Abstract
Background
Severity in irritable bowel syndrome (IBS) is associated to impaired quality of life and fatigue. Fecal microbiota transplantation (FMT) induces significant relief in gastro-intestinal related complaints. The objective was to evaluate the effect of FMT on the secondary endpoints: IBS-related quality of life and fatigue in patients with non-constipated IBS.
Method
In this double-blind randomized placebo-controlled, parallel-group, single-center study, we enrolled patients with non-constipated IBS, defined by the ROME 3 criteria. We randomly assigned participants (2:1) in blocks of six to active or placebo FMT. Responder in fatigue and quality of life were defined as a decrease of 20 points in total Fatigue Impact Scale score, and improvement of 14 points in the IBS-quality of life questionnaire, respectively. In a modified-intention-to-treat population, we excluded participants who did not undergo treatment or who were diagnosed with any other disease by pinch biopsies during the treatment procedure.
Findings
Between Jan1, and Oct 30, 2015, we recruited 90 participants and randomly assigned them to active treatment (n = 60) or placebo (n = 30). Three participants did not undergo FMT and four were excluded after diagnosis of microscopic colitis, leaving 83 for final modified intention-to-treat analysis (55 in the active treatment group and 28 in the placebo group). Significant improvement in QoL (Odds ratio (OR) 3,801; confidence interval (CI) = 1,309–11,042 p = 0.011) and fatigue (OR = 4,398; CI = 1,175–16,468 and p = 0,020) was found at six months. Absence of other self reported functional disorders and presence of depression at baseline is suggested to predict a lasting effect of FMT in QoL and fatigue, respectively.
Interpretation
FMT induced significant relief in quality of life and fatigue. Results suggest a lasting effect of FMT in subgroups that should be further investigated in future studies. Funding Helse Nord, Norway and the Norwegian Centre of Rural Medicine, University of Tromsø, Norway.
Keywords: Irritable bowel syndrome, Fecal microbiota transplantation, Quality of life, Fatigue, Bacterio therapy, Clinical trial
Research in context.
Evidence before this study
We searched PubMed for manuscripts published in English from inception and until Sept 20,2019, with the terms ‘’irritable bowel’’ in combination with ‘’fecal transplantation’’, ‘’fecal bacterio therapy’’, ‘’randomized controlled trial’’, ‘’dysbiosis’’, ‘’quality of life’’, ‘’fatigue’’, or ‘’microbiota’’. We identified five randomized controlled trials assessing the effect of fecal microbiota transplantation (FMT) in irritable bowel syndrome (IBS) with diverging results. Among others, differences in route and number of FMT administration, outcome measures, processing of transplants and criteria for inclusion of donors and FMT recipients can explain the lack of consistency in the results. Only one RCT with a single colonoscopic administration of FMT was identified (Holster et al. 2019, Clinical and Translational Gastroenterology). This study found a significant effect of FMT on IBS related quality of life in the donor FMT group, but not in the placebo group. There was not a significant difference between groups, but the study included only 17 patients. We could not find any studies assessing the effect of FMT on fatigue in IBS.
Added value of this study
The data show that FMT may improve quality of life and fatigue in IBS, in particular in the subgroups with no excessive functional comorbidity and self reported depression, respectively. The study also highlight fatigue as a part of IBS symptomatology, and available for therapeutic interventions.
Implications of all the available evidence
The study, combined with our previous reported result, show that there is a consistent effect of FMT in bowel related complaints, quality of life and fatigue in IBS. In future studies an effort should be made to determine which IBS subgroups benefit from FMT treatment.
Alt-text: Unlabelled box
1. Introduction
Irritable bowel syndrome (IBS) is a functional gut disorder characterized by abdominal pain related to abnormal frequency and consistency of bowel movements. In clinical practice and studies, participants are often categorized by the phenotypes: IBS with diarrhea, IBS with constipation and mixed IBS (i.e., IBS with alternating diarrhea and constipation).
IBS is associated with substantial costs to patients, healthcare system and society in terms of increased health care expenditures, loss of work productivity and decrease in quality of life (QoL) [1], [2], [3]. Patients are found willing to give up 10–15 years of their life expectancy for an immediate cure [2]. The severity of IBS is correlated negatively with QoL and positively with healthcare seeking [4]. We recently published the main results from the REFIT study, a double blind placebo-controlled trial on fecal microbiota transplantation (FMT) in moderate to severe non-constipated IBS. We found a beneficial effect on gastro-intestinal related complaints with number needed to treat of five [5].
The European Medicines Agency recommends assessing the treatment effect by abdominal pain/discomfort along with abnormalities in defecation [6]. However, the symptom burden in IBS is diverse and extends beyond these gastro-intestinal complaints [4]. Fatigue is experienced in ninety percent of IBS patients, one of the most pronounced domains with decrements in health related QoL, and is moreover found as an independent predictor for referral to the secondary health care [1,7].
In this analysis of secondary endpoints from the REFIT study we aim of to evaluate the effect of FMT on IBS-related QoL and fatigue.
2. Methods
2.1. Study design and patient population
This was the secondary endpoints of a randomized, double blind, placebo-controlled, parallel group, single-center trial (NCT02154867) and the patient population and study design has been previously described [5]. Briefly, individuals between 18–75 years of age with a diagnosis of non-constipated IBS (based on Rome 3 criteria) with moderate to severe IBS by the IBS-SSS (cut-off 175 IBS-SSS score) were eligible for the study.
Participants were randomized in blocks of six for active or placebo FMT (4:2). Non-study personnel generated the randomization sequence using a randomization website. Placebo and active transplants were prepared by the same procedure and standardized to be identical in appearance and temperature at assigning of treatment. Placebo was participants’ own feces obtained, processed and frozen during the inclusion assessment. Active treatment was processed donor feces. If frozen; processed and frozen 2–4 weeks before treatment and thawed at the day for allocation and treatment assignment. If fresh; collected and processed the same day as allocation and treatment assignment. It was predetermined if the active treatment in each block was fresh or frozen. We balanced the use of fresh and frozen active transplants to a ratio of 1:1. Transplants was made of 50–80 g of faces homogenized in 200 mL of isotonic saline and 50 mL of 85% glycerol and filtered.
After a bowel lavage, all participants underwent FMT at the University Hospital of North Norway, Harstad, within 2–4 weeks after the initial assessment. Treatment was delivered through the working channel of a colonoscope to the cecum. Participants, investigators and outcome assessors were kept blind to the allocation and intervention.
2.2. Outcomes
Secondary outcomes were to evaluate the effect of donor FMT vs. autologous FMT on fatigue (by the fatigue impact scale) at three six and twelve months and quality of life (by the irritable bowel quality of life) at six and twelve months.
2.2.1. The fatigue impact scale (FIS)
FIS is a 40 item questionnaire that assess the individuals’ attribution of functional limitations to their subjective experience of fatigue in an overall score with three subdomains (cognitive, physical and social fatigue) [8]. Each item is scored on a 5-point Likert response scale (0 = ‘’no problem’’ 1 = ‘’small problem’’ 2 = ‘’moderate problem’’, 3 = ‘’big problem’’ 4 = ‘’extreme problem’’). Higher scores indicate increased level of subjective experienced fatigue (range, minimum score 0 and maximum score 160). The Norwegian version of FIS is validated for use in IBS [8]. A conservative measure of minimal clinical important difference, validated in patients with multiple sclerosis, is a decrease of 20 in total score [9]. FIS was administrated 2–4 weeks before treatment, and at three, six and twelve months after.
2.2.2. IBS-Quality of life questionnaire (IBS-QoL)
Quality of life was assessed using a validated 34-item questionnaire (IBS-QoL) with seven subdomains (dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual and relationships). Each item is scored on a 5-point Likert response scale (1 = ‘’not at all’’ 2 = ‘’slightly’’ 3 = ‘’moderately’’ 4 = ‘’quite a bit’’ and 5 = extremely’’). Data were transformed to a sum score (range, minimum score 0 and maximum score 100). Transformation involved reversing all scores so that higher score indicated higher QoL, and then subtracting the lowest possible raw score from the actual raw score, dividing by possible raw score range, and multiplying by 100 [10]. IBS-QoL was administrated 2–4 weeks before treatment, and at six and twelve months after treatment. Minimally clinical important difference is an improvement of 14 in total IBS-QoL transformed score [11].
2.2.3. Metadata
In a self-assessment questionnaire before FMT treatment, we asked patients to report disorders that are associated to the severity in IBS [4]. This included other functional disorders (fibromyalgia, chronic fatigue syndrome, jaw and pelvic pain syndromes) and mood disorders (anxiety and depression). To assess the effect of diet on the results including the intake of FODMAPS (i.e., fermentable oligosaccharides, disaccharides, monosaccharaides and polyols) and probiotics, participants registered a 5-day prospective dietary record at baseline and after 3 months. The records were analyzed using the software Dietician Net Pro (Diet and Nutrition Data, Bromma, Sweden) [5]. In addition, all participants reported a complete list of medications pre FMT and at 12 months post FMT to detect any changes in medications that could affect the outcome measure. Participants were also asked to report any use of antibiotics during the follow up period from baseline and until 12 months after treatment. We also assessed intestinal complaints by the irritable bowel symptom severity score (IBS-SSS) before treatment and at 1, 3, 6 and 12 months. Those results have been reported previously [5].
2.3. Statistical analysis
The statistical analysis plan for the secondary endpoints was not adequately elaborated in the study protocol as we intended to use the same statistical analysis for the secondary as we did for the primary endpoints previously reported in the Lancet Gastroenterology and Hepatology [5]. In the secondary endpoints (as in the primary endpoint) we compared the proportion of participants who responded to active treatment with the proportion of participants who responded to placebo by cross tabs using Chi Square. A responder in IBS-Qol was defined as an improvement in total score of 14 or more, and a responder in FIS was defined as a decrease of 20 or more in total FIS score. Since fresh and frozen donor feces were randomized to placebo as one active group it was not appropriate to compare fresh vs. frozen vs. placebo in the primary analysis.
In a post hoc analysis of IBS QoL, we compared the effect between placebo, fresh and frozen donor FMT in the recorded time course in a repeated measures analysis of variance (RM-ANOVA) and explored other factors that might predict the treatment effect. These included IBS-subtype, functional comorbidity (from the self-assessment questionnaire at baseline). We also tested for potential confounding. Each of the following variables was entered into the model one at a time to test stability of the fit (and removed again if no effects were detected: sex, age, antibiotics during study, use of loperamide during study, change in FODMAP intake based on dietary records, and mood disorders (anxiety and depression)). Finally, we did a doubly multivariate RM-ANOVA on the subdomains of the QoL to assess the significance of each subdomain to the change in total score.
In a post hoc analysis of FIS we first did a RM-ANOVA with the same factors associated to a treatment effect in IBS-QoL (fresh, frozen and placebo, IBS-subtype and functional comorbidity). No significant effects were found. We then did a RM-ANOVA with treatment group (fresh, frozen and placebo) and depression (from the self-assessment questionnaire at baseline) as predictors, and found one significant term. In the confounder control each of the following variables was entered into the model one at a time to test stability of the fit (and removed again if no effects were detected: sex, age, antibiotics during study, use of loperamide during study, and change in FODMAP intake based on dietary records, functional comorbidity and IBS-subtype). When stability of the fit was established, fresh and frozen was combined in to one active group in a new RM-ANOVA with active vs. placebo and depression as predictors. Once again terms not significant was removed and the confounder control was repeated with the same confounders as above. Finally, we did a doubly multivariate RM-ANOVA on the subdomains of the FIS to assess the significance of each subdomain on the total score. All data were analyzed using SPSS, version 25 (SPSS Inc, Chicago, IL, USA).
3. Results
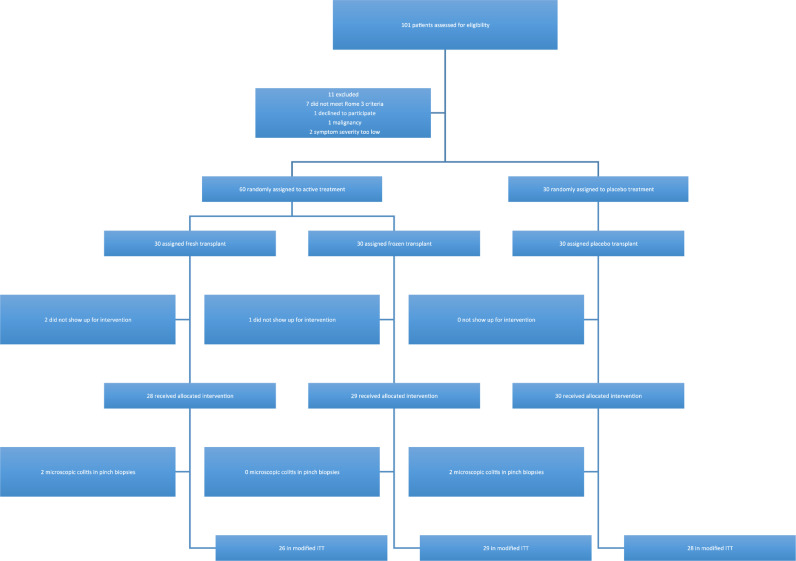
One-hundred-and-one individuals from primary care were assessed for eligibility (between Jan1, and Oct 30, 2015); of those, 90 were included and 83 remained for the final analysis; four were excluded after being diagnosed with microscopic colitis after the intervention colonoscopy and three did not show up on the day of intervention (Fig. 1) [5]. Baseline demographics and disease characteristics are found in Table 1 (earlier reported in the Lancet Gastroenterology and Hepatology 2018). All baseline demographics were similar between groups.
Fig. 1.
Study flow chart.
Table 1.
Baseline characteristics and demographics.
| Placebo (n = 28) | Active (n = 55) | Fresh (n = 26) | Frozen (n = 29) | |
|---|---|---|---|---|
| Age (years) | 45 (34 to 57) | 44 (33 to 54) | 44 (35 to 54) | 43 (26 to 54) |
| Sex | ||||
| Women | 19 (68%) | 36 (65%) | 18 (69%) | 18 (62%) |
| Men | 9 (32%) | 19 (35%) | 8 (31%) | 11 (38%) |
| IBS subtype | ||||
| IBS-M | 15 (54%) | 24 (44%) | 12 (46%) | 12 (41%) |
| IBS-D | 13 (46%) | 31 (56%) | 14 (54%) | 17 (59%) |
| Time with IBS (years) | 10 (6 to 16) | 10 (5 to 19) | 15 (4 to 19) | 10 (5 to 22) |
| Depression Ŧ | 5 (18%) | 9 (16%) | 5 (19%) | 4 (14%) |
| Functional comorbidity* | 9 (32%) | 14 (26%) | 7 (27%) | 7 (24%) |
| FIS at inclusion | ||||
| Total score | 61 (32 to 96) | 42 (16 to 78) | 42 (26 to 79) | 42 (16 to 80) |
| Score below threshold MCII | 6 (21%) | 15 (27%) | 6 (23%) | 9 (31%) |
| Score in IBS with depression | 102 (79 to 129) | 109 (56 to 123) | 71 (25 to 123) | 114 (90 to 144) |
| Score in IBS without depression | 51 (20 to 80) | 40 (15 to 60) | 40 (22 to 64) | 38 (15 to 58) |
| IBS QoL at inclusion | ||||
| Total score | 46 (39 to 60) | 60 (39 to 74) | 61 (33 to 70) | 58 (44 to 76) |
| Score below threshold for MCII | 1 (4%) | 2 (4%) | 1 (4%) | 1 (4%) |
| Score in IBS with functional comorbidity | 38 (24 to 46) | 56 (35 to 66) | 60 (33 to 65) | 52 (36 to 78) |
| Score in IBS without functional comorbidity | 56 (44 to 66) | 61 (44 to 76) | 62 (32 to 79) | 60 (49 to 75) |
| FODMAP before FMT (g/day) Ț | 0,0 (−4 to 4,7) | 0,0 (−6,9 to 4,9) | – | – |
Data are median (IQR) or n (%), IBS = irritable bowel syndrome, FODMAP = fermentable oligosaccharides, disaccharides, monosaccharaides, and polyols, MCII = minimally clinically important improvement, FIS = fatigue impact scale, IBS-QoL = irritable bowel quality of life.
Self reported at inclusion; includes fibromyalgia, chronic fatigue syndrome, jaw- and pelvic pain syndromes, ŦSelf reported at inclusion, ŢCalculated from the 5-day dietary record.
There were no significant differences in baseline overall and subdomain score between groups in FIS and QoL-score except for the subdomain dysphoria in the QoL questionnaire (active 58,9 ± 23,8 vs. placebo 47,5 ± 25,0 p = 0,046). Mean scores with standards deviations and p-values for the difference between groups by independent sample t-tests are found in appendix, Table 1.
A significantly greater proportion of patients in the active treatment group achieved minimal clinically important improvement in the IBS QoL score from baseline to 6 months compared to the placebo group, (85% vs. 61%, Odds ratio (OR) 3801; confidence interval (CI) = 1309–11,042 and p = 0,011;). The corresponding difference at 12 months was not significant (78% vs. 61%, OR = 2319; CI = 0,860–6254 and p = 0,093;). A significantly greater proportion of patients in the active treatment group achieved minimal clinically important difference in the FIS score from baseline to 6 months (active treatment 35% and placebo 11%, OR = 4398; CI = 1175–16,468 and p = 0,020) but not at 3 months (active treatment 31% and placebo 18%, OR = 2058; CI = 0,669–6330 and p = 0,203) nor at 12 months (donor treatment 31% and placebo 32%, OR = 0,944; CI = 0,355–2511 and p = 0,909;).
In a post-hoc RM-ANOVA analysis of IBS-QoL the terms found significant to predict the treatment effect were IBS-subtype*other functional disorders (Partial Eta Squared (ηp2) = 0,112 and p = 0,023) and other functional disorders*treatment group (ηp2 = 0,077 and p = 0,019). No potential confounding factors had a significant effect by itself nor changed the conclusions of the model.
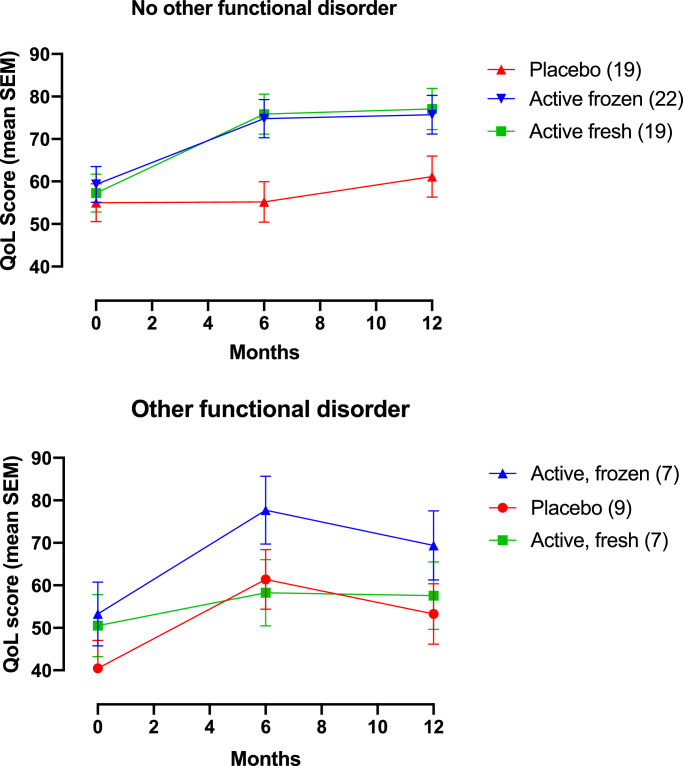
In the RM-ANOVA we further explored the difference between fresh vs. frozen vs. placebo in the subgroups with and without additional self-reported functional disorders. Estimated marginal means and confidence intervals for the interaction treatment group*other functional disorders are provided in the appendix (Table 2) Important differences in the treatment effect between subgroups were found (Fig. 2A and B). The subgroup without other functional disorder (Fig. 2A), given active treatment (fresh or frozen), shows a profound response from baseline to six months that sustain to twelve months. Same effect is not found in the corresponding placebo group with a small improvement in QoL only from six to twelve months. The participants with other functional disease (Fig. 2B), show a transient treatment effect in both active groups (fresh or frozen) very similar to the placebo group. Finally, we did a post hoc analysis of the individual components of the IBS-QoL using the same variables as in the reduced RM-ANOVA model in a doubly multivariate RM-ANOVA (appendix, Table 2; ηp2 and p-value for the effect on each sub domain). Treatment group (fresh vs. frozen vs. placebo) combined with other functional disorder (treatment group*other functional disorders) had a significant effect on the total score by the subdomains interference with activity, body image, and relationships.
Fig. 2.
The repeated time course of the treatment effect in the fresh, frozen and placebo group when functional comorbidity and treatment group are combined, in the term treatment group*functional comorbidity, as predictors. The time-course is in estimated marginal means by the IBS-QoL with the standard error in each time point. Fig. 2a is the treatment effect in the subgroup with no other self-reported functional disorders at baseline. Fig. 2b is the treatment effect in the subgroup with functional comorbidity (other than IBS) at baseline. (Number of participants in corresponding treatment group).
In a post-hoc analysis of FIS we did a repeated measures ANOVA with treatment group (fresh vs. frozen vs. placebo), IBS-subtype and functional comorbidity as predictors. No significant terms were found when the model was reduced. Because fatigue is prevalent in depression, we did a new RM-ANOVA with depression and treatment group as predictors. After removing terms not significant we were left with the term treatment group (fresh vs. frozen vs. placebo)*depression (p = 0,001) as predictor of the treatment effect. None of the potential confounding factors had a significant effect by itself.
In the treatment groups fresh, frozen and placebo there were respectably 5, 4 and 5 participants with self-reported depression at baseline. Because of small sample size in each arm, and the fact that the study was designed to compare active (fresh and frozen combined) to placebo, it was appropriate to combine the fresh and frozen donor FMT group in to one active group and compare it to placebo. Only the term treatment group (fresh and frozen combined)*depression had once again a significant effect on the treatment response (ηp2 = 0,104 and p = 0,005) in the new RM-ANOVA analysis. None of the confounders had a significant effect by itself.
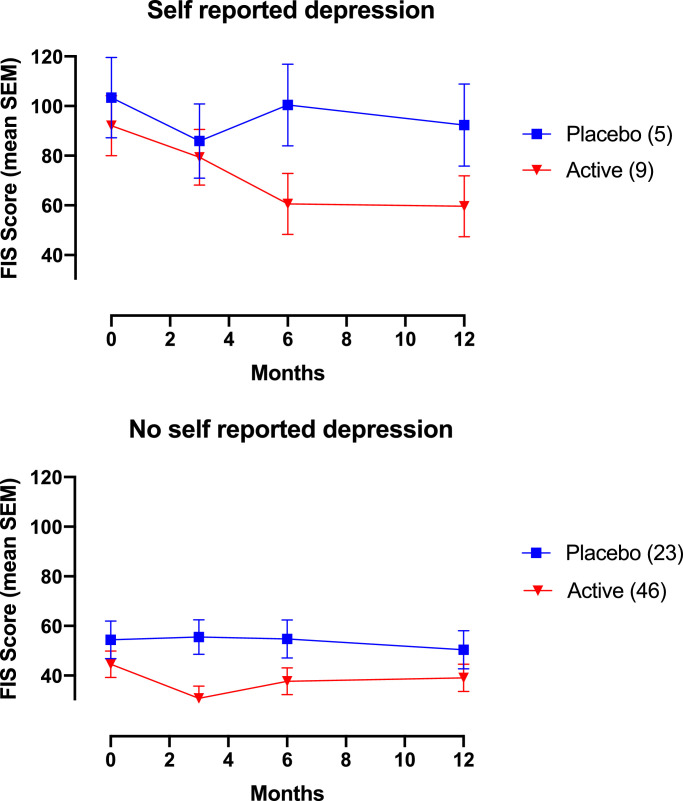
In the RM-ANOVA we further explored the difference in treatment response between the subgroup with and without self-reported depression at baseline and found important distinctions (Fig. 3A and B). Estimated marginal means and confidence intervals for the interaction treatment group*depression are provide in the appendix (Table 4) The subgroup with self-reported depression (3A) at baseline shows a treatment response that sustains from baseline and to three, six and twelve months, whereas the placebo response in the same subgroup is low. The subgroup without self-reported depression (3B) shows a treatment effect from baseline to three months that relapses and becomes almost indistinguishable from the effect in the corresponding placebo group.
Fig. 3.
The repeated time course of the treatment effect active (fresh and frozen combined) and placebo group when depression and treatment group are combined, in the term treatment group*depression, as predictors. The time-course is in estimated marginal means by the FIS with the standard error at each time point. Fig. 2a is the treatment effect in the subgroup with self reported depression at baseline. Fig. 2b is the treatment effect in the subgroup without self reported depression at baseline. (Number of participants in corresponding treatment group).
Finally, we did a breakdown of the individual components of the FIS using the same predictors as in the reduced RM-ANOVA analysis in a doubly multivariate RM-ANOVA (appendix Table 5, ηp2 and p-value for the effect on each sub domain). Treatment group (active vs. placebo with fresh and frozen donor FMT as one group) combined with depression (treatment group*depression) had a significant effect on the total FIS score by all three subdomains (physical, cognitive and social).
4. Discussion
We have presented secondary outcome results from our previously published RCT on FMT in IBS. Results show a clinical effect on QoL and fatigue six months after treatment, with waning effect from six to twelve months. In addition, results mirror our earlier reported findings of the treatment effect on gastrointestinal complaints by the IBS-SSS [5]. Consistency is found in the time course of the treatment response from baseline and until 12 months, and in terms of the predictors that determines the FMT effect. This supports the suggestion that IBS may entail pathophysiologic subgroups extending beyond the current phenotypic subtyping based on stool frequency and consistency [12,13]. Treatment group alone did not have a significant effect by itself on the time-course of the treatment response in QoL, nor in fatigue. This shows that the additional variables (other functional disorders, IBS-subtype and depression) associated with the treatment effect are important. In this context, and for a further discussion, it is very interesting how certain predictors for the FMT effect suggest subgroups with sustainable treatment response that do not only relate to IBS subtype.
Numbers needed to treat (NNT) for an improvement in QoL, six months after treatment, is five and equal to the NNT for relief in gastrointestinal complaints by the IBS-SSS previously reported in these patients [5]. The breakdown of the RM-ANOVA shows that the subdomains most responsive to treatment were interference with activity, body image and relationships (appendix, Table 2). Interference with activity includes bothered by how much time spent on the toilet, staying near toilet and worrying about losing control of bowels, getting less done, avoiding stressful situations, strenuous activity and long trips because of bowel problems. Body image includes limitations on what to wear, feeling fat, sluggish and unclean because of bowel problems. Relationships include limitations in interactions with strangers, uncomfortable talking about and feeling that the closes relationships are affected by bowel problems.
In our previously reported results (the effect of FMT on gastro intestinal complaints by the IBS-SSS), the difference in treatment response between fresh and frozen donor FMT (favoring frozen), was clearly due to the confounding effect from participants with additional functional disorders [5]. Same pattern is found in the RM-ANOVA of the treatment response in QoL (Fig. 2A and B). Comorbidity with other functional disorders is associated with a high placebo response and waning treatment effect on QoL (Fig. 2B), whereas IBS without functional comorbidity is associated with a lasting treatment effect and a less pronounced placebo effect (Fig. 2A). As a high and lasting placebo response often is an issue in clinical trials testing the treatment effect in IBS, this finding should be investigated in future trials [14,15]. Other studies have previously reported an over-representation of somatization disorder in the subgroup of IBS with concomitant somatic comorbidity [16]. A dominating placebo response and lack of a long-term effect in the subgroup with other functional disorders may be attributed to a somatization tendency were FMT have very little effect. Results suggest a lasting FMT effect in IBS without functional comorbidity, whereas IBS with other functional disorders have less benefit. This claim, however, warrants further studies. We hypothesize that additional presence of self-reported functional disorders is a surrogate measure for somatization tendency.
A significant minimal clinically relevant difference in fatigue between active and placebo treatment was only found six months after treatment. However, there were only 73% in the active and 76% in the placebo group with a fatigue score higher than the threshold for minimal clinically relevant difference [2]. Thus making the current dataset less sensitive for changes in FIS score compared to that of QoL and gastro-intestinal complaints in this and the previous report, respectively [5]. In addition, the RM-ANOVA suggests that it is mainly the participants with self-reported depression at baseline that experiences a sustaining decrease in fatigue by FMT. A significant difference between active and placebo by the chi square analysis is found six months after treatment when the effect peaked in this subgroup (Fig. 3A). The breakdown of the RM-ANOVA showed responsiveness to treatment in all three subdomains (physical, cognitive and social fatigue) (appendix, Table 3).
Our findings support previous studies suggesting that depression in IBS originates from the gut and not the brain in a subgroup of IBS. In about half of cases IBS symptoms are found to start first and psychological distress developing later [13]. Moreover, a randomized controlled trial from 2017 found an improvement in depression score and altered brain activity in IBS from treatment with a probiotic [17]. The RM-ANOVA of IBS with depression (from the interaction treatment group*depression) show a lasting treatment effect from baseline and until twelve months that supports benefit of FMT for fatigue in this subgroup particularly (Fig. 3A). Fatigue, or lack of energy, is one of the hallmarks for depressive disorders [18]. This study also points to a link between fatigue and depression in IBS, as the mean fatigue score at baseline was approximately twice as high in IBS with self-reported depression compared to IBS without (Table 1). It is elusive whether the effect on fatigue is an improvement in depression, or improvement in fatigue as a symptom of IBS. It is important to bear in mind the small sample size of IBS with depression with nine (16,4%) and five (17,9%) participants in the active and placebo group respectively (Table 1). Therefore, our notions of a possible treatment effect on self-reported depression in IBS should be explored in future studies.
The IBS cohort in this study confirms previous findings with fatigue as a common complaint in IBS, and it is the first study that shows improvement of fatigue in IBS from targeting the microbiota by FMT [19]. We hypothesize that fatigue in this study was a surrogate measure for depression as this was the only predictor, in addition to treatment group, that determined the treatment effect. There is support in the literature for the involvement of the microbiota in depression, which could explain why a treatment effect mainly was found in the subgroup with self-reported depression.
We have identified four other randomized controlled trials testing the effect of FMT in IBS [20], [21], [22], [23]. Capsulated FMT has not shown any benefits [20,21]. Whereas one study with nasojejunal FMT [23] and one with colonoscopic [22] favored donor FMT compared to placebo (autologous) by a mean improvement in self-reported adequate relief and decrease in gastrointestinal symptom rating-IBS respectively. However, the differences were not significant. The two studies had a lower number of participants than ours, which suggest that the lack of significant findings were caused by underpowered trials.
Route of administration could have an effect on the outcome. The bacterial population increase from the stomach to the colon [24]. In addition, the fermentation of FODMAP's takes place in the colon. This fermentation process is suggested to be involved in IBS pathophysiology [25,26]. Upper delivery of transplants [23], and capsulated FMT [20,21] may lead to an increase of the bacterial population in parts of the digestive system that is not favorable, causing symptom aggravation. In addition, pre-processing from passing through the digestive system may have an impact on how the transplant engraft and influence the colonic fermentation. We, and Holster et al. [22], delivered the transplants to the colon so that the microbiota could engraft in its natural habitat without any pre-processing from the digestive system. The pooled effect of donor FMT by colonoscopic delivery was found significantly in favor of donor FMT in a recent review [27]. In addition, transplants were not prepared by the same technique, did not have the same content (e.g. glycerol concentration), the freeze thaw cycle transplants were exposed to was probably not the same, neither was the amount of feces in transplants, the number of administrations differed, bowel lavage before treatment was not performed in all the studies and the study populations were selected by different criteria. These are important differences that could influence the treatment effect. Finally, only 14 of 664 genera conform to a core microbiota of the gut, so the donor microbiota was probably very different between studies [28]. However, a full comparison on how our results relates to different studies needs a more thorough discussion that is beyond the scope of this manuscript.
The main strength of this study is the thorough characterization of participants at baseline and the long-term follow up that allowed for new findings regarding possible sustainable effects of FMT treatment in subgroups. This study has a number of weaknesses. Of the most important we highlight; first, we do not yet have analysis of the microbiota to support the findings. Second, we used a mix of feces from two donors and we can, therefore, not investigate if there was a donor specific effect. Third, present results are based on secondary endpoints that were not elaborated with a complete statistical analysis plan. However and as planned for, the same statistical analysis as for the reporting of the primary endpoint is applied [5]. This is also consistent with earlier reporting of IBS-related QoL [29]. Forth, change in diet (including probiotics) and/or medications may have influenced the results, however point measures of these parameters did not reveal any change over time as earlier reported [5]. The safety profile is not assessed in this study, but in our previous reported results there were not found any serious adverse events related to the fecal matter in the transplants [5]
5. Conclusion
In conclusion the findings of the FMT effect in QoL and FIS is consistent with the effect on gastro-intestinal complaints earlier reported. The effect on QoL is significant at six months, but not maintained at twelve months. The effect on fatigue is significant at six, but not at three and twelve months. Additional analysis suggests a FMT effect that is maintained until twelve months in subgroups of IBS for both QoL and fatigue. However, the findings must be confirmed in larger studies.
Authors contributions
Funding acquistion: PHJ, PCV, FH, RG; Conceptualization: PHJ, GH, RG PCV; Data curation: PHJ, FH, PCV; Formal anlysis: PHJ, RG; Investigation: PHJ, PCV, FH, RG; Methodology: PHJ, FH, PCV, RG; Project administration: PHJ, RG, FH; Writing orgifinal draft: PHJ, RG, FH, PCV; Writing review and editing: PHJ, PCV, RG, PC.
Declaration of Competing Interest
We declare no competing interests.
Appendix
Table A1, Table A2, Table A3, Table A4, Table A5.
Table A1.
Baseline IBS-QoL and FIS score, including corresponding subdomain score. Data are mean score (standard deviation). The p-value is the difference between active and placebo score compared in an independent sample T-test.
| Active | Placebo | P-value | |
|---|---|---|---|
| IBS-QoL total score | 57,7 (19,1) | 49,2 (20,6) | 0,067 |
| Dysphoria | 58,9 (23,8) | 47,5 (25,0) | 0,046 |
| Interference with activity | 49,6 (22,1) | 40,1 (24,0) | 0,074 |
| Body image | 57,4 (25,1) | 47,8 (25,0) | 0,102 |
| Health worry | 63,5 (16,5) | 58,3 (16,5) | 0,182 |
| Food avoidance | 41,1 (26,9) | 39,6 (27,8) | 0,816 |
| Social reaction | 64,5 (22,5) | 58,7 (24,6) | 0,818 |
| Sexual | 60,9 (28,8) | 58,5 (30,6) | 0,141 |
| Relationships | 68,0 (21,8) | 58,9 (25,5) | 0,093 |
| FIS total score | 52,3 (40,3) | 63,2 (40,0) | 0,249 |
| Cognitive | 13,2 (11,1) | 15,4 (8,9) | 0,380 |
| Physical | 13,1 (10,4) | 15,5 (10,9) | 0,326 |
| Social | 26,0 (20,2) | 32,3 (21,1) | 0,190 |
Table A2.
Estimated marginal means (and confidence interval) from the interaction treatment group (fresh vs. frozen vs. placebo)*other functional disorders.
| Fresh donor FMT | Frozen donor FMT | Placebo | Fresh donor FMT | Frozen donor FMT | Placebo FMT | |
|---|---|---|---|---|---|---|
| With baseline functional disorders | Without baseline functional disorders | |||||
| Baseline | 51 (36–65) | 53 (38–68) | 40 (27–54) | 57 (48–66) | 59 (51–68) | 55 (46–64) |
| 6m | 58 (43–74) | 78 (62–94) | 61 (47–75) | 76 (66–85) | 75 (66–84) | 55 (46–65) |
| 12M | 58 (42–73) | 69 (53–86) | 53 (39–68) | 77 (67–87) | 76 (67–85) | 61 (52–71) |
Table A3.
A doubly multivariate repeated-measures ANOVA of all seven subdomains in IBS-QoL based on the same terms as in the repeated time course of the treatment effect. Partial Eta Squared and (p-value) are corrected by the Greenhouse-Geisser method for all seven subdomains. Patient group (fresh donor vs. frozen donor vs. placebo autologous FMT) combined with other functional disorders (patient group*other functional disorders) have significant effect in the subdomains interference with activity, body image and relationships. *<0,0005.. IBS-QoL = irritable bowel quality of life.
| Dysphoria | Interference with activity | Body image | Health worry | Food avoidance | Social reaction | Sexual | Relationships | |
|---|---|---|---|---|---|---|---|---|
| Time | 0,266 (*) | 0,296 (*) | 0,270 (*) | 0,222 (*) | 0,167 (*) | 0,211 (*) | 0,060 (0,013) | (0,153) (*) |
| Patient group* Other functional disorders |
0,059 (0,318) | 0,116 (0,026) | 0,136 (0,008) | 0,079 (0,129) | 0,081 (0,124) | 0,081 (0,126) | 0,089 (0,086) | 0,137 (0,006) |
| Other functional disorders* IBS-subtype |
0,061 (0,060) | 0,069 (0,043) | 0,068 (0,040) | 0,052 (0,093) | 0,022 (0,484) | 0,056 (0,078) | 0,062 (0,054) | 0,098 (0,005) |
Table A4.
Estimated marginal means (and confidence interval) from the interaction treatment group (active vs. placebo)*depression.
| Active and baseline depression | Placebo and baseline depression | Active without depression | Placebo without depression | |
|---|---|---|---|---|
| Baseline | 92 (68–119) | 103 (71–135) | 45 (34–55) | 54 (39–69) |
| 3 months | 79 (57–101) | 86 (56–116) | 31 (21–41) | 56 (42–70) |
| 6 months | 61 (36–85) | 100 (68–133) | 38 (27–49) | 55 (39–70) |
| 12 months | 60 (35–84) | 92 (59–125) | 39 (28–50) | 50 (35–66) |
Table A5.
A doubly multivariate repeated-measures ANOVA of all three subdomains in FIS based on the same terms as in the repeated time laps of the treatment effect. Partial Eta Squared and (P-values) are corrected by the Greenhouse-Geisser method for all seven subdomains. Patient group (fresh and frozen donor FMT combined vs. placebo autologous FMT) combined with other functional disorders (patient group*depression) have significant effect in the subdomains interference with activity, body image and relationships. FIS = fatigue impact scale.
| Physical | Cognitive | Social | |
|---|---|---|---|
| Time | 0,059 (0,004) | 0,043 (0,027) | 0,062 (0,005) |
| Patient group*depression | 0,102 (0,004) | 0,096 (0,009) | 0,094 (0,011) |
References
- 1.Gralnek I.M., Hays R.D., Kilbourne A., Naliboff B., Mayer E.A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119(3):654–660. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 2.Canavan C., West J., Card T. Review article: the economic impact of the irritable bowel syndrome. Aliment Pharmacol Ther. 2014;40(9):1023–1034. doi: 10.1111/apt.12938. [DOI] [PubMed] [Google Scholar]
- 3.Nellesen D., Yee K., Chawla A., Lewis B.E., Carson R.T. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J Manag Care Pharm. 2013;19(9):755–764. doi: 10.18553/jmcp.2013.19.9.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drossman D a, Chang L., Bellamy N., Gallo-Torres H.E., Lembo A., Mearin F. Severity in irritable bowel syndrome: a rome foundation working team report. Am J Gastroenterol. 2011;106(10):1749–1759. doi: 10.1038/ajg.2011.201. quiz 1760. [DOI] [PubMed] [Google Scholar]
- 5.Johnsen P.H., Hilpüsch F., Cavanagh J.P., Leikanger I.S., Kolstad C., Valle P.C. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3(1):17–24. doi: 10.1016/S2468-1253(17)30338-2. [DOI] [PubMed] [Google Scholar]
- 6.EMA. Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome [Internet]. 2014. p. CPMP/EWP/785/97 Rev. 1. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/09/WC500173457.pdf. [Epub ahead of print].
- 7.Simrén M., Abrahamsson H., Svedlund J., Björnsson E.S. Quality of life in patients with irritable bowel syndrome seen in referral centers versus primary care: the impact of gender and predominant bowel pattern. Scand J Gastroenterol. 2001;36(5):545–552. doi: 10.1080/003655201750153476. [DOI] [PubMed] [Google Scholar]
- 8.Lind R., Berstad A., Hatlebakk J., Valeur J. Chronic fatigue in patients with unexplained self-reported food hypersensitivity and irritable bowel syndrome: validation of a Norwegian translation of the fatigue impact scale. Clin Exp Gastroenterol. 2013;6(1):101–107. doi: 10.2147/CEG.S45760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordin Å., Taft C., Lundgren-Nilsson Å., Dencker A. Minimal important differences for fatigue patient reported outcome measures - A systematic review. BMC Med Res Methodol. 2016;16 doi: 10.1186/s12874-016-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patrick D.L., Drossman D.A., Frederick I.O., Dicesare J., Puder K.L. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 11.Drossman D., Morris C.B., Hu Y., Toner B.B., Diamant N., Whitehead W.E. Characterization of health related quality of life (HRQOL) for patients with functional bowel disorder (FBD) and its response to treatment. Am J Gastroenterol. 2007;102(7):1442–1453. doi: 10.1111/j.1572-0241.2007.01283.x. [DOI] [PubMed] [Google Scholar]
- 12.Holtmann G.J., Ford A.C., Talley N.J. Pathophysiology of irritable bowel syndrome. The Lancet Gastroenterology and Hepatology. 2016 doi: 10.1016/S2468-1253(16)30023-1. [DOI] [PubMed] [Google Scholar]
- 13.Ford A.C., Lacy B.E., Talley N.J. Irritable bowel syndrome. N Engl J Med. 2017;376(26):2566–2578. doi: 10.1056/NEJMra1607547. [DOI] [PubMed] [Google Scholar]
- 14.Ford A.C., Moayyedi P. Meta-analysis: factors affecting placebo response rate in the irritable bowel syndrome. Aliment Pharmacol Ther. 2010 doi: 10.1111/j.1365-2036.2010.04328.x. [DOI] [PubMed] [Google Scholar]
- 15.Patel S.M., Stason W.B., Legedza A., Ock S.M., Kaptchuk T.J., Conboy L. The placebo effect in irritable bowel syndrome trials: a meta-analysis. Neurogastroenterology and Motility. 2005 doi: 10.1111/j.1365-2982.2005.00650.x. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead W.E., Palsson O.S., Levy R.R., Feld A.D., Turner M., Von Korff M. Comorbidity in irritable bowel syndrome. Am J Gastroenterol. 2007;102(12):2767–2776. doi: 10.1111/j.1572-0241.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 17.Pinto-Sanchez M.I., Hall G.B., Ghajar K., Nardelli A., Bolino C., Lau J.T. Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–459. doi: 10.1053/j.gastro.2017.05.003. e8. [DOI] [PubMed] [Google Scholar]
- 18.Demyttenaere K., De Fruyt J., Stahl S.M. The many faces of fatigue in major depressive disorder. International Journal of Neuropsychopharmacology. 2005 doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- 19.Frändemark Jakobsson Ung E, Törnblom H., Simrén M., Jakobsson S. Fatigue: a distressing symptom for patients with irritable bowel syndrome. Neurogastroenterol Motil. 2017;29(1) doi: 10.1111/nmo.12898. [DOI] [PubMed] [Google Scholar]
- 20.Aroniadis O.C., Brandt L.J., Oneto C., Feuerstadt P., Sherman A., Wolkoff A.W. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2019 doi: 10.1016/S2468-1253(19)30198-0. [DOI] [PubMed] [Google Scholar]
- 21.Halkjær S.I., Christensen A.H., Lo B.Z.S., Browne P.D., Günther S., Hansen L.H. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018 doi: 10.1136/gutjnl-2018-316434. [DOI] [PubMed] [Google Scholar]
- 22.Holster S., Lindqvist C.M., Repsilber D., Salonen A., de Vos W.M., König J. The effect of allogenic versus autologous fecal microbiota transfer on symptoms, visceral perception and fecal and mucosal microbiota in irritable bowel syndrome. Clin Transl Gastroenterol. 2019 doi: 10.14309/ctg.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holvoet T., Joossens M., Jerina B., Christiaens E., Heyerick L., Verhasselt B. 617 - Fecal Microbiota transplantation in irritable bowel syndrome with predominant abdominal bloating: results from a double blind, placebo-controlled clinical trial. Gastroenterology. 2018;154(6) doi: 10.1053/j.gastro.2020.07.013. S-130. [DOI] [PubMed] [Google Scholar]
- 24.Dethlefsen L., Eckburg P.B., Bik E.M., Relman D.A. Assembly of the human intestinal microbiota. Trends in Ecology and Evolution. 2006 doi: 10.1016/j.tree.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Gibson P.R., Shepherd S.J. Evidence-based dietary management of functional gastrointestinal symptoms: the FODMAP approach. J Gastroenterol Hepatol. 2010 Feb;25(2):252–258. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 26.Schumann D., Klose P., Lauche R., Dobos G., Langhorst J., Cramer H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: a systematic review and meta-analysis. Nutrition. 2018;45:24–31. doi: 10.1016/j.nut.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Ianiro G., Eusebi L.H., Black C.J., Gasbarrini A., Cammarota G., Ford A.C. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019 doi: 10.1111/apt.15330. [DOI] [PubMed] [Google Scholar]
- 28.Pittayanon R., Lau J.T., Yuan Y., Leontiadis G.I., Tse F., Surette M. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157(1):97–108. doi: 10.1053/j.gastro.2019.03.049. [DOI] [PubMed] [Google Scholar]
- 29.Cash B.D., Pimentel M., Rao S.S.C., Weinstock L., Chang L., Heimanson Z. Repeat treatment with rifaximin improves irritable bowel syndrome-related quality of life: a secondary analysis of a randomized, double-blind, placebo-controlled trial. Therap Adv Gastroenterol. 2017;10(9):689–699. doi: 10.1177/1756283X17726087. [DOI] [PMC free article] [PubMed] [Google Scholar]