Abstract
Background
Albendazole is an orally administered broad-spectrum anthelmintic. Currently it is mostly used in the treatment of soil transmitted helminthes, hydatidosis and neurocysticercosis caused Taenia solium.
Aim of the study. To develop and optimize a formulation of chewable albendazole tablet with improved dissolution rate.
Methodology
This study was specifically focused on formulation development which passes compatibility studies and optimization of the developed formulation. The formulations were evaluated on assay, dissolution, friability, hardness, weight variation, disintegration and similarity in comparison with the reference product on the market. Analysis was required to be undertaken by High performance thin layer chromatography (HPTLC) analytical methods. Design of Expert version 7 software was used for selection or making scientific decisions in selecting the best composition of the best formulation.
Results
Five formulations out of ten (F-6, F-7, F-8, F-9 and F10) had all parameters in acceptable range. On optimization, one formulation with independent variables, Sodium Laury Sulphate (SLS) 1.911%, polyvinyl pyrrolidone (PVP-K30) 3.128%, and Sodium Cross carmellose (CCM) 4.95% was selected out of ten predictions made with Design expert version 7.0. It was found that assay of the best formulation is 99.23% which was within the in-house assay specification 95–105%. Dissolution single point in 30 min was found to be 91.5% disintegration between 2-5 min and friability 0.45%.The optimized formulation was tested and found to be within the acceptable limits. The formulation was comparable to the reference product on the market with similarity factor (f2) 62 and difference factor (f1) of 6 at pH1.2.
Conclusion
A new generic albendazole tablet with improved dissolution rate was formulated, developed and optimized by using a wet granulation method.
Keywords: Pharmaceutical chemistry, Design of expert version 7, High performance thin layer chromatography, Optimezed formulation
Pharmaceutical chemistry, Design of expert version 7; High performance thin layer chromatography; Optimezed formulation.
1. Introduction
Soil transmitted worms infestation are the one which mostly affect thousands of rural, impoverished villages throughout the tropical and subtropical are often chronically infected with several different species of parasitic worm; that is, they are polyparasitized [1, 2, 3]. Adverse health consequences impair childhood educational performance, reduce school attendance [4].
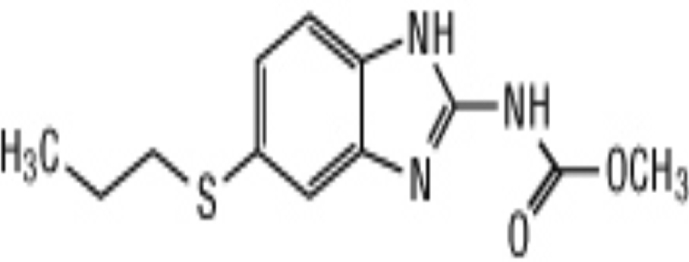
WHO recommended surgery and albendazole to be a treatment options for the management of hepatic and pulmonary echinococcosis caused by Echinococcus granulosus [5]. Larval cysts in the brain cause a form of cysticercosis called neurocysticercosis which can lead to seizures [6]. Albendazole is a treatment of choice for neurocysticercosis, the dosage for albendazole is 15 mg/kg per day for ten days. Chemically, it is methyl 5-(propylthio)-2-benzimidazolecarbamate. Its molecular formula is C12H15N3O2S. Its molecular weight is 265.34 [7] and structural formula of albendazole is shown in Figure 1.
Figure 1.
Chemical structure of Albendazole.
Drugs of low aqueous solubility (e.g. Albendazole) frequently present problems in relation to their formulation and bioavailability. The solution process will precede absorption unless the drug is administered as a solution, but even solutions may precipitate in the stomach contents or in blood, and the precipitated drug will then have to re-dissolve before being absorbed [8].
Generic albendazole tablets from developing countries showed poor dissolution rate compared to the innovator product [9], it create a need for developing generic product of a chewable albendazole tablet with improved dissolution rate. Previous studies shows that chewable albendazole tablet can be prepared by use of non-aqueous granulation and wet granulation method [13, 18].
This scientific work comes with a generic formulation with good in-vitro availability (Dissolution rate) comparable to the innovator product GSK as an indicative for good bioavailability as a solution for the problem of poor bioavailability noted in some generic albendazole tablets. The developed formulation has also demonstrated quality in other parameters including disintegration time of not more than 15 Minutes and friability of not more than 1%, Assay within 90–110%, therefore in line with the Pharmacopoeia specifications.
2. Materials and methods
2.1. Equipment and software
The instruments including a tablet press (EKO1 made in Germany) and Turbular mixer made in Switzerland, a densitometer with TLC scanner 3 operated with Wincats (version 1.4.3) planar chromatograph software as data manager and integrator, Linomat 5, a semi automatic applicator with Hamilton syringe of 100 μL capacity for sample application and CAMAG developing tank single rectangular with internal dimension (21.6 × 11.2 × 6) cm all were made fromCAMAG, Muntez, Switzerland were used. The TLC (5 × 10) cm, HPTLC (10 × 10) and (20 × 10) cm plate pre coated with silica gel 60 F 254 were made from Merck, Darmstadt, Germany. The D-Optimal design in the design expert version 7 software was used to generate and evaluate the trial batches.
Near Infrared spectrophotometer (Lab Spec 5000 Asdinc-USA), and analytical balance (new classic MS Mettler Toledo Germany). The equipment include: Monsanto type tablet hardness tester (IEC, Mumbai, India), Roche Friabilator (electro lab, Bangalore, India), single pan balance (Shimadzu, AX200, Japan), Disintegration Apparatus USP (Electrolab, Bangalore, India), graduated cylinder (Fisher Scientific, Germany), sieve analyzer (Endecott‟s, Germany), glass bottles (Fisher Scientific, Germany), ERWEKA TBH machine (Heusenstamm, Germany).
2.2. Reagents, and chemicals
Analytical grade and HPLC grade reagents were used for analytical part of this study during the pre-formulation and formulation steps. Methanol was made from Scharlau S.L, Sentmenat, Spain and Techno Pharmchem Bahadurggarh, Haryana, India. Glacial acetic acid was made from Scharlau S.L, Sentmenat, Spain. Ethylacetate was made from Techno Pharmchem Bahadurggarh, Haryana, India and Fisher Scientific, Leicestershire, UK. Distilled water was in-house prepared at MUHAS Pharm R&D Dar es Salaam, Tanzania by reverse osmosis using RO- Purification System equipment made from Millipore® France. Excipients included microcrystalline cellulose made from FMC BioPolymer, Philadelphia, sodium carboxymethyl cellulose and polyvinylpyrrolidone cross-linked made from Associate Co. Ltd, Shenzhen, China and magnesium stearate was made from Shandong Liaocheng Ehua Medicine Co. Ltd, China.
2.3. Materials and selection of excipients
Selections of excipients were based on excipients commonly found in medicines available on the market. Excipients which were used in the compatibility studies include Microcrystalline Cellulose (MCC) Microcrystalline Cellulose (Avicel PH101), Anhydrous Lactose, magnesium stearate, Sodium lauryl Sulphate (SLS), Croscamellose Sodium (CCM), Sodium starch glycolate (SSG), Sodium saccharin, Crosspovidone (CP) and polyvinyl pyrrolidone k-30 (PVP K-30). These are some of the common excipients.
Excipient Compatibility studies were conducted as follows; 200mg of excipient was mixed with 200mg of Albendazole powder (1:1 binary mixture) in order to prepare samples for detection of any incompatibility. The samples were stored at stability chamber (40±2 °C, RH 75%), oven (50 °c) and room temperature (30 ± 2 °C).
Then the samples were physically observed for caking, Liquefaction, Discoloration, Odor and Gel formation at 0, 7, 14, 21, 30, 60 and 90 days of storage.
Assessment of compatibility was performed by use of NIR spectra. At the interval of 0, 7, 14, 21, 30, 60 and 90 days under stated conditions, NIR spectra of samples of albendazole-excipient blends were prepared. The results of day 0 were used as a baseline to compare with the other days. Assay was performed by use of HPTLC.
2.4. Preparation of powder blend/mix
The active ingredient and the excipients were passed through sieve and then weighed according to each formulation requirement. The active ingredient and excipients were mixed/blended for 10 min after which evaluation of blend characteristics were performed.
2.4.1. Evaluation of powder mix
The powder mixtures for each of the formulations were evaluated for powder flow before compression by use of the following methods;
-
i)
Carr's compressibility index
Calculation of Tapped Density and Poured Density was done as follows;
For Poured Density, an accurately weighed sample of powder blend was carefully added to the measuring cylinder with the aid of funnel. Then the volume was noted. The volume of the packing was determined in an apparatus consisting of a graduated cylinder. Poured Density (Du) was determined by the formula [10, 11];
The Tapped Density (TD) was determined by use of the above procedure but after tapping the graduated cylinder until there is no further change in volume. Tapped Density was determined by the formula;
2.5. Compression of the powder blend/mix
The mix/blend was granulated by using a wet granulation method and then compressed at a constant compression force following which tablets evaluation were performed.
2.5.1. Tablets characterization
Tablets of each formulation were evaluated with respect to the following characteristics.
-
i)
Weight variation/Uniformity of mass
20 tablets were weighed individually, the average weight calculated and comparing the individual tablets weights to the average.
The tablets meet the test if no more than 2 tablets are outside the percentage limit and if no tablet differs by more than 2 times the percentage limit.
-
ii)
Tablet hardness
10 tablets were crushed by a hardness tester, one at a time while maintaining the same direction with respect to application of force. The hardness tester was cleaned to remove all the fragments of the tablets before each determination [12].
-
iii)
Friability
This test was performed by a Roche friabilator. Samples of ten pre-weighed tablets were placed in a friabilator which was operated for 100 revolutions.
The tablets were then being dusted and reweighed. Tablets that lose less than 0.5–1.0% of their weight were considered acceptable. Also, if capping occurs during friability testing, the tablets were rejected [13].
-
iv)
Tablet disintegration
One tablet was placed in each of the 6 tubes of the disintegration test device and the basket rack was positioned in a 1 L beaker of water at 37 °C + 2 °C. The time it took for all particles to pass through the 10 nm-mesh screen was noted [14].
-
v)
Dissolution (comparative Dissolution)
The in vitro drug release studies were performed using USP dissolution apparatus Type II (paddle) using 900ml of 0.1N Hydrochloric acid as the dissolution medium. The temperature of the dissolution medium was maintained at 37 ± 0.5 °C and the paddle was rotated at 50 rpm. Aliquots were withdrawn and the samples were suitably diluted and absorbance of the solutions was determined at the wavelengths of maximum and minimum absorbance at about 308nm and 350nm, in a UV visible spectrophotometer.
A simple model independent approach which uses a difference factor (f 1) and a similarity factor (f 2) was used to compare the dissolution profiles. The difference factor (f1) calculates the percent (%) difference between the two curves at each time point and is a measurement of the relative error between the two curves:
where n is the number of time points, Rt is the dissolution value of the reference (prechange) batch at time t, and Tt is the dissolution value of the test (post change) batch at time t.
The similarity factor (f2) is a logarithmic reciprocal square root transformation of the sum of squared error and is a measurement of the similarity in the percent (%) dissolution between the two curves.
For the dissolution profiles to be considered similar, the f 1 values should be close to 0, and f 2 values should be close to 100 [15].
-
vi)
Assay
Procedures.
-
i)
Standard solution preparation:
Weighed amount of 30.4 mg of albendazole standard into a 20 mls volumetric flask and small amount of glacial acetic acid was added in order to dissolve the powder then glacial acetic acid was added to make up the volume up to the mark, 1ml of the resulted solution was transferred into a 20ml volumetric flask and then methanol was added to make up the volume.
-
ii)
Test sample preparation:
Twenty albendazole tablets were weighed and grinded in a mortar and pestle, a powder which contains 30.4mg of albendazole was weighed into a 20 mls volumetric flask. Small amount of glacial acetic acid was added into a 20 ml volumetric flask in order to dissolve the powder, glacial acetic acid was again added to make up the volume then 1ml of the resulted solution was transferred into a 20ml volumetric flask and then add methanol to make up the volume.
-
iii)
Blank Preparation: methanol was used as a blank solution
-
iv)
Preparation of mobile phase:
Mobile phase with the following composition below was prepared in a 50 ml volumetric flask; Ethyl Acetate: Toluene: Glacial Acetic Acid (8:12:1).
-
v)
Saturation of Chamber:
Chamber was saturated for 20 min with the mobile phase above with the aid of filter paper.
HPTLC software was set in such a way that spotting of blank, standard and sample solutions was done automatically on a TLC silica F 60 254 plate of appropriate size with a mark at 70mm from the bottom to indicate the limit of solvent front..
After spotting, the plates were developed in the saturated chambers and then allow them to dry. Thenthe plates were scanned at 247nm UV light.
-
vi)
Calculation
Areas under the curves for standard and samples was read and used to calculate percentage assay.
3. Results and discussion
3.1. Preformulation studies
During pre-formulation studies albendazole remains stable showing no changes of the physical and chemical properties and this has been proved by the results obtained from identification and assay by high performance thin layer chromatography method in which the amount determined was more than 95% as required by our internal specification.
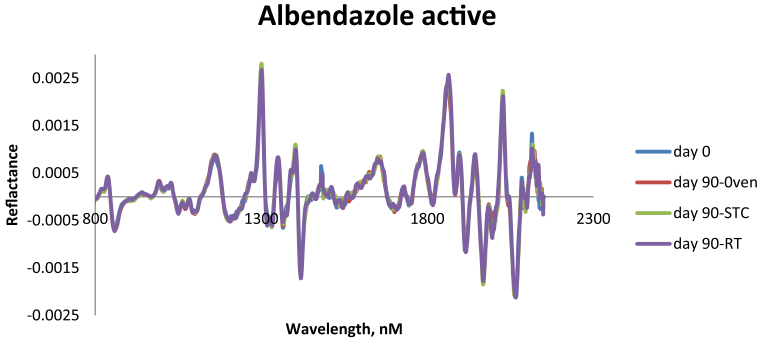
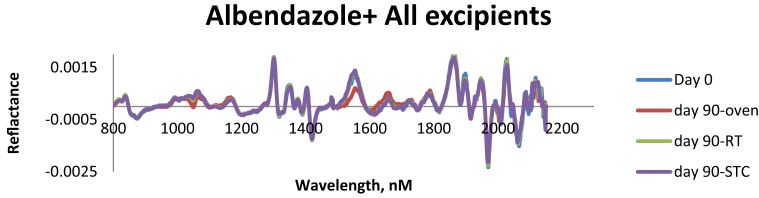
NIR overlay plot for albendazole active ingredients and NIR overlay plot for albendazole mixed with all excipients showing reflectance vs. wavelength. NIR overlay spectra of sample mixtures subjected to all preformulated conditions (40 ± 2 °C/75 ± 5% RH- climatic chamber, 50 °C-Oven and uncontrolled room conditions with 30 ± 2 °C) from day 0–90 show that albendazole remains stable and has no interactions with the excipients used throughout the study period as shown in Figures 2 and 3 respectively.
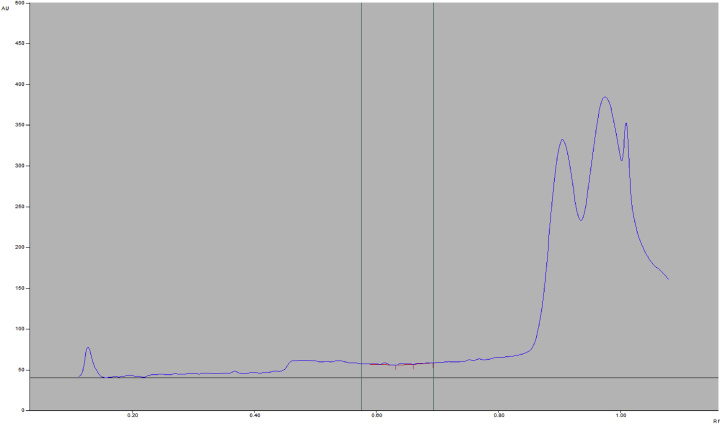
Figure 2.
A typical densitogram of 5 μL application of Blank (methanol) mobile phase: ethyl acetate: Toluene: Glacial acetic acid (8:12:1v/v) acquired by UV detection at 247nm.
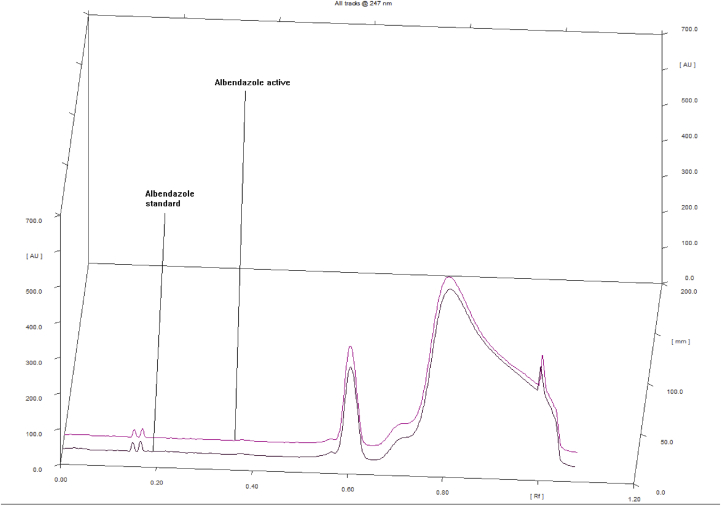
Figure 3.
A typical 3D diagram showing no interference of a peak between albendazole active stored in a room condition (day 90) and albendazole standard.
High performance thin layer chromatography have ability of detecting impurities coming from degradation products and also HPTLC is very selective and specific as shown in Figures 4 and 5, results has demonstrated that the RF values of albendazole sample were similar with the RF value of the reference standard as shown in the Figure 5.
Figure 4.
A NIR overlay plot for albendazole active ingredients showing reflectance vs wavelength of the sample mixtures kept to a preformulated conditions (40 ± 2 °C/75 ± 5% RH- climatic chamber, 50 °C-Oven and Room uncontrolled room conditions with 30 ± 2 °C).
Figure 5.
A NIR overlay plot for albendazole mixed with all excipients showing reflectance vs wavelength of the sample mixtures kept to a preformulated conditions (40 ± 2 °C/75 ± 5% RH- climatic chamber, 50 °C-Oven and Room uncontrolled room conditions with 30 ± 2 °C) from day 0–90.
Moreover, High performance thin layer chromatography (HPTLC) was also used for determining the amount of albendazole in all preformulated samples stored in all conditions (RT, STC and Oven). All samples which did not comply with the specification were due to analytical error like weighing and measurement of the sample during analysis as shown in Table 1.
Table 1.
Drug content of samples subjected into pre-formulation studies as from day 0–90. The acceptance limits for assay 95–105% (In-house specifications).
| Details | Room temperature (30 ± 2 °C) | Stability chamber (40±2 °C, RH 75%) | Oven (50Oc) | |||
|---|---|---|---|---|---|---|
| Day 0 | Day 90 | Day 0 | Day 90 | Day 0 | Day 90 | |
| Albendazole active | 99.37% | 107.6% | 99.37% | 99% | 99.37% | 99.5% |
| Albendazole + MCC | 101.19% | 96% | 101.19% | 103.7% | 101.19% | 104.78% |
| Albendazole + CP | 109.82% | 93% | 109.82% | 102.91% | 109.82% | 105.58% |
| Albendazole + SLS | 95% | 96.61 | 95% | 107.71 | 95% | 98% |
| Albendazole + PVP K30 | 96% | 103.22% | 96% | 96.36% | 96% | 95% |
| Albendazole + Lactose | 112.68% | 94% | 112.68% | 102.91% | 112.68% | 106.88% |
| Albendazole + Saccharin | 105.79% | 95.68% | 105.79% | 105.44% | 105.79% | 95% |
| Albendazole + SSG | 99.26% | 95% | 99.26% | 100.87% | 99.26% | 101.77% |
| Albendazole + All excipients | 102.56% | 105.67% | 102.56% | 110.42% | 102.56% | 101.61% |
| Albendazole + Magnesium sulphate | 97% | 101.74% | 97% | 105.27% | 97% | 98.4% |
| Albendazole + CCM | 116.94% | 95% | 116.94% | 97.83% | 116.94% | 106.72% |
3.2. Formulation development studies
Selection of the excipients to be used during formulation studies depends on the compatibility of those excipients to the active pharmaceutical ingredient [16],availability, physical properties like particle size example MCC-PH 102 was selected because of its large particle size compared to MCC-PH 101 which is an added advantage during granulation process [17]. Another reason used for excipient selection was that the most commonly used excipients in different albendazole formulations as shown in Table 2 was selected and also role of those excipients in the formulation to improve solubility and finally to improve dissolution of the final formulation example SLS was used as a solubilizing agent.
Table 2.
The proposed formulation included the following ingredients.
| SN | Ingredient | Function |
|---|---|---|
| 1 | Albendazole | Active ingredient |
| 2 | Polyvinylpyrrolidone (PVP) K30 | Binder |
| 3 | Crosscarmellose (CCM) | Disintegrant |
| 4 | Saccharin | Sweetener |
| 5 | Microcrystalline cellulose (Avicel PH 102) | Filler |
| 6 | Sodium Lauryl Sulphate (SLS) | Solubilizing agent |
| 7 | Magnesium stearate | Lubricant |
The percentage composition of each ingredient was proposed by the Design Expert Software (version 7.0) Different formulation trials were randomized and presented in Table 3.
Table 3.
Percentage compositions of excipients in different formulation trials.
| S/N | F.1 | F.2 | F.3 | F.4 | F.5 | F.6 | F.7 | F.8 | F.9 | F.10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Albendazole Active | 50% | 50% | 50% | 50% | 50% | 50% | 50% | 50% | 50% | 50% |
| 2 | MCC | 38% | 38% | 38% | 38% | 38% | 38% | 38% | 38% | 38% | 38% |
| 3 | Mg Stearate | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% |
| 4 | Na Saccharin | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% | 1% |
| 5 | CCM | 4.5% | 2.5% | 3.51% | 4.35% | 3.5% | 5% | 4.95% | 3.23% | 3.73% | 4.14% |
| 6 | PVP K30 | 5% | 5% | 5% | 3.15% | 5% | 2.5% | 3.12% | 4.48% | 3.76% | 4.19% |
| 7 | SLS | 0.5% | 2.5% | 1.49% | 2.5% | 1.5% | 2.5% | 1.91% | 2.28% | 2.5% | 1.65% |
Results of powder characterization shown in Table 4 above shows that direct compression cannot be employed because of the poor flow ability of the powdered mixture, a wet granulation method was used to improve flow ability of the powdered mixture and this has been shown in Table 5.
Table 4.
Results of hausner's ratio and Carr's index of a trial formulation.
| Hausner's ratio | Carr's index | Comments on flow properties | |
|---|---|---|---|
| First trial | 1.51 | 34% | Very poor |
Table 5.
Results for Hausner and Carr's Index ratios.
| Formulation | Hausner's ratios | Type of Flow | Carr's Index | Type of Flow |
|---|---|---|---|---|
| F.1 | 1.15 | Good | 13% | Good |
| F.2 | 1.12 | Good | 11% | Good |
| F.3 | 1.18 | Fair | 15% | Good |
| F.4 | 1.15 | Good | 13.26% | Good |
| F.5 | 1.18 | Good | 15% | Good |
| F.6 | 1.22 | Passable | 20% | Fair |
| F.7 | 1.19 | Fair | 15% | Good |
| F.8 | 1.16 | Good | 14% | Good |
| F.9 | 1.19 | Fair | 16% | Fair |
| F.10 | 1.17 | Good | 15% | Good |
Wet granulation method was used during formulation development to improve dissolution rate of a chewable albendazole tablets similar to the study of formulating and characterization of albendazole chewable tablets [18]. Preparation of microparticles by using spray drying technique, Spherical crystallization, Solid dispersion, nanotechnology and complexation of albendazole with cyclodextrin both of these methods were used to improve dissolution rate [19, 20, 21].
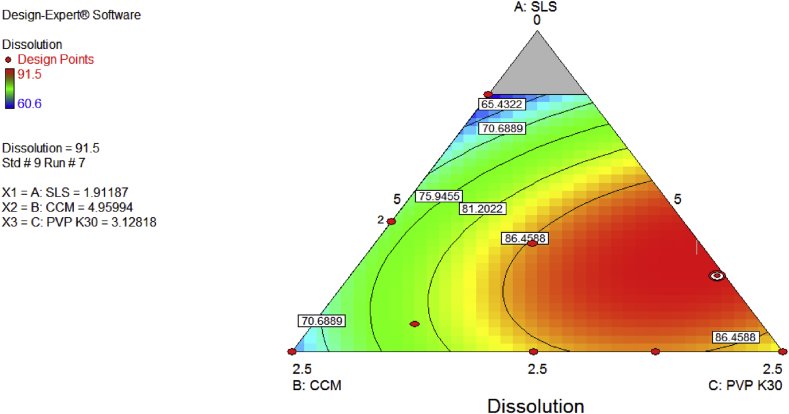
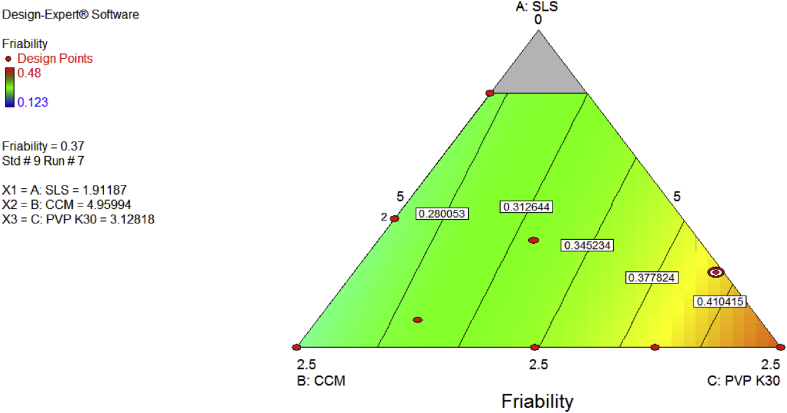
It has been found that increase in both solubilising agent and super disintegrants increase the friability of albendazole tablets but this is different when PVP K-30 (binder) is increased the friability reduced as predicted by design expert version 7 and shown in Figure 6.
Figure 6.
Contour diagram indicating optimization of dependent variable and prediction of dissolution.
All the Formulations showed satisfactory results as shown in Table 6 with respect to hardness, friability, weight variation, assay and in vitro disintegration time except for the percentage release, only five formulations (F 6, F7, F8, F9 and F10) releases more than eighty percent (80%).
Table 6.
Tablet hardness, Friability, Weight variation (%Rsd) disintegration time, dissolution and assay.
| Formulation | Tablet hardness (kg/cm2) | Friability (%) | Weight variation (%Rsd) | Disintegration time (Minutes) | Dissolution (%release) in 30 Minutes | Assay (%) |
|---|---|---|---|---|---|---|
| F.1 | 7.5 | 0.25 | 1.09 | 6:50 | 60.6 | 101.50 |
| F.2 | 7.6 | 0.18 | 1.07 | 10:42 | 64.4 | 99.27 |
| F.3 | 7.03 | 0.37 | 1.42 | 6:05 | 69.1 | 100.10 |
| F.4 | 7 | 0.37 | 1.58 | 3:00 | 82.5 | 99.85 |
| F.5 | 7.8 | 0.123 | 1.92 | 5:50 | 76.3 | 100.20 |
| F.6 | 5.8 | 0.48 | 1.2 | 2:15 | 85.7 | 99.45 |
| F.7 | 7 | 0.37 | 1.13 | 2:50 | 91.5 | 99.23 |
| F.8 | 6.9 | 0.476 | 1.49 | 4 | 85.6 | 102.16 |
| F.9 | 7.8 | 0.23 | 1.21 | 5 | 86.1 | 100.15 |
| F.10 | 7.04 | 0.385 | 1.07 | 5:50 | 85.7 | 99.25 |
Hardness of the tablets depends on the compression force during compression of the granules, hardness reduce the friability of tablet and increase disintegration time. Increase in the composition of the super disintegrant (CCM) reduces the disintegration time [22, 23]. It was found that the higher the amounts of super disintegrant together with solubilizing agent (SLS) improve the dissolution rate of albendazole tablet as predicted by Design Expert Software (version 7) and shown in Figure 7 and Table 7 and this is similar to the study of formulating and characterizing albendazole chewable tablets [18]. This proves the effect of solubilizing agent in improving the solubility of poor substance like albendazole molecule.
Figure 7.
Contour diagram indicating optimization of the independent variables and prediction of friability.
Table 7.
Design constraints in formulation development and optimization of albendazole tablets.
| Low≤ | Constraint | ≤ High |
|---|---|---|
| 0.5≤ | A:SLS | ≤2 |
| 0.5≤ | B: PVP K30 | ≤5 |
| 0.5≤ | C:CCM | ≤5 |
| A + B + C | =10 |
It has been found that use of Sodium Lauryl sulphate (SLS), Crosscarmellose (CCM) together with Polyvinyl pyrrolidone K-30 helps to improve dissolution rate of albendazole tablets. Moreover, it was found that the dissolution rate of the drug was affected by the polymer type and the ratio of albendazole to polymer. The highest dissolution of albendazole was obtained with HPMC in 1:1 ratio and with both PVA and PVP in ratio of 1: 4 microparticles [20].
Formulation (F7) was selected among five formulations (F6, F7, F8, F9 and F10) which release more than 85% as according to United State Pharmacopeia.
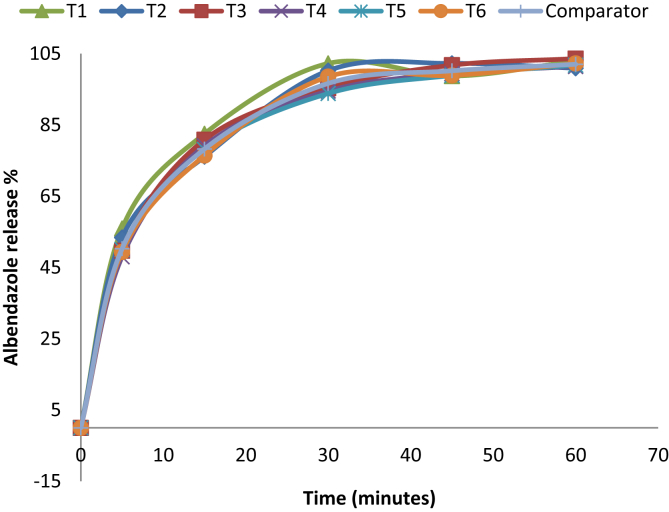
Albendazole new generic formulation is similar with innovator product in terms of percentage release as shown Table 8 also with the similarity factor (f2) of 62 and the difference (f1) is 6 as presented in Table 9, according to the acceptance criteria similarity factor (f2) and difference factor (f1) should be within this range 50–100% and 0–15% respectively.
Table 8.
Percentage release of the innovator product (comparator) and new generic formulation in hydrochloric acid buffer of pH1.2
| Time, Minutes | % Release Zentel | % Release new Formulation |
|---|---|---|
| 0 | 0 | 0 |
| 5 | 38.8 | 43.8 |
| 15 | 77.4 | 78.9 |
| 30 | 89.3 | 97.5 |
| 45 | 95.2 | 100 |
| 60 | 98.6 | 102.2 |
Table 9.
Difference factor (f1) and Similarity factor (f2) in terms of percentage release at pH 1.2
| Difference factor (f1) | Similarity factor (f2) | |
|---|---|---|
| Albendazole | 6 | 62 |
Albendazole tablets release more at a pH 1.2 as compared to pH 4.5 as shown in a Figure 8 and even very low release it is almost negligible at a pH 6.8 and this is explained by change in solubility of albendazole as you change acidity to alkalinity of the dissolution media, albendazole precipitate as you change pH from acidity to alkalinity.
Figure 8.
Comparison of percentage release (Dissolution) between new albendazole formulation & zentel/innovator at pH 1.2.
4. Conclusion
The developed generic albendazole tablet was found to be similar to the reference product on the market in terms of quality parameters, therefore can be produced as a generic formulation by an interested local pharmaceutical industry in the country.
5. Recommendation
Stability and bioequivalence studies are recommended to be performed if this new generic albendazole formulation is to be marketed in our local regions. Also Government financial support is needed to improve research activities for the development of the nation.
Declarations
Author contribution statement
Emmanuel Kimaro: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Eliangiringa Kaale: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mary Temu: Conceived and designed the experiments; Analyzed and interpreted the data.
Prosper Tibalinda, Raphael Shedafa: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Hotez P.J., Molyneux D.H., Fenwick A., Ottesen E., Sachs S.E., Sachs J.D. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS , tuberculosis , and malaria A comprehensive pro-poor health policy and strategy for the developing world. 2006;3(5):576–584. doi: 10.1371/journal.pmed.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotez P.J. Neglected diseases and poverty in "The Other America": the greatest health disparity in the United States? PLoS Negl Trop Dis. Public Libr. Sci. 2007 Dec 26;1(3):e149. doi: 10.1371/journal.pntd.0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crompton D.W.T., Nesheim M.C. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu. Rev. Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 4.Miguel E., Kremer M. worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica. 2004;72(1):159–217. [Google Scholar]
- 5.WHO/OIE Manual on Echinococcosis in Humans and Animals: a Public Health Problem of Global Concern. 2001. [Google Scholar]
- 6.Murrell KD, Dorny P, Flisser A, Geerts S, Kyvsgaard NC, Mcmanus D, et al. WHO/FAO/OIE Guidelines for the Surveillance, Prevention and Control of Taeniosis/cysticercosis Editor: Associate Editors.
- 7.Cavalcanti N.C.T., Sousa G.D., Tabosa M.A.M., Sobrinho J.L.S., Leal L.B., de Santana D.P. Assay and physicochemical characterization of the antiparasitic albendazole. Braz. J. Pharm. Sci. 2012;48(2):281–290. [Google Scholar]
- 8.Alexander T., Florence D.A. Physicochemical principles of pharmacy. J. Chem. Inf. Model. 2013;53(9):1689–1699. [Google Scholar]
- 9.Galia E., Horton J., Dressman J.B. Albendazole generics — a comparative in vitro study. Pharm. Res. 1999 doi: 10.1023/a:1018907527253. 16: 1871. 16: 1871. [DOI] [PubMed] [Google Scholar]
- 10.Srinath K.R. Formulation and evaluation of effervescent paracetamol tablets. Int. J. Pharm. Res. Dev. 2011;3(3):76–104. 12. [Google Scholar]
- 11.Wells J. Pharmaceutical preformulation; the physical chemical properties of drug substances. In: Aulton M.E., editor. Pharmaceutics. The Science of Dosage Form Design. second ed. Churchil Livingston; 2002. [Google Scholar]
- 12.British Pharmacopoeia. 2013. Method 2.9.8. [Google Scholar]
- 13.Sharma Kuchi Shishir Chandra, Kumar Y. Kranthi, Ranjith Reddy Kondeti. Effect of drug release on albendazole chewable tablets by using different formulation techniques. Int. J. Pharm. Sci. Res. 2014;5(10):4543–4547. [Google Scholar]
- 14.Agency E.M. 2008. ICH Q4B Annex 5 - Disintegration Test General Chapter. September. [Google Scholar]
- 15.Solid R., Dosage O. 1997. Guidance for Industry Guidance for Industry Dissolution Testing of Immediate. August. [Google Scholar]
- 16.Narang A. Drug excipient interactions. In: Narang A., Boddu S., editors. Excipient Applications in Formulation Design and Drug Delivery. Springer; Cham: 2015. [Google Scholar]
- 17.Hindi S.S.Z. Microcrystalline Cellulose : the inexhaustible treasure for. Pharmaceut. Ind. 2017;4(1):17–24. [Google Scholar]
- 18.Sinica D.P., Anusha V., Palanichamy S., Sugumar M., Rajesh M., Parasakthi N. Formulation and characterization of albendazole chewable tablets. Der Pharm. Sin. 2012;3(2):211–216. [Google Scholar]
- 19.Elsamaligy Samar, ShaymaaKhater Wedadsakran. Improvement of solubility dissolution rate by ternary Solid dispersion technique. Am. J. Pharm. Health Res. 2014 [Google Scholar]
- 20.Alanazi F.K., El-badry M., Ahmed M.O., Ibrahim A. Improvement of albendazole dissolution by preparing microparticles using spray-drying technique. Sci. Pharm. 2007;75:63–79. [Google Scholar]
- 21.Prashnant Rajaiya, Mishra Rakesh, Tanaji Nandgude, Poddar Sushilkumar. Asian Journal of Biomedical and Pharmaceutical Sciences; 2016. Solubility and dissolution enhancement of albendazole by spherical crystallization. [Google Scholar]
- 22.Naga Aparna T., Sambasiva Rao A. Traditional and emerging disintegrants - a review. Int. J. Pharm. Sci. Rev. Res. 2013;22(1):205–212. [Google Scholar]
- 23.Hari Har Prasad M., Duraivel S. Effect of different binders and super disintegrants on formulation of glimepiride immediate release tablets by wet granulation method. Int. J. Pharm. Chem. Res. 2012;4(4):44–47. [Google Scholar]