volume due to PM. LPE also prevented the increase in corneal irregularity scores and decreased apoptosis induced by PM. These results show that LPE may relieve DES symptoms and protect the ocular surface from the damage caused by PM exposure.
Damage to the corneal and conjunctival epithelia also involves the disturbance of mucin expression and loss of goblet cells [34]. Mucins are high-molecular weight glycoproteins that are expressed on the ocular epithelial surface [35]. They are classified into three categories based on their amino acid sequences: transmembrane (MUC1, MUC4, MUC16, etc.) and gel-forming (MUC5AC, etc.), soluble (MUC7 and MUC9), and unclassified (MUC8 and MUC11) types [36].
Ocular surface membrane-associated mucins are found in the mucin layer of the corneal and conjunctival epithelia; the gel-forming mucins are mainly found in the water layer. Mucins play a role in keeping the ocular surface wet [36] and protecting it from pathogens, allergens, and extracellular molecules [37]. The gel-forming mucins are produced by goblet cells in the conjunctival epithelium. Goblet cells maintain homeostasis within the conjunctival epithelium and are important in DES development.
The alteration of ocular mucins is also related to DES progression [38]. In addition, it is reported that PM exposure impacts the corneal MUC4 layer [31] and the density of conjunctival goblet cells [39]. In this study, LPE thickened the MUC4 layer and increased the conjunctival goblet cell density under PM exposure. These results revealed that LPE may have an ability to protect and recover the disruption of mucin induced by PM.
LPE includes steroid saponins (such as spicatoside A) and flavonoids [20, 21]. Among the bioactive compounds, spicatoside A is reported to have antioxidative and anti-inflammatory effects [40–42] and can induce an increase in the production and secretion of mucins [43]. In addition, the combination of various flavonoids and saponins is expected to provide eye protection. Further experiments will aim to demonstrate the mechanisms behind the effects of individual bioactive compounds and the LPE-induced mucin upregulation under PM exposure.
5. Conclusion
In conclusion, our results show that LPE treatment improved DES, which was confirmed based on changes in tear volume, corneal irregularity, apoptosis, MUC4 levels, and goblet cell numbers. The results suggest that LPE has the potential to relieve DES through its ocular protective effects.
Acknowledgments
This study was supported by the “R&D Program for Forest Science Technology (project no. 2017035A00-1919-BA01)” of Korea Forest Service (Korea Forestry Promotion Institute), Korea Institute of Oriental Medicine (project no. KSN1812080), and “NST (National Research Council of Science & Technology)-KIOM (Korea Institute of Oriental Medicine) Postdoctoral Research Fellowship for Young Scientists.”
Data Availability
All data and materials used are described in the article. Upon request, the corresponding author can provide further information.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Loxham M., Nieuwenhuijsen M. J. Health effects of particulate matter air pollution in underground railway systems—a critical review of the evidence. Particle and Fibre Toxicology. 2019;16(1):p. 12. doi: 10.1186/s12989-019-0296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin S.-P., Li Z., Choi E. K., et al. Urban particulate matter in air pollution penetrates into the barrier-disrupted skin and produces ROS-dependent cutaneous inflammatory response in vivo. Journal of Dermatological Science. 2018;91(2):175–183. doi: 10.1016/j.jdermsci.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Martinelli N., Olivieri O., Girelli D. Air particulate matter and cardiovascular disease: a narrative review. European Journal of Internal Medicine. 2013;24(4):295–302. doi: 10.1016/j.ejim.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Hamra G. B., Guha N., Cohen A., et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environmental Health Perspectives. 2014;122(11):906–911. doi: 10.1289/ehp.140809210.1289/ehp/1408092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He Y., Gao Z., Guo T., et al. Fine particulate matter associated mortality burden of lung cancer in Hebei Province, China. Thoracic Cancer. 2018;9(7):820–826. doi: 10.1111/1759-7714.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowe B., Xie Y., Li T. T., Yan Y., Xian H., Al-Aly Z. Estimates of the 2016 global burden of kidney disease attributable to ambient fine particulate matter air pollution. BMJ Open. 2019;9(5) doi: 10.1136/bmjopen-2018-022450.e022450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan G., Li J., Yang Q., et al. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Scientific Reports. 2018;8(1):p. 17828. doi: 10.1038/s41598-018-36181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mimura T., Ichinose T., Yamagami S., et al. Airborne particulate matter (PM2.5) and the prevalence of allergic conjunctivitis in Japan. Science of the Total Environment. 2014;487:493–499. doi: 10.1016/j.scitotenv.2014.04.057. [DOI] [PubMed] [Google Scholar]
- 9.Torricelli A. A. M., Novaes P., Matsuda M., et al. Correlation between signs and symptoms of ocular surface dysfunction and tear osmolarity with ambient levels of air pollution in a large metropolitan area. Cornea. 2013;32(4):E11–E15. doi: 10.1097/ICO.0b013e31825e845d. [DOI] [PubMed] [Google Scholar]
- 10.Han J. Y., Kang B., Eom Y., Kim H. M., Song J. S. Comparing the effects of particulate matter on the ocular surfaces of normal eyes and a dry eye rat model. Cornea. 2017;36(5):605–610. doi: 10.1097/ICO.0000000000001171. [DOI] [PubMed] [Google Scholar]
- 11.Grubbs J. R., Jr., Tolleson-Rinehart S., Huynh K., Davis R. M. A review of quality of life measures in dry eye questionnaires. Cornea. 2014;33(2):215–218. doi: 10.1097/ICO.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulsen A. J., Cruickshanks K. J., Fischer M. E., et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. American Journal of Ophthalmology. 2014;157(4):799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrand K. F., Fridman M., Stillman I. Ö., Schaumberg D. A. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 Years and older. American Journal of Ophthalmology. 2017;182:90–98. doi: 10.1016/j.ajo.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 14.Stapleton F., Alves M., Bunya V. Y., et al. TFOS DEWS II epidemiology report. The Ocular Surface. 2017;15(3):334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Cui Y.-H., Hu Z.-X., Gao Z.-X., et al. Airborne particulate matter impairs corneal epithelial cells migration via disturbing FAK/RhoA signaling pathway and cytoskeleton organization. Nanotoxicology. 2018;12(4):312–324. doi: 10.1080/17435390.2018.1440651. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q., Li K., Li D., Zhang Y., Liu X., Wu K. Effects of fine particulate matter on the ocular surface: an in vitro and in vivo study. Biomedicine & Pharmacotherapy. 2019;117:p. 109177. doi: 10.1016/j.biopha.2019.109177. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Shafy H. I., Mansour M. S. M. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum. 2016;25(1):107–123. doi: 10.1016/j.ejpe.2015.03.011. [DOI] [Google Scholar]
- 18.Jung S. J., Lee S. H. Association between three heavy metals and dry eye disease in Korean adults: results of the Korean national health and nutrition examination survey. Korean Journal of Ophthalmology. 2019;33(1):26–35. doi: 10.3341/kjo.2018.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hur J., Lee P., Kim J., Kim A. J., Kim H., Kim S. Y. Induction of nerve growth factor by butanol fraction of Liriope platyphylla in C6 and primary astrocyte cells. Biological & Pharmaceutical Bulletin. 2004;27(8):1257–1260. doi: 10.1248/bpb.27.1257. [DOI] [PubMed] [Google Scholar]
- 20.Lee K.-A., Park J.-S. Antioxidative activity of beverage with water and ethanol extracts of maegmundong (Liriope platyphylla) Korean Journal of Food and Cookery Science. 2014;30(6):785–791. doi: 10.9724/kfcs.2014.30.6.785. [DOI] [Google Scholar]
- 21.Kim S. H., Kim H. K., Yang E. S., et al. Optimization of pressurized liquid extraction for spicatoside A in Liriope platyphylla. Separation and Purification Technology. 2010;71(2):168–172. doi: 10.1016/j.seppur.2009.11.016. [DOI] [Google Scholar]
- 22.Park H. R., Lee H., Park H., Jeon J. W., Cho W.-K., Ma J. Y. Neuroprotective effects of Liriope platyphylla extract against hydrogen peroxide-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. BMC Complementary and Alternative Medicine. 2015;15(1):p. 171. doi: 10.1186/s12906-015-0679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H., Lee J., Han H.-S., et al. Immunomodulatory effects of Liriope platyphylla water extract on lipopolysaccharide-activated mouse macrophage. Nutrients. 2012;4(12):1887–1897. doi: 10.3390/nu4121887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li W. J., Cheng X. L., Liu J., et al. Phenolic compounds and antioxidant activities of Liriope muscari. Molecules. 2012;17(2):1797–1808. doi: 10.3390/molecules17021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang T.-J., Tsai Y.-C., Chiang S.-Y., et al. Anti-viral effect of a compound isolated from Liriope platyphylla against hepatitis B virus in vitro. Virus Research. 2014;192:16–24. doi: 10.1016/j.virusres.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Kim J. E., Yun W. B., Lee M. L., et al. Synergic laxative effects of an herbal mixture of Liriope platyphylla, Glycyrrhiza uralensis, and cinnamomum cassia in loperamide-induced constipation of Sprague Dawley rats. Journal of Medicinal Food. 2019;22(3):294–304. doi: 10.1089/jmf.2018.4234. [DOI] [PubMed] [Google Scholar]
- 27.Kim M.-J., Yoo Y.-C., Sung N.-Y., et al. Anti-inflammatory effects of Liriope platyphylla in LPS-stimulated macrophages and endotoxemic mice. The American Journal of Chinese Medicine. 2016;44(6):1127–1143. doi: 10.1142/S0192415x16500634. [DOI] [PubMed] [Google Scholar]
- 28.Lee E. H., Go J., Kim J. E., et al. Therapeutic effect of hydrocolloid membrane containing Liriope platyphylla extracts on the burn wounds of SD rats. Journal of Life Science. 2015;25(5):523–532. doi: 10.5352/JLS.2015.25.5.523. [DOI] [Google Scholar]
- 29.Kim H. C., Kim S., Kim B. U., et al. Recent increase of surface particulate matter concentrations in the Seoul Metropolitan Area, Korea. Scientific Reports. 2017;7(1):p. 4710. doi: 10.1038/s41598-017-05092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Losacco C., Perillo A. Particulate matter air pollution and respiratory impact on humans and animals. Environmental Science and Pollution Research. 2018;25(34):33901–33910. doi: 10.1007/s11356-018-3344-9. [DOI] [PubMed] [Google Scholar]
- 31.Hyun S. W., Kim J., Park B., et al. Apricot kernel extract and amygdalin inhibit urban particulate matter-induced keratoconjunctivitis sicca. Molecules. 2019;24(3):p. 650. doi: 10.3390/molecules24030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H., Yiu S. C. Dry eye disease: a review of diagnostic approaches and treatments. Saudi Journal of Ophthalmology. 2014;28(3):173–181. doi: 10.1016/j.sjopt.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mantelli F., Massaro-Giordano M., Macchi I., Lambiase A., Bonini S. The cellular mechanisms of dry eye: from pathogenesis to treatment. Journal of Cellular Physiology. 2013;228(12):2253–2256. doi: 10.1002/jcp.24398. [DOI] [PubMed] [Google Scholar]
- 34.Lemp M. A. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye WorkShop (2007) The Ocular Surface. 2007;5(2):75–92. doi: 10.1016/S1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 35.Mantelli F., Argüeso P. Functions of ocular surface mucins in health and disease. Current Opinion in Allergy and Clinical Immunology. 2008;8(5):477–483. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corrales R. M., Narayanan S., Fernández I., et al. Ocular mucin gene expression levels as biomarkers for the diagnosis of dry eye syndrome. Investigative Opthalmology & Visual Science. 2011;52(11):8363–8369. doi: 10.1167/iovs.11-7655. [DOI] [PubMed] [Google Scholar]
- 37.Davidson H. J., Kuonen V. J. The tear film and ocular mucins. Veterinary Ophthalmology. 2004;7(2):71–77. doi: 10.1111/j.1463-5224.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stephens D. N., McNamara N. A. Altered mucin and glycoprotein expression in dry eye disease. Optometry and Vision Science. 2015;92(9):931–938. doi: 10.1097/OPX.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 39.Torricelli A. A. M., Matsuda M., Novaes P., et al. Effects of ambient levels of traffic-derived air pollution on the ocular surface: analysis of symptoms, conjunctival goblet cell count and mucin 5AC gene expression. Environmental Research. 2014;131:59–63. doi: 10.1016/j.envres.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Ramalingam M., Kim S.-J. Pharmacological activities and applications of spicatoside A. Biomolecules & Therapeutics. 2016;24(5):469–474. doi: 10.4062/biomolther.2015.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hur J., Lee P., Moon E., et al. Neurite outgrowth induced by spicatoside A, a steroidal saponin, via the tyrosine kinase A receptor pathway. European Journal of Pharmacology. 2009;620(1–3):9–15. doi: 10.1016/j.ejphar.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Kou J., Sun Y., Lin Y., et al. Anti-inflammatory activities of aqueous extract from radix ophiopogon japonicus and its two constituents. Biological & Pharmaceutical Bulletin. 2005;28(7):1234–1238. doi: 10.1248/bpb.28.1234. [DOI] [PubMed] [Google Scholar]
- 43.Park S. H., Lee H. J., Ryu J., et al. Effects of ophiopogonin D and spicatoside A derived from Liriope Tuber on secretion and production of mucin from airway epithelial cells. Phytomedicine. 2014;21(2):172–176. doi: 10.1016/j.phymed.2013.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials used are described in the article. Upon request, the corresponding author can provide further information.