Abstract
Recent advances in recombinant adeno-associated virus (rAAV) gene therapy for choroideremia show gene replacement to be a promising approach. It is, however, well known that contact of vector solution with plastic materials in the surgical device may result in non-specific adsorption with resulting loss of physical titer and/or level of protein expression and activity. Here we assessed the biocompatibility and stability of rAAV2-REP1 (Rab Escort Protein-1) before and following passage through the injection device over a period of time to mimic the clinical scenario. Three identical devices were screened using two concentrations of vector: high (1E+12 DNase-resistant particles [DRP]/mL) and low (1E+11 DRP/mL), to mimic high- and low-dose administrations of vector product. The low dose was prepared using either formulation buffer that contained 0.001% of a non-ionic surfactant (PF68) or balanced salt solution (BSS). We observed significant losses in the genomic titer of samples diluted with BSS for all time points. The addition of 0.001% PF68 did not, however, affect rAAV physical titer, or REP1 protein expression and biological activity. Hence we observed that neither the genomic titer nor the biological activity of a rAAV2-REP1-containing solution was affected following passage through the surgical device when PF68 was present as a surfactant and this was maintained over a period up to 10 h.
Keywords: AAV, choroideremia, retinal gene therapy, prenylation, surfactant, biocompatibility, potency assay
Introduction
Inherited retinal diseases are degenerative disorders of the retina that affect 1:4,000 individuals worldwide.1 One of them is choroideremia (CHM; OMIM Phenotype MIM: 303100), an X-linked disease with a prevalence of around 1:50,000 in which afflicted males may lose their vision by the fifth decade of life.2 The faulty gene in choroideremia is CHM (OMIM Gene/Locus MIM: 300390), located on the X chromosome at position Xq21.2. The CHM gene encodes Rab Escort Protein-1 (REP1), a ubiquitously expressed protein that regulates intracellular trafficking pathways by prenylation of Rab GTPases.3,4 The impairment of trafficking pathways in the retinal pigment epithelium (RPE) specialized cell layer disrupts cell homeostasis and causes retinal degeneration in choroideremia patients.5
Choroideremia is considered a prime candidate for gene augmentation therapy, where a working healthy copy of the CHM gene is delivered.5 In fact, previous reports have shown that subretinal delivery of a recombinant adeno-associated virus serotype 2 (rAAV2) carrying the human CHM gene (rAAV2-REP1) is safe and can sustain and improve visual acuity in a cohort of predominantly late-stage patients.6, 7, 8 An ongoing phase 3 clinical trial (ClinicalTrials.gov: NCT03496012) will look at efficacy in a wider range of disease manifestations.
The surgical delivery to the subretinal space remains, however, one of the least predictable variables in the entire procedure of choroideremia gene therapy. Therefore, the vector losses in the standardized surgical device (loading syringe [19G] and delivery syringe [23G with 41G tip]) must be reduced to a minimum so that the dose delivered is maximal.9,10 Moreover, concerns exist that the contact of the vector solution with the system may result in non-specific vector adsorption with resulting loss of physical titer and/or level of expression and activity of the transgene.11 Literature reports have shown that the use of 0.001% Pluronic-F68 (PF68), a US Food and Drug Administration (FDA)-approved non-ionic surfactant, prevents rAAV vector losses due to adsorption to the surfaces of the materials used in preparation of the dilution, the loading syringe, and the surgical delivery equipment.9,11 In previous choroideremia gene therapy trials, the subretinal dose of rAAV2-REP1 was 0.1 mL of a 1E+12 genome particles (gp)/mL solution containing 0.001% PF68 (1E+11 gp in total) (ClinicalTrials.gov: NCT01461213, NCT02077361, NCT02553135, NCT02671539, and NCT02407678).8,12, 13, 14 In preparation for a phase 3 trial where high and low dose will be administered to patients randomized to the study group, we sought to investigate the biocompatibility and stability of rAAV2-REP1 following passage through the injection system over a period of time to mimic the clinical scenario.
Results
This study entailed three phases of work: (1) preparation of rAAV2-REP1 solutions and passage through the drug delivery device under simulated clinical conditions, (2) measurement of rAAV2-REP1 titer to look for any adsorptive losses, and (3) assessment of the rAAV2-REP1 biological activity to identify losses in vector potency.
Preparation of rAAV2-REP1 Solutions and Passage through the Device
One stock solution of research grade rAAV2-REP1 at 5E+12 DRP/mL was diluted to prepare one working solution of rAAV2-REP1 at 1E+12 DRP/mL (nominal) using formulation buffer containing 0.001% PF68 (commercial name TMN200), to mimic the current clinical scenario. Samples were taken and retained for analysis (Table 1). This solution at 1E+12 DRP/mL was then diluted 1:10 using formulation buffer or balanced salt solution (BSS; AMO Endosol, #15020) to a working concentration of 1E+11 DRP/mL. BSS is an isotonic solution for use in irrigating tissues of the eyes and is routinely used as a pharmaceutical diluent. One single dilution was prepared for each condition, which was used to load three replicate syringes. All dilutions were performed under aseptic conditions in sterile polypropylene tubes (Eppendorf Microcentrifuge tube Biopur Safe-Lock, individually sealed).
Table 1.
Experimental Design in which Samples from Two Doses of rAAV2-REP1 Were Collected at Baseline and Over Time Using Three Replicate Loading Syringes (19G Needle) and Three Replicate Dosing Syringes (23G with 41G Teflon Tip) Kept at 4°C and 23°C, Respectively
| Dose (DRP/mL) | Diluent | Replicate | Baseline | 4°C |
23°C |
|||
|---|---|---|---|---|---|---|---|---|
| 6 h |
90 min |
90+90 min |
||||||
| Loading | Dosing |
Dosing |
||||||
| Injected | Syringe | Injected | Syringe | |||||
| 1E+12 | 0.001% PF68 in FB | 1 | a | a | Xa | Xa | a | a |
| 2 | a | a | Xa | Xa | a | a | ||
| 3 | a | a | Xa | Xa | a | a | ||
| 1E+11 | 0.001% PF68 in FB | 1 | a | a | Xa | Xa | a | a |
| 2 | a | a | Xa | Xa | a | a | ||
| 3 | a | a | Xa | Xa | a | a | ||
| 1E+11 | BSS | 1 | X | Xa | Xa | Xa | Xa | Xa |
| 2 | X | Xa | Xa | Xa | Xa | Xa | ||
| 3 | X | Xa | Xa | Xa | Xa | Xa | ||
Low-dose samples (1E+11 DRP/mL) were prepared in either PF68 0.001% formulation buffer (0.001% PF68 in FB) or BSS.
Samples used for cell transduction and measurement of REP1 expression and biological activity.
Three replicate loading syringes (sterile 1 mL BD Luer-Lok #309628, fitted with a sterile 19G BD Microlance needle attached) were loaded with one of each rAAV2-REP1 working solutions (Table 1). Each solution was partially dispensed through the devices by depressing the plunger at the selected time points. All samples were collected into sterile polypropylene tubes as per Table 1 and kept on ice for a maximum of 20 min before being transferred to an ultralow freezer for storage until for further analyses.
Baseline samples of vector were collected immediately after loading. The loading syringes and their remaining samples were then kept at 4°C for approximately 6 h, which was considered the likely maximum delay from vector thawing to the start of surgery. After this period of time, additional vector solution from each syringe was dispensed and collected (loading sample). The vector solutions were then transferred from the loading syringe to a new dosing syringe (sterile 1 mL BD Luer-Lok, #309628) by inserting the 19G needle through the open tip of the latter. Care was taken to avoid bubbles or contact between the needle tip and rubber bung during the loading process. Once loaded with vector solution, the dosing syringes were fitted with a 23G subretinal injection needle with a 41G Teflon tip (DORC, The Netherlands). Following full priming of the needle, dosing syringes were kept at room temperature (RT; 23°C) for 90 min, after which vector solution from each syringe was ejected through the 41G tip (dosing injected) by depressing the plunger. The needles were then removed and additional vector solution dispensed directly through the syringe (dosing syringe). Once the needle was attached again and fully primed, dosing syringes containing the remaining vector solution were kept for 90 min further, and dosing samples were collected as before (“injected” and “syringe” at 90+90 min). This time (180 min) was estimated to be the maximal surgical hold prior to subretinal injection once the vector solution had been thawed and fully loaded into a syringe.
Measurement of rAAV2-REP1 Physical Titer
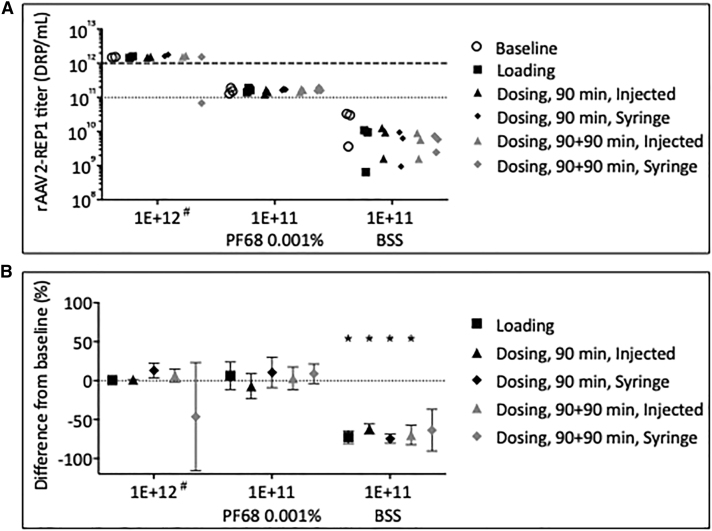
To determine whether any adsorptive losses had occurred during the preparation and passage of rAAV2-REP1 through the surgical devices, we measured the physical titer from all samples collected in the first phase of this study by qPCR, and the individual values (in DRP/mL) for all samples are plotted in Figure 1A. Due to failure of one of the qPCR analysis runs to meet assay specification criteria, only data from two out of three samples collected for high dose (1E+12 DRP/mL) are shown; for all low-dose samples (1E+11 DRP/mL), three replicates are shown. The titers measured from all samples diluted with the PF68 0.001% formulation buffer cluster around the nominal titer, except for one dosing syringe at 90+90 min value that is anomalously low (6.83E+10 DRP/mL). All samples diluted with BSS showed an 85.9% drop in titer at baseline, compared with the samples prepared with the PF68 0.001% formulation buffer (n = 3, 1.57E+11 DRP/mL versus 2.23E+10 DRP/mL). A two-way ANOVA then confirmed there is no statistical significance between BSS-diluted samples collected over time (p > 0.9999; dilution and time points as factors; Bonferroni’s multiple comparison test for the effect of the time points within each dilution). These data indicate that non-specific adsorption was immediate and maintained in all subsequent time points assessed with no further losses.
Figure 1.
Titer of rAAV2-REP1 Vector Samples following Dilution and Passage through Surgical Devices at Several Time Points and Temperatures
(A) Sample titers were determined by qPCR and plotted as individual values (in DRP/mL). Dotted lines mark the nominal titers for both high (1E+12 DRP/mL) and low doses (1E+11 DRP/mL). (B) Plot of the difference of the mean titer to baseline at each time point for all samples collected. Symbols represent mean of three replicates ± SD, except for 1E+12 DRP/mL, where only two replicates were considered. #Only two replicates were analyzed for high dose (1E+12 DRP/mL). A two-way ANOVA found BSS-diluted samples to have a significant low titer compared with both high-dose samples and low-dose samples prepared in formulation buffer (*0.01 < p < 0.0001; dilution and time points as factors; Dunnett’s multiple comparison test for the effect of the dilution within each time point; dosing syringe at 90+90 min time point excluded from analysis because of high CV).
The mean physical titer for samples at each time point was then plotted as a difference to baseline to look at vector losses more accurately (Figure 1B). Within high-dose samples (1E+12 DRP/mL), differences from baseline varied between +13.1% and −46.3% (dosing, 90 min; syringe and dosing, 90+90 min; syringe, respectively). The result from the dosing syringe at 90+90 min was considered anomalous due to high coefficient of variation (CV) between samples (CV = 129.3%). The differences from baseline for low-dose samples prepared in the PF68 0.001% formulation buffer varied between −7.1% and +10.6% (dosing, 90 min, and injected and syringe, respectively). Low-dose samples prepared with BSS show an average drop of −71.9% ± 5.4% of baseline for all time points (n = 5, mean ± SD). A further two-way ANOVA confirmed this difference as statistically significant when compared with both high-dose samples and low-dose samples prepared in formulation buffer (0.01 < p < 0.0001; dilution and time points as factors; Dunnett’s multiple comparison test for the effect of the dilution within each time point; dosing syringe at 90+90 min time point excluded from analysis because of high CV).
The data obtained at this phase of the study indicate that losses of rAAV2-REP1 samples prepared with PF68 0.001% formulation buffer were minimal and/or within experimental error/variation. In contrast, samples prepared with BSS showed significant losses of vector, which implies this diluent is not suitable to retain viral titer following passage of the solution through the surgical devices.
Assessment of the rAAV2-REP1 Biological Activity
The third and last phase of this study was the assessment of rAAV2-REP1 biological activity to determine whether the vector solution that had been through the surgical devices in the PF68 0.001% formulation buffer had lost potency. In view of the large drop in titer observed in the samples prepared using BSS as diluent, which resulted in vector losses, REP1 expression and the correlative biological activity were assumed to be reduced. Further analyses of these samples were not performed. Moreover, for optimal experimental design and due to restricted number in sample loading on each SDS-PAGE gel, samples collected from the dosing syringes after 90 min at room temperature (injected and syringe) were also excluded from this type of analysis.
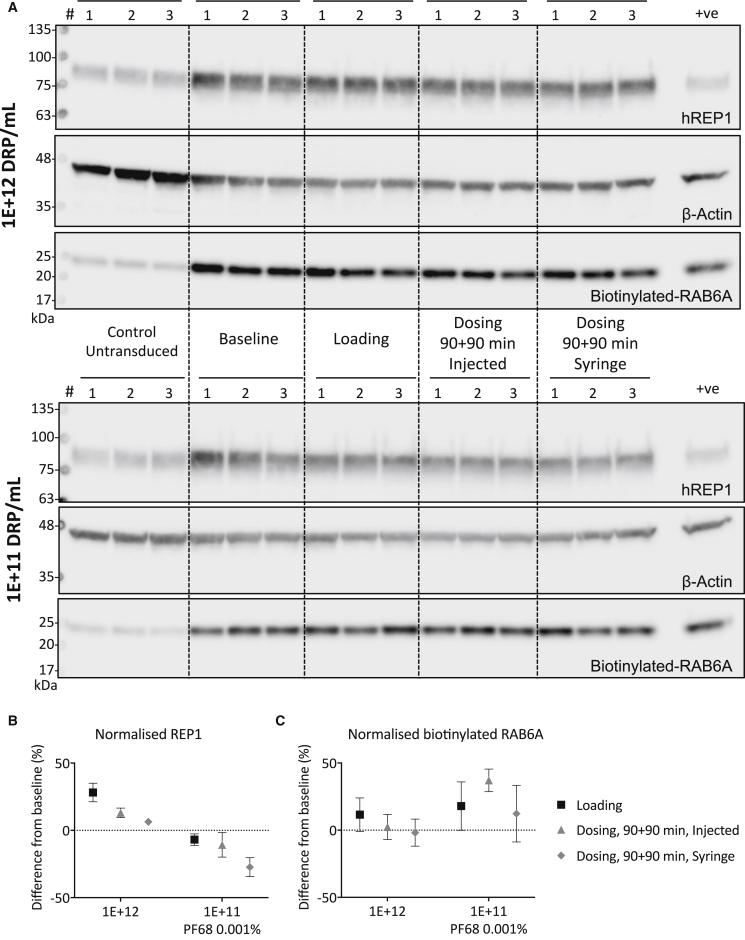
In order to determine whether the formulation buffer adversely affected rAAV transduction, we used high-dose and low-dose samples (1E+12 and 1E+11 DRP/mL) in the PF68 0.001% formulation buffer to transduce 293 cells at an MOI of 10,000 DRP/cell. Cells were harvested at 5 days post-transduction, and cytosolic fractions of cell lysates were used in an in vitro prenylation reaction using RAB6A as a substrate.15 The results of the immunoblot detection of REP1 expression, expression of actin as loading control, and biotinylated RAB6A are depicted in Figure 2. As expected, REP1 expression was higher in transduced samples, compared with untransduced controls, for both high-dose (Figure 2A, top panel) and low-dose samples (Figure 2A, bottom panel). Furthermore, the levels of biotinylated RAB6A were increased in the transduced samples in direct proportion to the REP1 expression levels in all conditions tested. These results are in line with previous findings15 and allow us to conclude that the biological activity of rAAV2-REP1 is not impacted when PF68 is present at a concentration of 0.001%.
Figure 2.
Biological Activity of rAAV2-REP1 Samples Containing PF68 following Passage through the Surgical Device
(A) 293 cells were transduced with rAAV2-REP1 samples prepared in PF68 0.001% formulation buffer (high and low doses) at MOI 10,000 DRP/cell. Sample replicates for each condition (baseline, loading and dosing 90+90 min, syringe and injected) were run in parallel for both high dose (top panel) and low dose (bottom panel). Protein expression (human REP1 and β-actin) and biotin incorporation in RAB6A were detected in prenylation reaction products following SDS-PAGE and immunoblot analysis. Positive (+ve) control: untransduced cell lysate spiked with recombinant fish REP1 (25 nM). (B and C) Semi-quantification data (band density values normalized to actin as loading control) are plotted as percentage of difference to baseline for each dose; REP1 expression (B) and biotinylated-RAB6A (C). Symbols are mean of three replicates ± SD. A two-way ANOVA confirmed the levels of biotinylated Rab substrate did not vary significantly from baseline in either high- or low-dose samples (p > 0.5; dilution and time points as factors; Bonferroni’s multiple comparison test for the effect of the time points within each dilution).
Analysis of the band density values allowed for semi-quantification of immunoblot data by normalization to actin as loading control, and results are shown as percentage of difference from baseline for both REP1 (Figure 2B) and biotinylated RAB6A (Figure 2C). Within high-dose samples (1E+12 DRP/mL), difference from baseline varied between +28.2% ± 6.9% and +6.4% ± 2.2% for normalized REP1, and between +11.6% ± 12.5% and −1.8% ± 10.0% for biotinylated RAB6A (n = 3, mean ± SD; loading and dosing, 90+90 min, and syringe, respectively). For the low-dose samples, difference from baseline varied between −6.9% ± 4.3% and −27.3% ± 7.0% for normalized REP1 (loading and dosing, 90+90 min, and syringe, respectively), and between +37.1% ± 18.0% and +12.3% ± 21.0% for biotinylated RAB6A (dosing, 90+90 min, and injected and syringe, respectively) (n = 3, mean ± SD). A two-way ANOVA confirmed the levels of biotinylated Rab substrate did not vary significantly from baseline in either high- or low-doses samples (p > 0.5; dilution and time points as factors; Bonferroni’s multiple comparison test for the effect of the time points within each dilution). These data corroborated the data obtained by qPCR for the samples diluted with the PF68 0.001% formulation buffer and suggested there were no detrimental effects on REP1 expression and function following passage of the vector solution through the surgical device.
Discussion
In this work, we show for the first time that both the biocompatibility and stability of rAAV2-REP1 are maintained following passage of the vector solution containing PF68 through the injection system used for human retinal gene therapy.
The first report of the beneficial effects of PF68 as a surfactant in AAV solutions used in retinal gene therapy dates back to 2008 when Bennicelli et al.11 demonstrated reversal of blindness in an animal model of LCA2 using AAV2.RPE65. That study also included data on vector recovery in the presence or absence of PF68. The authors measured the titer of AAV2.RPE65 solution diluted to the target concentration in PBS supplemented or not with PF68 and passed through three devices, and found an average 66% loss of vector without PF68.11 Further studies showed that functional activity of AAV2.RPE65 was also maintained for up to 20 h.11 The formulation buffer containing PF68 was then used in two LCA gene therapy trials16,17 that reported improvements in vision.18,19 These results backed the approval of AAV2.RPE65 as a prescription gene therapy product by the FDA in 2017 (LUXTURNA) and the European Commission in 2018. LUXTURNA is supplied with a diluent containing 0.001% poloxamer 188, the non-proprietary equivalent of PF68.
In case of choroideremia gene therapy, PF68 was added to the formulation buffer of rAAV2-REP1 used in a phase 1/2 trial to prevent vector losses.6 Although the biocompatibility of rAAV2-REP1 has never been studied previously, there is a report testing the impact of 0.001% PF68 in the biocompatibility of rAAV2/8 preparations put through the same customized surgical device used in other choroideremia gene therapy trials (ClinicalTrials.gov: NCT01461213, NCT02077361, NCT02553135, NCT02671539, NCT02407678, and NCT03496012).9 Similar to previous findings with AAV2.RPE65, Fischer et al.9 reported that twice as many vector genomes are lost when loading rAAV8-based viral vector solutions in the injection system without adding 0.001% PF68. In that case, however, PBS and not BSS was used as a diluent.
BSS is routinely used as a pharmaceutical diluent, but there is a concern that following 1:10 dilution of rAAV2-REP1 for preparation of low-dose surgical administrations, the final concentration of PF68 present in the diluted product may not be present in sufficient quantities to prevent adsorption to the surfaces of the materials used in preparation of the dilution, the loading syringe, and the surgical delivery equipment. No literature reports have been located that have assessed this level of PF68. Due to the loss of rAAV2-REP1 vector observed in the low-dose samples prepared with BSS, we discourage the use of BSS as a diluent for rAAV retinal gene therapy and recommend that all vector dilutions are prepared using a formulation buffer containing 0.001% PF68.
For samples diluted with formulation buffer, the data obtained in both physical titer and potency assays suggest that there was no loss of rAAV2-REP1 vector or its biological activity following holding in the injection system for approximately 6 h at 4°C (nominal) and approximately 3.75 h at RT (nominal) and after passing through the injection system. One of the high-dose replicates failed to meet assay specification criteria for the determination of the titer, which most likely originated from experimental error during downstream sample processing. Therefore, only two replicates were considered for analysis. Between these two, a large difference in the measured titer from baseline was observed in the high-dose “dosing, syringe” after 90+90 min at RT. This result is considered anomalous for the following reasons: (1) the corresponding injected sample (“dosing, injected”) collected at the same time point showed a +6.6% difference in titer from baseline; (2) the data obtained from the corresponding sample after 90 min at RT showed a +13% difference in measured titer from baseline, suggesting no downward trend on storage; (3) the data obtained from the diluted low-dose (1E+11 DRP/mL) samples (injected and syringe) collected after 90+90 min at RT showed differences in the measured titer of +1.4% and +7.5%, respectively; and (4) the matching data for this sample in the potency assay showed differences from baseline of +6.6% and −1.8% for REP1 expression and biological activity, respectively. When planning future similar studies, care should be taken to include five replicate samples and extra sampling material for each time point to account for variation caused by experimental error during sample processing.
The data presented here show that the use of BSS to dilute rAAV2-REP1 without surfactant PF68 results in immediate losses of the product following passage through the surgical device currently in use, as measured by its genomic titer. On the other hand, the use of TMN200 containing PF68 prevents vector losses, as confirmed by qPCR and REP1 protein expression levels. Moreover, the biological activity of REP1 is maintained as shown by the results of the prenylation assay run in vitro. Hence inclusion of this surfactant in the formulation buffer at a concentration of 0.001% ensures biocompatibility and stability of rAAV2-REP1 even at a lower dilution, over a period up to 10 h. In case the delivery system or any devices were to change in the future, studies should be performed using an approved product to validate the findings described in this manuscript.
Materials and Methods
rAAV Vector Production
The recombinant AAV2/2 viral vector containing the CHM transgene under the control of a CAG promoter was produced at the Nationwide Children’s Hospital (OH, USA) following a standard protocol.20 The viral stock was prepared in formulation buffer (TMN200) containing 20 mM Tris (pH 8.0), 1 mM MgCl2, 200 mM NaCl, and 0.001% PF68 in water for injections at a concentration of 4.95E+12 DRP/mL.
qPCR for rAAV Titration
Vector samples were DNase treated, and the viral capsids were lysed with Proteinase K to release the genomic DNA. Replicates of each sample were subjected to qPCR using a TaqMan-based primer/probe set specific for the CAG promoter. Due to the number of samples it was not possible to assess all samples together on the same plate and/or on the same day. However, where possible, samples were grouped such that samples from a defined condition/concentration were assessed on the same plate to allow for direct comparison of any adsorptive losses with these samples. In addition, each test plate assessed included an internal trending control with acceptance limits around this control to provide additional assurance of comparable performance between runs. All samples were assessed using the same primer/probe sequences, equipment, reagents, and consumables. Some of the lot numbers of the reagents were different between runs. All qPCR plates used were polypropylene and frosted plates in a 96-well format, compatible with Applied Biosystems 96-well Real-Time PCR systems and thermal cyclers (reference #4316813). A standard curve was produced by taking the average for each point in the linear range of the standard plasmid dilution series and plotting the log copy number against the average cycle threshold (Ct) value for each point. The titer of each rAAV preparation was calculated from the standard curve and expressed as DRP per milliliter.
Cell Culture and Preparation of Total Cell Lysates
HEK293 cells (293, #85120602; Culture Collections, Public Health England, Salisbury, UK) were cultured in MEM supplemented with L-glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL), 1% non-essential amino acids, and 10% fetal bovine serum. Cells were maintained at 37°C in a 5% CO2 environment. Cells were seeded in six-well plates at a density of 9.5E+5 cells/well on the day prior to transduction. Cell transduction was performed at 10,000 MOI of rAAV2-REP1 (i.e., DRP/cell), and media were changed at 3 days post-transduction. Cell lysates were prepared in prenylation buffer (50 mM HEPES, 50 mM NaCl, 2 mM MgCl2, 1 mM DTT [pH 7.5]) at 5 days post-transduction as previously described.15 Total protein content was determined using the Bradford method according to the manufacturer’s instructions (Quick Start Bradford 1× Dye Reagent; Bio-Rad, Hertfordshire, UK), and sample values were extrapolated from a standard curve using a sigmoidal four parameter logistic (4-PL) regression.
In Vitro Prenylation and Immunoblot Analysis
The prenylation reactions were set up using 20 μg of total cell lysate, recombinant rat GGT-II (2 μM; Jena Biosciences, Jena, Germany), recombinant human RAB6A (4 μM; Jena Biosciences, Jena, Germany), and biotin-labeled geranyl pyrophosphate (B-GPP, 5 μM; Jena Biosciences, Jena, Germany) as lipid donor in prenylation buffer. All reactions were supplemented with fresh guanosine 5′-diphosphate (GDP; 20 mM; Merck Millipore, Watford, UK) and DTT (1 mM; Thermo Fisher Scientific, Loughborough, UK). Positive controls were prepared using untransduced cell lysate spiked with a recombinant REP1 protein (fish His-REP1; Jena Biosciences, Jena, Germany). The reactions were incubated for 2 h at 37°C and then stopped by addition of Laemmli sample buffer. Reaction products were subjected to SDS-PAGE and immunoblot analysis as per the protocol previously described in Patrício et al.15 Membranes were incubated separately for detection of human REP1 (MABN52; 1:2,500; Merck Millipore, Watford, UK) and β-actin (AM4302; 1:50,000; Thermo Fisher Scientific, Loughborough, UK), which were detected using a horseradish peroxidase (HRP)-labeled secondary antibody (1:10,000). The incorporation of biotinylated lipid donor into the RAB6A substrate was detected by direct incubation with streptavidin-HRP (1:10,000; Thermo Fisher Scientific, Loughborough, UK).
Statistical Analysis
All statistical analysis was done using Prism 7 for Windows (San Diego, CA, USA).
Author Contributions
Conceptualization: M.I.P., C.I.C., C.B., A.R.B., C.M.-F.D.C., R.E.M. Methodology: M.I.P., C.I.C., C.B., A.R.B., C.M.-F.D.C., R.E.M. Formal Analysis: M.I.P., C.I.C., C.B., R.E.M. Investigation: M.I.P., A.R.B., C.M.-F.D.C. Writing – Original Draft: M.I.P. Writing – Review & Editing: M.I.P., C.I.C., C.B., A.R.B., C.M.-F.D.C., R.E.M. Supervision: C.I.C., R.E.M. Funding Acquisition: R.E.M.
Conflicts of Interest
M.I.P. and R.E.M. are coinventors on a pending patent for “prenylation assay” filed on behalf of Nightstar Therapeutics (now Biogen, Inc.), a gene therapy company established by the University of Oxford and based at 9-10 Midford Place, London W1T 5BJ, UK. A.R.B. is a consultant for Nightstar Therapeutics. C.I.C. is a former employee at Nightstar Therapeutics. C.B. is an employee at Nightstar Therapeutics. R.E.M. is a founder of Nightstar Therapeutics. R.E.M. receives research funding from Nightstar Therapeutics through the University of Oxford.
Acknowledgments
This research was funded by Nightstar Therapeutics, the Medical Research Council, the Royal College of Surgeons of Edinburgh and the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
References
- 1.Lipinski D.M., Thake M., MacLaren R.E. Clinical applications of retinal gene therapy. Prog. Retin. Eye Res. 2013;32:22–47. doi: 10.1016/j.preteyeres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Barnard A.R., Groppe M., MacLaren R.E. Gene therapy for choroideremia using an adeno-associated viral (AAV) vector. Cold Spring Harb. Perspect. Med. 2014;5:a017293. doi: 10.1101/cshperspect.a017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cremers F.P.M., van de Pol D.J.R., van Kerkhoff L.P.M., Wieringa B., Ropers H.-H. Cloning of a gene that is rearranged in patients with choroideraemia. Nature. 1990;347:674–677. doi: 10.1038/347674a0. [DOI] [PubMed] [Google Scholar]
- 4.Andres D.A., Seabra M.C., Brown M.S., Armstrong S.A., Smeland T.E., Cremers F.P.M., Goldstein J.L. cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell. 1993;73:1091–1099. doi: 10.1016/0092-8674(93)90639-8. [DOI] [PubMed] [Google Scholar]
- 5.Patrício M.I., Barnard A.R., Xue K., MacLaren R.E. Choroideremia: molecular mechanisms and development of AAV gene therapy. Expert Opin. Biol. Ther. 2018;18:807–820. doi: 10.1080/14712598.2018.1484448. [DOI] [PubMed] [Google Scholar]
- 6.MacLaren R.E., Groppe M., Barnard A.R., Cottriall C.L., Tolmachova T., Seymour L., Clark K.R., During M.J., Cremers F.P., Black G.C. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–1137. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards T.L., Jolly J.K., Groppe M., Barnard A.R., Cottriall C.L., Tolmachova T., Black G.C., Webster A.R., Lotery A.J., Holder G.E. Visual Acuity after Retinal Gene Therapy for Choroideremia. N. Engl. J. Med. 2016;374:1996–1998. doi: 10.1056/NEJMc1509501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue K., Jolly J.K., Barnard A.R., Rudenko A., Salvetti A.P., Patrício M.I., Edwards T.L., Groppe M., Orlans H.O., Tolmachova T. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat. Med. 2018;24:1507–1512. doi: 10.1038/s41591-018-0185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer M.D., Hickey D.G., Singh M.S., MacLaren R.E. Evaluation of an Optimized Injection System for Retinal Gene Therapy in Human Patients. Hum. Gene Ther. Methods. 2016;27:150–158. doi: 10.1089/hgtb.2016.086. [DOI] [PubMed] [Google Scholar]
- 10.Xue K., Groppe M., Salvetti A.P., MacLaren R.E. Technique of retinal gene therapy: delivery of viral vector into the subretinal space. Eye (Lond.) 2017;31:1308–1316. doi: 10.1038/eye.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennicelli J., Wright J.F., Komaromy A., Jacobs J.B., Hauck B., Zelenaia O., Mingozzi F., Hui D., Chung D., Rex T.S. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimopoulos I.S., Hoang S.C., Radziwon A., Binczyk N.M., Seabra M.C., MacLaren R.E., Somani R., Tennant M.T.S., MacDonald I.M. Two-Year Results After AAV2-Mediated Gene Therapy for Choroideremia: The Alberta Experience. Am. J. Ophthalmol. 2018;193:130–142. doi: 10.1016/j.ajo.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Fischer M.D., Ochakovski G.A., Beier B., Seitz I.P., Vaheb Y., Kortuem C., Reichel F.F.L., Kuehlewein L., Kahle N.A., Peters T. Changes in retinal sensitivity after gene therapy in choroideremia. Retina. 2018 doi: 10.1097/IAE.0000000000002360. Published online October 9, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Lam B.L., Davis J.L., Gregori N.Z., MacLaren R.E., Girach A., Verriotto J.D., Rodriguez B., Rosa P.R., Zhang X., Feuer W.J. Choroideremia Gene Therapy Phase 2 Clinical Trial: 24-Month Results. Am. J. Ophthalmol. 2019;197:65–73. doi: 10.1016/j.ajo.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Patrício M.I., Barnard A.R., Cox C.I., Blue C., MacLaren R.E. The Biological Activity of AAV Vectors for Choroideremia Gene Therapy Can Be Measured by In Vitro Prenylation of RAB6A. Mol. Ther. Methods Clin. Dev. 2018;9:288–295. doi: 10.1016/j.omtm.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauswirth W.W., Aleman T.S., Kaushal S., Cideciyan A.V., Schwartz S.B., Wang L., Conlon T.J., Boye S.L., Flotte T.R., Byrne B.J., Jacobson S.G. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.-F., Tillman A., Wittes J., Pappas J., Elci O., McCague S. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobson S.G., Cideciyan A.V., Roman A.J., Sumaroka A., Schwartz S.B., Heon E., Hauswirth W.W. Improvement and decline in vision with gene therapy in childhood blindness. N. Engl. J. Med. 2015;372:1920–1926. doi: 10.1056/NEJMoa1412965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zolotukhin S., Byrne B.J., Mason E., Zolotukhin I., Potter M., Chesnut K., Summerford C., Samulski R.J., Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]