Abstract
Boswellia serrata is a widely used herb in Indian systems of medicine and is well known for its potential medicinal properties. A chromatographic method was developed for the analysis and quantification of six boswellic acid marker compounds, i.e., keto boswellic acid (1), 3-O-Acetyl 11-keto β-boswellic acid (2), ɑ-Boswellic acid (3), β-Boswellic acid (4), 3-O-Acetyl-ɑ-boswellic acid (5) and 3-O-Acetyl-β-boswellic acid (6) in commercial herbal products containing B. serrata as an ingredient. Combining UPLC with Q-Tof-MS/MS makes the better identification of secondary metabolites and adulterants in the herbal formulations containing B. serrata in rapid time using fragmentation approach than the traditional approaches. In this study quantification of boswellic acids with UPLC-PDA method was performed as per the pharmacopeia guidelines. Furthermore, minor phytochemical constituents were identified and characterized with the help of LC-Q-Tof-MS/MS fragmentation data and various isoforms of boswellic acids and tirucallic acids in B. serrata oleo-gum-resin extract were identified.
Keywords: Boswellia serrata, Herbal formulations, Quantification, UPLC-Q-tof-MSe, Boswellic acids, Tirucallic acids
Graphical abstract
Highlights
-
•
Development of UPLC-PDA method followed by Tof-MS/MS analysis for secondary metabolites characterization.
-
•
Quantification of six boswellic acids in crude extract & commercial herbal products available in the market.
-
•
Comprehensive analysis of crude extract using UPLC-Q-Tof-MSe.
-
•
Identification 16 secondary metabolites from Tof-MS/MS fragmentation pattern.
1. Introduction
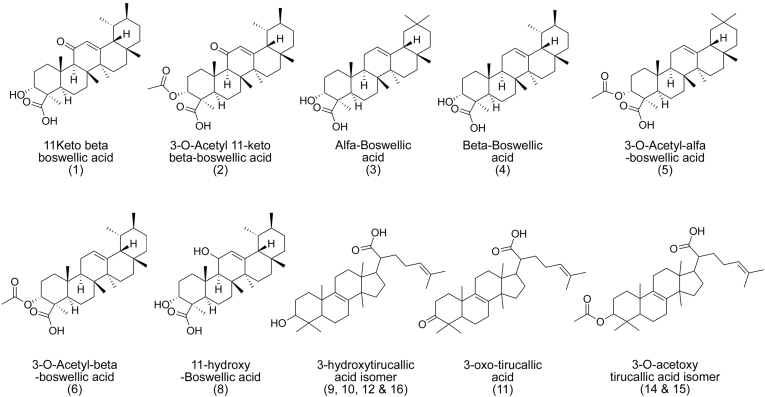
The genus Boswellia (Fam: Burseraceae) grows on calcareous soil at high altitudes, slopes and ridges of hills. Oleo-gum-resin is collected from wild trees by making incisions in bark of the trunks and the gum resin exudes from incision point and solidifies into irregular mass as a result of exposure to open air [1]. Gum resin of Boswellia serrata, commonly known as Indian frankincense, has been used in Asian and African folk medicine for treatment of arthritis [2]. Terpenoids are by far the most abundant metabolites of this gum resin and boswellic acids in particular have been identified as active principles [[2], [3], [4]] whose chemical structures are given in Fig. 1. B. serrata gum resin has been reported potential therapeutics for a wide range of ailments including hepatoprotective, anti-inflammatory [5], anticancer [6], beneficial in liver fibrosis [7] and antibacterial activity [8]. Toxicological studies performed on animals with boswellia extract indicate that their inclusions in herbal formulation are safe [9].
Fig. 1.
Chemical structures of standard boswellic acid (1–6) and putatively identified compounds (7–16).
This resin gum is being used widely in Ayurvedic herbal formulations which were prepared in accordance with the authorized methodology provided in Ayurvedic formulary of India [10]. The increased demand for frankincense has put pressure on supply of natural resources which ultimately results in use of substandard materials or substitution and adulteration. The content of six boswellic acids [keto boswellic acid (1), 3-O-Acetyl 11-keto β-boswellic acid (2), α-Boswellic acid (3), β-Boswellic acid (4), 3-O-Acetyl-ɑ-boswellic acid (5) and 3-O-Acetyl-β-boswellic acid (6)] had been used as the standard index to appraise the quality of boswellia gum resin and its products [4]. To control the adulteration and maintain quality and efficacy of product, analytical tools play a major role. Consequently, several analytical [[11], [12], [13], [14], [15]] and bio-analytical methods [[16], [17], [18]] reported in the literature include high performance liquid chromatography (HPLC) and high-performance thin layer chromatography (HPTLC) for quantification of the above-mentioned markers. Keeping in view of the growing interest on herbal products, continuous studies were undertaken in our laboratory pertaining to their standardization and demonstrated the potential utility of the methods emphasizing the need to effectively and quantitatively identify the active ingredients [19,20]. In the present work, we developed a new, simple, rapid and specific reversed-phase UPLC-PDA method for the quantification of six markers (1–6) from crude resin and this method was successfully applied to determine these compounds in fourteen different marketed herbal formulations. Furthermore, optimized method was coupled to Q-Tof-MS/MS system for metabolite profiling and to characterize minor/unknown constituents from crude resin extract. Our study led to the identification of 16 tri terpenoids from crude extracts through detailed mass fragmentation study. To the best of our knowledge, metabolite profiling and identification of constituents from crude extracts using UPLC-Q-Tof-MS/MS is being reported for the first time.
2. Materials and methods
2.1. B. serrata resin gum material and samples
The B. serrata resin gum exudates were collected from Devgiri and Karhal region of Uttar Pradesh, India in spring season 2016. Four resin extract (methanolic) samples (S1–S4) and fourteen commercially available herbal drug formulations of capsules (#BS C1–C6), tablets (#BS T1-T3), powders (#BS P1–P2), gel formulations (#BS G1-G2) and tea fusion material (#BS F1) claimed to contain B. serrata were purchased from local suppliers and online market. The specimens of all samples were deposited at Natural Products Laboratory, CSIR-IICT, Hyderabad, India. Out of 14 marketed samples, eight are single herb formulations containing B. serrata gum resin as active constituent and remaining six formulations were polyherbal in nature, and B. serrata gum resin was one of the constituent along with other plant extracts in different concentrations.
2.2. Chemicals and standards
The reference standards keto boswellic acid (1), 3-O-Acetyl 11-keto β-boswellic acid (2), α-Boswellic acid (3), β-Boswellic acid (4), 3-O-Acetyl-ɑ-boswellic acid (5) and 3-O-Acetyl-β-boswellic acid (6) were purchased from Sigma-Aldrich (St. Louis, MO, USA). The purity of reference standards was >98% (Confirmed with HPLC and MS). Acetonitrile and formic acid were obtained from BioSolv (ULC/MS Grade) (Dieuze, France). Ammonium hydroxide solution (25%), methanol and Milli Q water were procured from Merck KGaALi Chrosolv LC-MS grade (Darmstadt, Germany).
2.3. Preparation of standard stock and working solutions
Accurately weighed 2 mg of individual reference standards were dissolved in methanol to obtain concentration of 2.0 mg/mL. Mixture of six reference standards was prepared by the addition process to appropriate concentrations for construction of calibration curve by UPLC-PDA.
2.4. Preparation of resin material and commercial samples
Accurately weighed 1g of the sample was transferred into conical flask with 10 mL of MeOH and sonicated for about 45 min. Supernatant liquid was collected from samples after centrifugation process. Extraction process was repeated for three times to ensure effective extraction without any loss of analytes. Finally, supernatant liquid was diluted up to 50 mL with MeOH. Samples were taken about 20 mg/mL concentration, filtered through 0.20 μm PVDF membrane filters and used as stock solution throughout validation process. In addition, the process was also extended to UPLC-Q-Tof-MS/MS to characterize secondary metabolites in the methanolic gum extract which could be useful for species differentiation.
Solid dosage forms (capsules, tablets and powder) were pulverized with mortar and pestle in individual to the appropriate amount weighed and extracted. For the semi-solid dosage form (Gel), adequate amount was weighed and subjected for extraction process. Same extraction process was followed for commercially available formulations.
2.5. Chromatographic and mass spectrometric instrumental conditions
Sample analysis was performed with Waters acquity UPLC H-Class system (Waters Corp., Milford, U.S.A) which includes quaternary solvent manager (QSM), sampler manager-FTN with ANSI 2X48 well plate, thermostatic controlled column compartment with PDA eλ detector. UPLC column was acquity BEH C18 (100 mm × 2.1 mm, 1.9 μm) (Waters, Ireland). The column and auto sampler temperatures were maintained at 45 °C and 10 °C, respectively. The flow rate was 0.4 mL/min and mobile phase consisted of water with 0.1% formic acid (A) and acetonitrile with 0.1% ammonium hydroxide (B). The gradient program was as follows: 0 min, 70 %B; 3.50 min, 75 %B; 7.00 min, 80 %B; 14.00 min, 95 %B. Column was equilibrated with initial mobile phase composition for 3.00 min before injection of each sample. Needle wash solution (strong wash, acetonitrile/water: 80/20, v/v) and seal wash solution (weak wash, acetonitrile /water: 10/90, v/v) were used. Injection volume was set at 3 μL throughout validation process. Mass spectrometric analysis was performed with Waters Xevo G2-XS Q-Tof-MS/MS (Waters, Manchester, U.K) with ESI source with Z-Spray technology using following parameters: capillary voltage, 3.0 kV; sample cone, 30 V; source temperature, 120 °C; desolvation temperature, 350 °C; cone gas flow rate, 50 L/h; desolvation gas (N2) flow rate, 1000 L/h; argon as CID gas for MS/MS experiments. To ensure accuracy and reproducibility, all analyses were performed using lock spray. Leu-enkephalin (YGGFL) (5 ng/mL) was used as lock mass generating reference ion in negative mode at m/z 554.2615 and was introduced by a lock spray at 10 μL/min for accurate mass acquisition. The data were processed with MassLynx v4.1 SCN937 workstation.
2.6. Method validation
Developed UPLC-PDA method was validated in terms of LOD, LOQ, system suitability, linearity, precision and accuracy as per ICH Q2 R1 Guidelines [21].
LOD and LOQ of individual analytes were determined by signal-to-noise ratio at lower concentration which was equal to 3:1 and 10:1, respectively. Linearity of the developed method was determined by plotting calibration curves with different concentrations of analytes versus peak area. From calibration data regression equation and correlation coefficients were calculated via regression analysis.
The system suitability was observed by repetition of standard analytes mixture with specific concentration in triplicate with developed method parameters. Precision of method was evaluated in terms of intra and inter-day precision by analyzing #BS T1 & #BS C1 samples for a week by following the same extraction procedure in triplicate manner. Further the samples were subjected to accuracy study. Accuracy was determined by analyzing samples to which reference analytes were added at three different concentrations followed by extraction and analysis.
3. Results and discussion
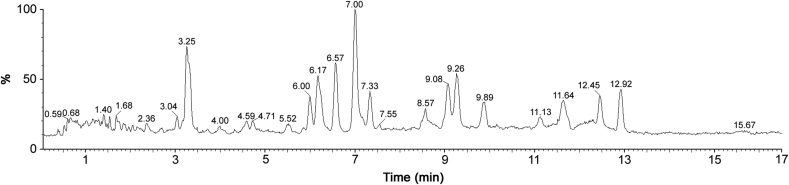
Ultra-performance liquid chromatography-diode array detection (UPLC-PDA) coupled to an electro spray ionization time of flight mass spectrometer (ESI-MS) has emerged as a new technique for identification and elucidation of metabolites in complex extracts. Therefore, in continuation of previous studies on fragmentation patterns on natural products [22,23], we extended UPLC/PDA/ESI-Q-Tof-MS analysis of crude resin extract of B. serrata for detailed characterization of secondary metabolites. Acquired total ion chromatograms (TIC) were analyzed in ESI negative ionization mode and the results are presented in Table 1, Figs. S1–S16 and Fig. 2. The metabolites assignments were made based on their MS spectral data (accurate mass and fragmentation pattern), retention times, comparison to the standard compounds (In-house library) and search in public online databases (DNP, Reaxys and SciFinder), respectively. Our studies led to the identification of fourteen compounds from methanolic extract of B. serrata resin. Majority of compounds in B. serrata are triterpeniods belonging to boswellic acids and tirucallic acid type metabolites. This can be easily distinguishable through mass fragmentation mechanism using CID-MS/MS. Based on developed analytical method, we could identify seven (9–12 & 14–16) isoforms of tirucallic acids along with six well characterized boswellic acids shown in Table 1 and Fig. S17.
Table 1.
LC-Q-Tof-MSe data for Boswellia serrata gum resin extract (methanolic).
| S No | Retention time (min) | HRMS |
Error (Δppm) | Fragment ions | Fragments formula [M − H]- | λmax (nm) | Name of the compound (Putatively identified) | |
|---|---|---|---|---|---|---|---|---|
| m/z | Formula [M − H]- | |||||||
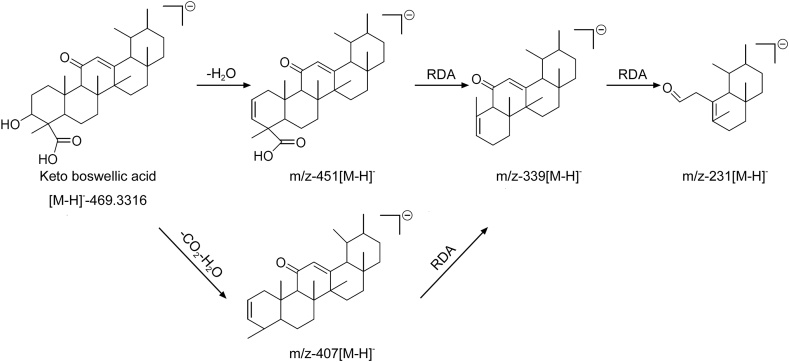
| 1 | 3.20 | 469.3316 | C30H45O4 | −0.4 | 407.3308 | C29H43O | 250.42 | Keto boswellic acid |
| 391.3000 | C28H39O | |||||||
| 353.2880 | C25H37O | |||||||
| 339.2719 | C24H35O | |||||||
| 271.2066 | C19H27O | |||||||
| 231.1762 | C16H23O | |||||||
| 2 | 6.13 | 511.3420 | C32H47O5 | −0.6 | 485.3627 | C31H49O4 | 228.42 | 3-O-Acetyl 11-keto β-boswellic acid |
| 467.3571 | C31H47O3 | |||||||
| 439.3624 | C30H47O2 | |||||||
| 3 | 8.41 | 455.3521 | C30H47O3 | −0.9 | 437.3412 | C30H45O2 | 225.42 | ɑ-Boswellic acid |
| 409.3462 | C29H45O | |||||||
| 361.2930 | C27H37 | |||||||
| 233.1931 | C16H25O | |||||||
| 4 | 8.91 | 455.3527 | C30H47O3 | 0.4 | 409.3466 | C29H45O | 225.42 | β-Boswellic acid |
| 367.3059 | C26H39O | |||||||
| 361.2906 | C27H37 | |||||||
| 283.2123 | C20H27O | |||||||
| 233.1919 | C16H25O | |||||||
| 5 | 12.30 | 497.3637 | C32H49O4 | 1.2 | 423.3326 | C29H43O2 | 226.42 | 3-O-Acetyl-ɑ-boswellic acid |
| 6 | 12.79 | 497.3633 | C32H49O4 | 0.4 | 439.3222 | C29H43O3 | 226.42 | 3-O-Acetyl-β-boswellic acid |
| 423.3318 | C29H43O2 | |||||||
| 285.1173 | C17H17O4 | |||||||
| 233.1944 | C16H25O | |||||||
| 7 | 3.42 | 513.3595 | C32H49O5 | 2.9 | 487.3420 | C30H47O5 | 190.42, 226.42 | Unknown |
| 443.3571 | C29H47O3 | |||||||
| 415.3665 | C28H47O2 | |||||||
| 371.2997 | C25H39O2 | |||||||
| 249.1881 | C16H25O2 | |||||||
| 235.1749 | C15H23O2 | |||||||
| 209.1549 | C13H21O2 | |||||||
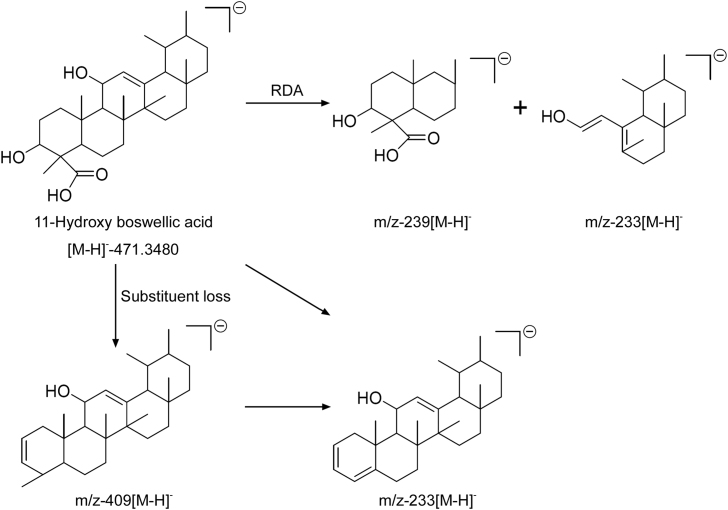
| 8 | 4.60 | 471.3480 | C30H47O4 | 1.3 | 409.3516 | C29H45O | 225.42 | 11-hydroxy-boswellic acid (new) |
| 393.3176 | C28H41O | |||||||
| 377.2855 | C27H37O | |||||||
| 253.1658 | C18H21O | |||||||
| 233.1917 | C16H25O | |||||||
| 9 | 5.93 | 455.3523 | C30H47O3 | −0.4 | 425.3062 | C28H41O3 | 191.42, 226.42 | 3-hydroxytirucallic acid isomer |
| 373.2748 | C24H37O3 | |||||||
| 339.2690 | C24H35O | |||||||
| 10 | 6.49 | 455.3523 | C30H47O3 | −0.4 | 373.2744 | C24H37O3 | 224.42 | 3-hydroxytirucallic acid isomer |
| 355.2648 | C24H35O2 | |||||||
| 341.2894 | C24H37O | |||||||
| 339.2757 | C24H35O | |||||||
| 269.1953 | C19H25O | |||||||
| 11 | 6.95 | 453.3361 | C30H45O3 | −1.8 | 371.2587 | C24H35O3 | 190.42, 222.42 | 3-oxo-tirucallic acid |
| 353.2510 | C24H33O2 | |||||||
| 339.2696 | C24H35O | |||||||
| 309.2245 | C22H29O | |||||||
| 269.1947 | C19H25O | |||||||
| 255.1791 | C18H23O | |||||||
| 12 | 7.27 | 455.3531 | C30H47O3 | 1.5 | 373.2746 | C24H37O3 | 224.42 | 3-hydroxytirucallic acid isomer |
| 355.2758 | C24H35O2 | |||||||
| 341.2864 | C24H37O | |||||||
| 281.2542 | C18H33O2 | |||||||
| 13 | 8.67 | 485.3637 | C31H49O4 | 1.2 | 441.3382 | C29H45O3 | 225.42 | Unknown |
| 397.3553 | C28H45O | |||||||
| 381.3212 | C27H41O | |||||||
| 365.2928 | C26H37O | |||||||
| 337.3018 | C25H37 | |||||||
| 229.1437 | C12H21O4 | |||||||
| 14 | 9.21 | 497.3635 | C32H49O4 | 1.1 | 437.3427 | C30H45O2 | 225.42 | 3-O-acetoxy tirucallic acid isomer |
| 415.2854 | C26H39O4 | |||||||
| 397.2770 | C26H37O3 | |||||||
| 355.2649 | C24H35O2 | |||||||
| 15 | 9.80 | 497.3636 | C32H49O4 | 1.0 | 437.3431 | C30H45O2 | 225.42 | 3-O-acetoxy tirucallic acid isomer |
| 415.2856 | C26H39O4 | |||||||
| 397.2798 | C26H37O3 | |||||||
| 355.2671 | C24H35O2 | |||||||
| 16 | 11.45 | 455.3527 | C30H47O3 | 0.4 | 437.3432 | C30H45O2 | 226.42 | 3-hydroxytirucallic acid isomer |
| 409.3477 | C29H45O | |||||||
| 377.3214 | C28H41 | |||||||
| 361.2947 | C27H37 | |||||||
| 237.1601 | C18H21 | |||||||
| 175.1109 | C12H15O | |||||||
Fig. 2.
LC-MS (TIC) chromatogram of gum resin extract (methanolic) of B. serrata.
3.1. Chromatographic method development
Two isoforms of tirucallic acids in B. serrata extract could be identified applying our TIC methodology. It is important to mention here that tirucallic acids present in boswellia have been reported to inhibit 5-lipoxygenase [24] and cyclooxygenase [25] enzymes, which may significantly contribute to the overall anti-inflammatory activities. Therefore, provision is warranted while preparing recommendation for standardization of B. serrata formulations.
In the present study, chromatographic separation was performed by using several reversed phase columns such as Acquity BEH C18 (100 mm × 2.1 mm, 1.7 μm), X Select Phenyl-Hexyl (100 mm × 2.1 mm, 1.9 μm) and Hypersil gold C18 (150 mm × 2.1 mm, 1.9 μm) to achieve a short run time, symmetric peak shapes and better chromatographic efficiency. This was investigated by appropriate changes in mobile phase composition (aqueous and organic phase) and flow rate. The standard boswellic acids were mid polar in nature and therefore suitable combination of aqueous and organic solvents was optimized with 0.1% formic acid and 0.1% NH4OH in both aqueous and organic phase. Mobile phase was operated at a flow rate of 0.4 mL/min. For better resolution between the polar and non-polar metabolites, gradient program was applied where gradual change in aqueous and organic solvents composition led to enhanced peak purity and resolution.
3.2. Chromatographic method validation
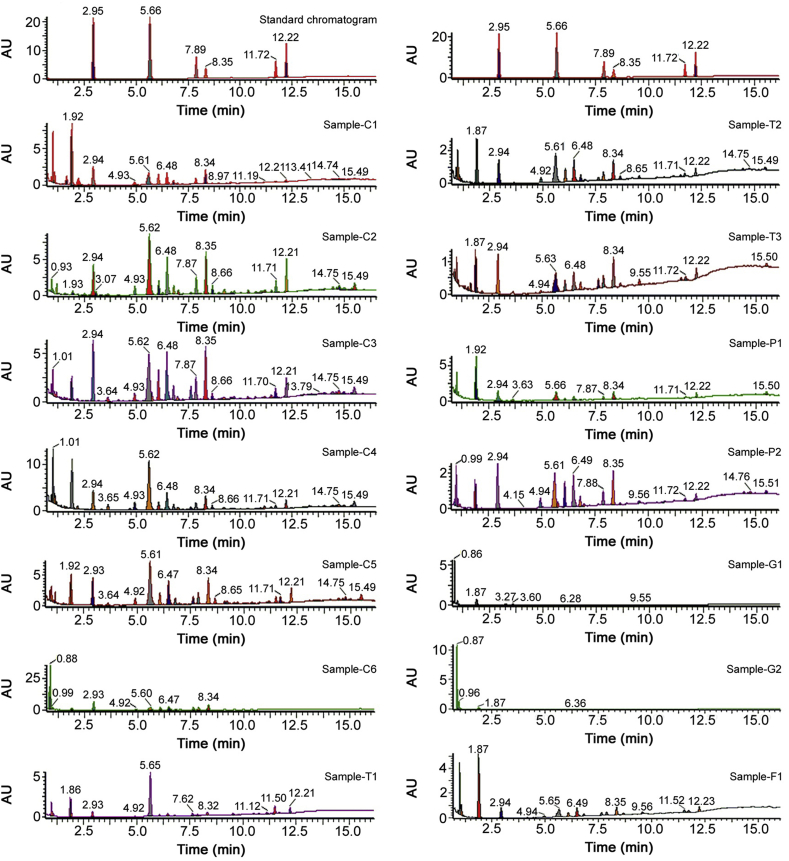
Initially, six reference analytes of boswellic acids (BA), namely, keto boswellic acid (1), 3-O-Acetyl-11-keto β-boswellic acid (2), ɑ-Boswellic acid (3), β-Boswellic acid (4), 3-O-Acetyl-ɑ-boswellic acid (5) and 3-O-Acetyl-β-boswellic acid (6), were quantified in crude resin using UPLC-PDA method and validated in terms of its linearity, LOD, LOQ, precision and recovery. Validation results are presented in Table 2, Table 3 and Table 1S. Linearity of six standard solutions at different concentrations (1, 5, 10, 50, 100 and 500 μg/mL) were analyzed. LOD and LOQ for standard analytes are given in Table 2. Precision data (Intra and inter day) in terms of % RSD and % recovery of analytes are shown in Table 3, Table 4, respectively. All the reference standards were found to be stable at room temperature for 72 h. Subsequently, applicability of method for quantification of marker compounds was demonstrated with fourteen commercial samples containing single or polyherbal formulations belonging to different classes viz tablets (#BS T1-T3), powder (#BS P1–P2), capsules (#BS C1–C6), semi-solid dosage [Gel (#BS G1-G2)] and tea fusion material (#BS F1), respectively. Selected formulations consisted of B. serrata as an important ingredient. PDA chromatogram of methanolic gum extract and commercial herbal formulations were recorded in the rage of 200–400 nm and quantitative analysis was performed at 210 nm. Reference standards (1–6) present in resin extracts and commercial formulations were identified by comparing retention time, peak purity and UV spectra of compounds in standard mixture. UPLC-PDA chromatograms at 210 nm of resin extract and commercial herbal formulations are shown in Fig. 3 and quantitative data for compounds (1–6) from different formulations are presented in Table 5.
Table 2.
Linearity, LOD and LOQ data for six boswellic acid standards.
| S No | Analyte | Retention time (min) | Linearity (μg/mL) | Regression equation | r2 | LOD (μg/mL) | LOQ (μg/mL) | %RSD |
|---|---|---|---|---|---|---|---|---|
| 1 | Keto boswellic acid | 2.95 | 1–500 | y=18138x – 17092 | 0.999 | 0.10 | 0.50 | 0.44 |
| 2 | 3-O-Acetyl 11-keto β-boswellic acid | 5.66 | 1–500 | y=20645x – 49509 | 0.999 | 0.10 | 0.50 | 1.26 |
| 3 | ɑ-Boswellic acid | 7.89 | 1–500 | y=6705x – 14231 | 0.999 | 0.50 | 1.00 | 3.72 |
| 4 | β-Boswellic acid | 8.35 | 1–500 | y=3251x – 6391 | 0.999 | 0.50 | 1.00 | 2.61 |
| 5 | 3-O-Acetyl-ɑ-boswellic acid | 11.72 | 1–500 | y=4942x – 13251 | 0.999 | 0.40 | 1.00 | 3.42 |
| 6 | 3-O-Acetyl-β-boswellic acid | 12.22 | 1–500 | y=9085x – 23136 | 0.999 | 0.40 | 1.00 | 1.28 |
Table 3.
Precision data (n=3) for analytes subjected to quantification (Mean conc. (μg/mL) ± %RSD).
| Analyte | Nominal conc. (μg/mL) | Intra-day | Inter-day |
|---|---|---|---|
| Keto boswellic acid | 1 | 0.91 ± 2.67 | 0.90 ± 3.72 |
| 50 | 51.22 ± 0.91 | 50.08 ± 2.70 | |
| 500 | 495.84 ± 2.37 | 487.14 ± 0.65 | |
| 3-O-Acetyl 11-keto β-boswellic acid | 1 | 0.89 ± 4.41 | 0.92 ± 3.79 |
| 50 | 49.82 ± 1.26 | 50.24 ± 1.33 | |
| 500 | 492.71 ± 0.97 | 495.82 ± 3.13 | |
| ɑ-Boswellic acid | 1 | 0.94 ± 3.72 | 0.90 ± 3.55 |
| 50 | 46.43 ± 1.15 | 45.09 ± 2.41 | |
| 500 | 492.36 ± 1.84 | 482.91 ± 2.55 | |
| β-Boswellic acid | 1 | 0.91 ± 4.42 | 0.95 ± 4.61 |
| 50 | 46.22 ± 1.19 | 47.26 ± 2.80 | |
| 500 | 488.61 ± 0.74 | 489.52 ± 1.56 | |
| 3-O-Acetyl-ɑ-boswellic acid | 1 | 0.90 ± 5.10 | 0.91 ± 3.54 |
| 50 | 48.52 ± 2.65 | 45.66 ± 1.57 | |
| 500 | 491.05 ± 0.95 | 493.19 ± 3.36 | |
| 3-O-Acetyl-β-boswellic acid | 1 | 0.91 ± 5.04 | 0.92 ± 4.29 |
| 50 | 46.25 ± 4.65 | 51.82 ± 3.23 | |
| 500 | 493.19 ± 1.49 | 483.07 ± 0.81 |
Table 4.
% Recovery data (n=3) for single herbal formulations (Mean ± %RSD).
| Sample code | KBA | AKBA | ɑ-BA | β-BA | 3-O-ɑ-ABA | 3-O-β-ABA |
|---|---|---|---|---|---|---|
| S1 | 94.23 ± 3.12 | 93.15 ± 0.87 | 93.85 ± 1.87 | 95.20 ± 0.81 | 94.58 ± 0.97 | 98.52 ± 2.70 |
| S2 | 98.52 ± 2.17 | 102.11 ± 1.75 | 96.45 ± 1.41 | 98.85 ± 1.58 | 102.82 ± 1.71 | 98.74 ± 0.85 |
| S3 | 99.81 ± 0.52 | 94.61 ± 2.85 | 96.58 ± 3.40 | 99.20 ± 1.85 | 101.63 ± 0.91 | 100.02 ± 0.75 |
| S4 | 102.06 ± 2.15 | 98.62 ± 2.32 | 96.52 ± 0.75 | 102.45 ± 0.19 | 98.17 ± 2.03 | 102.11 ± 1.76 |
| #BS C1 | 96.53 ± 3.60 | 101.21 ± 1.82 | 94.87 ± 3.64 | 95.94 ± 3.74 | 94.75 ± 2.61 | 99.23 ± 1.06 |
| #BS C2 | 93.52 ± 1.44 | 94.91 ± 2.99 | 97.52 ± 1.57 | 102.49 ± 0.36 | 98.63 ± 0.62 | 94.50 ± 4.01 |
| #BS C3 | 98.56 ± 3.01 | 99.86 ± 3.21 | 93.85 ± 0.82 | 93.11 ± 0.82 | 103.15 ± 3.78 | 95.00 ± 3.60 |
| #BS C5 | 94.66 ± 0.92 | 96.61 ± 2.59 | 98.30 ± 0.93 | 100.34 ± 3.67 | 93.12 ± 3.92 | 98.62 ± 1.45 |
| #BS T1 | 94.15 ± 1.61 | 95.73 ± 1.31 | 103.66 ± 2.41 | 95.83 ± 2.81 | 96.54 ± 2.73 | 101.85 ± 2.66 |
| #BS T2 | 101.28 ± 1.79 | 99.57 ± 2.52 | 94.52 ± 1.20 | 98.60 ± 1.68 | 95.68 ± 0.49 | 102.16 ± 0.42 |
| #BS P1 | 95.49 ± 2.56 | 98.28 ± 2.74 | 99.83 ± 1.88 | 95.27 ± 2.77 | 95.10 ± 1.82 | 98.66 ± 1.20 |
| #BS P2 | 96.45 ± 1.09 | 102.63 ± 3.11 | 101.05 ± 0.63 | 101.83 ± 0.46 | 92.91 ± 3.75 | 93.19 ± 2.84 |
S1–S4: Methanolic extracts, #BS C1–C5: Capsule formulations, #BS T1-T2: Tablet formulations, #BS P1–P2: Powder formulations.
Fig. 3.
UPLC-PDA chromatograms of herbal formulations samples containing B. serrata as active component or one of the active component in comparison with boswellic acids standard mixture (1–6).
Table 5.
Content % of boswellic acids (BA) (1–6) in various herbal formulations; Keto boswellic acid (1), 3-O-Acetyl 11-keto β-boswellic acid (2), ɑ-Boswellic acid (3), β-Boswellic acid (4), 3-O-Acetyl-ɑ-boswellic acid (5) and 3-O-Acetyl-β-boswellic acid (6).
| Sample code | Label claim (boswellia gum resin/extract) | Poly/Single | 1 | 2 | 3 | 4 | 5 | 6 | Total BA |
|---|---|---|---|---|---|---|---|---|---|
| S1 | – | – | 0.94 ± 0.02 | 0.26 ± 0.01 | 0.28 ± 0.01 | 1.22 ± 0.05 | 0.61 ± 0.02 | 0.25 ± 0.01 | 3.10 |
| S2 | – | – | 2.81 ± 0.01 | 0.77 ± 0.01 | 0.82 ± 0.01 | 3.10 ± 0.07 | 0.38 ± 0.01 | 0.61 ± 0.02 | 8.49 |
| S3 | – | – | 0.82 ± 0.01 | 0.24 ± 0.01 | 0.27 ± 0.01 | 1.36 ± 0.02 | 0.12 ± 0.01 | 0.20 ± 0.01 | 3.02 |
| S4 | – | – | 1.43 ± 0.02 | 0.47 ± 0.01 | 0.58 ± 0.01 | 2.64 ± 0.03 | 0.27 ± 0.02 | 0.42 ± 0.01 | 5.82 |
| #BS C1 | 250 mg (Capsule) | Single | 1.02 ± 0.02 | 3.21 ± 0.05 | 1.06 ± 0.03 | 4.25 ± 0.19 | 0.51 ± 0.01 | 0.73 ± 0.01 | 10.80 |
| #BS C2 | 500 mg (Capsule) | Single | 1.44 ± 0.01 | 1.69 ± 0.01 | 1.48 ± 0.03 | 7.16 ± 0.05 | 0.87 ± 0.03 | 0.84 ± 0.01 | 13.50 |
| #BS C3 | 500 mg (Capsule) | Single | 1.08 ± 0.01 | 2.78 ± 0.23 | 1.51 ± 0.05 | 7.59 ± 0.01 | 1.24 ± 0.02 | 1.86 ± 0.01 | 16.08 |
| #BS C4 | 300 mg (Capsule) | Poly | 0.66 ± 0.02 | 0.55 ± 0.01 | 0.52 ± 0.01 | 2.55 ± 0.08 | 0.14 ± 0.01 | 0.13 ± 0.01 | 4.58 |
| #BS C5 | 350 mg (Capsule) | Single | 1.07 ± 0.01 | 2.33 ± 0.03 | 1.27 ± 0.02 | 5.51 ± 0.08 | 0.84 ± 0.02 | 0.97 ± 0.01 | 12.01 |
| #BS C6 | 250 mg (Capsule) | Poly | 1.49 ± 0.01 | 0.81 ± 0.03 | 1.14 ± 0.09 | 5.06 ± 0.02 | 0.17 ± 0.04 | 0.22 ± 0.01 | 8.91 |
| #BS T1 | 75 mg (Tablet) | Single | 0.15 ± 0.01 | 1.27 ± 0.01 | 0.11 ± 0.02 | 0.54 ± 0.02 | 0.12 ± 0.01 | 0.28 ± 0.01 | 2.45 |
| #BS T2 | 125 mg (Tablet) | Single | 0.37 ± 0.01 | 0.58 ± 0.01 | 0.33 ± 0.01 | 1.63 ± 0.03 | 0.21 ± 0.01 | 0.19 ± 0.01 | 3.32 |
| #BS T3 | 240 mg (Tablet) | Poly | 0.30 ± 0.01 | 0.24 ± 0.01 | 0.22 ± 0.01 | 1.22 ± 0.01 | 0.10 ± 0.01 | 0.12 ± 0.01 | 2.19 |
| #BS P1 | -(Powder) | Single | 0.39 ± 0.01 | 0.41 ± 0.01 | 0.23 ± 0.01 | 1.66 ± 0.01 | 0.15 ± 0.01 | 0.24 ± 0.01 | 3.09 |
| #BS P2 | -(Powder) | Single | 0.62 ± 0.01 | 0.60 ± 0.01 | 0.51 ± 0.02 | 2.52 ± 0.02 | 0.18 ± 0.01 | 0.15 ± 0.01 | 4.59 |
| #BS G1 | 7.5 mg (Gel) | Poly | 0.14 ± 0.01 | ND | ND | ND | ND | ND | 0.14 |
| #BS G2 | 100 mg (Gel) | Poly | ND | ND | ND | ND | ND | ND | ND |
| #BS F1 | Tea Fusion | Poly | 0.20 ± 0.01 | 0.23 ± 0.01 | 0.17 ± 0.01 | 0.83 ± 0.01 | 0.13 ± 0.01 | 0.16 ± 0.01 | 1.72 |
ND: Not Detected; S1–S4: Methanolic extracts, #BS C1–C6: Capsule formulations, #BS T1-T3: Tablet formulations, #BS P1–P2: Powder formulations,. #BS G1-G2: Gel formulations and #BS F1: Tea fusion material.
As per the Indian and European pharmacopeias [2,4], the herbal preparations containing B. serrata must have minimum of 1.0% 11-keto-β-boswellic acid (1) and 1.0% acetyl-11-keto-β-boswellic acid (2) (w/w, dried drug) as active principles. From our study, it was found that authentic gum resin extract showed all reference standards in required quantity. However, analysis of commercial formulations indicated presence of markers (1–6) in the range of 0.14%–16.08% of dried material. Content of total boswellic acids (1–6) was found to be maximum of 16.08% in #BS C3 sample. It is important to mention that essential markers, KBA (1) and AKBA (2), were found to be in minimum of 0.14% and 0.23% and maximum of 1.49% & 3.21%, respectively. Commercial sample (#BS G1) showed only one reference standard, KBA (1).
Overall, our analysis of commercial samples containing Boswellia serrata showed that only four samples (#BS C1–C3, C5) could meet pharmacopeia guidelines (Fig. 1 containing compounds 1 and 2 with specified limits), hence warranting proper standardization of herbal formulations according to pharmacopeia guidelines for optimum presence of bioactive principles.
3.3. Q-Tof-MS/MS fragmentation study
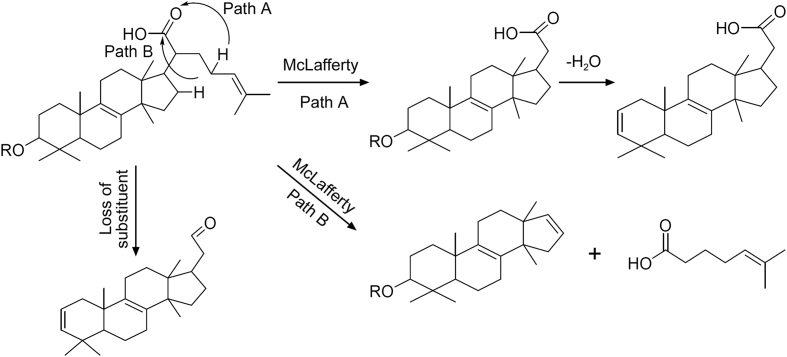
Boswellic acids follow retro Diels-Alder reaction (RDA) at C-ring either on/or before loss of small molecules. Another class of triterpeniods called tirucallic acid and its derivatives following McLafferty rearrangement (MFR) at C-21 acid followed by breakage of bond between C-20 and C-17 as shown in Fig. 4. Based on these key fragments, compounds were identified and depicted in Table 1. Key fragmentation pattern and mechanism are shown in Fig. 4, Fig. 5, Fig. 6 and Figs. S1–S16. Although CID-MS/MS cannot discriminate between stereoisomers, CID-MS/MS coupling with UPLC resolves stereoisomer’s separation and alleged identification. This hyphenation helped us in separating 3-hydroxy tirucallic acid isomers (9, 10, 12 & 16) with the same key fragmentation (m/z 373 & m/z 83) formed through McLafferty rearrangement (MFR) at C-21. The compounds, ɑ-Boswellic acid (3) and β-Boswellic acid (4), were clearly separated in TIC with key fragment ion (m/z 237 and m/z 218) through RDA reaction and also matched with standard compounds along with other isoforms of boswellic acids shown in Table 1.
Fig. 4.
Proposed common fragmentation of tirucallic acid type molecules.
Fig. 5.
Proposed common fragmentation of boswellic acid (peak 1) type molecules.
Fig. 6.
Proposed common fragmentation of boswellic acid (peak 8, new) type molecules.
In detail, peaks at retention time (Rt) 5.93, 6.49, 7.27 and 11.45 min with end absorption m/z 455 [M − H]- in ESI negative mode and m/z 437.3432 [M-H-H2O]- along with m/z 373 and m/z 83 formed through McLafferty rearrangement (MFR) were identified as 3-hydroxy-8,9,24,25-tetradehydrotirucallic acid [C30H48O3] isomers (9, 10, 12 and 16). On the other hand, peaks at Rt 9.21 and 9.80 min with end absorption m/z 497.3635 [M − H]- and 437 [M-AcOH-H]- along with m/z 416 and m/z 83 by McLafferty rearrangement (MFR) in ESI negative mode were identified as 3-O-acetoxy tirucallic acid isomer(14 and 15). Peak at Rt 6.95 min with end adsorption m/z 453.3361[M − H]- and m/z 425[M-H-CO]- along with m/z 310 and m/z 83 formed though MFR was characterized as 3-oxo-tirucallic acid (11). Similarly, boswellic acids (1–6) were also identified at Rt 3.20 (1), 6.13 (2), 8.41 (3), 8.91 (4), 12.30 (5) and 12.79 (6) min based on their fragmentation patterns along with RDA and also clearly matched their reference standards. In addition, peak at Rt 4.60 with molecular ion 471[M − H]- and 409 [M-H-CH2O3]- along with fragment ion at m/z 233 formed through RDA as shown in Fig. 6, clearly supports the position of hydroxyl at C-11 and was identified as 11-hydroxy boswellic acid (8) as a new compound.
4. Conclusion
A simple and efficient UPLC-PDA method was developed for quantification of six boswellic acids in B. serrata gum resin extract and various commercial herbal formulations containing B. serrata as one of the ingredients. The method was validated in terms of LOD, LOQ, precision and recovery. From quantitative data, it was observed that there was a wide variation in content of boswellic acids from the commercial formulations. The LC-Q-Tof-MS/MS technique was applied for the characterization of metabolites including boswellic acids and tirucallic acids in the B. serrata extract. The MS/MS fragmentation of boswellic acids and tirucallic acids would be a valuable tool for quality control assessment of commercially available formulations containing B. serrata.
Acknowledgments
The authors thank Dr. S. Chandrasekhar, Director, CSIR-IICT, for the financial grant under MLP-0030. Bandi Siva thank CSIR for financial support (IICT Communication No. IICT/Pubs/2018/183gs5).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2019.09.007.
Contributor Information
Ashok Kumar Tiwari, Email: tiwari@csiriict.in.
Katragadda Suresh Babu, Email: suresh@iict.res.in.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wichtl M M., Brinckmann J.A., Lindenmaier M.P. Herbal Drugs and Phytopharmaceuticals. A Handbook for Practice on a Scientific Basis. Medpharm Scientific Publishers; Germany: 2004. Olibanum; pp. 418–420. [Google Scholar]
- 2.Indian Pharmacoepia; Kunduru . Indian Pharmacopoeia Commission; 2010. Herbs and Herbal Products, Herbal Monographs; p. p33. [Google Scholar]
- 3.Ammon H.P.T. Boswellic acids and their role in chronic inflammatory diseases. Adv. Exp. Med. Biol. 2016;928:291–327. doi: 10.1007/978-3-319-41334-1_13. [DOI] [PubMed] [Google Scholar]
- 4.European Pharmacopoeia . seventh ed. European Directorate for the Quality of Medicines (EDQM); Strasburg, France: 2010. (PhEur 7.0) pp. 1152–1153. [Google Scholar]
- 5.Engels G. Frankincense, herbal gram. J. Am. Bot. Coun. 2010;88:1–4. http://cms.herbalgram.org/herbalgram/issue88/HG88herbprofile.html [Google Scholar]
- 6.Ranjbarnejad T., Saidijam M., Moradkhani S. Methanolic extract of Boswellia serrata exhibits anti-cancer activities by targeting microsomal prostaglandin E synthase-1 in human colon cancer cells, Prostaglandins Other Lipid. Mediations. 2017;131:1–8. doi: 10.1016/j.prostaglandins.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed H.H., El-Alfy N.Z., Mahmoud M.F. Boswellia serrata oleo-gum resin: a natural remedy for retrogradation of liver fibrosis in rats. Der.Pharmacia. Lettre. 2015;7:134–144. [Google Scholar]
- 8.Gaurea S.H., Bapat U.C. Study of antibacterial activity of resins of Boswellia serrata roxb ex colebr., Commiphora mukul (hooks ex- stocks) engl., Gardenia resinifera roth. And Shorea robusta gaertn. Int. J. Pharm. Pharm. Sci. 2016;8:29–31. [Google Scholar]
- 9.Lalithakumari K., Krishnaraju A.V., Sengupta K. Safety and toxicological evaluation of a novel, standardized 3-O-Acetyl-11-keto-beta-Boswellic Acid (AKBA)-enriched Boswellia serrata extract (5-Loxin(R)) Toxicol. Mech. Methods. 2006;16:199–226. doi: 10.1080/15376520600620232. [DOI] [PubMed] [Google Scholar]
- 10.The Ayurvedic Pharmacoepia of India . first ed. vol. IV. Department of Ayush, Government of India; New Delhi: 1997. (Ministry of Family and Welfare). [Google Scholar]
- 11.Badmanaban R., Vala S.G., Patel C.N. Validated HPTLC method for the simultaneous estimation of 11-Keto-b-Boswellic acid, 3-O-Acetyl-11-Keto-β-Boswellic acid and glucosamine in combined tablet dosage form. Inventi Impact Pharm Anal. Qual. Assur. 2013;3:175–179. [Google Scholar]
- 12.Rehman N.U., Ali L., Al-Harrasi A. Quantification of AKBA in Boswellia sacrausing NIRS coupled with PLSR as an alternative method and cross-validation by HPLC. Phytochem. Anal. 2018;29:137–143. doi: 10.1002/pca.2721. [DOI] [PubMed] [Google Scholar]
- 13.Al-Harrasi A., Rehman N.U., Mabood F. Application of NIRS coupled with PLS regression as a rapid, non-destructive alternative method for quantification of KBA in Boswellia sacra. Spectrochim. Acta. A Mol. Biomol. Spec. 2017;184:277–285. doi: 10.1016/j.saa.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Mannino G., Occhipinti A., Maffei M.E. Quantitative determination of 3-O-Acetyl-11-Keto-β-Boswellic Acid (AKBA) and other boswellic acids in Boswellia sacra Flueck (syn. B. carteri Birdw) and Boswellia serrata Roxb. Molecules. 2016;21:1–8. doi: 10.3390/molecules21101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C., Sun L., Tian R.T. Combination of quantitative analysis and chemometric analysis for the quality evaluation of three different frankincenses by ultra-high-performance liquid chromatography and quadrupole time of flight mass spectrometry. J. Sep. Sci. 2015;38:3324–3330. doi: 10.1002/jssc.201500326. [DOI] [PubMed] [Google Scholar]
- 16.Ivanova S.A., Pozharitskaya O.A., Karlina M.V. Application of HPLC in pharmacokinetic studies of triterpenoids of Boswellia serrata Roxb. extract. Voprosy Biol. Meditsinskoi Farmatsevticheskoi Khimii. 2008;6:59–63. [Google Scholar]
- 17.Frank A., Unger M. Analysis of frankincense from various Boswellia species with inhibitory activity on human drug metabolizing cytochrome P450 enzymes using liquid chromatography mass spectrometry after automated on-line extraction. J. Chromatogr., A. 2006;1112:255–262. doi: 10.1016/j.chroma.2005.11.116. [DOI] [PubMed] [Google Scholar]
- 18.Wang H., Zhang C., Wu Y. Comparative pharmacokinetic study of two boswellic acids in normal and arthritic rat plasma after oral administration of Boswellia serrata extract or Huo Luo Xiao Ling Dan by LC-MS. Biomed. Chromatogr. 2014;28:1402–1408. doi: 10.1002/bmc.3182. [DOI] [PubMed] [Google Scholar]
- 19.Kumar K., Ramesh B., Rao V.P.R. Validated high performance thin layer chromatographic method for the determination of Dibenzyl Cyclooctadiene lignans from Schisandra grandiflora. J. Planar Chromatogr. 2014;27:460–465. [Google Scholar]
- 20.Rao V.P.R., Kumar K., Sarma V.U.M. Estimation of costunolide and dehydrocostus lactone in Saussurea lappa and its polyherbal formulations followed by their stability studies using HPLC-DAD. Pharmacogn. Mag. 2015;11:180–190. doi: 10.4103/0973-1296.149736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Conference on Harmonization Validation of analytical procedures: text and methodology Q2(R1), Published November 2005. Available at https://www.ich.org/-fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1_Guideline.pdf.
- 22.Kumar K., Siva B., Rama Rao N. Rapid identification of limonoids from Cipadessa baccifera and Xylocarpus granatum using ESI-Q-Tof-MS/MS and their structure-fragmentation study. J. Pharm. Biomed. Anal. 2018;152:224–233. doi: 10.1016/j.jpba.2017.12.050. [DOI] [PubMed] [Google Scholar]
- 23.Kumar K., Siva B., Sarma V.U.M. UPLC-MS/MS quantitative analysis and structural fragmentation study of five Parmotrema lichens from the Eastern Ghats. J. Pharm. Biomed. Anal. 2018;156:45–57. doi: 10.1016/j.jpba.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Boden S.E., Schweizer S., Bertsche T. Stimulation of leukotriene synthesis in intact polymorphonuclear cells by the 5-lipoxygenase inhibitor 3-oxo-tirucallic acid. Mol. Pharmacol. 2001;60:267–273. doi: 10.1124/mol.60.2.267. [DOI] [PubMed] [Google Scholar]
- 25.Ali S.I., Zhang C.R., Mohamed A.A. Major constituents of Boswellia carteri resin exhibit cyclooxygenase enzyme inhibition and antiproliferative activity. Nat. Prod. Commun. 2013;8:1365–1366. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.