Abstract
Oncolytic virotherapy uses replication-competent virus as a means of treating cancer. Whereas this field has shown great promise as a viable treatment method, the limited spread of these viruses throughout the tumor microenvironment remains a major challenge. To overcome this issue, researchers have begun looking at syncytia formation as a novel method of increasing viral spread. Several naturally occurring fusogenic viruses have been shown to possess strong oncolytic potential and have since been studied to gain insight into how this process benefits oncolytic virotherapy. Whereas these naturally fusogenic viruses have been beneficial, there are still challenges associated with their regular use. Because of this, engineered/recombinant fusogenic viruses have also been created that enhance nonfusogenic oncolytic viruses with the beneficial property of syncytia formation. The purpose of this review is to examine the existing body of literature on syncytia formation in oncolytics and offer direction for potential future studies.
Keywords: syncytia, oncolytic virotherapy, fusogenic virus, fusion protein
Main Text
Roughly 1.6 million new cases of cancer are diagnosed each year, leading to an ever-increasing number of patients in need of viable treatment options.1 Whereas there have been many advances in cancer treatment, most patients ultimately still undergo chemotherapy and/or radiation therapy as their standard of care. Unfortunately, these treatments are associated with varying amounts of success, and many patients experience either refractory or relapsing disease. Due to these underwhelming results, medicine has long been searching for more efficient solutions to the problem of cancer.
One solution that has recently shown great promise is the field of oncolytic virotherapy (OV).2 This treatment uses cancer-tropic viruses to infect and subsequently eliminate a wide range of malignancies. The power of the oncolytic strategy is 2-fold. First, it combines a multimodal therapeutic approach, which is both rapid, through the direct cellular death caused from viral infections, as well as long term, through the initiation of an adaptive immune response against both viral and tumor antigens.3, 4, 5, 6 Second, genetic engineering allows for “arming” of the oncolytic genomes to maximize phenotypes, which are associated with improved treatment efficacy.7
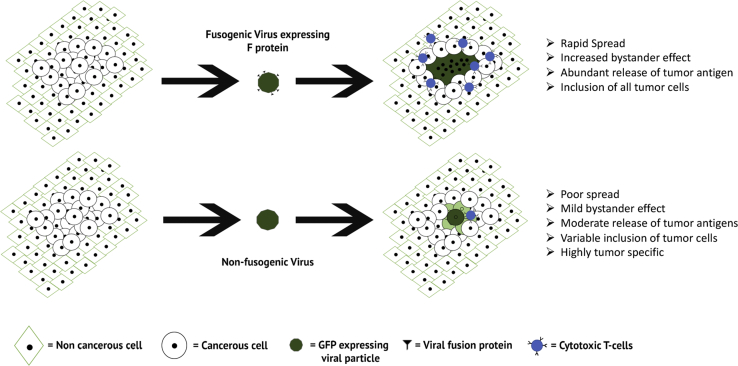
One phenotype, which has been suggested to improve oncolytic potential, is the ability of a virus to form syncytia. Syncytia are multinucleated cells created by the fusion of membranes from neighboring cells (Figure 1). Syncytia appear naturally during development, in the trophoblast, as well as in the development of the embryo.8,9 In other instances, however, such a phenotype results in either abnormal cell death or the spread of an infectious pathogen, such as a virus.10, 11, 12, 13 In 2002, a seminal paper by Fu and Zhang14 proposed that the induction of syncytia during OV might improve therapeutic efficacy both by enhancing viral spread within the tumor microenvironment as well as by inducing bystander killing of noninfected tumor cells. Since then, a number of naturally syncytia-forming viruses have been studied as potential oncolytic candidates.15 Additionally, a variety of nonsyncytia-forming viruses have been genetically engineered to induce artificially cell-cell fusion. Both types of viruses spread through de novo infection as well as fusion of infected and uninfected cells, which theoretically increases both their dissemination throughout the tumor and their overall efficacy. The purpose of this review is to discuss the capacity of different oncolytic viruses to form syncytia and how this ability influences each virus’s therapeutic potential.
Figure 1.
Schematic of Syncytia-Mediated Viral Spread
During traditional viral infections, spread occurs slowly by repeated infections of single cells following production of new infectious progeny. In syncytia-mediated viral spread, dissemination is facilitated by the expression of the fusion protein on the infected cells, which in turn, binds to various receptors on the neighboring cells. By spreading through the interaction of the fusion protein, dissemination is both more rapid and no longer limited to cells that express the viral receptor. This results in infection of more tumor cells than with a nonfusogenic virus.
Natural Syncytia Viruses
Several viral families have evolved the ability to form syncytia between individual infected cells and neighboring uninfected cells. During infection, this phenomenon is facilitated by a viral fusion protein (often termed F) that mediates its function either with or without the presence of additional viral proteins.16,17 A number of these naturally occurring fusogenic viruses have been studied as oncolytic agents, including Newcastle disease virus (NDV), Sendai virus (SV), respiratory syncytial virus (RSV), and measles. In addition to these viruses, other viral families can induce membrane fusion between viral particles and cellular membranes without causing subsequent syncytia formation. For the purpose of this review, however, we will limit our discussion to viruses for which infection has been definitively shown to result in direct cell-cell fusion.
Newcastle Disease Virus
NDV represents one of the first oncolytic viruses to show clinical potential and has been studied for more than 60 years.18, 19, 20 NDV differentiated itself from other early oncolytic candidates both for its ability to infect a majority of human cancers, without the presence of a tumor-specific receptor,21 and for its lack of pathogenicity in humans.22 This allowed NDV to be used against a plethora of cancers with relative success.23, 24, 25, 26 From an oncolytic standpoint, at least part of the efficacy of NDV is achieved through virally induced apoptosis.27 Unfortunately, few studies have defined the mechanism through which NDV induces this pathway; however, it has been suggested that it is a cytotoxic effect of viral syncytial formation.28,29 It has been known for many years that NDV encodes a fusion complex that is normally involved in the fusion of the virion with a host cell during infection.30 For NDV, this fusion complex is made up of both the viral F (fusion) and HN (neuraminadase) proteins, with the F protein being initially transcribed as an inactive form (F0) and subsequently cleaved into the active polypeptides F1 and F2 by cellular proteases.21,31 Whereas the major evolutionary role of this fusion complex is most likely during viral entry, studies have also described high multiplicity NDV infections resulting in syncytia formation between infected and noninfected cells.32 Interestingly, during these infections, the relationship between F and HN plays a significant role in determining the outcomes of syncytia formation. The presence of both proteins results in efficient formation of stable syncytia, whereas mutations in one or both of these proteins can result in hyperfusogenic phenotypes that increase oncolytic potential.33, 34, 35 This suggests that the role of syncytia formation during oncolytic NDV treatment might vary based on the ratio of F to HN during a given infection. Some studies have attempted to bypass this issue by generating mutated forms of NDV that are hyperfusogenic due to an altered multibasic cleavage component of the F0 protein.36, 37, 38, 39 In these studies, the introduced mutations increased therapeutic efficacy by eliminating the need for the HN attachment protein. The above studies build off of an earlier study that shows that a mutation of lysine to arginine resulted in a mutated NDV that can mediate cell:cell fusion in the absence of HN.40 However it is important to mention that whereas this mutation allows for F to induce cell:cell fusion without HN, this occurs less efficiently than with HN present. This HN independence promoted NDV infection by decreasing the specificity of F protein binding, allowing for inclusion of a more heterogeneous tumor population. Surprisingly, these works also show that this increased fusion potential does not increase toxicity to healthy cells. These finding are significant since they provide evidence that virally induced fusion can be modified while still maintaining the cancer-specific tropism of oncolytic viruses in terms of the virus being able to replicate preferentially in tumor cells. Although this work establishes that oncotropic specificity can be maintained during syncytia formation, however, the molecular basis for this specificity was not determined.
In addition to the F:HN ratio, it has also been shown that the outcome of the fusogenicity of NDV depends on tumor cells’ resistance to apoptosis.41 Under normal conditions, NDV-induced syncytial formation rapidly activates apoptotic pathways, resulting in cellular death.42, 43, 44, 45 Although this is might seem an attractive outcome during OV, there is a fine balance in terms of cellular death and viral replication.46 In the case of fusogenic NDV, it is plausible that the increase in cellular death occurs too rapidly, thus preventing completion of the replication cycle. In contrast, expression of the anti-apoptotic protein B cell lymphoma-extra large (BCL-xL) during infection inhibits acute apoptotic cell death, which allows for more sustained viral replication.41,47 Over time, this decreased cell death actually results in improved viral spread and increased release of tumor antigens.48 These data suggest that the upregulation of BCL-xL might promote increased oncolytic potential of fusogenic NDV over time. Interestingly, it has previously been reported that the upregulation of BCL-xL is able to confer resistance to both multidrug and radiation therapy through a similar anti-apoptotic mechanism.49,50 By hijacking the tumors own cell-survival pathways, fusogenic NDV might therefore become an attractive treatment option for patients who have failed traditional therapies.
Sendai Virus
SV is a member of the same viral family as NDV, and the two viruses likely possess similar oncolytic characteristics.51 Compared to NDV, however, the oncolytic potential of SV is less well studied, although it also appears to have potential against a wide range of cancers.52,53 Like NDV, SV forms syncytia upon infection, which results in the induction of apoptotic cell death.52,54 Additionally, SV also encodes an accessory glycoprotein (G) HN, which aids in both virus:cell as well as cell:cell fusion,55, 56, 57 although it has been suggested that this protein is less important for the latter.58
Importantly, recent studies have directly addressed the role that syncytia formation plays in SV-based therapy.53,54,59 A publication by Hasegawa et al.53 demonstrates that the use of a fusogenic SV strain can increase both spread and cytotoxicity from viral infection by as much as four times over that of a nonfusogenic counterpart across a range of glioblastoma (GB) tumor lines. These results were then translated to an in vivo model using a virus also encoding an interferon (IFN)-β transgene, which resulted in significantly reduced tumor volume and improved overall survival compared to both mock and nonfusogenic controls.53 The authors of this work suggest that the efficacy of this fusogenic virus might be partially due to potential synergy between syncytia formation, which increased spread of the fusogenic virus, and the INF-β transgene, which enhanced overall anti-tumor immunity. In contrast, another possible explanation to why fusogenic SV shows increased efficacy was offered in a study by Suzuki et al.60 In this study, the research team found the presence of the SV-F protein itself resulted in an upregulation of interleukin-6 (IL-6) following treatment.60 Critically, IL-6 is associated with the inhibition of T-regulatory (Treg) cells, which are known to downregulate adaptive immune responses during immunotherapy. The fact that the F protein of SV can trigger IL-6 upregulation could therefore explain part of the increased efficacy seen with fusogenic SV. Whether this is an isolated phenomenon or whether all fusion proteins produce similar effects, however, remains to be determined.
Respiratory Syncytial Virus
RSV was originally identified as an oncolytic virus due to the degree of sensitivity that it exhibits toward IFN. As cancer cells have frequently lost the ability to respond to IFN, this allows for highly oncotropic infections using RSV.61 As with NDV and SV, the RSV-F protein is transcribed in an inactive form that then undergoes additional processing to the active form. In the case of RSV, F0 is cleaved by furin to produce the active complex consisting of two subunits: F1 a C-terminal membrane-anchored subunit, and F2, the N-terminal subunit. Like SV, RSV fusogenicity is not dependent on the presence of an HN protein, although this virus does encode a glycoprotein (G that can enhance its fusion capacity.62, 63, 64, 65, 66, 67, 68, 69 Theoretically, the ability of RSV to initiate fusion with neighboring cells, independent of coexpressed attachment proteins, could make this virus a more versatile inducer of syncytia formation; unfortunately, the direct role that syncytia formation plays in RSV-based oncolytics remains poorly defined
A 2015 paper by Choi et al.70 demonstrates that infection with RSV resulted in a reduction in the growth of some, but not all, hepatocellular carcinoma cell lines and that syncytia formation was specifically present in the cell lines that showed a decrease in cellular viability. A second study obtained similar results in a variety of skin carcinoma cell lines; however, this work also showed that infection with RSV resulted in increased apoptotic cell death that correlated with syncytia formation.71 Unfortunately, neither paper directly examines if there is a mechanistic relationship between syncytia formation and the inhibition of cancer cell growth; however, RSV-dependent syncytial formation has been shown to result in activation of p53, which could explain the apparent correlation.72,73
Measles Virus
The oncolytic potential of genetically modified measles virus (MV) has been known since the 1970s.74,75 Since then, the virus has been used against a wide range of cancers from many different tissue types, including lymphoma, leukemia, gliomas, and osteosarcoma.76, 77, 78, 79 MV is able to infect cells when its fusion complex interacts with one of three receptors on the target cell: CD46, signaling lymphocyte activation molecule (SLAM), or nectin-4.80, 81, 82, 83 MV then induces cell-to-cell fusion via expression of the MV-F protein and its interaction with the hemagglutinin protein H.84 Similar to other fusogenic viruses, MV-F is initially translated as an inactive form that cannot interact with the H protein to create the fusion complex unless MV-F is cleaved by the appropriate proteases during vesicular trafficking.85, 86, 87
A 2015 paper by Studebaker et al.88 shows the positive effect that fusogenicity has on oncolytic MV treatment of a typical teratoid rhabdoid tumor. Whereas this is a rare cancer subset, this work is interesting because it offers two key findings relevant to the use of syncytia forming oncolytic agents. First is that even a low MOI is sufficient to reduce tumor cell viability drastically after infection. Second is that the use of fusogenic virus in vivo can improve survival in both localized and metastatic tumor models. Similar results were also reported by the same group in the context of medulloblastoma.89 Together, these results show that the enhanced efficacy of fusogenic viruses is not strictly due to improvements in direct viral infection and spread alone but rather a combination of these mechanisms, along with the cytotoxic effects of noninfected cells, known as the bystander effect. Whereas the mechanism(s) mediating this bystander killing are likely multifactorial, efficacy does require viral replication and is associated with both increased cytotoxicity correlated with syncytial formation as well as activation of the Toll-like receptor 2 (TLR-2) antiviral signaling pathways.79 Critically, this work suggested that activation of TLR-2 signaling was caused by the mere presence of the H protein in the fusion complex.90, 91, 92 Similar to the induction of IL-6 by the SV-F protein, this result suggests that the presence of a fusion complex could itself be inflammatory, independent of either viral replication or cellular lysis. Interestingly, the authors of this work further suggest that in the case of MV, sensing of the F protein might be enhanced by the evolutionary interaction of the immune system with the human MV.93,94 Whether a similar effect is seen with the use of nonhuman oncolytic viruses is therefore a question that future studies should address.
Although both of the previous papers discuss how fusogenic MV can be used against fully differentiated tumor cell lines, tumor-initiating cells (TICs) pose a much greater challenge in terms of therapeutic outcomes and techniques.95, 96, 97 These cells are often resistant to most conventional treatments and are a primary cause of relapse for cancer patients.6,98,99 It is therefore interesting to note that MV-induced syncytia can apparently include gliomal TICs based on the inclusion of the CD133 marker.78 This work demonstrates a critical characteristic of syncytial-forming viruses in that they can often form syncytia with cells near them regardless of these cells’ susceptibility to direct viral infection. This allows syncytia-forming oncolytic viruses to spread to both differentiated tumor cells and TICs, offering a much greater therapeutic potential. Critically, the inclusion of normally noninfectible TICs in MV-induced syncytia appears at odds with the previously discussed retention of oncotropism following modification of virally induced fusion. Future studies are therefore needed to define the breadth and specificity of the fusogenic phenotype both in vitro and in vivo.
Engineered Syncytia Viruses
With recent advances in molecular cloning and improved understanding of viral genomes, it is now possible to increase the oncolytic potential of many viruses through the addition or removal of specific genes. The goal of these changes is to increase or decrease phenotypes associated with strong oncolytic potential, including various aspects of the immune response (natural killer [NK] cell inhibition, CD8+ T cell activation, checkpoint blockade inhibition, etc.), lysis of infected cells, or spread within the tumor. One approach scientists have begun studying is to insert the fusogenic proteins of naturally occurring syncytia viruses into the proven backbones of nonfusogenic oncolytic viruses in order to enhance the spread of these viruses through the tumor. This combination allows for oncolytic viruses to benefit from the increased spread and lytic potential caused by syncytial formation while maintaining the inherent oncolytic properties of their nonfusogenic backbones. A variety of common vectors and fusion proteins have been used during these studies.
Recombinant Vesicular Stomatitis Virus
Vesicular stomatitis virus (VSV) is a cattle pathogen that is largely nonpathogenic in humans. Similar to RSV, VSV has been shown to replicate preferentially in tumor cells due to its restriction by functional IFN responses.100,101 Whereas VSV can cause membrane fusion between the virion and the host cell membranes, true syncytia formation is normally prevented by the presence of the fusion glycoprotein G, which is capable of initiating fusion only at acidic pH.102 In addition to its natural oncolytic potential, VSV is also frequently used to create recombinant viruses, since the virus offers significant freedom to add therapeutic genes to the genome without compromising other aspects of the viral biology.103
One example of such a recombinant virus is that of the VSV-H construct that encodes both the MV-F and H proteins into the VSV genome.104 This virus maintains the IFN-restricted replication properties of VSV,105 while adding the CD46-specific fusion mechanics of MV. Interestingly however, the new recombinant virus possesses greater all-around oncolytic capacity than either wild-type parental virus. This increased capacity is partially due to the replacement of the endogenous VSV-G protein with that of the MV fusion complex. With this replacement, VSV-induced membrane fusion is no longer limited by the pH dependence characteristic of the G fusion protein, giving the recombinant virus a more general fusion ability than either wild-type vector.102,106,107 This increased ability to produce syncytia results in the generation of significantly larger plaques than those seen with wild-type VSV, as well as syncytia formation that is independent of CD46 receptor density.108 A second recombinant VSV encoding only MV-F displayed a similar phenotype but also had improved cytotoxic effects against TICs, again independent of CD46-based viral entry, suggesting a second possible mechanism through which improved oncolytic potential might be obtained.109
A 2017 study by Le Boeuf et al.110 shows another recombinant VSV possessing a fusion-associated small transmembrane (FAST) protein. FAST proteins are F proteins that have been isolated from reovirus and are attractive from a genetic engineering perspective, since they are the smallest viral fusion proteins that allow for easy insertion into other oncolytic genomes.111 The results of this paper demonstrate that recombinant VSVs, which encode FAST proteins, are able to induce syncytial growth in vitro. The study then goes on to demonstrate that treatment with the recombinant virus leads to a reduction in the growth of established tumors in vivo. Additionally, VSV-FAST treatment also reduced the size and number of lesions in both metastatic breast and colon cancer models.110 This finding was not seen with nonfusogenic control viruses, suggesting that the ability of the recombinant virus to form syncytia may have induced an increased adaptive immune response, a hypothesis that was supported by VSV-FAST treatment, causing an increase in activated CD8+ and CD4+ T cells in both treated and nontreated tumors. Although this activation would start mainly in the treated tumors, as the adaptive immune response to the tumor escalates, these cells would localize into the metastatic lesions as well. This enhanced immune activation has been previously attributed to the rapid release of antigens that occurs during immunogenic death of the syncytia;15,112 however, our review suggests that other potential mechanisms, such as IL-6 or TLR activation, might also be involved.
Insertion of NDV-F has also been shown to improve the oncolytic potential of VSVs.113 In this work, mutated NDV-F was used, which allows for the induction of fusion, independent of the NDV-HN glycoprotein.113 Treatment with this virus resulted in both prolonged survival and increased viral infection in both metastatic liver and lung models compared to treatment with nonrecombinant controls. Importantly, as with the work on NDV, this study also demonstrates that altering virally fusogenicity does not compromise cancer tropism. In this case, the authors explicitly looked at the normal tissue surrounding the treated tumor to determine potential toxicities associated with introducing a fusogenic viral construct in an in vivo model; while robust infection of malignant tissue was observed, these studies demonstrated that infection was still excluded from surrounding normal cells. A later study using this same recombinant virus confirmed this result in metastatic colorectal cancer models.114
Recombinant Herpes Simplex Virus
Herpes simplex virus (HSV) is a member of the herpes virus family that is typically associated with dermal lesions (cold sores) in humans. Although certain strains of HSV can acutely form syncytia at physiological pH, the majority of HSV oncolytic trials, including the ones that led to the recent Food and Drug Administration (FDA) approval of Iymlygic,115 have been done using nonfusogenic recombinant HSV vectors armed with immune-modulating transgenes. In addition to the extensive body of oncolytic literature on nonfusogenic HSV, however, several studies have looked at the possibility of enhancing the normally low fusogenicity of HSV by adding fusogenic proteins into the HSV genome.
One such fusogenic virus is the recombinant HSV-GALV,116, 117, 118 which combines the oncolytic HSV backbone with the gibbon ape leukemia virus (GALV) fusion protein. Although GALV has poor oncolytic potential alone, the virus is hyperfusogenic, and insertion of its F protein into oncolytic HSV has been reported to generate a fusogenic construct with significantly increased oncolytic potential.119 Studies using this virus have shown that the addition of the GALV-F protein increases the death of infected tumor cells in vitro by up to 54%. Critically, as with work on fusogenic MV, these studies also observed substantial death of uninfected cells both in vivo and in vitro across several tumor models, including colorectal adenocarcinoma, GB astrocytoma, and lung epidermoid carcinoma. Since infection of 100% of malignant cells during oncolytic treatment has proven impossible to obtain, this enhanced bystander effect might represent a major mechanism through which fusogenic viruses achieved enhanced efficacy.
Fusogenic HSV was also used to study the potential impact of encoding two distinct fusion mechanisms into a single oncolytic virus.120 This work used a hyperfusogenic HSV backbone into which the authors additionally encoded the F glycoprotein of GALV.14 The proposed rationale for this approach was to combat the potential development of fusion resistance in treated tumors. Whether the development of this resistance is a problem during fusogenic OV remains unclear; however, the doubly fusogenic viruses did display enhanced efficacy in models of both breast and ovarian cancer. This improved efficacy correlated with increased cell death, release of virus into the tumor microenvironment at far greater levels (over 90% of the viral progeny), and higher rates of infection in vivo. Unfortunately, whereas the double fusion viral constructs resulted in overall impressive tumor control compared to untreated and nonfusion viral construct groups,107 no comparison to single fusogenic HSV constructs was included. Without this comparison, it is difficult to say how much added effect the addition of the second fusion protein has on the outcome of this model or if one such protein would have yielded the same results.
Finally, recombinant fusogenic HSVs have also been used to study the immunological ramifications of inducing syncytia formation.61,121,122 Similar to the work with VSV, these studies have generally shown that treatment with fusogenic HSVs induces more substantial and more effective anti-tumor immune responses than treatment with nonfusogenic controls. Critically, these results were found using HSVs encoding multiple distinct F proteins, which implies that the improved anti-tumor immunity is not specific to a single fusion protein, but instead represents an inherent benefit of syncytial induction. No mechanism for this enhanced immune activity has been discovered to date; however, one proposal is that it is related to fusogenic viruses improved with bystander killing. In this model, more cells being killed results in more antigens from the tumor able to be presented on antigen-presenting cells. This results in improved adaptive immunity, particularly against subpopulations of the tumor that were previously uninfectable by the virus. Alternatively, it has also been proposed that the formation of unique antigen-rich vesicles might play a role.61,123 These vesicles, known as syncytiosomes, are released at an increased rate from syncytial bodies and are then uptaken by antigen-presenting cells, resulting in increased cross presentation of tumor-associated antigens.61 Unfortunately, neither of these hypotheses has yet to be definitively proven in the context of OV. Therefore, whereas multiple studies point to syncytial formation inducing improved anti-tumor immunity, this phenomenon remains poorly understood and should be a focus of future studies.111,124
Recombinant Adenovirus
Adenovirus (Ad) is a member of the Adenoviridae family, which is characterized as nonenveloped, double-stranded DNA viruses. Oncolytic Ad has shown promising results across a variety of tumor models with many of the studies focusing on a common human variant, Ad subtype 5 (Ad5).125 Although Ad5 is normally, completely nonfusogenic, it can be made to induce syncytia by the addition of exogenous F proteins. In the case of Ad5, many of these studies have encoded various fusion proteins into replication-defective variants of the virus as a form of gene therapy. As this is not a true form of oncolytics, we have excluded these works form our current review; however, it should be noted that many of these papers show increased efficacy using fusogenic, nonreplicating Ad vectors, which supports the conclusion that formation of syncytia alone can be a potent mediator of anti-tumor efficacy.
The earliest study combining replication-competent Ad and syncytia formation used the unique approach of oncolytic Ad5 together with plasmid DNA encoding the GALV-F protein to induce syncytia formation. Similar to results with actual recombinant fusogenic viruses, this study found the exogenous addition of F protein increased both viral spread and cytotoxicity in vitro and impressively, translated into complete cures of both small and large established tumors in an in vivo model.126 Later studies confirmed that this efficacy could be duplicated in multiple cell lines when the GALV-F protein was inserted directly into the Ad genome,119,127,128 while still maintaining oncolytic safety. Interestingly, in contrast to previous studies using GALV-F, the efficacy of this fusogenic Ad appears to be strictly due to improved viral spread, since the immunological responses induced following treatment did not differ with the addition of the F protein.
Conclusions
Syncytia are defined as the fusion of multiple cells into a single multinucleated cell body. From an evolutionary perspective, the likely intention of virally induced syncytia formation is to increase the spread of a virus. Thus, the induction of this process represents a novel solution to one of the biggest challenges facing OV. By utilizing fusogenic viruses for OV, scientists have proven that forcing syncytia formation both can and will kill tumor cells regardless of tumor type. Although these results show the promise of syncytial-forming viruses, many questions remain about the mechanisms involved following therapy. In particular, the mechanisms mediating tumor specificity during fusogenic infection and the ability of these viruses to induce bystander killing and enhanced anti-tumor immunity remain to be determined. Additionally, although a variety of fusion proteins have been shown to enhance nonfusogenic oncolytic viruses, whether any of these has an inherent advantage compared to the others remains unclear. Therefore, whereas multiple studies have conclusively shown the promise of inducing syncytia formation during OV, additional mechanistic work is likely needed to maximize this strategy in the future.
Author Contributions
C.B. researched and prepared manuscript. E.B. oversaw the project and prepared the manuscript.
Conflicts of interest
The authors declare no competing interests.
References
- 1.Miller K.D., Siegel R.L., Lin C.C., Mariotto A.B., Kramer J.L., Rowland J.H., Stein K.D., Alteri R., Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Russell S.J., Peng K.W. Viruses as anticancer drugs. Trends Pharmacol. Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beryshnikov A.Iu., Bukhman V.M., Svet-Moldavskiĭ G.Ia., Kadagidze Z.G. [Use of stress on the reticuloendothelial system and hyperthemia for enhancement of tumor heterogenization by viruses and direct viral oncolysis] Vopr. Virusol. 1973;18:51–53. [PubMed] [Google Scholar]
- 4.Lapointe J.R.P., Portelance V. Growth retardation and prevention of Ehrlich solid tumor by Clostridium perfringens type A spores and culture supernatant. Cancer Res. 1978;38:2295–2300. [PubMed] [Google Scholar]
- 5.Chiocca E.A., Rabkin S.D. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol. Res. 2014;2:295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toda M. Immuno-viral therapy as a new approach for the treatment of brain tumors. Drug News Perspect. 2003;16:223–229. doi: 10.1358/dnp.2003.16.4.829334. [DOI] [PubMed] [Google Scholar]
- 7.Sze D.Y.R., Reid T.R., Rose S.C. Oncolytic virotherapy. J. Vasc. Interv. Radiol. 2013;24:1115–1122. doi: 10.1016/j.jvir.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Ogle B.M., Cascalho M., Platt J.L. Biological implications of cell fusion. Nat. Rev. Mol. Cell Biol. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 9.Shemer G., Podbilewicz B. The story of cell fusion: big lessons from little worms. BioEssays. 2003;25:672–682. doi: 10.1002/bies.10301. [DOI] [PubMed] [Google Scholar]
- 10.Roumier T., Castedo M., Perfettini J.L., Andreau K., Métivier D., Zamzami N., Kroemer G. Mitochondrion-dependent caspase activation by the HIV-1 envelope. Biochem. Pharmacol. 2003;66:1321–1329. doi: 10.1016/s0006-2952(03)00480-5. [DOI] [PubMed] [Google Scholar]
- 11.Sylwester A., Murphy S., Shutt D., Soll D.R. HIV-induced T cell syncytia are self-perpetuating and the primary cause of T cell death in culture. J. Immunol. 1997;158:3996–4007. [PubMed] [Google Scholar]
- 12.Symeonides M., Murooka T.T., Bellfy L.N., Roy N.H., Mempel T.R., Thali M. HIV-1-Induced Small T Cell Syncytia Can Transfer Virus Particles to Target Cells through Transient Contacts. Viruses. 2015;7:6590–6603. doi: 10.3390/v7122959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bracq L., Xie M., Benichou S., Bouchet J. Mechanisms for Cell-to-Cell Transmission of HIV-1. Front. Immunol. 2018;9:260. doi: 10.3389/fimmu.2018.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu X., Zhang X. Potent systemic antitumor activity from an oncolytic herpes simplex virus of syncytial phenotype. Cancer Res. 2002;62:2306–2312. [PubMed] [Google Scholar]
- 15.Higuchi H., Bronk S.F., Bateman A., Harrington K., Vile R.G., Gores G.J. Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: implications for gene therapy. Cancer Res. 2000;60:6396–6402. [PubMed] [Google Scholar]
- 16.Garner O.B., Aguilar H.C., Fulcher J.A., Levroney E.L., Harrison R., Wright L., Robinson L.R., Aspericueta V., Panico M., Haslam S.M. Endothelial galectin-1 binds to specific glycans on nipah virus fusion protein and inhibits maturation, mobility, and function to block syncytia formation. PLoS Pathog. 2010;6:e1000993. doi: 10.1371/journal.ppat.1000993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe S., Shirogane Y., Suzuki S.O., Ikegame S., Koga R., Yanagi Y. Mutant fusion proteins with enhanced fusion activity promote measles virus spread in human neuronal cells and brains of suckling hamsters. J. Virol. 2013;87:2648–2659. doi: 10.1128/JVI.02632-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prince A.M., Ginsberg H.S. Studies on the cytotoxic effect of Newcastle disease virus (NDV) on Ehrlich ascites tumor cells. I. Characteristics of the virus-cell interaction. J. Immunol. 1957;79:94–106. [PubMed] [Google Scholar]
- 19.Prince A.M., Ginsberg H.S. Studies on the cytotoxic effect of Newcastle disease virus (NDV) on Ehrlich ascites tumor cells. II. The mechanism and significance of in vitro recovery from the effect of NDV. J. Immunol. 1957;79:107–112. [PubMed] [Google Scholar]
- 20.Adams W.R., Prince A.M. An electron microscopic study of incomplete virus formation; infection of Ehrlich ascites tumor cells with chick embryo-adapted Newcastle disease virus (NDV) J. Exp. Med. 1957;106:617–626. doi: 10.1084/jem.106.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beier R., Hermiston T., Mumberg D. Isolation of more potent oncolytic paramyxovirus by bioselection. Gene Ther. 2013;20:102–111. doi: 10.1038/gt.2012.13. [DOI] [PubMed] [Google Scholar]
- 22.Lam H.Y., Yeap S.K., Pirozyan M.R., Omar A.R., Yusoff K., Suraini A.A., Abd-Aziz S., Alitheen N.B. Safety and clinical usage of newcastle disease virus in cancer therapy. J. Biomed. Biotechnol. 2011;2011:718710. doi: 10.1155/2011/718710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman A.I., Zakay-Rones Z., Gomori J.M., Linetsky E., Rasooly L., Greenbaum E., Rozenman-Yair S., Panet A., Libson E., Irving C.S. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. 2006;13:221–228. doi: 10.1016/j.ymthe.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Ahlert T., Sauerbrei W., Bastert G., Ruhland S., Bartik B., Simiantonaki N., Schumacher J., Häcker B., Schumacher M., Schirrmacher V. Tumor-cell number and viability as quality and efficacy parameters of autologous virus-modified cancer vaccines in patients with breast or ovarian cancer. J. Clin. Oncol. 1997;15:1354–1366. doi: 10.1200/JCO.1997.15.4.1354. [DOI] [PubMed] [Google Scholar]
- 25.Cassel W.A., Garrett R.E. Newcastle Disease Virus as an Antineoplastic Agent. Cancer. 1965;18:863–868. doi: 10.1002/1097-0142(196507)18:7<863::aid-cncr2820180714>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Sinkovics J.G., Horvath J.C. Newcastle disease virus (NDV): brief history of its oncolytic strains. J. Clin. Virol. 2000;16:1–15. doi: 10.1016/s1386-6532(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 27.Sui H., Wang K., Xie R., Li X., Li K., Bai Y., Wang X., Bai B., Chen D., Li J., Shen B. NDV-D90 suppresses growth of gastric cancer and cancer-related vascularization. Oncotarget. 2017;8:34516–34524. doi: 10.18632/oncotarget.16563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ravindra P.V., Tiwari A.K., Ratta B., Chaturvedi U., Palia S.K., Chauhan R.S. Newcastle disease virus-induced cytopathic effect in infected cells is caused by apoptosis. Virus Res. 2009;141:13–20. doi: 10.1016/j.virusres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Shobana R., Samal S.K., Elankumaran S. Prostate-specific antigen-retargeted recombinant newcastle disease virus for prostate cancer virotherapy. J. Virol. 2013;87:3792–3800. doi: 10.1128/JVI.02394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bratt M.A., Gallaher W.R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc. Natl. Acad. Sci. USA. 1969;64:536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y., Canine B.F., Hatefi A. HSV-TK/GCV cancer suicide gene therapy by a designed recombinant multifunctional vector. Nanomedicine (Lond.) 2011;7:193–200. doi: 10.1016/j.nano.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cram L.S., Forslund J.C., Jett J.H. Quantification of cell fusion by twenty-one strains of Newcastle disease virus using flow microfluorometry. J. Gen. Virol. 1978;41:27–36. doi: 10.1099/0022-1317-41-1-27. [DOI] [PubMed] [Google Scholar]
- 33.Rangaswamy U.S., Wang W., Cheng X., McTamney P., Carroll D., Jin H. Newcastle Disease Virus Establishes Persistent Infection in Tumor Cells In Vitro: Contribution of the Cleavage Site of Fusion Protein and Second Sialic Acid Binding Site of Hemagglutinin-Neuraminidase. J. Virol. 2017;91:e00770-17. doi: 10.1128/JVI.00770-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Leeuw O.S., Koch G., Hartog L., Ravenshorst N., Peeters B.P. Virulence of Newcastle disease virus is determined by the cleavage site of the fusion protein and by both the stem region and globular head of the haemagglutinin-neuraminidase protein. J. Gen. Virol. 2005;86:1759–1769. doi: 10.1099/vir.0.80822-0. [DOI] [PubMed] [Google Scholar]
- 35.Kim S.H., Subbiah M., Samuel A.S., Collins P.L., Samal S.K. Roles of the fusion and hemagglutinin-neuraminidase proteins in replication, tropism, and pathogenicity of avian paramyxoviruses. J. Virol. 2011;85:8582–8596. doi: 10.1128/JVI.00652-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altomonte J., Marozin S., Schmid R.M., Ebert O. Engineered newcastle disease virus as an improved oncolytic agent against hepatocellular carcinoma. Mol. Ther. 2010;18:275–284. doi: 10.1038/mt.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigil A., Park M.S., Martinez O., Chua M.A., Xiao S., Cros J.F., Martínez-Sobrido L., Woo S.L., García-Sastre A. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 2007;67:8285–8292. doi: 10.1158/0008-5472.CAN-07-1025. [DOI] [PubMed] [Google Scholar]
- 38.Song K.Y., Wong J., Gonzalez L., Sheng G., Zamarin D., Fong Y. Antitumor efficacy of viral therapy using genetically engineered Newcastle disease virus [NDV(F3aa)-GFP] for peritoneally disseminated gastric cancer. J. Mol. Med. (Berl.) 2010;88:589–596. doi: 10.1007/s00109-010-0605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamarin D., Martínez-Sobrido L., Kelly K., Mansour M., Sheng G., Vigil A., García-Sastre A., Palese P., Fong Y. Enhancement of oncolytic properties of recombinant newcastle disease virus through antagonism of cellular innate immune responses. Mol. Ther. 2009;17:697–706. doi: 10.1038/mt.2008.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sergel T.A., McGinnes L.W., Morrison T.G. A single amino acid change in the Newcastle disease virus fusion protein alters the requirement for HN protein in fusion. J. Virol. 2000;74:5101–5107. doi: 10.1128/jvi.74.11.5101-5107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansour M., Palese P., Zamarin D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J. Virol. 2011;85:6015–6023. doi: 10.1128/JVI.01537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molouki A., Yusoff K. NDV-induced apoptosis in absence of Bax; evidence of involvement of apoptotic proteins upstream of mitochondria. Virol. J. 2012;9:179. doi: 10.1186/1743-422X-9-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elankumaran S., Rockemann D., Samal S.K. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J. Virol. 2006;80:7522–7534. doi: 10.1128/JVI.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lam K.M., Vasconcelos A.C., Bickford A.A. Apoptosis as a cause of death in chicken embryos inoculated with Newcastle disease virus. Microb. Pathog. 1995;19:169–174. doi: 10.1006/mpat.1995.0055. [DOI] [PubMed] [Google Scholar]
- 45.Lam K.M. Apoptosis in chicken embryo fibroblasts caused by Newcastle disease virus. Vet. Microbiol. 1995;47:357–363. doi: 10.1016/0378-1135(95)00111-5. [DOI] [PubMed] [Google Scholar]
- 46.Kaminskyy V., Zhivotovsky B. To kill or be killed: how viruses interact with the cell death machinery. J. Intern. Med. 2010;267:473–482. doi: 10.1111/j.1365-2796.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 47.Polčic P., Mentel M., Gavurníková G., Bhatia-Kiššová I. To keep the host alive - the role of viral Bcl-2 proteins. Acta Virol. 2017;61:240–251. doi: 10.4149/av_2017_302. [DOI] [PubMed] [Google Scholar]
- 48.Kvansakul M. Viral Infection and Apoptosis. Viruses. 2017;9:356. doi: 10.3390/v9120356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Datta R., Manome Y., Taneja N., Boise L.H., Weichselbaum R., Thompson C.B., Slapak C.A., Kufe D. Overexpression of Bcl-XL by cytotoxic drug exposure confers resistance to ionizing radiation-induced internucleosomal DNA fragmentation. Cell Growth Differ. 1995;6:363–370. [PubMed] [Google Scholar]
- 50.Minn A.J., Rudin C.M., Boise L.H., Thompson C.B. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood. 1995;86:1903–1910. [PubMed] [Google Scholar]
- 51.Gao H., Gong X.C., Chen Z.D., Xu X.S., Zhang Q., Xu X.M. Induction of apoptosis in hormone-resistant human prostate cancer PC3 cells by inactivated Sendai virus. Biomed. Environ. Sci. 2014;27:506–514. doi: 10.3967/bes2014.082. [DOI] [PubMed] [Google Scholar]
- 52.Matveeva O.V., Guo Z.S., Senin V.M., Senina A.V., Shabalina S.A., Chumakov P.M. Oncolysis by paramyxoviruses: preclinical and clinical studies. Mol. Ther. Oncolytics. 2015;2:150017. doi: 10.1038/mto.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasegawa Y., Kinoh H., Iwadate Y., Onimaru M., Ueda Y., Harada Y., Saito S., Furuya A., Saegusa T., Morodomi Y. Urokinase-targeted fusion by oncolytic Sendai virus eradicates orthotopic glioblastomas by pronounced synergy with interferon-β gene. Mol. Ther. 2010;18:1778–1786. doi: 10.1038/mt.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matveeva O.V., Kochneva G.V., Netesov S.V., Onikienko S.B., Chumakov P.M. Mechanisms of Oncolysis by Paramyxovirus Sendai. Acta Naturae. 2015;7:6–16. [PMC free article] [PubMed] [Google Scholar]
- 55.Aroeti B., Henis Y.I. Accumulation of Sendai virus glycoproteins in cell-cell contact regions and its role in cell fusion. J. Biol. Chem. 1991;266:15845–15849. [PubMed] [Google Scholar]
- 56.Lamb R.A. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197:1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- 57.Russell C.J., Jardetzky T.S., Lamb R.A. Membrane fusion machines of paramyxoviruses: capture of intermediates of fusion. EMBO J. 2001;20:4024–4034. doi: 10.1093/emboj/20.15.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merz D.C., Scheid A., Choppin P.W. Importance of antibodies to the fusion glycoprotein of paramyxoviruses in the prevention of spread of infection. J. Exp. Med. 1980;151:275–288. doi: 10.1084/jem.151.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kinoh H., Inoue M., Komaru A., Ueda Y., Hasegawa M., Yonemitsu Y. Generation of optimized and urokinase-targeted oncolytic Sendai virus vectors applicable for various human malignancies. Gene Ther. 2009;16:392–403. doi: 10.1038/gt.2008.167. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki H., Kurooka M., Hiroaki Y., Fujiyoshi Y., Kaneda Y. Sendai virus F glycoprotein induces IL-6 production in dendritic cells in a fusion-independent manner. FEBS Lett. 2008;582:1325–1329. doi: 10.1016/j.febslet.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 61.Bateman A.R.H., Harrington K.J., Kottke T., Ahmed A., Melcher A.A., Gough M.J., Linardakis E., Riddle D., Dietz A., Lohse C.M. Viral fusogenic membrane glycoproteins kill solid tumor cells by nonapoptotic mechanisms that promote cross presentation of tumor antigens by dendritic cells. Cancer Res. 2002;62:6566–6578. [PubMed] [Google Scholar]
- 62.Mastrangelo P., Hegele R.G. RSV fusion: time for a new model. Viruses. 2013;5:873–885. doi: 10.3390/v5030873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weissenhorn W., Dessen A., Calder L.J., Harrison S.C., Skehel J.J., Wiley D.C. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]
- 64.Walsh E.E., Hruska J. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J. Virol. 1983;47:171–177. doi: 10.1128/jvi.47.1.171-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schlender J., Zimmer G., Herrler G., Conzelmann K.K. Respiratory syncytial virus (RSV) fusion protein subunit F2, not attachment protein G, determines the specificity of RSV infection. J. Virol. 2003;77:4609–4616. doi: 10.1128/JVI.77.8.4609-4616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Techaarpornkul S., Barretto N., Peeples M.E. Functional analysis of recombinant respiratory syncytial virus deletion mutants lacking the small hydrophobic and/or attachment glycoprotein gene. J. Virol. 2001;75:6825–6834. doi: 10.1128/JVI.75.15.6825-6834.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karron R.A., Buonagurio D.A., Georgiu A.F., Whitehead S.S., Adamus J.E., Clements-Mann M.L., Harris D.O., Randolph V.B., Udem S.A., Murphy B.R., Sidhu M.S. Respiratory syncytial virus (RSV) SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA. 1997;94:13961–13966. doi: 10.1073/pnas.94.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feldman S.A., Audet S., Beeler J.A. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J. Virol. 2000;74:6442–6447. doi: 10.1128/jvi.74.14.6442-6447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teng M.N., Collins P.L. Identification of the respiratory syncytial virus proteins required for formation and passage of helper-dependent infectious particles. J. Virol. 1998;72:5707–5716. doi: 10.1128/jvi.72.7.5707-5716.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi S.H., Park B.K., Lee K.-W., Chang J., Lee Y., Kwon H.-J. Effect of respiratory syncytial virus on the growth of hepatocellular carcinoma cell-lines. BMB Rep. 2015;48:565–570. doi: 10.5483/BMBRep.2015.48.10.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salimi V., Tavakoli-Yaraki M., Mahmoodi M., Shahabi S., Gharagozlou M.J., Shokri F., Mokhtari-Azad T. The Oncolytic Effect of Respiratory Syncytial Virus (RSV) in Human Skin Cancer Cell Line, A431. Iran. Red Crescent Med. J. 2013;15:62–67. doi: 10.5812/ircmj.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eckardt-Michel J., Lorek M., Baxmann D., Grunwald T., Keil G.M., Zimmer G. The fusion protein of respiratory syncytial virus triggers p53-dependent apoptosis. J. Virol. 2008;82:3236–3249. doi: 10.1128/JVI.01887-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffmann D., Grunwald T., Bayer W., Wildner O. Immune-mediated anti-neoplastic effect of intratumoral RSV envelope glycoprotein expression is related to apoptotic death of tumor cells but not to the size of syncytia. World J. Gastroenterol. 2008;14:1842–1850. doi: 10.3748/wjg.14.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasquinucci G. Possible effect of measles on leukaemia. Lancet. 1971;1:136. doi: 10.1016/s0140-6736(71)90869-5. [DOI] [PubMed] [Google Scholar]
- 75.Zygiert Z. Hodgkin’s disease: remissions after measles. Lancet. 1971;1:593. doi: 10.1016/s0140-6736(71)91186-x. [DOI] [PubMed] [Google Scholar]
- 76.Kernahan J., McQuillin J., Craft A.W. Measles in children who have malignant disease. Br. Med. J. (Clin. Res. Ed.) 1987;295:15–18. doi: 10.1136/bmj.295.6589.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grote D., Russell S.J., Cornu T.I., Cattaneo R., Vile R., Poland G.A., Fielding A.K. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- 78.Allen C., Opyrchal M., Aderca I., Schroeder M.A., Sarkaria J.N., Domingo E., Federspiel M.J., Galanis E. Oncolytic measles virus strains have significant antitumor activity against glioma stem cells. Gene Ther. 2013;20:444–449. doi: 10.1038/gt.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Domingo-Musibay E., Allen C., Kurokawa C., Hardcastle J.J., Aderca I., Msaouel P., Bansal A., Jiang H., DeGrado T.R., Galanis E. Measles Edmonston vaccine strain derivatives have potent oncolytic activity against osteosarcoma. Cancer Gene Ther. 2014;21:483–490. doi: 10.1038/cgt.2014.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dörig R.E., Marcil A., Chopra A., Richardson C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 81.Naniche D., Varior-Krishnan G., Cervoni F., Wild T.F., Rossi B., Rabourdin-Combe C., Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noyce R.S., Bondre D.G., Ha M.N., Lin L.T., Sisson G., Tsao M.S., Richardson C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011;7:e1002240. doi: 10.1371/journal.ppat.1002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tatsuo H., Ono N., Tanaka K., Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 84.Mühlebach M.D., Leonard V.H., Cattaneo R. The measles virus fusion protein transmembrane region modulates availability of an active glycoprotein complex and fusion efficiency. J. Virol. 2008;82:11437–11445. doi: 10.1128/JVI.00779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashiguchi T., Kajikawa M., Maita N., Takeda M., Kuroki K., Sasaki K., Kohda D., Yanagi Y., Maenaka K. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc. Natl. Acad. Sci. USA. 2007;104:19535–19540. doi: 10.1073/pnas.0707830104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colf L.A., Juo Z.S., Garcia K.C. Structure of the measles virus hemagglutinin. Nat. Struct. Mol. Biol. 2007;14:1227–1228. doi: 10.1038/nsmb1342. [DOI] [PubMed] [Google Scholar]
- 87.Saphire E.O., Oldstone M.B. Measles virus fusion shifts into gear. Nat. Struct. Mol. Biol. 2011;18:115–116. doi: 10.1038/nsmb0211-115. [DOI] [PubMed] [Google Scholar]
- 88.Studebaker A.W., Hutzen B., Pierson C.R., Shaffer T.A., Raffel C., Jackson E.M. Oncolytic measles virus efficacy in murine xenograft models of atypical teratoid rhabdoid tumors. Neuro-oncol. 2015;17:1568–1577. doi: 10.1093/neuonc/nov058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Studebaker A.W., Kreofsky C.R., Pierson C.R., Russell S.J., Galanis E., Raffel C. Treatment of medulloblastoma with a modified measles virus. Neuro-oncol. 2010;12:1034–1042. doi: 10.1093/neuonc/noq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bieback K., Lien E., Klagge I.M., Avota E., Schneider-Schaulies J., Duprex W.P., Wagner H., Kirschning C.J., Ter Meulen V., Schneider-Schaulies S. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gilliet M., Cao W., Liu Y.J. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 92.Kumar H., Kawai T., Akira S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009;388:621–625. doi: 10.1016/j.bbrc.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 93.Iankov I.D., Blechacz B., Liu C., Schmeckpeper J.D., Tarara J.E., Federspiel M.J., Caplice N., Russell S.J. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol. Ther. 2007;15:114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- 94.Liu C., Russell S.J., Peng K.W. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol. Ther. 2010;18:1155–1164. doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wicha M.S. Cancer stem cells and metastasis: lethal seeds. Clin. Cancer Res. 2006;12:5606–5607. doi: 10.1158/1078-0432.CCR-06-1537. [DOI] [PubMed] [Google Scholar]
- 96.Wicha M.S. Identification of murine mammary stem cells: implications for studies of mammary development and carcinogenesis. Breast Cancer Res. 2006;8:109. doi: 10.1186/bcr1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wicha M.S., Liu S., Dontu G. Cancer stem cells: an old idea--a paradigm shift. Cancer Res. 2006;66:1883–1890, discussion 1895–1896. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 98.Tong Y., Qian W. Targeting cancer stem cells with oncolytic virus. Stem Cell Investig. 2014;1:20. doi: 10.3978/j.issn.2306-9759.2014.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allen C., Paraskevakou G., Liu C., Iankov I.D., Msaouel P., Zollman P., Myers R., Peng K.W., Russell S.J., Galanis E. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin. Biol. Ther. 2008;8:213–220. doi: 10.1517/14712598.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balachandran S., Barber G.N. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 101.Hastie E., Grdzelishvili V.Z. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J. Gen. Virol. 2012;93:2529–2545. doi: 10.1099/vir.0.046672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roche S., Albertini A.A., Lepault J., Bressanelli S., Gaudin Y. Structures of vesicular stomatitis virus glycoprotein: membrane fusion revisited. Cell. Mol. Life Sci. 2008;65:1716–1728. doi: 10.1007/s00018-008-7534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Durham N.M., Mulgrew K., McGlinchey K., Monks N.R., Ji H., Herbst R., Suzich J., Hammond S.A., Kelly E.J. Oncolytic VSV Primes Differential Responses to Immuno-oncology Therapy. Mol. Ther. 2017;25:1917–1932. doi: 10.1016/j.ymthe.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ayala-Breton C., Russell L.O., Russell S.J., Peng K.W. Faster replication and higher expression levels of viral glycoproteins give the vesicular stomatitis virus/measles virus hybrid VSV-FH a growth advantage over measles virus. J. Virol. 2014;88:8332–8339. doi: 10.1128/JVI.03823-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Willmon C.L., Saloura V., Fridlender Z.G., Wongthida P., Diaz R.M., Thompson J., Kottke T., Federspiel M., Barber G., Albelda S.M., Vile R.G. Expression of IFN-beta enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 2009;69:7713–7720. doi: 10.1158/0008-5472.CAN-09-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roche S., Bressanelli S., Rey F.A., Gaudin Y. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science. 2006;313:187–191. doi: 10.1126/science.1127683. [DOI] [PubMed] [Google Scholar]
- 107.Roche S., Rey F.A., Gaudin Y., Bressanelli S. Structure of the prefusion form of the vesicular stomatitis virus glycoprotein G. Science. 2007;315:843–848. doi: 10.1126/science.1135710. [DOI] [PubMed] [Google Scholar]
- 108.Anderson B.D., Nakamura T., Russell S.J., Peng K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- 109.Kleinlützum D., Hanauer J.D.S., Muik A., Hanschmann K.M., Kays S.K., Ayala-Breton C., Peng K.W., Mühlebach M.D., Abel T., Buchholz C.J. Enhancing the Oncolytic Activity of CD133-Targeted Measles Virus: Receptor Extension or Chimerism with Vesicular Stomatitis Virus Are Most Effective. Front. Oncol. 2017;7:127. doi: 10.3389/fonc.2017.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Le Boeuf F., Gebremeskel S., McMullen N., He H., Greenshields A.L., Hoskin D.W., Bell J.C., Johnston B., Pan C., Duncan R. Reovirus FAST Protein Enhances Vesicular Stomatitis Virus Oncolytic Virotherapy in Primary and Metastatic Tumor Models. Mol. Ther. Oncolytics. 2017;6:80–89. doi: 10.1016/j.omto.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boutilier J., Duncan R. The reovirus fusion-associated small transmembrane (FAST) proteins: virus-encoded cellular fusogens. Curr. Top. Membr. 2011;68:107–140. doi: 10.1016/B978-0-12-385891-7.00005-2. [DOI] [PubMed] [Google Scholar]
- 112.Inoue H., Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ. 2014;21:39–49. doi: 10.1038/cdd.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ebert O., Shinozaki K., Kournioti C., Park M.-S., García-Sastre A., Woo S.L.C. Syncytia induction enhances the oncolytic potential of vesicular stomatitis virus in virotherapy for cancer. Cancer Res. 2004;64:3265–3270. doi: 10.1158/0008-5472.can-03-3753. [DOI] [PubMed] [Google Scholar]
- 114.Yamaki M., Shinozaki K., Sakaguchi T., Meseck M., Ebert O., Ohdan H., Woo S.L. The potential of recombinant vesicular stomatitis virus-mediated virotherapy against metastatic colon cancer. Int. J. Mol. Med. 2013;31:299–306. doi: 10.3892/ijmm.2012.1205. [DOI] [PubMed] [Google Scholar]
- 115.Pol J., Kroemer G., Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. OncoImmunology. 2015;5:e1115641. doi: 10.1080/2162402X.2015.1115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simpson G.R., Han Z., Liu B., Wang Y., Campbell G., Coffin R.S. Combination of a fusogenic glycoprotein, prodrug activation, and oncolytic herpes simplex virus for enhanced local tumor control. Cancer Res. 2006;66:4835–4842. doi: 10.1158/0008-5472.CAN-05-4352. [DOI] [PubMed] [Google Scholar]
- 117.Simpson G.R., Coffin R.S. Construction and characterization of an oncolytic HSV vector containing a fusogenic glycoprotein and prodrug activation for enhanced local tumor control. Methods Mol. Biol. 2009;542:551–564. doi: 10.1007/978-1-59745-561-9_29. [DOI] [PubMed] [Google Scholar]
- 118.Fu X., Tao L., Jin A., Vile R., Brenner M.K., Zhang X. Expression of a fusogenic membrane glycoprotein by an oncolytic herpes simplex virus potentiates the viral antitumor effect. Mol. Ther. 2003;7:748–754. doi: 10.1016/s1525-0016(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 119.Guedan S., Grases D., Rojas J.J., Gros A., Vilardell F., Vile R., Mercade E., Cascallo M., Alemany R. GALV expression enhances the therapeutic efficacy of an oncolytic adenovirus by inducing cell fusion and enhancing virus distribution. Gene Ther. 2012;19:1048–1057. doi: 10.1038/gt.2011.184. [DOI] [PubMed] [Google Scholar]
- 120.Nakamori M., Fu X., Meng F., Jin A., Tao L., Bast R.C., Jr., Zhang X. Effective therapy of metastatic ovarian cancer with an oncolytic herpes simplex virus incorporating two membrane fusion mechanisms. Clin. Cancer Res. 2003;9:2727–2733. [PubMed] [Google Scholar]
- 121.Todo T., Rabkin S.D., Sundaresan P., Wu A., Meehan K.R., Herscowitz H.B., Martuza R.L. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum. Gene Ther. 1999;10:2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 122.Hoffmann D., Bayer W., Wildner O. Therapeutic immune response induced by intratumoral expression of the fusogenic membrane protein of vesicular stomatitis virus and cytokines encoded by adenoviral vectors. Int. J. Mol. Med. 2007;20:673–681. [PubMed] [Google Scholar]
- 123.Zhu B., Yang J.R., Fu X.P., Jiang Y.Q. Anti-tumor effects of gene therapy with GALV membrane fusion glycoprotein in lung adenocarcinoma. Cell Biochem. Biophys. 2014;69:577–582. doi: 10.1007/s12013-014-9835-5. [DOI] [PubMed] [Google Scholar]
- 124.Bateman A., Bullough F., Murphy S., Emiliusen L., Lavillette D., Cosset F.L., Cattaneo R., Russell S.J., Vile R.G. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 2000;60:1492–1497. [PubMed] [Google Scholar]
- 125.Ulasov I.V., Zhu Z.B., Tyler M.A., Han Y., Rivera A.A., Khramtsov A., Curiel D.T., Lesniak M.S. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum. Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 126.Ahmed A., Jevremovic D., Suzuki K., Kottke T., Thompson J., Emery S., Harrington K., Bateman A., Vile R. Intratumoral expression of a fusogenic membrane glycoprotein enhances the efficacy of replicating adenovirus therapy. Gene Ther. 2003;10:1663–1671. doi: 10.1038/sj.gt.3302064. [DOI] [PubMed] [Google Scholar]
- 127.Chen H.H., Cawood R., El-Sherbini Y., Purdie L., Bazan-Peregrino M., Seymour L.W., Carlisle R.C. Active adenoviral vascular penetration by targeted formation of heterocellular endothelial-epithelial syncytia. Mol. Ther. 2011;19:67–75. doi: 10.1038/mt.2010.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guedan S., Gros A., Cascallo M., Vile R., Mercade E., Alemany R. Syncytia formation affects the yield and cytotoxicity of an adenovirus expressing a fusogenic glycoprotein at a late stage of replication. Gene Ther. 2008;15:1240–1245. doi: 10.1038/gt.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]