Abstract
As a traditional Chinese medicine, the root of Astragalus membranaceus var. mongholicus (AMM) or A. membranaceus (AM) has been widely used in China and other Asian countries for thousands of years. Till now, the flavonoids, phenolic acids and saponins are considered as the main active components contributing to their therapeutic effect in these plants. In order to clarify the distribution and contents of these compounds in different organs of these plants, a rapid and sensitive analytical method for simultaneous determination of 25 active compounds including seven types (i.e. dihydroflavones, isoflavane, isoflavones, flavones, pterocarpans, phenolic acid and saponins) within 10 min was established using ultra-pressure liquid chromatography coupled with tandem mass spectrometry (UPLC–MS/MS). Then, the established method was fully validated and successfully applied to the determination of the contents of these analytes in different parts (root, rhizome, stem, leaf and flower) of AMM and AM. The results indicated that the contents of the same type of compounds in two different species plants were significantly different. Moreover, the obvious differences were also found for the distribution and contents of different type of compounds in five organs of the same species. The present study could provide necessary information for the rational development and utilization of AMM and AM resource.
Keywords: Astragalus membranaceus var. mongholicus, Astragalus membranaceus, UPLC–MS/MS, Flavonoid, Saponin
Highlights
-
•
A sensitive UPLC–MS/MS method was established for analysis of Astragalus plants.
-
•
Total 25 analytes such as flavonoids, phenolic acids and saponins were determined.
-
•
The contents of these analytes in different parts of the two plants were compared.
-
•
A scientific information for the utilization of two Astragalus plants was provided.
1. Introduction
Astragali Radix (AR, Huangqi in Chinese), the root of Astragalus membranaceus var. mongholicus (Bge.) Hsiao (AMM) or A. membranaceus (Fisch.) Bge. (AM), has been widely used as crude drug and medicinal material in Northeast, North, and Northwest of China as well as in Mongolia and Korea [1]. As a valuable medicinal herb, AR has been widely used as a tonic and diuretic for thousands of years in China [2]. Recent studies reveal that AR exhibits wide biochemical and pharmacological activities, such as immunomodulating [3,4], antihyperglycemic [5], anti-inflammatory [6], antioxidant [7,8] and antiviral activities. At present, AR is mainly used in the treatment of nephritis, diabetes and cancer, and the saponins, flavonoids and polysaccharides contained in the herb are considered as the main components contributing to its clinical therapeutic effect [1,9]. Thus, these compounds have been tentatively assigned as the chemical markers for the quality control of AR, and a variety of analytical methods have been developed for characterizing the flavonoids and saponins of AR [[10], [11], [12], [13], [14], [15], [16]]. Among them, high performance liquid chromatography (HPLC) is more frequently used. However, the long analysis time and lower sensitivity, especially for the HPLC-ELSD for determination of the saponins [10,11], prevent the method from being used for the simultaneous determination of multiple compounds especially some trace constituents in a short time.
The successful application of ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) in the qualitative and quantitative determination of multiple components in complex samples, especially in medical herbs, provides a suitable way to determine different types of compounds in a single run [[17], [18], [19]]. Recently, there are reports that UPLC-MS/MS method was used for the determination of the active compounds in AMM and AM [20,21]. However, only isoflavonoids and saponins were analyzed in these methods, and the other types of flavonoids, such as flavones, dihydroflavones and pterocarpans which exist in the herbs with the significant activities, were little involved. It is well known that the curative effect of traditional Chinese medicine is considered as the synergic activities of their multiple bioactive compounds [22,23]. Thus, it is necessary to establish an analytical method that is rapid, sensitive, and capable of simultaneously measuring various pharmacologically active components in these Aatragalus species, which could be used for the quality control of the herbal drug.
In light of this, an accurate, fast and highly sensitive UPLC-MS/MS method was established for the first time and applied to the simultaneous detection of 25 compounds including eleven flavones, two dihydroflavones, five isoflavones, two pterocarpans, one phenolic acid and four saponins in different organs of AMM and AM. The proposed method would be helpful in further enriching the quality control of AR, and the results of this study would provide more information about the types and contents of active compounds in different organs of these two Aatragalus species, which is also helpful for the efficient utilization of the disused organs of these plants.
2. Experimental
2.1. Chemicals, reagents and materials
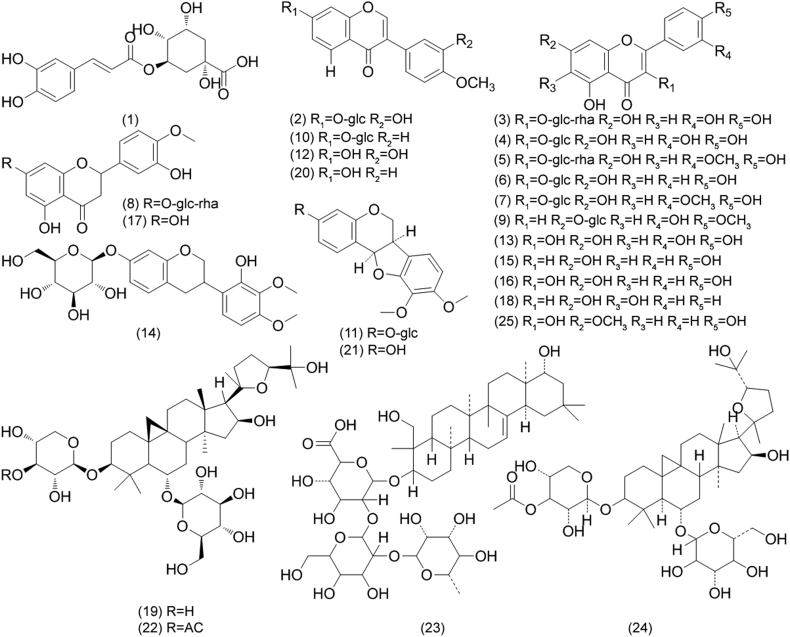
Reference chemicals of chlorogenic acid (99.28%), calycosin-7-O-β-D-glucoside (99.17%), rutin (98.81%), hyperoside (99.40%), isorhamnetin-3-O-neohespeidoside (99.43%), astragalin (99.55%), isorhamnetin-3-O-β-D-glucoside (99.39%), hesperidin (99.38%), diosmetin-7-O-β-d-glucopyranoside (98.66%), ononin (99.40%), (−)-methylinissolin-3-O-β-D-glucoside (99.31%), calycosin (99.52%), quercetin (98.78%), isomucronulatol-7-O-β-D-glucoside (99.62%), apigenin (99.50%), kaempferol (98.88%), hesperetin (99.71%), baicalein (99.64%), astragaloside IV (99.10%), formononetin (99.40%), (−)-methylnissolin (99.40%), astragaloside II (99.12%), soyasaponin I (98.72%), isoastragaloside II (98.90%) and rhamnocitrin (99.37%) were purchased from Nanjing Long Wave Biological Science and Technology Co., Ltd. (Nanjing, China). Their structures are presented in Fig. 1.
Fig. 1.
Chemical structures of the compounds analyzed in the plants of Astragalus membranaceus var. mongholicus and A. membranaceus. chlorogenic acid (1), calycosin-7-O-β-D-glucoside (2), rutin (3), hyperoside (4), isorhamnetin-3-O-neohespeidoside (5), astragalin (6), isorhamnetin-3-O-β-D-glucoside (7), hesperidin (8), diosmetin-7-O-β-d-glucopyranoside (9), ononin (10), (−)-methylinissolin-3-O-β-D-glucoside (11), calycosin (12), quercetin (13), isomucronulatol-7-O-β-D-glucoside (14), apigenin (15), kaempferol (16), hesperetin (17), baicalein (18), astragaloside IV (19), formononetin (20), (−)-methylnissolin (21), astragaloside II (22), soyasaponin I (23), isoastragaloside II (24) and rhamnocitrin (25).
Acetonitrile and methanol were HPLC-grade from Merck (Darmstadt, Germany). Ultra-pure water was prepared by a Millipore Direct Q5 purification system (Millipore, Bedford, MA, USA). All other used chemicals were of analytical grade, and obtained from TCI Chemical Reagent Co., Ltd. (Shanghai, China).
Three AMM and AM plants were collected from Dingxi County (Gansu, China) in August 2018, and originally identified by Prof. Jin-Ao Duan (Nanjing University of Chinese Medicine). After collection, the whole plant was divided into root, rhizome, stem, leaf and flower, and then dried at 40 °C.
2.2. Standard solutions preparation
Mixed standard stock solution containing the reference compounds of chlorogenic acid (1), calycosin-7-O-β-D-glucoside (2), rutin (3), hyperoside (4), isorhamnetin-3-O-neohespeidoside (5), astragalin (6), isorhamnetin-3-O-β-D-glucoside (7), hesperidin (8), diosmetin-7-O-β-d-glucopyranoside (9), ononin (10), (−)-methylinissolin-3-O-β-D-glucoside (11), calycosin (12), quercetin (13), isomucronulatol-7-O-β-D-glucoside (14), apigenin (15), kaempferol (16), hesperetin (17), baicalein (18), astragaloside IV (19), formononetin (20), (−)-methylnissolin (21), astragaloside II (22), soyasaponin I (23), isoastragaloside II (24) and rhamnocitrin (25) was prepared in methanol. The working standard solutions were obtained by diluting the standard solution with methanol to form a series of appropriate concentrations within the ranges: (1) 14.77–1.513 × 104 ng/mL, (2) 21.97–2.250 × 104 ng/mL, (3) 13.42–1.375 × 104 ng/mL, (4) 12.93–1.325 × 104 ng/mL, (5) 12.57–1.288 × 104 ng/mL, (6) 14.52–1.488 × 104 ng/mL, (7) 9.003–9.250 × 103 ng/mL, (8) 15.50–1.588 × 104 ng/mL, (9) 11.84–1.213 × 103 ng/mL, (10) 13.79–1.413 × 104 ng/mL, (11) 11.96–1.013 × 104 ng/mL, (12) 33.81–3.463 × 104 ng/mL, (13) 13.54–1.388 × 104 ng/mL, (14) 9.887–1.013 × 104 ng/mL, (15) 15.01–1.538 × 104 ng/mL, (16) 11.59–1.188 × 104 ng/mL, (17) 16.11–1.650 × 104 ng/mL, (18) 12.08–1.238 × 104 ng/mL, (19) 15.38–1.575 × 104 ng/mL, (20) 15.01–1.538 × 104 ng/mL, (21) 14.03–1.438 × 104 ng/mL, (22) 13.54–1.388 × 104 ng/mL, (23) 15.99–1.638 × 104 ng/mL, (24) 15.38–1.575 × 104 ng/mL, (25) 11.35–1.163 × 104 ng/mL. All these solutions were stored at 4 °C until use, and filtered through a 0.22 μm membrane before injection.
2.3. Preparation of sample solutions
The samples were pulverized into homogeneous powders (40 mesh) and stored at room temperature prior to analysis. An PMB-53 automatic moisture meter (ADAM equipment Co., Ltd., Wuhan, China) was used to determine the moisture content of the samples. The dried powder (1.0 g) of sample was accurately weighted into a 100 mL conical flask with stopper, and 50 mL of 75% methanol was added. Subsequently, ultrasonication (40 kHz) was performed at room temperature for 60 min, and then the same concentration of solvent was added to compensate for the lost weight during the extraction. The solution was filtered through 0.22 μm membrane and 1 μL of aliquot of the filtrates was injected into the UPLC system for analysis. Three replicates were performed on the independent samples.
2.4. Chromatographic conditions and instrumentation
The separation was performed on a Waters Acquity UPLC system (Waters, Corp., Milford, MA, USA), and an Acquity UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm) was applied for all analyses. Formic acid (0.1%) (A) in water and acetonitrile (B) were used as mobile phases with the flow rate of 0.4 mL/min under a gradient program: 10%–15% (B) initial to 2 min, 15%–40% (B) from 2 to 8 min, and 40%–90% (B) from 8 to 10 min. The column temperature was maintained at 30 °C, and the equilibration of 1 min was applied between individual runs.
An AB SCIEX Triple Quad 6500 plus (AB SCIEX, USA) equipped with an electrospray ionization source (ESI) was used for the detection of the MS signals of the analytes. ESI-MS was used in both positive and negative ion multiple reaction monitoring (MRM) modes with the following parameters: the capillary voltage operated at 5 kV, the flow rate of cone gas set at 50 L/h and the source temperature at 150 °C, the flow rate and the temperature of desolvation gas set as 1000 L/h and 550 °C. The cone voltage (CV) and collision energy (CE) were set to match the MRM of each marker. Data were acquired with MultiQuant software. Table 1 shows the summary of MS/MS detection parameters.
Table 1.
Retention time and related MS information of the 25 analytes detected on the UPLC–MS/MS.
| Analytes | RTa (min) | Ion mode | Precursor ion (m/z) | Product ionsb (m/z) | DP (V) | CE (V) | CXP (eV) |
|---|---|---|---|---|---|---|---|
| 1 chlorogenic acid | 2.17 | ESI− | 352.933 [M−H]− | 190.8, 84.9 | −55 | −26 | −11 |
| 2 calycosin-7-O-β-D-glucoside | 3.84 | ESI+ | 447.019 [M+H]+ | 285.0, 269.9 | 231 | 23 | 26 |
| 3 rutin | 3.89 | ESI− | 608.990 [M−H]− | 300.3, 270.9 | −175 | −46 | −31 |
| 4 hyperoside | 4.01 | ESI− | 462.990 [M−H]− | 299.7, 270.8 | −195 | −36 | −23 |
| 5 isorhamnetin-3-O-neohespeidoside | 4.11 | ESI+ | 625.013 [M+H]+ | 478.9, 316.9 | 26 | 31 | 18 |
| 6 astragalin | 4.64 | ESI− | 447.109 [M−H]− | 283.8, 254.9 | −120 | −32 | −10 |
| 7 isorhamnetin-3-O-β-D-glucoside | 4.74 | ESI+ | 478.965 [M+H]+ | 316.8, 301.9 | 71 | 19 | 18 |
| 8 hesperidin | 4.96 | ESI+ | 611.042 [M+H]+ | 302.9, 153.0 | 136 | 31 | 24 |
| 9 diosmetin-7-O-β-d-glucopyranoside | 5.12 | ESI+ | 462.911 [M+H]+ | 300.9, 285.9 | 136 | 27 | 34 |
| 10 ononin | 5.71 | ESI+ | 432.060 [M+H]+ | 269.6, 268.8 | 276 | 21 | 18 |
| 11 (−)-methylinissolin-3-O-β-D-glucoside | 6.18 | ESI− | 461.098 [M−H]− | 299.1, 268.8 | −35 | −18 | −21 |
| 12 calycosin | 6.38 | ESI+ | 284.993 [M+H]+ | 225.0, 212.9 | 156 | 47 | 24 |
| 13 quercetin | 6.42 | ESI− | 300.859 [M−H]− | 178.9, 150.8, 120.9 | −90 | −28 | −17 |
| 14 isomucronulatol-7-O-β-D-glucoside | 6.44 | ESI− | 463.013 [M−H]− | 301.0, 120.9 | −135 | −24 | −19 |
| 15 apigenin | 7.31 | ESI+ | 271.023 [M+H]+ | 153.1, 119.1 | 171 | 49 | 14 |
| 16 kaempferol | 7.56 | ESI− | 284.798 [M−H]− | 184.6, 92.7 | −165 | −36 | −5 |
| 17 hesperetin | 7.7 | ESI+ | 303.019 [M+H]+ | 177.0, 153.0 | 171 | 27 | 20 |
| 18 baicalein | 7.99 | ESI+ | 271.018 [M+H]+ | 123.0, 68.9 | 216 | 43 | 14 |
| 19 astragaloside IV | 8.28 | ESI+ | 768.294 [M+H]+ | 446.2, 195.0 | 191 | 105 | 24 |
| 20 formononetin | 8.53 | ESI+ | 269.969 [M+H]+ | 238.0, 198.2 | 11 | 39 | 28 |
| 21 (−)-methylnissolin | 8.81 | ESI+ | 302.019 [M+H]+ | 168.1, 167.1 | 136 | 21 | 20 |
| 22 astragaloside II | 8.98 | ESI+ | 828.181 [M+H]+ | 315.3, 175.0 | 141 | 21 | 16 |
| 23 soyasaponin I | 9.11 | ESI− | 942.089 [M−H]− | 204.9, 59.0 | −15 | −62 | −21 |
| 24 isoastragaloside II | 9.17 | ESI+ | 828.255 [M+H]+ | 807.3, 438.3, 143.2 | 186 | 10 | 25 |
| 25 rhamnocitrin | 9.47 | ESI+ | 300.974 [M+H]+ | 258.0, 107.1 | 201 | 41 | 30 |
Retention times.
Most abundant product ion, with the quantification ion being bolded.
2.5. Method validation
2.5.1. Calibration curves, limits of detection (LOD) and quantification (LOQ)
A calibration curve was used to determine the calculated concentration of the samples. In order to estimate the LOD and LOQ, the blank solution (75% methanol) was injected six times into the UPLC system for analysis. LOD and LOQ can be calculated using the following equation as per ICH guidelines: LOD = 3.3 (N/S) and LOQ = 10 (N/S), where N is the standard deviation of the blank solvent response and S is the slope of the corresponding calibration curve.
2.5.2. Precision, repeatability and accuracy
For the precision, repeatability and accuracy tests, the QC sample which was prepared by mixing the different parts of AMM in the same amount was used and the solutions were prepared as the procedure of Section 2.3. In order to determine the precision of the method, the intra-day and inter-day variations were selected, which were investigated by determining the QC sample in 6 replicates during a single day and duplicating the experiment on 3 consecutive days. The variations of the peak areas were chosen to determine the precision of the method, expressed as the percentage relative standard deviations (RSD). The repeatability was determined by measuring six replicates of QC samples and variations were expressed by RSD. The stability of the sample was evaluated by comparing the average concentration of the sample solutions stored at room temperature for 0, 2, 4, 8, 12, and 24 h with those of the freshly prepared calibration. The accuracy of the method was evaluated using a recovery test which was performed by adding known amounts of the 25 standards at low (80% of the known amounts), medium (the same as the known amounts) and high (120% of the known amounts) levels into a certain amount (0.5 g) of QC sample separately. Then, the spiked samples were extracted and analyzed according the methods mentioned above, and the formula: recovery (%)=(observed amount−original amount)/spiked amount × 100%, was used to calculate the average recovery percentage.
2.6. Identification and quantification
Target peaks were identified by comparing the UPLC retention time and mass/charge ratios (m/z) with their standards. The linear calibration plots of peak areas and concentration were used for quantitative analysis.
3. Results and discussion
3.1. Optimization of the extraction conditions
In order to obtain the optimal extraction conditions, the extraction methods, extraction solvents and extraction time were investigated. Since it is reported that the extraction efficiency of flavonoids, phenolic acids and saponins from natural plants with methanol is superior to that of ethanol [11,14,16], different concentrations of methanol were compared, and the contents of the 25 compounds were used as indicators for evaluating the extraction efficiency. The results showed that 75% aqueous methanol was the optimum extraction solution. The ultrasonic extraction and reflux extraction were compared, and the result showed that the extraction efficiency had no significant difference between the two extraction methods, but ultrasonication was easy to operate. In addition, the ultrasonic extraction time (30, 60 and 90 min) was investigated and 60 min was found to be adequate and appropriate for the analysis. According to the previous studies and our results, ultrasonic extraction with 75% aqueous methanol for 60 min was finally determined as the optimal extraction conditions.
3.2. Optimization of the chromatography and mass spectrometry system
In order to obtain the optimum conditions for mass spectrometry, all analytes were detected separately in direct infusion mode in full positive and negative ionization modes using full-scan MS method. For the isoflavones (compounds 2, 10, 12 and 20), dihydroflavones (compounds 8 and 17) and most of the saponins (compounds 19, 22 and 24), a stronger response in the positive ion mode than in the negative was found. For the flavones, those compounds containing methoxyl groups, such as 5, 7, 9 and 25, showed a relative higher response in positive ion mode than those in negative. Nevertheless, compared to the positive ion mode, some compounds such as chlorogenic acid, rutin, hyperoside, and astragalin obtained a higher response from the negative ion mode, which made them accurate and easy to detect with lower content levels in the Astragalus plants and identify each peak by confirming the molecular ions or quasi-molecular ions. The chemical structures of 25 components were characterized based on their retention behavior and mass spectrometry information such as quasi-molecular ions and fragment ions.
To obtain better sensitivity, the most abundant product ions were chosen as the quantitative ions. At the same time, another product ion with typical characteristics was selected to further identify the analyzed compounds. For example, flavonoid glycosides (such as compounds 2–9, 10, 11 and 14) could cause a glycosylic loss. For the flavonoids analyzed, such as compounds 12, 13, 15–18, 20, 21 and 25, some common features such as CO and CO2 losses were observed. As for the saponins (compounds 19, 22–24), the fragments of losing 2 to 3 molecular glycosyl groups were observed. The 25 compounds in AMM and AM were identified and quantified under optimized UPLC and MS/MS conditions. Table 1 shows the MS information for each analyte, including quasi-molecular ions, product ions, quantitative ions, CV and CE.
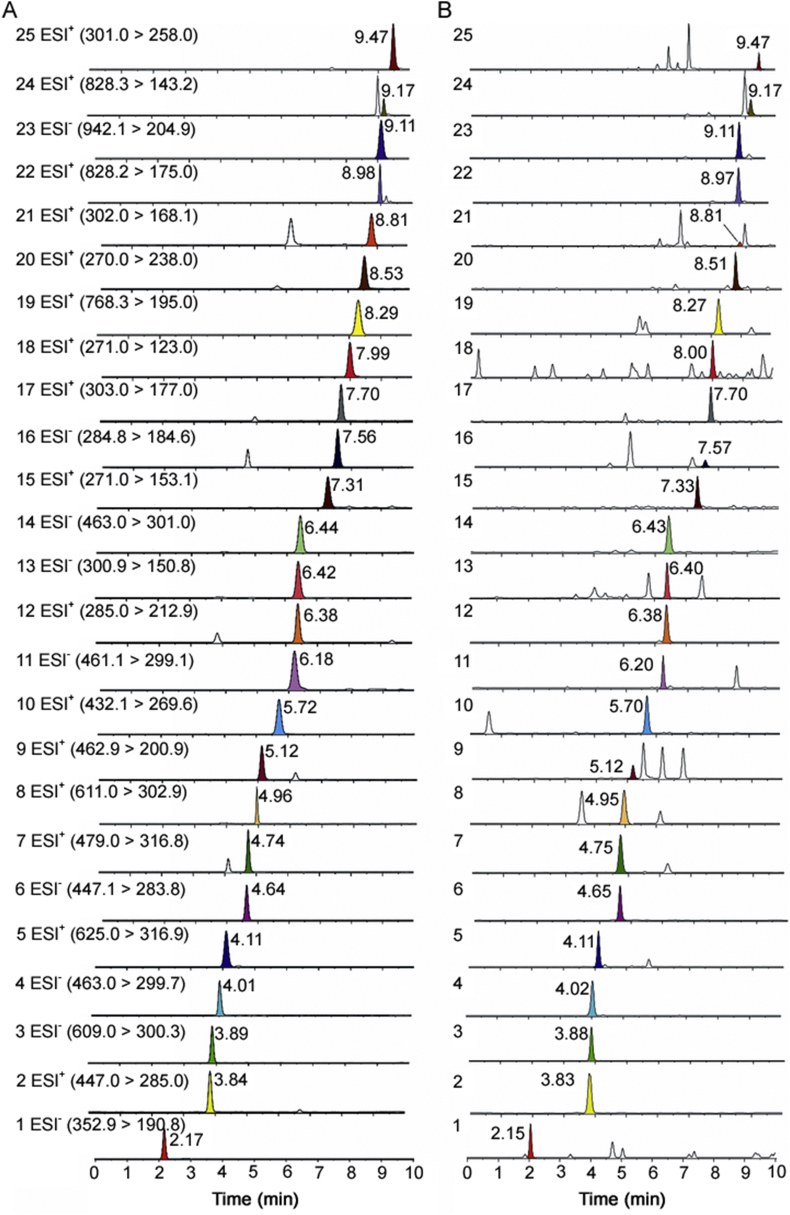
In order to achieve optimal separation in a short analysis time, the UPLC conditions such as chromatographic column, mobile phase and gradient program were preliminarily optimized. By comparing the analytical columns of Acquity HSS T3 (100 mm × 2.1 mm, 1.8 μm) and Acquity BEH C18 (100 mm × 2.1 mm, 1.7 μm), better resolution of adjacent peaks within shorter time was obtained by the BEH C18 column. For the Acquity HSS T3 column, although all compounds could be separated, the chromatographic peaks were asymmetric. Thus, the Acquity BEH C18 column was chosen as analytical column in this study. In addition, the mobile phases of acetonitrile-acid aqueous solution, acetonitrile-water and methanol-water were tested for good chromatographic behavior and appropriate ionization. Acetonitrile–0.1% aqueous formic acid (v/v) solution was finally selected owing to its best separation and ionization for the compounds analyzed. The typical MRM chromatograms are shown in Fig. 2.
Fig. 2.
Typical UPLC–MS/MS chromatograms (MRM) of 25 markers of mixed standard stock solution (A) and QC sample (B). Chlorogenic acid (1), calycosin-7-O-β-D-glucoside (2), rutin (3), hyperoside (4), isorhamnetin-3-O-neohespeidoside (5), astragalin (6), isorhamnetin-3-O-β-D-glucoside (7), hesperidin (8), diosmetin-7-O-β-d-glucopyranoside (9), ononin (10), (−)-methylinissolin-3-O-β-D-glucoside (11), calycosin (12), quercetin (13), isomucronulatol-7-O-β-D-glucoside (14), apigenin (15), kaempferol (16), hesperetin (17), baicalein (18), astragaloside IV (19), formononetin (20), (−)-methylnissolin (21), astragaloside II (22), soyasaponin I (23), isoastragaloside II (24) and rhamnocitrin (25).
3.3. Analytical method validation
The proposed UPLC–MS/MS method was validated by determining the linearity, LOD, LOQ, intra-day and inter-day precisions, stability, and accuracy. As shown in Table 2, all calibration curves exhibited good linearity (r2 > 0.9924) within the test ranges, and the overall LODs and LOQs were in the range of 0.05–1.94 ng/mL and 0.15–5.88 ng/mL, respectively. The acceptable precisions, repeatability and stability of the 25 analytes with the RSD values less than 4.83% were also observed (Table 3). The overall recoveries laid between 94.24% and 103.24% with RSDs less than 4.55%. The above results suggested that the proposed method was precise and accurate. Notably, the LODs value of this method is pg level, which is ten or even hundreds times lower than those of the previously reported methods including HPLC-DAD-ELSD, HPLC coupled with pulsed amperometric detection and HPLC-DAD-MS [10,13,16,24]. In addition, the analysis time was shortened synchronously by the method provided in this assay. More valuable, this method is effective for simultaneous qualification and quantification of various types of compounds.
Table 2.
Linearity, LOD and LOQ data of the 25 analytes by UPLC–MS/MS.
| Analytesa | Calibration curves | R2 | Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|
| 1 | y = 4.694 × 107 x – 2.844 × 104 | 0.9960 | 14.77–3781 | 1.18 | 3.58 |
| 2 | y = 2.207 × 109 x + 3.386 × 105 | 0.9978 | 21.97–5625 | 0.25 | 0.76 |
| 3 | y = 1.409 × 108 x – 1.767 × 104 | 0.9975 | 13.42–3437 | 1.43 | 4.33 |
| 4 | y = 5.046 × 108 x – 1.526 × 105 | 0.9997 | 12.93–3312 | 0.34 | 1.03 |
| 5 | y = 9.617 × 109 x – 3.25 × 106 | 0.9933 | 12.57–3218 | 0.06 | 0.18 |
| 6 | y = 4.699 × 108 x – 9.081 × 105 | 0.9953 | 14.52–3718 | 0.70 | 2.12 |
| 7 | y = 9.332 × 109 x – 1.006 × 105 | 0.9961 | 9.03–2312 | 0.11 | 0.33 |
| 8 | y = 1.022 × 109 x – 2.396 × 104 | 0.9995 | 15.50–3968 | 0.28 | 0.85 |
| 9 | y = 5.724 × 109 x + 5.254 × 104 | 0.9999 | 11.84–3031 | 0.13 | 0.39 |
| 10 | y = 1.155 × 109 x + 7.420 × 104 | 0.9684 | 13.79–3531 | 0.09 | 0.27 |
| 11 | y = 2.424 × 108 x – 2.028 × 105 | 0.9984 | 11.96–3062 | 0.05 | 0.15 |
| 12 | y = 2.565 × 109 x – 1.232 × 105 | 0.9985 | 33.81–8656 | 0.21 | 0.64 |
| 13 | y = 4.571 × 108 x – 1.569 × 105 | 0.9930 | 13.54–3468 | 0.91 | 2.76 |
| 14 | y = 2.565 × 109 x – 1.232 × 105 | 0.9956 | 9.89–2531 | 0.17 | 0.52 |
| 15 | y = 6.865 × 108 x – 2.800 × 105 | 0.9992 | 15.01–3843 | 0.33 | 1.00 |
| 16 | y = 7.750 × 105 x + 7.177 × 102 | 0.9944 | 11.59–2968 | 1.68 | 5.09 |
| 17 | y = 1.221 × 109 x – 7.151 × 105 | 0.9982 | 16.11–4125 | 0.43 | 1.30 |
| 18 | y = 9.945 × 108 x – 5.402 × 105 | 0.9995 | 12.08–3093 | 0.50 | 1.52 |
| 19 | y = 9.139 × 106 x + 3.203 × 104 | 0.9985 | 15.38–3937 | 1.31 | 3.97 |
| 20 | y = 4.601 × 108 x + 1.885 × 104 | 0.9968 | 15.01–3843 | 0.12 | 0.36 |
| 21 | y = 2.174 × 108 x + 2.735 × 104 | 0.9924 | 14.03–3593 | 0.88 | 2.67 |
| 22 | y = 8.741 × 107 x + 2.657 × 104 | 0.9973 | 13.54–3468 | 0.05 | 0.15 |
| 23 | y = 1.051 × 106 x – 8.457 × 102 | 0.9990 | 15.99–4093 | 1.94 | 5.88 |
| 24 | y = 2.529 × 108 x – 1.173 × 105 | 0.9977 | 15.38–3937 | 0.17 | 0.52 |
| 25 | y = 2.539 × 109 x – 1.450 × 106 | 0.9957 | 11.35–2906 | 0.36 | 1.09 |
The No. of analytes is the same as that in Table 1.
Table 3.
Precision, repeatability, stability and recovery results of the 25 analytes.
| Analytesa | Precision (%, n = 6) |
Repeatability (%, n = 6) | Stability (%, n = 6) | Recovery (%, n = 3) |
||
|---|---|---|---|---|---|---|
| Intra-day | Inter-day | Mean | RSD | |||
| 1 | 1.86 | 2.82 | 1.68 | 1.13 | 100.83 | 1.95 |
| 2 | 3.04 | 4.70 | 1.11 | 4.27 | 100.42 | 4.55 |
| 3 | 2.42 | 3.11 | 3.51 | 4.21 | 97.47 | 2.42 |
| 4 | 2.48 | 3.89 | 3.95 | 2.88 | 99.82 | 3.53 |
| 5 | 2.00 | 2.89 | 3.29 | 2.72 | 102.38 | 2.90 |
| 6 | 3.55 | 3.66 | 1.45 | 4.08 | 96.49 | 1.11 |
| 7 | 2.20 | 4.54 | 4.46 | 3.85 | 98.86 | 3.28 |
| 8 | 2.45 | 4.69 | 4.83 | 2.89 | 96.54 | 3.81 |
| 9 | 1.46 | 3.60 | 4.05 | 2.35 | 103.24 | 2.97 |
| 10 | 1.12 | 3.45 | 2.66 | 2.67 | 99.00 | 1.03 |
| 11 | 1.99 | 2.90 | 0.71 | 3.11 | 100.01 | 2.17 |
| 12 | 1.96 | 2.08 | 3.90 | 3.37 | 97.21 | 4.23 |
| 13 | 2.10 | 4.03 | 2.18 | 3.88 | 102.44 | 2.42 |
| 14 | 1.86 | 2.81 | 1.13 | 1.68 | 94.24 | 1.38 |
| 15 | 1.80 | 3.67 | 4.23 | 2.97 | 97.78 | 3.53 |
| 16 | 4.00 | 4.52 | 1.38 | 3.93 | 100.32 | 3.90 |
| 17 | 0.92 | 1.04 | 1.91 | 3.45 | 97.60 | 2.66 |
| 18 | 1.28 | 2.77 | 3.07 | 2.60 | 99.91 | 2.27 |
| 19 | 2.42 | 3.82 | 3.01 | 4.27 | 96.82 | 2.43 |
| 20 | 2.52 | 4.69 | 2.39 | 2.03 | 99.60 | 3.20 |
| 21 | 1.24 | 3.57 | 2.70 | 4.56 | 99.82 | 1.79 |
| 22 | 1.49 | 1.99 | 1.54 | 4.83 | 100.56 | 2.14 |
| 23 | 1.34 | 2.59 | 1.63 | 2.68 | 102.83 | 3.55 |
| 24 | 2.48 | 2.97 | 2.00 | 3.78 | 99.38 | 3.81 |
| 25 | 2.53 | 3.07 | 1.26 | 2.68 | 95.35 | 1.28 |
The No. of analytes is the same as that in Table 1.
3.4. Quantitative analysis of AMM and AM
To clarify the distribution and contents of the bioactive compounds in different organs of these two Astragalus plants, the proposed UPLC-MS/MS method was subsequently used to determine the 25 analytes in their roots, stems, leaves and flowers. The results (Table 4) showed that there were remarkable differences in dihydroflavones, isoflavane, isoflavones, flavones, pterocarpans, phenolic acids and saponins contents, not only in different organs of the same species, but also in the same organ of different species.
Table 4.
Contents (μg/g DW) of the 25 compounds analyzed in root, rhizome, stem, leaf and flower of two Astragalus plants.
| Analytesa | Root |
Rhizome |
Stem |
Leaf |
Flower |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMM | AM | AMM | AM | AMM | AM | AMM | AM | AMM | AM | |
| 1 | 35.20 ± 1.03 | 34.09 ± 0.12 | 34.85 ± 4.28 | 35.52 ± 2.46 | 36.95 ± 5.45 | 37.38 ± 1.45 | 36.26 ± 4.57 | 35.82 ± 3.85 | 37.30 ± 6.86 | 38.10 ± 3.56 |
| 2 | 226.3 ± 11.0 | 235.2 ± 3.06 | 62.80 ± 4.09 | nd | 32.73 ± 2.86 | nd | 11.45 ± 1.02 | 2.261 ± 0.03 | nd | nd |
| 3 | 7.927 ± 0.408 | 7.294 ± 0.236 | 7.849 ± 0.129 | 9.292 ± 0.13 | 16.93 ± 1.34 | 24.01 ± 2.42 | 52.34 ± 4.24 | 187.7 ± 8.8 | 312.3 ± 10.2 | 1309 ± 32 |
| 4 | 21.37 ± 1.17 | 22.44 ± 1.85 | 21.40 ± 2.00 | 34.17 ± 3.06 | 196.9 ± 19.5 | 286.2 ± 21.1 | 677.3 ± 33.3 | 2169 ± 114 | 1071 ± 41.1 | 2327 ± 209 |
| 5 | 19.74 ± 1.19 | 18.72 ± 0.93 | 21.13 ± 1.29 | 19.45 ± 2.3 | 49.98 ± 4.42 | 20.68 ± 3.35 | 36.61 ± 3.58 | 22.64 ± 1.99 | 38.28 ± 2.49 | 30.96 ± 2.35 |
| 6 | 110.6 ± 10.32 | 106.6 ± 9.24 | 109.9 ± 7.39 | 110.6 ± 8.23 | 149.9 ± 12.3 | 128.3 ± 7.2 | 219.9 ± 16.3 | 319.5 ± 11.1 | 222.9 ± 8.3 | 337.1 ± 14.8 |
| 7 | 14.55 ± 1.31 | 2.250 ± 0.309 | 24.13 ± 1.70 | 4.717 ± 0.01 | 487.1 ± 18.0 | 25.70 ± 2.84 | 1158 ± 23 | 310.7 ± 20.6 | 243.1 ± 12.1 | 98.98 ± 9.02 |
| 8 | 1.934 ± 0.106 | 1.440 ± 0.090 | 2.109 ± 0.125 | 2.838 ± 0.10 | 10.30 ± 0.74 | 8.962 ± 0.50 | 15.79 ± 1.54 | 105.0 ± 7.34 | 211.7 ± 11.4 | 732.9 ± 17.3 |
| 9 | 4.285 ± 0.971 | nd | 7.322 ± 1.001 | 6.467 ± 0.56 | 200.7 ± 16.2 | 13.63 ± 2.71 | 158.2 ± 17.3 | 28.88 ± 3.07 | 66.83 ± 3.85 | 29.24 ± 1.95 |
| 10 | 18.34 ± 0.52 | 1.632 ± 0.005 | 9.517 ± 0.043 | nd | nd | nd | nd | nd | nd | nd |
| 11 | 49.87 ± 2.43 | 46.98 ± 1.93 | 49.98 ± 0.56 | 49.89 ± 0.44 | 153.3 ± 8.64 | 53.00 ± 4.78 | 138.8 ± 10.0 | 56.99 ± 3.50 | 77.38 ± 4.06 | 72.76 ± 2.75 |
| 12 | 82.11 ± 5.64 | nd | nd | nd | nd | nd | 73.73 ± 4.77 | 729.4 ± 14.3 | 628.8 ± 12.2 | 62.98 ± 2.63 |
| 13 | 20.27 ± 0.70 | 19.33 ± 0.42 | 19.82 ± 1.33 | 19.80 ± 0.05 | 22.14 ± 0.77 | 21.95 ± 1.02 | 22.56 ± 3.27 | 32.99 ± 0.64 | 69.56 ± 1.84 | 40.27 ± 2.29 |
| 14 | 35.58 ± 1.32 | 22.95 ± 0.31 | 18.12 ± 0.81 | 3.106 ± 0.04 | 6.991 ± 0.91 | 3.097 ± 0.01 | 43.64 ± 3.73 | 5.204 ± 0.04 | 6.150 ± 0.05 | 5.118 ± 0.10 |
| 15 | 23.66 ± 1.53 | 22.67 ± 0.63 | 23.85 ± 0.84 | 23.67 ± 1.62 | 24.77 ± 1.47 | 24.51 ± 1.64 | 25.04 ± 0.72 | 29.61 ± 1.69 | 29.76 ± 2.73 | 32.66 ± 0.84 |
| 16 | 5.899 ± 0.245 | 5.713 ± 0.240 | 80.90 ± 15.63 | nd | 164.1 ± 17.2 | 6.102 ± 0.92 | 915.9 ± 10.3 | 454.4 ± 25.4 | 617.8 ± 16.3 | 673.8 ± 39.0 |
| 17 | 33.42 ± 1.06 | 32.30 ± 0.63 | 33.23 ± 2.00 | 33.85 ± 1.31 | 34.86 ± 1.06 | 36.46 ± 2.12 | 33.92 ± 0.25 | 36.12 ± 1.31 | 39.23 ± 0.74 | 35.44 ± 1.00 |
| 18 | 31.41 ± 2.58 | 30.10 ± 2.01 | 31.92 ± 1.94 | 31.20 ± 0.99 | 32.92 ± 0.36 | 32.30 ± 0.97 | 31.50 ± 2.54 | 31.79 ± 1.22 | 32.67 ± 2.28 | 32.80 ± 1.53 |
| 19 | 381.5 ± 14.5 | 455.7 ± 8.6 | 35.14 ± 2.01 | 19.37 ± 0.05 | nd | nd | 216.3 ± 12.1 | 255.7 ± 7.8 | 81.70 ± 4.23 | 49.02 ± 0.02 |
| 20 | 15.65 ± 1.25 | 6.037 ± 0.069 | 7.954 ± 0.414 | 5.841 ± 0.08 | 7.718 ± 0.00 | 10.98 ± 1.06 | 8.739 ± 0.06 | 53.51 ± 4.62 | 8.286 ± 0.63 | 109.6 ± 4.95 |
| 21 | 27.86 ± 3.34 | 28.08 ± 1.43 | 13.16 ± 0.68 | 6.938 ± 0.07 | 8.691 ± 0.85 | 5.583 ± 0.00 | 17.25 ± 3.73 | 6.683 ± 0.93 | 17.70 ± 0.77 | 7.462 ± 0.08 |
| 22 | 273.3 ± 9.2 | 323.6 ± 6.61 | 71.75 ± 4.06 | 6.579 ± 0.275 | nd | 29.14 ± 0.99 | 4.161 ± 0.14 | nd | nd | nd |
| 23 | 215.7 ± 9.7 | 291.2 ± 11.6 | 119.0 ± 6.8 | 88.48 ± 2.74 | 92.03 ± 1.74 | 80.35 ± 3.29 | 163.2 ± 11.6 | 57.46 ± 5.57 | 450.6 ± 7.71 | 728.2 ± 12.6 |
| 24 | 45.35 ± 3.19 | 53.99 ± 2.07 | 42.81 ± 3.24 | 32.07 ± 2.42 | nd | 161.6 ± 8.9 | 32.01 ± 0.13 | nd | nd | nd |
| 25 | 32.67 ± 0.42 | 34.02 ± 1.85 | 34.67 ± 0.95 | 32.68 ± 0.64 | 45.13 ± 1.09 | 34.91 ± 1.16 | 35.75 ± 2.69 | 35.27 ± 0.75 | 38.73 ± 2.66 | 34.55 ± 1.93 |
| Total | 1734 ± 24 | 1802 ± 121 | 883.3 ± 28.8 | 576.5 ± 14.4 | 1774 ± 88 | 1005 ± 34 | 4128 ± 64 | 4967 ± 100 | 4302 ± 87 | 6789 ± 245 |
nd: Not detected.
AMM: Astragalus membranaceus var. mongholicus (Bge.) Hsiao. AM: A. membranaceus (Fisch.) Bge.
The No. of analytes is the same as that in Table 1.
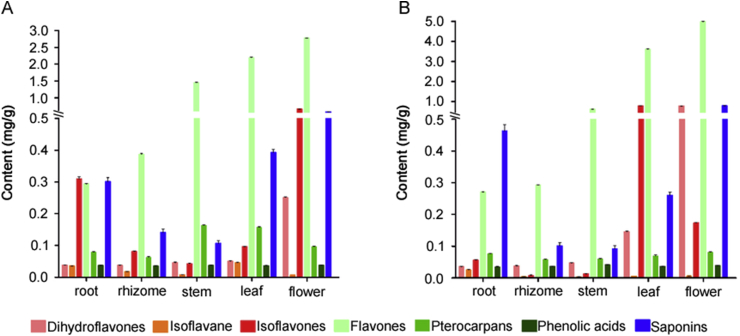
For the two species, the total contents of 25 investigated compounds in flower were found to be the highest (4.302 mg/g dw for AMM and 6.788 mg/g dw for AM) in whole plants, followed by leaves. The rhizomes were found to be the lowest part for these compounds, which were only 0.8833 mg/g dw and 0.5765 mg/g dw for AMM and AM, respectively.
According to the content of each analyte, the distribution and contents of different type of compounds showed significant differences among the five organs of AMM and AM. As shown in Table 4 and Fig. 3, dihydroflavones, isoflavones and flavones were mainly distributed in the above ground parts, such as flowers and leaves, while isoflavane and saponins were mainly distributed in the medicinal parts (root). In addition, pterocarpans and phenolic acids were uniformly distributed in these five different organs.
Fig. 3.
The distribution and content of different types of compounds in different organs of A. membranaceus var. mongholicus (A) and A. membranaceus (B). Dihydroflavones (hesperidin, hesperetin), Isoflavanes (isomucronulatol-7-O-β-D-glucoside), Isoflavones (calycosin-7-O-β-D-glucoside, ononin, calycosin, formononetin), Flavones (rutin, hyperoside, kaempferol, astragalin, isorhamnetin-3-O-β-D-glucoside, diosmetin-7-O-β-d-glucopyranoside, rhamnocitrin, quercetin, apigenin, baicalein, isorhamnetin-3-O-neohespeidoside), Pterocarpans ((−)-methylnissolin, (−)-methylinissolin-3-O-β-D-glucoside), Phenolic acids (chlorogenic acid) and Saponins (astragaloside IV, astragalosides II, soyasaponin I, isoastragaloside II).
Hyperoside (4), kaempferol (16) and soyasaponin I (23) were found to be the most abundant in the flowers, and were even a thousand times higher than in the roots. However, the contents of calycosin-7-O-β-D-glucoside (2), astragaloside IV (19) and astragaloside II (22) in the roots were higher. Compared to calycosin-7-O-β-D-glucoside, its aglucon (calycosin, 12) was found mainly present in the leaf and flower. It is worth noting that some flavonoids, such as rutin (3), hyperoside (4), hesperidin (8), and kaempferol (16), show a tendency that from the root, rhizome, stem, leaf to flower, the closer to the top of the plants, the higher these flavonoid contents. In addition, the flower was found to be another part of these plants rich in saponins (AMM 0.532 mg/g dw, AM 0.777 mg/g dw).
In addition, the contents of the same type of compounds in two different species plants were significantly different. For example, the contents of isoflavane (14) and some isoflavones (2 and 10) in AMM were much more than those in AM. However, for hesperidin (8) in flowers and leaves, and saponins (19, 22, 23 and 24) in root, their contents were significantly higher in AM than in AMM. It is well known that the biosynthesis of flavonoids, phenolic acids and saponins are all catalyzed by the corresponding enzymes [25,26]. Thus, the difference in the content and distribution of different types of compounds may be owing to the various activities of corresponding enzyme in different organs of AMM and AM, which requires further exploration. In addition, the results may be helpful for the rational utilization of the bioactive constituents in AMM and AM resources. For example, the flowers could be used as the raw materials for the flavonoids and isoflavones extraction, while the stems and leaves may be the better materials of pterocarpans.
4. Conclusion
In this paper, a UPLC-MS/MS method for simultaneous determination of 25 analytes including seven types of compounds (dihydroflavones, isoflavones, borneol, phenolic acid and saponin) within 10 min was established, and validated as a rapid, sensitive and accurate approach. Then, the proposed method was successfully applied in the analysis of these analytes in different organs of AMM and AM. The results showed a significant difference in the distribution and content of the compounds analyzed, which provides information for the rational utilization of these two Astragalus plants resources. In addition, the approach provided in the study would be a suitable method for the quality control of these plant products.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81473538, 81873189), the Key R & D Program of Ningxia Hui Autonomous Region, China (2017BY079, 2018ZWYQ0077), and China Agricultural Research System (CARS-21).
Footnotes
Peer review under responsibility of Xi'an Jiaotong University.
Contributor Information
Sheng Guo, Email: guosheng@njucm.edu.cn.
Jin-Ao Duan, Email: dja@njucm.edu.cn.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Fu J., Wang Z., Huang L. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi) Phytother Res. 2014;28:1275–1283. doi: 10.1002/ptr.5188. [DOI] [PubMed] [Google Scholar]
- 2.Sun H., Kang B., Chai Z. Characterization of root-associated microbiota in medicinal plants Astragalus membranaceus and Astragalus mongholicus. Ann. Microbiol. 2017;67:587–599. [Google Scholar]
- 3.Bedir E., Pugh N., Calis I. Immunostimulatory effects of cycloartane-type triterpene glycosides from Astragalus species. Biol. Pharm. Bull. 2000;23:834–837. doi: 10.1248/bpb.23.834. [DOI] [PubMed] [Google Scholar]
- 4.Wang T., Sun Y., Jin L. Enhancement of non-specific immune response in sea cucumber (Apostichopus japonicus) by Astragalus membranaceus and its polysaccharides. Fish Shellfish Immunol. 2009;27:757–762. doi: 10.1016/j.fsi.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Chan J.Y., Lam F.C., Leung P.C. Antihyperglycemic and antioxidative effects of a herbal formulation of Radix Astragali, Radix Codonopsis and Cortex Lycii in a mouse model of type 2 diabetes mellitus. Phytother, Res. 2010;23:658–665. doi: 10.1002/ptr.2694. [DOI] [PubMed] [Google Scholar]
- 6.Choi H.S., Joo S.J., Yoon H.S. Quality characteristic of Hwangki(Astragalus membranaceus) Chungkukjang during fermentation. Kor. J. Food Preserv. 2007;14:356–363. [Google Scholar]
- 7.Rui L., Chen W.C., Wang W.P. Antioxidant activity of Astragalus polysaccharides and antitumour activity of the polysaccharides and siRNA. Carbohydr. Polym. 2010;82:240–244. [Google Scholar]
- 8.Yu D.H., Bao Y.M., Wei C.L. Studies of chemical constituents and their antioxidant activities from Astragalus mongholicus Bunge, Biomed. Environ. Sci. 2005;18:297–301. [PubMed] [Google Scholar]
- 9.Auyeung K.K., Han Q.B., Ko J.K. Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016;44:1–22. doi: 10.1142/S0192415X16500014. [DOI] [PubMed] [Google Scholar]
- 10.Yu Q.T., Qi L.W., Li P. Determination of seventeen main flavonoids and saponins in the medicinal plant Huang-qi (Radix astragali) by HPLC-DAD-ELSD. J. Sep. Sci. 2007;30:1292–1299. doi: 10.1002/jssc.200600422. [DOI] [PubMed] [Google Scholar]
- 11.Qi L.W., Yu Q.T., Yi L. Simultaneous determination of 15 marker constituents in various Radix Astragali preparations by solid-phase extraction and high-performance liquid chromatography. J. Sep. Sci. 2008;31:97–106. doi: 10.1002/jssc.200700286. [DOI] [PubMed] [Google Scholar]
- 12.Ying L., Liu Y., Wang Y. Phytochemical analysis of an antiviral fraction of Radix astragali using HPLC–DAD–ESI–MS/MS. J. Nat. Med. 2010;64:182–186. doi: 10.1007/s11418-009-0381-1. [DOI] [PubMed] [Google Scholar]
- 13.Qi L.W., Cao J., Li P. Qualitative and quantitative analysis of Radix Astragali products by fast high-performance liquid chromatography-diode array detection coupled with time-of-flight mass spectrometry through dynamic adjustment of fragmentor voltage. J. Chromatogr. A. 2008;1203:27–35. doi: 10.1016/j.chroma.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Zhang X., Zhao Y. Comparative chemical analysis of Radix astragali and Radix Hedysari by HPLC. Nat. Prod. Res. 2012;26:1935–1938. doi: 10.1080/14786419.2011.619188. [DOI] [PubMed] [Google Scholar]
- 15.Lee S.M., Jeong J.S., Kwon H.J. Quantification of isoflavonoids and triterpene saponins in Astragali Radix, the root of Astragalus membranaceus, via reverse-phase high-performance liquid chromatography coupled with integrated pulsed amperometric detection. J. Chromatogr. B. 2017;1070:76–81. doi: 10.1016/j.jchromb.2017.10.046. [DOI] [PubMed] [Google Scholar]
- 16.Kwon H.J., Park Y.D. Determination of astragalin and astragaloside content in Radix Astragali using high-performance liquid chromatography coupled with pulsed amperometric detection. J. Chromatogr. A. 2012;1232:212–217. doi: 10.1016/j.chroma.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Singh A., Bajpai V., Kumar S. Analysis of isoquinoline alkaloids from Mahonia leschenaultia and Mahonia napaulensis roots using UHPLC-Orbitrap-MSn and UHPLC-QqQLIT-MS/MS. J. Pharm. Anal. 2017;7:77–86. doi: 10.1016/j.jpha.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weng Z., Zeng F., Zhu Z. Comparative analysis of sixteen flavonoids from different parts of Sophora flavescens Ait. by ultra high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018;156:214–220. doi: 10.1016/j.jpba.2018.04.046. [DOI] [PubMed] [Google Scholar]
- 19.Liu M., Cao Y., Lv D. Effect of processing on the alkaloids in Aconitum tubers by HPLC-TOF/MS. J. Pharm. Anal. 2017;7:170–175. doi: 10.1016/j.jpha.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Liu J., Wang Y. Simultaneous determination of six active metabolites in Astragalus mongholicus (Fisch.) Bge. under salt stress by ultra-pressure liquid chromatography with tandem mass spectrometry. SpringerPlus. 2016;5:927. doi: 10.1186/s40064-016-2638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y., Liu J., Wu K.X. A rapid method for sensitive profiling of bioactive triterpene and flavonoid from Astragalus mongholicus and Astragalus membranaceus by ultra-pressure liquid chromatography with tandem mass spectrometry. J. Chromatogr. B. 2018;1085:110–118. doi: 10.1016/j.jchromb.2018.03.044. [DOI] [PubMed] [Google Scholar]
- 22.Li S.P., Zhao J., Yang B. Strategies for quality control of Chinese medicines. J. Pharm. Biomed. Anal. 2011;55:802–809. doi: 10.1016/j.jpba.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y., David B., Tu P. Recent analytical approaches in quality control of traditional Chinese medicines—a review. Anal. Chim. Acta. 2010;657:9–18. doi: 10.1016/j.aca.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 24.Tang D.Q., Zheng X.X., Chen X. Quantitative and qualitative analysis of common peaks in chemical fingerprint of Yuanhu Zhitong tablet by HPLC-DAD-MS/MS. J. Pharm. Anal. 2014;4:96–106. doi: 10.1016/j.jpha.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haralampidis K., Trojanowska M., Osbourn A.E. Biosynthesis of Triterpenoid saponins in plants. Adv. Biochem. Eng. Biotechnol. 2002;75:31–49. doi: 10.1007/3-540-44604-4_2. [DOI] [PubMed] [Google Scholar]
- 26.Holton T.A., Cornish E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell. 1995;7:1071–1083. doi: 10.1105/tpc.7.7.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]