Abstract
Objective:
To review the state of omics science specific to asthma and allergic diseases and discuss the current and potential applicability of omics in clinical disease prediction, treatment, and management
Data Sources:
Studies and reviews focused on the use of omics technologies in asthma and allergic disease research and clinical management were identified using PubMed
Study Selections:
Publications were included based on relevance, with emphasis placed on the most recent findings
Results:
Omics-based research is increasingly being used to differentiate asthma and allergic disease subtypes, identify biomarkers and pathological mediators, predict patient responsiveness to specific therapies, and monitor disease control. While most studies have focused on genomics and transcriptomics approaches, increasing attention is being placed on omics technologies that assess the effect of environmental exposures on disease initiation and progression. Studies utilizing omics data to identify biological targets and pathways involved in asthma and allergic disease pathogenesis have primarily focused on a specific omics subtype, providing only a partial view of the disease process.
Conclusions:
While omics technologies have advanced our understanding of the molecular mechanisms underlying asthma and allergic disease pathology, omics testing for these diseases are not standard of care at this point. Several important factors need to be addressed before these technologies can be effectively utilized in clinical practice. Use of clinical decision support systems and integration of these systems within electronic medical records will become increasingly important as omics technology becomes more widely utilized in the clinical setting.
Keywords: asthma, allergic disease, clinical management, genomics, epigenomics, transcriptomics, proteomics, metabolomics, lipidomics, microbiomics, exposomics, phenomics
Introduction
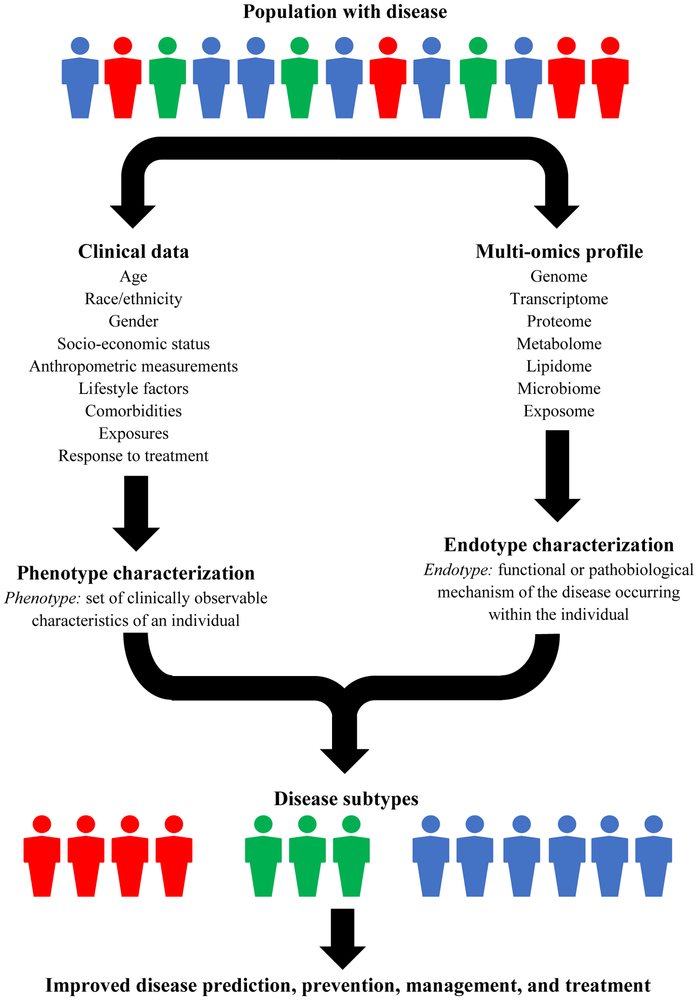
Most diseases are caused by a complex, multi-level combination of genomic, biological, and environmental factors, contributing to a high degree of variability in disease development, natural history, and response to therapy.1-3 Disease subtyping has emerged as a way of identifying sub-populations of individuals with similar disease features for improved diagnosis and treatment.3, 4 Traditionally, patients have been classified into groups according to their clinical characteristics (i.e., phenotypes). However, these classifications do not provide insight into the functional or pathobiological mechanisms of the disease within the individual (i.e., endotype).5 Omics is the comprehensive assessment of the molecules that comprise a cell, tissue, or organism.6 Integration of multi-omics data, such as genomics, proteomics, and metabolomics, along with clinical data allows for better understanding of disease pathogenesis and will be important for predicting, diagnosing, and treating diseases (Figure 1).7, 8
Figure 1.
Framework for integration of clinical and multi-omics data for improved disease subtyping within the disease population.
Asthma and allergic diseases, including allergic rhinitis and atopic dermatitis, are common diseases that often manifest early in life and persist into adulthood.9 The pathophysiology and expression of these diseases are influenced by interactions between susceptibility genes and exposure to environmental factors such as aeroallergens, secondhand smoke, and infections. Isolated use of traditional markers, such as lung function parameters and skin-prick testing, and clinical symptoms to diagnose specific subtypes and manage asthma and allergic diseases have been shown to be inadequate due to the heterogenous underlying pathophysiology of disease phenotypes.10 Integration of phenotyping and multi-omics endotyping can help differentiate asthma and allergic disease subtypes, identify biomarkers and pathological mediators, predict patient responsiveness to specific therapies, and monitor disease control.8, 11, 12
We aim to briefly review the state of omics science specific to asthma and allergic diseases and discuss the current and potential applicability of omics in clinical disease prediction, treatment, and management. Previous reviews have focused on omics as they relate to specific allergic diseases, primarily asthma.5, 13-17 Our review will be uniquely framed to focus on omics subtypes, referencing current applications of each subtype within the field of asthma and allergic diseases, including research applications.
Applied Omics in Asthma and Allergic Diseases
Genomics
Genomics is the study of variations in the deoxyribonucleic acid (DNA) and their association with disease onset, severity, exacerbation, response to therapeutic agents (pharmacogenomics), or patient prognosis (Table 1).16, 18 A large portion of the susceptibility to asthma and allergic diseases is attributed to genomic contributions.19 Many genes have been identified as contributing to the development of asthma and allergic diseases, including the well-replicated 17q21 locus (associated with childhood-onset wheeze) and filaggrin (FLG) gene (associated with atopic dermatitis).13, 16 However, these genes account for only a small proportion of the disease heritability. The missing heritability may be attributed to rare variants, gene-gene, or gene-environment interactions. Lack of replication across studies and unknown functional implications of genes implicated in asthma and allergic disease pathogenesis have hindered the use of genetic risk factors in predicting disease onset and exacerbation in the clinical setting.19, 20 Further assessment of polygenetic scores of several genes may help in prediction and prognosis of such complex diseases.
Table 1.
Features of omics data.
| Omics subtype | Measured biomarker | Sample type | Data collection technologies |
|---|---|---|---|
| Genomics | DNA | Any tissue that has a nucleusa | Genotyping arrays NGS Exome sequencing |
| Epigenomics | DNA methylation levels, post-translational histone modifications, non-coding RNA (e.g., microRNA) | Tissue-specific (sera, other bodily fluids, or tissues may be used) | NGS DNA methylation analysis with arrays Small RNA sequencing |
| Transcriptomics | RNA | Tissue-specific (sera, other bodily fluids, or tissues may be used) | Probe-based arrays RNA-Seq |
| Proteomics | Proteins | Tissue-specific (sera, other bodily fluids, or tissues may be used) | MS |
| Metabolomics | Metabolites | Tissue-specific (sera, other bodily fluids, or tissues may be used) | MS NMR Semiconductor metal oxide |
| Lipidomics | Lipids | Tissue-specific (sera, other bodily fluids, or tissues may be used) | MS NMR |
| Microbiomics | Microorganisms | Tissue-specific (sera, other bodily fluids, or tissues may be used) | NGS |
| Exposomics | Any biomarker of exposureb | Dependent on the biomarker(s) assessedc | Dependent on the biomarker(s) assessed |
| Phenomics | Disease states, symptoms, lab measurements, vitals | N/Ad | Extraction from electronic health records, surveys, physical exams, measurements, etc. |
DNA, deoxyribonucleic acid; NGS, next generation sequencing; RNA, ribonucleic acid; MS, mass spectrometry; NMR, nuclear magnetic resonance spectroscopy; N/A, not applicable.
Blood and saliva are commonly used.
Examples of biomarkers of environmental exposures include vitamins (diet), polycyclic aromatic hydrocarbons (pollutant), and organohalogens (pesticide).
Samples types may include sera, urine, saliva, exhaled gas, tissue, etc.
Phenomics studies use patient-level data extracted from electronic health records, surveys, physical exams, measurements, etc. These data are sometimes linked with genetic information.
The potential role of pharmacogenomics in the clinical management of asthma and allergic diseases is being increasingly recognized. As many susceptibility genes are shared across allergic diseases, targeted therapeutics may be used to treat multiple diseases.20, 21 Most pharmacogenomics studies of asthma to date have focused on the clinical response to commonly prescribed medications, such as bronchodilators, leukotriene modifiers, and inhaled corticosteroids (ICSs), through candidate-gene approaches.20, 22 Studies assessing the role of short- and long-acting bronchodilators have mainly focused on ADRB2 (adrenoceptor beta 2).23 Asthmatic patients with a homozygous genotype for a variant substituting at amino acid 16 within ADRB2 have been shown to have decreased lung function and increased exacerbation with regular short-acting beta agonist use. Studies assessing the response of these patients to long-acting beta agonists have been conflicting.22, 23 Variants in the arachidonate 5-lipoxygenase (ALOX5), leukotriene C4 synthase (LTC4S), leukotriene A4 hydrolase (LTA4H), and cysteinyl leukotriene receptor 2 (CYSLTR2) genes have been associated with response to leukotriene modifiers;22, 23 however, replication of these findings are needed.23 Although a large number of studies have assessed the genetic contribution to ICS response, findings have been inconsistent. Variants within the FCER2 (fc fragment of IgE receptor 2) gene have shown the most promising results, with asthmatic children with these variants showing poor ICS response.16 To increase clinical applicability or pharmacogenomic findings, research has shifted towards genome-wide association studies (GWAS). As a result, many novel therapies are being developed and evaluated.20
Epigenomics
While genomics is known to play a large role in susceptibility to asthma and allergic diseases, the increase in incidence and prevalence of these diseases observed globally over the past 70 years cannot be explained by genetic predisposition. Environmental and lifestyle factors must play an important role in the initiation and persistence of disease in predisposed individuals.24 Epigenomics is the study of potentially reversible modifications of the chromatin, measured by DNA methylation levels, histone modifications, or non-coding ribonucleic acid (RNA), due to normal cellular repair or environmental modifiers.24, 25 Epigenomics may be used to better understand the influence of environmental and lifestyle factors on the underlying mechanisms that contribute to the development of asthma and allergic diseases, which could aid in the development of preventive strategies for susceptible individuals.24
While epigenomics technology and methodologies are less developed than genomics and transcriptomics approaches,17 studies aimed at identifying epigenomic signatures within asthmatic and allergic disease populations are on the rise. The value of DNA methylation signatures as biomarkers of diagnosis and/or therapeutic response has been illuminated in studies of other complex diseases, such as cancer and autoimmune diseases, suggesting its potential in understanding modifiable alterations in DNA that predispose to asthma and allergic diseases. Epigenome-wide association studies (EWAS) could be used to identify individuals who are allergy-prone prior to disease onset.24 Epigenomic signatures can be inherited,17 and in utero exposure to farms, air pollution, and tobacco smoke have been shown to alter DNA methylation signatures associated with development of asthma and allergic disease early in life.16, 24, 26 DNA methylation signatures have also been shown to outperform conventional biomarkers, such as allergen-specific IgE levels and skin-prick tests, in the prediction of food allergy, suggesting that these signatures could potentially be used as a safe alternative to food challenges.24, 27
A limited number of studies have assessed the effect of asthma and allergic disease treatment on the epigenome.16, 24 Demethylation of FOXP3 (forkhead box P3), a gene that is only expressed by regulatory T cells and is reduced among allergic children, has been observed in children who develop tolerance for IgE-mediated cow’s milk allergy after dietary intervention and peanuts after oral immune-therapy.24, 28, 29 Epigenomic changes in response to ICS and biological agents have been identified but further replication is needed.16, 30 The use of DNA methylation inhibitors, such as 5-azacytidine, in patients with asthma and allergic diseases has been assessed; however, results are conflicting. In the future, the CRISPR/Cas9 gene-editing system could potentially be used to reverse environmentally-induced changes in the epigenome prior to disease onset.24, 30
Transcriptomics and Proteomics
Gene expression is a dynamic process that is highly influenced by many factors including age, gender, developmental stage, health status, tissue/cell type, time of day, and exposure to allergens, infections, and medications.16, 31 Transcriptomics and proteomics investigate two important aspects of gene expression, RNA (i.e., the molecular intermediate between DNA and proteins) and proteins (i.e., the primary functional product of DNA).18 Omics technologies are currently being used to identify differential gene expression patterns between those with and without disease, leading to mechanistic hypotheses and biomarker development.20, 31
In order to analyze the massive amount of data that is generated through gene expression analyses, sophisticated analytical methods are needed.16 Such techniques have been applied in large-scale projects such as the Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes (U-BIOPRED) consortium, which aimed to identify asthma endotypes through the characterization of gene expression profiles.16, 32 While transcriptomics approaches are now widely used is asthma and allergic disease research,13, 17 untargeted proteomics research has emerged more slowly which may be due in part to the diversity of technologies used.31
Transcriptomics and proteomics studies of asthma and allergic diseases have been most fruitful in identifying disease endotypes. Differences in gene expression patterns between childhood and adult-onset asthma suggest that distinct mechanisms underly disease onset.16 Sputum proteomics has been used to identify multiple sub-endotypes of eosinophil- and neutrophil-mediated asthma.17 Transcriptomics has also been used to investigate mechanisms of asthma and allergic disease severity, exacerbation, and response to treatment. Genes involved in bronchodilation, reduction of inflammation, interferon response, and expression of CD8+ T cells have been shown to be differentially expressed in children with severe asthma compared to children with mild asthma.31 Increased expression of immune cytokines and chemokines have been shown to correlate with disease severity and progression in atopic dermatitis.15 Studies aimed at understanding the underlying mechanisms of asthma exacerbations in children have identified several differentially expressed genes during exacerbation related to immune responses against viral infections and potential environmental exposures, such as smoke, pollutants, or allergens.16, 33 A few studies have assessed transcriptomics response to glucocorticosteroid treatment;34 however, further replication studies are needed. Although transcriptomics and proteomics studies in asthma and allergic disease have yet to yield new diagnostic tests or drugs, the growing sample size and robust design of ongoing studies show potential for clinical translation in the near future.31
Metabolomics
Metabolomics measures the total repertoire of low molecular weight products of cellular metabolism (e.g., amino acids, fatty acids, sugars, and lipids) present in cells, tissues, organs, and biological fluids.13, 17, 35 The identities, concentrations, and fluxes of these metabolites result from the complex interplay of the genome, gene expression, protein translation, and the environment.35 As many cellular processes are regulated by metabolites, they can act as indicators of homeostatic imbalances.36 Metabolomics can, thus, be used to improve our understanding of disease pathogenesis, assess biological responses to risk factors, identify susceptibility biomarkers, and monitor disease progression.35
Metabolomics approaches are particularly appealing for the study of asthma and allergic diseases, which are highly influenced by host-environment interactions.35 Though limited in number, metabolomics studies have provided unique and novel insights into allergy and asthma profiling at the molecular level. Most metabolomics studies have focused on biomarker discovery to identify metabolites that distinguish between asthma/allergic disease and healthy phenotypes and asthma severity.13, 17, 35 Noninvasive biomarkers, such as volatile organic compounds in exhaled breath, have shown promising roles in the diagnosis, monitoring, and treatment of asthmatics and could be especially useful for the assessment of airway inflammation in young children.37 However, validation of these biomarkers in independent cohorts of biological samples is needed to demonstrate clinical utility.17, 31 In addition to the global metabolic profiles of individuals with asthma and allergic diseases, metabolomics can provide deeper insights into the pathophysiology of distinct asthma and allergic disease phenotypes. Accurate prediction of phenotypes using metabolic biomarkers may have the greatest clinical impact, encouraging increased utilization of prospective study designs in metabolomics research.38
Lipidomics
Lipids have both structural and functional roles, acting as the primary component of cellular membranes, energy reservoirs, and mediators in cellular mechanisms such as signal transduction, immunity, and inflammation.39, 40 Over 40,000 lipids have been documented to date.40, 41 Lipidomics, a sub-field of metabolomics, aims to understand the structure and function of a given cell or organism’s lipidome and how lipoproteins are affected by diseases and treatments.39, 40
Although the emergence of more sophisticated techniques, such as mass spectrometry-based lipidomics, has facilitated the expansion of knowledge of the effects of lipids on asthma and allergic diseases, most studies have focused on targeted profiling of lipid mediators for treatment development. Lipid mediators, such as leukotrienes, platelet activating factor, prostaglandins, and sphingolipids, modulate the immune system in response to allergens and have, thus, been primary targets for therapeutic interventions. The leukotriene pathway, which promotes bronchial smooth muscle constriction and increases vascular permeability, has been the most successfully targeted, with two classes of drugs currently on the market, cysteinyl leukotriene receptor 1 (CysLTR1) antagonists and 5-lipoxygenase inhibitors.42 Clinical trials assessing the efficacy of antagonists of platelet activating factor, such as rupatadine which is not currently available in the United States, and prostaglandin receptor agonists/antagonists in patients with asthma and allergic diseases have been performed or are currently underway.42-44 While findings from animal studies assessing the efficacy of anti-sphingosine 1-phosphate compounds in allergic disease management have been promising, no clinical trials are currently underway.42, 45
Microbiomics
The microbiome broadly describes the microorganisms (including bacteria, viruses, and fungi) colonizing the human body, their genes, and their interactions with each other and their host.18, 46 It is exceedingly complex, composed of trillions of microorganisms whose composition varies across body sites, time, and between individuals and is highly influenced by environmental and dietary factors.18, 47 Alterations in the composition or metabolic activity of the microbiome can negatively impact immune function due to the intimate crosstalk between the two networks.47 The immunomodulatory mechanisms of microbial dysbiosis are beginning to be elucidated using omics technologies, such as shotgun metagenomics sequencing and metatranscriptiomics.47, 48 Whether alterations in the composition of the microbiome precede or follow immune responses in asthma and allergic diseases remains unclear; however, prospective birth cohort studies have begun to shed light on the temporal relationship.47
Microbiome-based strategies for prevention, treatment, and management of asthma and allergic diseases have focused on targets of innate immunity and therapies altering microbial communities, including prebiotics, probiotics, and microbial transplantation.48 Drugs targeting lung inflammation, such as macrolide antibiotics and corticosteroids, have shown beneficial effects for treatment of certain asthma phenotypes. However, the airway microbiome may modulate the response to these therapies, suggesting that microbiome phenotyping of individuals prior to administration may be beneficial for treatment effectiveness.48, 49 While findings from studies assessing the effectiveness of prebiotic (non-digestible fiber that stimulates the growth of beneficial microorganisms) and probiotic (live microorganisms) use in preventing asthma and allergic diseases have been promising, particularly for prevention of eczema,46-48 no recommendations have been made to date for pre- or probiotic use in patients with asthma or allergic diseases.47 Several clinical trials are currently underway to assess the effectiveness of microbiome transplants, including vaginal swabbing, skin creams, and oral encapsulated fecal microbiota, in preventing and treating asthma and allergic diseases.50
Exposomics
The exposome defines the totality of an individual’s external (e.g., climate, traffic, and pollutants) and internal (e.g., metabolism, inflammation, and aging) environmental exposures throughout their life course.51, 52 Unlike epigenomics, which is used to assess modifications to the genome specifically, exposomics comprehensively assesses multi-omics responses to environmental exposures. These response patterns, ideally characterized from longitudinal biomonitoring, could then be used to provide an individualized disease risk profile for targeted prevention. Using a more holistic approach in assessing the effect of environmental exposures on disease will likely be more informative in predicting complex diseases like asthma and allergies than assessing the separate effects of individual exposures.51
While a growing number of studies have assessed the role of specific environmental exposures in the pathogenesis of asthma and allergic diseases, challenges related to measurement harmonization, feasibility of exposure assessment, integration of multifactorial data, and methods of discovery analysis have hindered exposome-wide analyses in this field.51 In an effort to overcome some of these issues, large-scale initiatives, such as the Human Early-Life Exposome (HELIX) and EXPOsOMICS projects, have been launched with the goal of refining exposomics characterization.53, 54 Additionally databases, such as the World Health Organization’s Exposome-Explorer, can be used to identify biomarkers of environmental exposures for biomonitoring or studies of disease etiology.55
Phenomics
Phenomics is the systematic study of a large set of phenotypes used to describe an organism. In biomedical informatics, the phenome is defined as symptoms, physical findings, and disease diagnoses that describe patients for the purposes of medical care. Phenomics can be used to identify and describe disease subtypes or study pleiotropy (i.e., multiple phenotypes arising from the same genetic alteration).56
The electronic health record (EHR) is an important resource for the study of human phenomics. Compared to observational research cohorts that only capture a pre-specified set of phenotypes, the EHR contains information on a vast array of phenotypes that are pertinent to medical care.57 Many health care systems now link EHR and genetic information, obtained through biospecimen collection, which has led to the development of phenome-wide association studies (PheWAS).57-61 While originally designed to study the relationship between a large set of human phenotypes and a single genetic variant, PheWAS applications have since broadened to assess associations between phenotypes to identify comorbidities, subtypes, or health-service outcomes (e.g., length of hospital stay and treatment-related complications) related to a specific disease.57
A mounting body of evidence has demonstrated the utility of EHR data in genomics research. EHRs have been used to replicate known genetic associations with asthma first discovered in observational cohorts.58, 62 PheWAS has been used to identify novel asthma risk loci and discover pleiotropy of asthma-associated genetic variants with atopy and leukemia and allergic rhinitis-associated genetic variants with metabolic disease and diabetes.58-61 PheWAS has also been used to identify new therapeutic targets as well as predict adverse drug events. One study identified asthma as a potential side effect of drugs that inhibit PNPLA3 (patatin-like phospholipase domain containing 3), a potential therapeutic for liver disease.63
EHR-driven phenomics have also been used to study monogenic, or Mendelian, diseases. As variants within Mendelian disease-causing genes have been shown to contribute to complex diseases,64 assessment of the association between these variants and asthma and allergic disease risk is warranted. The Online Mendelian Inheritance in Man (OMIM), a catalog of monogenetic diseases, describes many diseases that are characterized, in part, by traits related to asthma and allergic diseases.65 A method called the phenotype-risk score (PheRS) was recently developed to capture Mendelian phenotype patterns using EHR data and inform on the contribution of rare variants to common diseases.64 Rare genetic variants may be used to link some patients’ asthma to Mendelian diseases with targeted therapies.
Conclusions and Future Perspectives
Clinical Decision Support Systems
Electronic Health Records
As omics technology becomes more widely utilized in the clinical setting, integration of omics data within EHRs will become increasingly important for interpretation and clinical decision support.66, 67 However, EHRs, which have traditionally been structured to provide a common workflow for health care providers and centralized documentation of billing and clinical monitoring and decisions, are not suited to accommodate omics data in their current form.66 Next-generation EHRs will need increased storage capacity, structured data formats to allow return of omics results to physicians and patients, links to reference sources to aid in the interpretation of results from Clinical Laboratory Improvement Act (CLIA)-certified clinical laboratories and genetic counselors, and systems for reprocessing archived data and updating interpretations as new scientific knowledge becomes available.66, 67 To begin this transition, initiatives, such as the Electronic Medical Records and Genomics (eMERGE) network, have been established to develop methods and best practices for integrating omics data into the EHR system and returning results to patients.68
Ethical, legal, and privacy considerations for data storage and sharing are also of concern with the integration of omics data in EHRs. While the federal Health Insurance Privacy and Accountability Act (HIPAA) imposed privacy standards on the use of protected health information, such as names and birth dates,69 omics data are not explicitly mentioned in the act and are, therefore, potentially vulnerable to privacy violations.66, 70 Frameworks for the storage and management of omics data and consent have not been widely implemented;66 however, some institutions have established guidelines for physical and technological security controls to help protect omics data.70, 71 Further, issues related to recontact of potentially affected patients once new scientific knowledge becomes available and return of results, particularly from genomics testing, to potentially affected family members need to be carefully assessed and strategized.66
Laboratory Formularies
The use of omics technologies in clinical care has been somewhat limited to those areas where data and validation have shown sufficient laboratory sensitivity, specificity, and reproducibility, as well as clinical validity and utility. The areas which have been brought forward as standard of care include cancer diagnosis and therapy, rare and/or inherited disease diagnosis and risk assessment, and pharmacogenomics.72 In an effort to provide more affordable, accessible, and high-quality healthcare in the United States, attention has been placed on reducing hospitalizations, readmissions, and therapeutic costs. However, management of diagnostic and screening testing is also important to healthcare reform as the number of these tests available to clinicians is rising and many are expensive and require extensive background knowledge for correct interpretation.73 To promote the appropriate utilization of laboratory testing some institutions have implemented laboratory formularies.74 These programs provide strategic guidance for ordering clinicians through evidence review and expert consultation of each test’s clinical utility, cost, and interpretation. Successful implementation requires ongoing collaboration of hospital administrators, clinical and laboratory staff, and information technology developers.73, 74 Although omics testing for asthma and allergic diseases are not standard of care at this point, use of a laboratory formulary structure to introduce and advance testing for these diseases would provide a structure to obtain strong evidence and appropriate utilization management.
Integrated Omics
The vast majority of studies utilizing omics data to identify biological targets and pathways involved in asthma and allergic disease pathogenesis have primarily focused on a specific omics subtype.13, 75 While important insights have been gained from these studies, they provide only a partial view of the disease process.13 Integration of multi-omics data provides a more comprehensive understanding of the underlying mechanisms of disease through identification of molecular interactions, intermediate phenotypes and processes, and upstream/downstream molecular targets.13, 76 However, amplification of issues related to data acquisition, harmonization, storage, quality, and analysis have slowed the integration and simultaneous assessment of multiple, multi-dimensional omics subtypes. As multi-omics approaches often require the collection and processing of larger volumes of multiple sample types, patient burden and cost-effectiveness also need to be weighed when performing these analyses.13, 18 Several large-scale consortia, such as the Mechanisms of the Development of ALLergy (MeDALL),9 U-BIOPRED,32 and Environmental influences on Child Health Outcomes-Children’s Respiratory and Environmental Workgroup (ECHO-CREW),77 have been established to study and develop approaches to facilitate clinical translation of multi-omics data for patients with asthma and allergic diseases.
Clinical Implementation: Current Challenges and Progress
While omics technologies have advanced our understanding of the molecular mechanisms underlying asthma and allergic disease pathology, these technologies are primarily being used as research tools at this time and several important factors need to be addressed before they can be effectively utilized in clinical practice. Validation and replication of findings from previous studies is essential, necessitating standardization of data collection, processing, and analysis.13, 18 Large-scale initiatives and data repositories, such as the UK Biobank78 and the Biologic Specimen and Trans-NIH BioMedical Informatics Coordinating Committee (BMIC),79 have facilitated the generation of robust biomedical datasets to improve research efficiency, increase collaboration, and facilitate validation of findings.67, 80 Institutions, such as the Food and Drug Administration81 and the American College of Medical Genetics and Genomics,82 have created guidelines for collection and processing of biospecimens and development of standard data storage formats and data interpretations.80 Additionally, standardized clinical diagnostic codes and phenotypic terminology have been established to allow consistent information exchange and comparability of diseases across populations.80
Infrastructures for clinical informatics and increased cross-disciplinary training for health professionals are also key for the successful application of omics technologies and have been highlighted in projects such as CASyM (Coordinating Action Systems Medicine).83-85 Many online and offline courses are now being offered to improve the analysis and interpretation of omics data.80 With a concerted collaborative effort from patients and experts with diverse backgrounds, including clinicians, bioinformaticians, medical laboratory scientists, lawyers, ethicists, and hospital administrators, it is possible for omics technologies to transform and improve patient health and the healthcare system.83, 84
Acknowledgements
We kindly thank Dr. Katherine Cahill, Dr. R. Stokes Peebles, Dr. Christian Rosas-Salazar, and Kaitlin Costello for their expert review of this manuscript.
Funding Source: This work was supported by the National Institutes of Health (grant number 5 T32 HL087738–14 which supports B.M.D., K24 AI 077930 to T.V.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of this agency.
Abbreviations/Acronyms:
- DNA
deoxyribonucleic acid
- FLG
filaggrin
- ICS
inhaled corticosteroid
- ADRB2
adrenoceptor beta 2
- ALOX5
arachidonate 5-lipoxygenase
- LTC4S
leukotriene C4 synthase
- LTA4H
leukotriene A4 hydrolase
- CYSLTR2
cysteinyl leukotriene receptor 2
- FCER2
fc fragment of IgE receptor 2
- GWAS
genome-wide association study
- RNA
ribonucleic acid
- EWAS
epigenome-wide association study
- FOXP3
forkhead box P3
- U-BIOPRED
Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes
- CysLTR1
cysteinyl leukotriene receptor 1
- HELIX
Human Early-Life Exposome
- EHR
electronic health record
- PheWAS
phenome-wide association study
- PNPLA3
patatin-like phospholipase domain containing 3
- OMIM
Online Mendelian Inheritance in Man
- PheRS
phenotype-risk score
- CLIA
Clinical Laboratory Improvement Act
- eMERGE
Electronic Medical Records and Genomics
- HIPAA
Health Insurance Privacy and Accountability Act
- MeDALL
Mechanisms of the Development of ALLergy
- ECHO-CREW
Environmental influences on Child Health Outcomes-Children’s Respiratory and Environmental Workgroup
- BMIC
Biologic Specimen and Trans-NIH BioMedical Informatics Coordinating Committee
- CASyM
Coordinating Action Systems Medicine
Footnotes
Conflicts of Interest: None
Clinical Trial Registration: Not applicable
References
- 1.Noell G, Faner R, Agusti A. From systems biology to P4 medicine: applications in respiratory medicine. European respiratory review : an official journal of the European Respiratory Society. 2018;27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higdon R, Earl RK, Stanberry L, et al. The promise of multi-omics and clinical data integration to identify and target personalized healthcare approaches in autism spectrum disorders. Omics : a journal of integrative biology. 2015;19:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saria S, Goldenberg A. Subtyping: What It is and Its Role in Precision Medicine. IEEE Intelligent Systems. 2015;30:70–75. [Google Scholar]
- 4.Merino J, Florez JC. Precision medicine in diabetes: an opportunity for clinical translation. Annals of the New York Academy of Sciences. 2018;1411:140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svenningsen S, Nair P. Asthma Endotypes and an Overview of Targeted Therapy for Asthma. Frontiers in medicine. 2017;4:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horgan RP, Kenny LC. ‘Omic’ technologies: genomics, transcriptomics, proteomics and metabolomics. The Obstetrician & Gynaecologist. 2011;13:189–195. [Google Scholar]
- 7.Chen R, Mias George I, Li-Pook-Than J, et al. Personal Omics Profiling Reveals Dynamic Molecular and Medical Phenotypes. Cell. 2012;148:1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simon D. Recent Advances in Clinical Allergy and Immunology. International Archives of Allergy and Immunology. 2018;177:324–333. [DOI] [PubMed] [Google Scholar]
- 9.Anto JM, Bousquet J, Akdis M, et al. Mechanisms of the Development of Allergy (MeDALL): Introducing novel concepts in allergy phenotypes. The Journal of allergy and clinical immunology. 2017;139:388–399. [DOI] [PubMed] [Google Scholar]
- 10.Diamant Z, Boot JD, Mantzouranis E, Flohr R, Sterk PJ, Gerth van Wijk R. Biomarkers in asthma and allergic rhinitis. Pulmonary pharmacology & therapeutics. 2010;23:468–481. [DOI] [PubMed] [Google Scholar]
- 11.Choi H, Song WM, Zhang B. Linking childhood allergic asthma phenotypes with endotype through integrated systems biology: current evidence and research needs. Reviews on environmental health. 2017;32:55–63. [DOI] [PubMed] [Google Scholar]
- 12.Park CS, Rhim T. Application of proteomics in asthma research. Expert review of proteomics. 2011;8:221–230. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh D, Bernstein JA, Khurana Hershey GK, Rothenberg ME, Mersha TB. Leveraging Multilayered “Omics” Data for Atopic Dermatitis: A Road Map to Precision Medicine. Frontiers in immunology. 2018;9:2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galeone C, Scelfo C, Bertolini F, et al. Precision Medicine in Targeted Therapies for Severe Asthma: Is There Any Place for “Omics” Technology? BioMed research international. 2018;2018:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliva M, Renert-Yuval Y, Guttman-Yassky E. The ‘omics’ revolution: redefining the understanding and treatment of allergic skin diseases. Current opinion in allergy and clinical immunology. 2016;16:469–476. [DOI] [PubMed] [Google Scholar]
- 16.Farzan N, Vijverberg SJ, Kabesch M, Sterk PJ, Maitland-van der Zee AH. The use of pharmacogenomics, epigenomics, and transcriptomics to improve childhood asthma management: Where do we stand? Pediatric pulmonology. 2018;53:836–845. [DOI] [PubMed] [Google Scholar]
- 17.Ivanova O, Richards LB, Vijverberg SJ, et al. What did we learn from multiple omics studies in asthma? Allergy. 2019. [DOI] [PubMed] [Google Scholar]
- 18.Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome biology. 2017;18:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunological reviews. 2011;242:10–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernandez-Pacheco N, Pino-Yanes M, Flores C. Genomic Predictors of Asthma Phenotypes and Treatment Response. Frontiers in Pediatrics. 2019;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Portelli MA, Hodge E, Sayers I. Genetic risk factors for the development of allergic disease identified by genome-wide association. Clin Exp Allergy. 2015;45:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holloway JW, Yang IA, Holgate ST. Genetics of allergic disease. Journal of Allergy and Clinical Immunology. 2010;125:S81–S94. [DOI] [PubMed] [Google Scholar]
- 23.Kersten ET, Koppelman GH. Pharmacogenetics of asthma: toward precision medicine. Current opinion in pulmonary medicine. 2017;23:12–20. [DOI] [PubMed] [Google Scholar]
- 24.Potaczek DP, Harb H, Michel S, Alhamwe BA, Renz H, Tost J. Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics. 2017;9:539–571. [DOI] [PubMed] [Google Scholar]
- 25.Holland N. Future of environmental research in the age of epigenomics and exposomics. Reviews on environmental health. 2017;32:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeVries A, Vercelli D. Early predictors of asthma and allergy in children: the role of epigenetics. Current opinion in allergy and clinical immunology. 2015;15:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martino D, Dang T, Sexton-Oates A, et al. Blood DNA methylation biomarkers predict clinical reactivity in food-sensitized infants. The Journal of allergy and clinical immunology. 2015;135:1319–1328.e1311–1312. [DOI] [PubMed] [Google Scholar]
- 28.Paparo L, Nocerino R, Cosenza L, et al. Epigenetic features of FoxP3 in children with cow’s milk allergy. Clinical epigenetics. 2016;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syed A, Garcia MA, Lyu SC, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). The Journal of allergy and clinical immunology. 2014;133:500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tost J A translational perspective on epigenetics in allergic diseases. The Journal of allergy and clinical immunology. 2018;142:715–726. [DOI] [PubMed] [Google Scholar]
- 31.Kan M, Shumyatcher M, Himes BE. Using omics approaches to understand pulmonary diseases. Respiratory Research. 2017;18:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Lung Foundation. U-BIOPRED: What is the project? Available from: https://www.europeanlung.org/en/projects-and-research/projects/u-biopred/what-is-the-project/. Accessed March 5, 2019.
- 33.Altman MC, Gill MA, Whalen E, et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nature Immunology. 2019;20:637–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ortiz RA, Barnes KC. Genetics of allergic diseases. Immunology and allergy clinics of North America. 2015;35:19–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turi KN, Romick-Rosendale L, Ryckman KK, Hartert TV. A review of metabolomics approaches and their application in identifying causal pathways of childhood asthma. The Journal of allergy and clinical immunology. 2018;141:1191–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmidt CW. Metabolomics: what’s happening downstream of DNA. Environmental health perspectives. 2004;112:A410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bannier M, van de Kant KDG, Jobsis Q, Dompeling E. Feasibility and diagnostic accuracy of an electronic nose in children with asthma and cystic fibrosis. Journal of breath research. 2019;13:036009. [DOI] [PubMed] [Google Scholar]
- 38.Kelly RS, Dahlin A, McGeachie MJ, et al. Asthma Metabolomics and the Potential for Integrative Omics in Research and the Clinic. Chest. 2017;151:262–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng C, Wen B, Hou G, et al. Lipidomics profiling reveals the role of glycerophospholipid metabolism in psoriasis. GigaScience. 2017;6:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lydic TA, Goo YH. Lipidomics unveils the complexity of the lipidome in metabolic diseases. Clinical and translational medicine. 2018;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LIPID MAPS Lipidomics Gateway. Available from: http://lipidmaps.org/resources/databases/index.php. Accessed June 5, 2019.
- 42.Schauberger E, Peinhaupt M, Cazares T, Lindsley AW. Lipid Mediators of Allergic Disease: Pathways, Treatments, and Emerging Therapeutic Targets. Current allergy and asthma reports. 2016;16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/results?cond=Asthma&term=prostaglandin&cntry=&state=&city=&dist=&Search=Search&type=Intr. Accessed June 5, 2019.
- 44.ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/results?cond=Allergy&term=platelet+activating+factor&type=&rslt=&age_v=&gndr=&intr=&titles=&outc=&spons=&lead=&id=&cntry=&state=&city=&dist=&locn=&strd_s=&strd_e=&prcd_s=&prcd_e=&sfpd_s=&sfpd_e=&l. Accessed August 8, 2019.
- 45.ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/results?term=sphingolipid&type=Intr&cond=Asthma. Accessed June 5, 2019.
- 46.Pascal M, Perez-Gordo M, Caballero T, et al. Microbiome and Allergic Diseases. Frontiers in immunology. 2018;9:1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokolowska M, Frei R, Lunjani N, Akdis CA, O’Mahony L. Microbiome and asthma. Asthma research and practice. 2018;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ozturk AB, Turturice BA, Perkins DL, Finn PW. The Potential for Emerging Microbiome-Mediated Therapeutics in Asthma. Current allergy and asthma reports. 2017;17:62. [DOI] [PubMed] [Google Scholar]
- 49.Kozik AJ, Huang YJ. The microbiome in asthma: Role in pathogenesis, phenotype, and response to treatment. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2019;122:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ClinicalTrials.gov. Available from: https://clinicaltrials.gov/ct2/results?cond=allergy&term=microbiota&cntry=&state=&city=&dist=&Search=Search&type=Intr. Accessed June 5, 2019.
- 51.Agache I, Miller R, Gern JE, et al. Emerging concepts and challenges in implementing the exposome paradigm in allergic diseases and asthma: a Practall document. Allergy. 2019;74:449–463. [DOI] [PubMed] [Google Scholar]
- 52.Wild CP. The exposome: from concept to utility. International journal of epidemiology. 2012;41:24–32. [DOI] [PubMed] [Google Scholar]
- 53.Maitre L, de Bont J, Casas M, et al. Human Early Life Exposome (HELIX) study: a European population-based exposome cohort. BMJ Open. 2018;8:e021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vineis P, Chadeau-Hyam M, Gmuender H, et al. The exposome in practice: Design of the EXPOsOMICS project. International Journal of Hygiene and Environmental Health. 2017;220:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neveu V, Moussy A, Rouaix H, et al. Exposome-Explorer: a manually-curated database on biomarkers of exposure to dietary and environmental factors. Nucleic Acids Res. 2017;45:D979–d984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bilder RM, Sabb FW, Cannon TD, et al. Phenomics: the systematic study of phenotypes on a genome-wide scale. Neuroscience. 2009;164:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denny JC, Bastarache L, Roden DM. Phenome-Wide Association Studies as a Tool to Advance Precision Medicine. Annual review of genomics and human genetics. 2016;17:353–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Namjou B, Marsolo K, Caroll RJ, et al. Phenome-wide association study (PheWAS) in EMR-linked pediatric cohorts, genetically links PLCL1 to speech language development and IL5-IL13 to Eosinophilic Esophagitis. Frontiers in genetics. 2014;5:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bloodworth MH, Rusznak M, Bastarache L, Wang J, Newcomb DC. Association of estrogen receptor alpha polymorphism rs1999805 with asthma. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2019;122:208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Claar DD, Larkin EK, Bastarache L, et al. A Phenome-Wide Association Study Identifies a Novel Asthma Risk Locus Near TERC. American journal of respiratory and critical care medicine. 2016;193:98–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bloodworth MH, Rusznak M, Bastarache L, Wang J, Denny JC, Peebles RS Jr. Association of ST2 polymorphisms with atopy, asthma, and leukemia. Journal of Allergy and Clinical Immunology. 2018;142:991–993.e993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James G, Reisberg S. An exploratory phenome wide association study linking asthma and liver disease genetic variants to electronic health records from the Estonian Biobank. 2019;14:e0215026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diogo D, Tian C, Franklin CS, et al. Phenome-wide association studies across large population cohorts support drug target validation. Nature Communications. 2018;9:4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bastarache L, Hughey JJ. Phenotype risk scores identify patients with unrecognized Mendelian disease patterns. 2018;359:1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Online Mendelian Inheritance in Man, OMIM®. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University; (Baltimore, MD: ). Available from: https://omim.org/. Accessed June 14, 2019. [Google Scholar]
- 66.Kho AN, Rasmussen LV, Connolly JJ, et al. Practical challenges in integrating genomic data into the electronic health record. Genetics in medicine : official journal of the American College of Medical Genetics. 2013;15:772–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu PY, Cheng CW, Kaddi CD, Venugopalan J, Hoffman R, Wang MD. -Omic and Electronic Health Record Big Data Analytics for Precision Medicine. IEEE Trans Biomed Eng. 2017;64:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gottesman O, Kuivaniemi H, Tromp G, et al. The Electronic Medical Records and Genomics (eMERGE) Network: past, present, and future. Genetics In Medicine. 2013;15:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.HIPAA Privacy Rule: MINIMUM NECESSARY. Standards for privacy of individually identifiable health information, Final Rule 2002, 45 CFR 164.502(b), 164.514(d). [Google Scholar]
- 70.Takai-Igarashi T, Kinoshita K, Nagasaki M, et al. Security controls in an integrated Biobank to protect privacy in data sharing: rationale and study design. BMC Medical Informatics and Decision Making. 2017;17:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.NIH security best practices for controlled-access data subject to the NIH Gemomic Data Sharing (GDS) policy; 2015. Available from: https://osp.od.nih.gov/wp-content/uploads/NIH_Best_Practices_for_Controlled-Access_Data_Subject_to_the_NIH_GDS_Policy.pdf.
- 72.Karczewski KJ, Snyder MP. Integrative omics for health and disease. Nature reviews. Genetics. 2018;19:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang YV, Smoller BR, Levy PC. Laboratory Formulary: A Model for High-Value Evidence-Based Medicine. Clinical chemistry. 2017;63:1299–1300. [DOI] [PubMed] [Google Scholar]
- 74.Zutter M, Field J, Bernard G. Improving care and cutting costs: Implementation of a laboratory formulary to facilitate better laboratory ordering practices: NEJM Catalyst; 2017. Available from: https://catalyst.nejm.org/laboratory-formulary-facilitate-ordering-practices/.
- 75.Pecak M, Korosec P, Kunej T. Multiomics Data Triangulation for Asthma Candidate Biomarkers and Precision Medicine. Omics : a journal of integrative biology. 2018;22:392–409. [DOI] [PubMed] [Google Scholar]
- 76.Forno E, Wang T, Yan Q, et al. A Multiomics Approach to Identify Genes Associated with Childhood Asthma Risk and Morbidity. Am J Respir Cell Mol Biol. 2017;57:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gern JE, Jackson DJ, Lemanske RF Jr., et al. The Children’s Respiratory and Environmental Workgroup (CREW) birth cohort consortium: design, methods, and study population. Respir Res. 2019;20:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.UK Biobank: Protocol for a large-scale prospective epidemiological resource. Protocol No: UKBB-PROT-09–6 (Main Phase): UK Biobank Coordinating Centre; 2007. Available from: www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf. [Google Scholar]
- 79.NIH Data Sharing Repositories. Available from: https://www.nlm.nih.gov/NIHbmic/nih_data_sharing_repositories.html. Accessed July 31, 2019.
- 80.Hulsen T, Jamuar SS, Moody AR, et al. From Big Data to Precision Medicine. Frontiers in medicine. 2019;6:34–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.E18 genomic sampling and management of genomic data guidance for industry: U.S. Department of Health and Human Services Food and Drug Administration; 2018. Available from: https://www.fda.gov/media/98596/download. [Google Scholar]
- 82.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in medicine : official journal of the American College of Medical Genetics. 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tebani A, Afonso C, Marret S, Bekri S. Omics-Based Strategies in Precision Medicine: Toward a Paradigm Shift in Inborn Errors of Metabolism Investigations. International journal of molecular sciences. 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benson M. Clinical implications of omics and systems medicine: focus on predictive and individualized treatment. Journal of internal medicine. 2016;279:229–240. [DOI] [PubMed] [Google Scholar]
- 85.Kirschner M. Final report summary- CASYM (Coordinating Action Systems Medcine- Implementation of systems medicine across Europe), Grant agreement ID: 305033. online: European Commision Community Research and Development Information Service; 2017. Available from: https://cordis.europa.eu/project/rcn/106187/reporting/en. [Google Scholar]