Abstract
The technique used for cancer monitoring is essential for effective cancer therapy. Currently, several methods such as diagnostic imaging and biochemical markers have been used for cancer monitoring, but these are invasive and show low sensitivity. A previous study reported that Caenorhabditis elegans sensitively discriminated patients with cancer from healthy subjects, based on the smell of a urine sample. However, whether C. elegans olfaction can detect the removal of cancerous tumours remains unknown. This study was conducted to examine C. elegans olfactory behaviour to urine samples collected from 78 patients before and after surgery. The diagnostic ability of the technique termed Nematode-NOSE (N-NOSE) was evaluated by receiver operating characteristic (ROC) analysis. The ROC curve of N-NOSE was higher than those of classic tumour markers. Furthermore, we examined the change in C. elegans olfactory behaviour following exposure to preoperative and postoperative samples. The results suggest that a reduction in attraction indicates the removal of the cancerous tumour. This study may lead to the development of a noninvasive and highly sensitive tool for evaluating postoperative cancer patients.
Keywords: Caenorhabditis elegans, olfaction, cancer, prognosis, biodiagnosis
Introduction
Cancer is the leading cause of death worldwide, and the number of people with cancer is increasing. The major treatments for cancer include surgery, chemotherapy, and radiotherapy.1-3 Among these methods, the prognosis of surgery depends on the complete removal of cancer tissue, as any remaining cancer cells have the potential to spread to other tissues and cause metastasis and recurrence.4-6 Therefore, postoperative observation is important for assessing surgical outcomes.
Several methods have been developed for postoperative evaluation of cancer conditions. Diagnostic imaging methods such as positron emission tomography–computed tomography and endoscopy have been used for follow-up examination after surgery;7,8 however, these methods can be invasive or costly, and their repeated application is limited.9 Classic biochemical markers, such as carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), have been used for postoperative monitoring of patients with cancer.10,11 Although these methods are relatively noninvasive, their sensitivity must be improved.12,13 Therefore, noninvasive, low-cost, and highly sensitive methods are needed for monitoring patients with cancer after surgery.
A recent study reported that the olfactory behaviour of the nematode Caenorhabditis elegans to human urine can be utilized to discriminate patients with cancer from healthy people.14 C. elegans showed an innate attractive behaviour towards urine from patients with cancer, whereas they avoided urine from healthy subjects. Attractive olfactory sensory neurons of C. elegans consistently responded to urine from patients with cancer, and aversive sensory neurons have a role in response to urine from healthy subjects.14 Based on the olfactory response of C. elegans, a novel cancer screening test was developed, named Nematode-NOSE (N-NOSE), which was referred to as the nematode scent detection test (NSDT) in a previous study.14 Both the sensitivity and specificity of N-NOSE, including those for early-stage cancers, were more than 90%. Nematode-NOSE can detect broad types of cancers including most gastrointestinal cancers.14
However, whether N-NOSE can be used to monitor the progress of cancer in the same patient remains unclear. The previous study indicated that C. elegans detected cancer-specific odours in urine from cancer patients, even when the cancer was in stage 0 or I,14 suggesting that C. elegans sensitively detect the potential for cancer development. As postoperative patients have a history of carcinogenesis and potential for recurrence, C. elegans may fail to show aversive behaviour to the urine samples clearly. We therefore hypothesized that the degree of attractive behaviour to postoperative urine is decreased compared to preoperative urine.
In this study, we investigated the ability of N-NOSE as a postoperative tool for monitoring the removal of cancer. We first performed receiver operating characteristic (ROC) analysis to compare the diagnostic performance of N-NOSE and conventional tumour markers, CEA and CA19-9. We next tested our hypothesis and examined alterations in C. elegans olfactory behaviour between preoperative and postoperative samples from the same patient. Our results demonstrated that the reduction in attractive olfactory behaviour for postoperative samples indicates the removal of cancer. This study may lead to the development of an effective tool for postoperative evaluation based on C. elegans olfaction.
Results
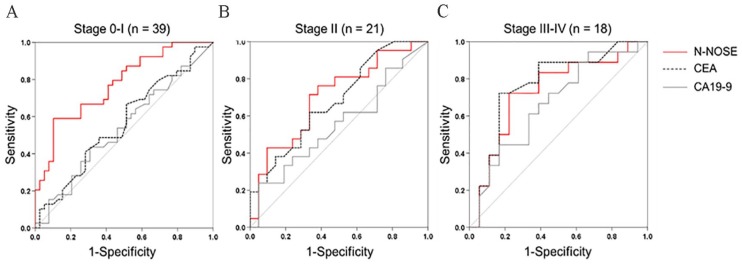
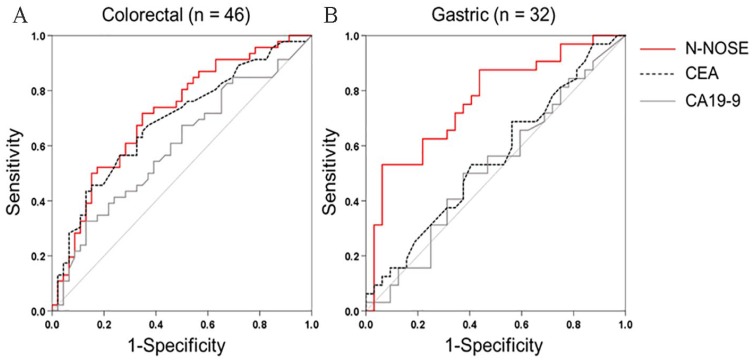
To examine the diagnostic performance of N-NOSE for monitoring the removal of cancer, we performed ROC analysis using preoperative and postoperative samples from the same patient (N = 78 patients; Table 1). Conventionally, ROC analysis has been used to quantify how accurately a medical diagnostic tool can discriminate between 2 groups (eg, cancer patients and healthy subjects). In this study, ROC analysis was used to quantify the differentiation between preoperative and postoperative samples to investigate whether chemotaxis index (see Methods) reflects the removal of cancer. Values obtained for N-NOSE, CEA, and CA19-9 are listed in Supplementary Table S1. Chemotaxis indices of each patient were listed in Supplementary Table S2. The area under the curve (AUC) indicates the usefulness of N-NOSE, AUC = 0.742, P < .001, 95% confidence interval (CI): 0.664-0.819, compared to that of classic tumour markers, CEA (AUC = 0.638, P = .003, 95% CI: 0.551-0.724) and CA19-9 (AUC = 0.570, P = .133, 95% CI: 0.480-0.660), for diagnosing the removal of cancer (Figure 1, Table 2). Furthermore, we examined the diagnostic ability of N-NOSE for different pathological stages and cancer types. In stages 0 and I cancer, the AUC of N-NOSE was higher than those of CEA and CA19-9 (Figure 2, Table 3). In addition, in the 2 types of cancer, the AUC of N-NOSE was higher than those of CEA and CA19-9 (Figure 3, Table 4). Images showing animals were attracted to the preoperative sample, but not to the postoperative are described in Supplementary Figure S1. These results suggest that N-NOSE detected the removal of cancer more sensitively than the classical tumour markers.
Table 1.
Patient characteristics.
| Characteristics | Colorectal cancer patients (N = 46) | Gastric cancer patients (N = 32) | Total (N = 78) |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 67.4 ± 12.1 | 67.0 ± 10.8 | 67.3 ± 11.5 |
| Range | 35-86 | 43-89 | 35-89 |
| Gender | |||
| Female | 19 | 8 | 27 |
| Male | 27 | 24 | 51 |
| Tumour stage | |||
| 0-I | 15 | 24 | 39 |
| II | 15 | 6 | 21 |
| III-IV | 16 | 2 | 18 |
Abbreviations: SD: standard deviation.
Figure 1.
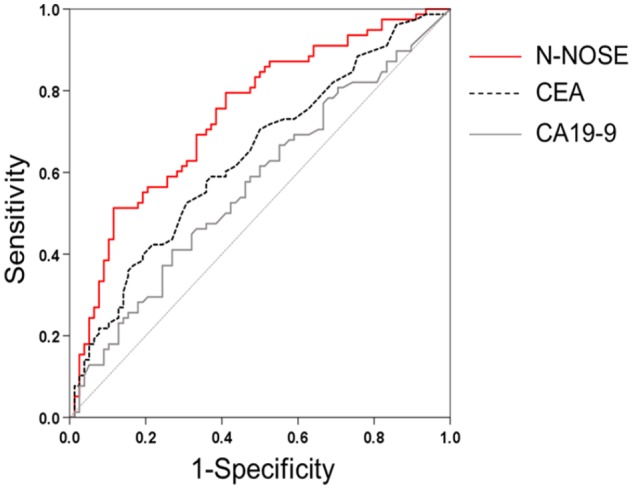
ROC curves depicting the diagnostic capability of N-NOSE.
Area under the ROC curve for N-NOSE (red line), CEA (black dotted line), and CA19-9 (grey line).
CA19-9 indicates carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; N-NOSE, Nematode-NOSE; ROC, receiver operating characteristic.
Table 2.
ROC analysis results.
| AUC | P-value | 95% confidence interval | |
|---|---|---|---|
| N-NOSE | 0.742 | <.001 | 0.664-0.819 |
| CEA | 0.638 | .003 | 0.551-0.724 |
| CA19-9 | 0.570 | .133 | 0.480-0.660 |
Abbreviations: AUC, area under the curve; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; N-NOSE, Nematode-NOSE; ROC, receiver operating characteristic.
The results correspond to those shown in Figure 1. AUC indicates area under the curve. The P-values indicate whether the value of AUC is significantly different from the value of 0.5 AUC. The n is the number of the chemotaxis indices or values of tumour markers (eg, the 78 preoperative chemotaxis indices versus 78 postoperative indices).
Figure 2.
ROC curves of N-NOSE by pathological stage.
Area under the ROC curve for N-NOSE (red line), CEA (black dotted line), and CA19-9 (grey line) in stages 0 and I (a), stage II (b), and stages III and IV (c).
CA19-9 indicates carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; N-NOSE, Nematode-NOSE; ROC, receiver operating characteristic.
Table 3.
Results of ROC analysis by pathological stage.
| AUC | P-value | 95% confidence interval | |
|---|---|---|---|
| N-NOSE | |||
| Stages 0 and I | 0.771 | <.001 | 0.669-0.874 |
| Stage II | 0.703 | .024 | 0.544-0.862 |
| Stages III and IV | 0.731 | .018 | 0.560-0.903 |
| CEA | |||
| Stages 0 and I | 0.560 | .358 | 0.432-0.689 |
| Stage II | 0.681 | .044 | 0.521-.842 |
| Stages III and IV | 0.769 | .006 | 0.605-0.932 |
| CA19-9 | |||
| Stages 0 and I | 0.537 | .576 | 0.408-0.666 |
| Stage II | 0.552 | .563 | 0.355-0.729 |
| Stages III and IV | 0.667 | .088 | 0.487-0.846 |
Abbreviations: AUC, area under the curve; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; N-NOSE, Nematode-NOSE; ROC, receiver operating characteristic.
The results correspond to those shown in Figure 2. The P-values indicate whether the value of AUC is significantly different from the value of .5 AUC. The n is the number of the chemotaxis indices or values of tumour markers.
Figure 3.
ROC curves of N-NOSE by cancer type.
Area under the ROC curve for N-NOSE (red line), CEA (black dotted line), and CA19-9 (grey line) in colorectal cancer (a) and gastric cancer (b).
CA19-9 indicates carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; N-NOSE, Nematode-NOSE; ROC, receiver operating characteristic.
Table 4.
Results of ROC analysis by cancer type.
| AUC | P-value | 95% confidence interval | |
|---|---|---|---|
| Colorectal cancer | |||
| N-NOSE | 0.716 | <.001 | 0.611-0.821 |
| CEA | 0.688 | .002 | 0.579-0.796 |
| CA19-9 | 0.597 | .109 | 0.481-0.714 |
| Gastric cancer | |||
| N-NOSE | 0.765 | <.001 | 0.647-0.882 |
| CEA | 0.554 | .456 | 0.413-0.696 |
| CA19-9 | 0.521 | .778 | 0.378-0.664 |
Abbreviations: AUC, area under the curve; CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; N-NOSE, Nematode-NOSE; ROC, receiver operating characteristic.
The results correspond to those shown in Figure 3. The P-values indicate whether the value of AUC is significantly different from the value of 0.5 AUC. The n is the number of the chemotaxis indices or values of tumour markers.
We next investigated whether N-NOSE can indicate the removal of cancer. To evaluate whether the removal of cancer induced a reduction in attractive behaviour, we analysed changes in the chemotaxis index using preoperative to postoperative samples from the same patient. Among the 78 samples, 59 samples showed a reduced chemotaxis index from preoperative to postoperative samples (Figure 4). One-sample t-test revealed that the differences of the preoperative and postoperative chemotaxis index were significantly different from 0 (mean ± standard error of the M: −0.066 ± 0.013, t = −5.053, P < .0001). These results suggest that decreased attractive olfactory behaviour of C. elegans can indicate the removal of cancerous tumours.
Figure 4.
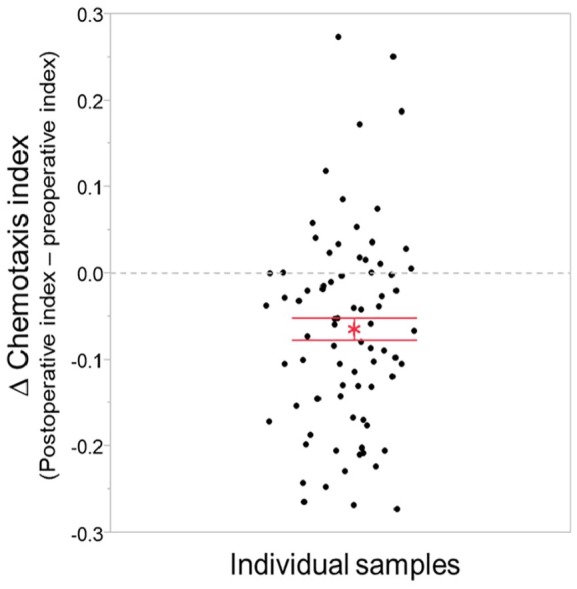
Change in chemotaxis index in individual samples.
Differences in chemotaxis indices were obtained by subtracting the preoperative chemotaxis index from the postoperative chemotaxis index in each sample. Among the 78 samples, 59 samples showed a decreased chemotaxis index. Red asterisk indicates the mean and error bar indicates standard error of the mean.
Discussion
We investigated the use of N-NOSE as a postoperative tool for monitoring the presence of cancer. We found that N-NOSE showed a higher area under the ROC curves than CEA and CA19-9. In the comparison of the area under ROC curve by pathological stage and cancer type, N-NOSE showed a higher area under the ROC than CEA and CA19-9. We then focused on changes in the chemotaxis index in individual samples. Among the 78 samples, 59 samples showed a decreased chemotaxis index in postoperative samples, supporting our hypothesis. These results indicate that N-NOSE is a useful surrogate marker for detecting the postoperative cancer status.
We investigated the diagnostic ability of N-NOSE to detect the removal of cancerous tumours. The ROC analysis revealed that N-NOSE was more accurate than CEA or CA19-9 (Figure 1). The detection ability of C. elegans olfaction was validated in 2 types of cancer (ie, colorectal and gastric cancer) and pathological stages (Figures 2 and 3, Tables 3 and 4). The results revealed that C. elegans can detect cancer regardless of the types and stages.14 In stages 0 and I cancers, CEA and CA19-9 showed nearly negative results, in which the classic tumour markers showed less than 0.6 AUC values (Figure 2A and Table 3) and then we could not use these markers as indicators of cancer removal. In contrast, N-NOSE could discriminate the preoperative and postoperative status even in stages 0 and I cancers. These results suggest that N-NOSE can detect cancer removal regardless of the number of cancer cells present. In gastric cancers, compared to colorectal cancers, N-NOSE showed a higher AUC than the classical tumour markers. This may be because more early-stage patients were included in the gastric cancer group (ie, 24 of 32 samples were from stages 0 and I cancer patients). Analysis of the changes in C. elegans olfaction also revealed the ability to detect cancer removal in the same individuals (Figure 4). Furthermore, the follow-up test using 3 samples showing recurrence supported the clinical usefulness of N-NOSE. In these 3 samples, the chemotaxis index was decreased following cancer removal and increased following recurrence (patient A; preoperative index = 0.027, postoperative index = −0.016, and recurrence index = 0.002, patient B; preoperative index = 0.095, postoperative index = −0.148, and recurrence index = 0.034, patient C; preoperative index = 0.027, postoperative index = 0.023, and recurrence index = 0.067, Supplementary Figure S2). For example, patient B was a female with stage II rectal cancer. Her CEA and CA19-9 levels were sustained within the normal limit during follow-up regardless of surgery or recurrence. In contrast, the chemotaxis index changed as described above, reflecting removal and recurrence. We also found that in several stage IV patients who underwent palliative surgery, the chemotaxis index changed according to their tumour volumes (Supplementary Figure S3), in which the tumour volume was measured according to revised RECIST guideline (version 1.1).15 The chemotaxis index decreased when the primary sites were removed and then increased when metastatic sites increased. These results suggest that N-NOSE has the potential to be a postoperative tool for monitoring cancer.
Our results suggest that the olfactory behaviour of C. elegans reflected the change in urinary cancer-specific odour caused by surgical removal. Previous studies showed that urinary chemical components, including volatile components, in patients with cancer different from those in control subjects,16-18 and the pattern of urinary components were changed by surgical therapy.19 Such changes were detected by C. elegans in this study. C. elegans showed dose-dependent olfactory behaviour, with attractive behaviour to attractants corresponding to the concentration of spotted odour samples.20-24 These results support those of the current study.
Our results showed that N-NOSE had a higher AUC than CEA and CA19-9 except for in the diagnosis of stages III and IV disease (Figure 2C, Table 3), although the classic tumour markers also showed decreased values in postoperative samples (Supplementary Table S1). The superiority of N-NOSE in the AUC may be explained by the distribution of values. The values for CEA and CA19-9 are individual-specific,25 and such dispersion may lead to relatively low diagnostic performance. Indeed, the ranges of values for CEA and CA19-9 in this study were 0.5 to 476.9 and 0.1 to 82 171.4, respectively. The values of CEA and CA19-9 indicate their concentrations in serum, and individual difference is directly reflected by the values. In contrast, in N-NOSE, the chemotaxis index is a normalized value that indicates the ratio of animals showing attractive behaviour to the spotted sample. Thus, the value showed a relatively low distribution compared to the classic tumour markers. Such characteristics may also contribute to diagnostic performance in our results. However, the normalization in chemotaxis index might account for the difference of AUC in our results, which might be noted as limitation. To compare the diagnostic abilities more impartially, other response of C. elegans to urine, such as olfactory neural response, could be utilized instead of behavioural response.
There were some limitations to this study. Our results suggest that the absolute value of the chemotaxis index might be unsuitable for indicating cancer removal. The animals did not show aversive behaviour to postoperative urine, with only 32 urine samples showing a negative chemotaxis index in postoperative samples compared to 63 samples showing a positive chemotaxis index preoperatively. This may be because postoperative patients have some differences from healthy subjects, such as a history of carcinogenesis and the potential for recurrence. In addition, patients with cancer utilize unique metabolic pathways that have residual effects on postoperative urine samples. Indeed, several reports showed that urine from patients with cancer have a specific pattern of bioactive molecules, such as DNA, miRNA, and extracellular vesicle proteins.1,26,27 Thus, C. elegans would fail to show aversive behaviour in postoperative samples. Furthermore, several postoperative samples showed an increased chemotaxis index (Figure 4). Studies are needed to identify the factors that increase the chemotaxis index in postoperative samples. The premise of ROC analysis might not be congruent with this study. The ROC curve relies on a measure of ‘true positives’ and ‘true negative’. Although we confirmed that the tumour was removed from the patient by intraoperative macroscopic findings, histopathological examination, and postoperative imaging inspection, the recurrence was found (Supplementary Figure S2). An alternative analysis, which is congruent with this study and enables to examine the resolution from preoperative to postoperative samples, might be needed. There may be a limitation that the method of N-NOSE can be optimized to make larger different chemotaxis indices between preoperative and postoperative samples. For example, in Supplementary Figure S2, patients A and C showed no significance, though the alteration of chemotaxis index could trace the condition of cancer (ie, decreased by removal of cancer and increased by recurrence). For the optimization of N-NOSE with more accuracy, other chemotaxis assay formats and devices could be utilized. In past C. elegans studies, several methods were used to investigate and examine the olfactory behaviour of C. elegans in detail, such as single animal assay,24 animal tracking system,24 and monitoring neural response in moving animals.21 Otherwise, identification of cancer-specific odourants and the olfactory receptor could also lead to optimization of N-NOSE. Based on the previous studies suggesting the cancer-specific odourants,16-18 method of N-NOSE assay could be enhanced more accurately. Furthermore, no individual-specific normalization of urine samples was performed due to following the previous study.14 The normalization of urine concentration might lead to the optimization. Those methods of optimization would help to brush up N-NOSE as a more accurate technique to monitor preoperative patients. The analysis of other types of cancer might be needed. In this study, only 2 types of cancer (ie, colorectal and gastric cancer) were tested. Although the previous study showed that N-NOSE can detect several types of cancer,14 more number of types of cancer should be analysed to investigate the capability of N-NOSE for prognosis.
In conclusion, we demonstrated that N-NOSE can reflect the removal of cancerous tumours using preoperative and postoperative urine samples collected from patients. Nematode-NOSE shows potential as a tool for monitoring cancer in patients as well as for detecting cancer.
Methods
Study populations
Urine samples were collected from 78 patients (age of mean ± SD = 67.3 ± 11.5, female:male = 27:51) who were diagnosed as cancer at the Nanpuh Hospital (Kagoshima, Japan) between June 2016 and February 2018. The characteristics of the patients are listed in Table 1. Measurement was performed within 60 days before and after surgery. Multiple cancers were excluded. Preoperative urine was collected before 26 ± 11 (mean ± SD) days, while postoperative urine was collected after 29 ± 9 (mean ± SD) days. In all patients, the pathologic stages were determined by histological diagnosis of the primary tumour. The classical tumour markers CEA and CA19-9 were also measured in the patients. Blood samples were collected before 26 ± 11 and after 28 ± 9 days of surgery. Collected urine samples were stored frozen until analysis by N-NOSE. The volume of tumour was measured according to revised RECIST guideline (version 1.1).15 Briefly, the targeted tumour was picked out up to 10 pieces, then the sum of the maximum lengths of the tumour pieces was calculated in the preoperative, postoperative, and recurred samples.
The study was approved by the Ethics Committee of Nanpuh Hospital, Kagoshima Kyosaikai, Public Interest, Inc. Association, Japan. Clinical examinations were performed according to the principles of the Declaration of Helsinki. Research consent was obtained in writing from each patient. Informed consent was obtained from all participants. The first author guarantees the accuracy and completeness of the data and analysis and of the study’s fidelity regarding technical and biostatistics protocols.
Measurement by N-NOSE
Nematode-NOSE detects cancer by sensing a distinguishable cancer odour in urine using the C. elegans olfactory system.14 The olfactory behaviour of C. elegans was evaluated by population assays, which was in accordance with the classical method by Bargmann et al.20 This method uses the chemotactic attraction or avoidance of C. elegans to odourants in urine. C. elegans (wild-type N2) were cultured at 20°C under well-fed and uncrowded conditions with the Escherichia coli strain NA22 as a food source. Chemotaxis assays with human urine were performed on 9-cm plates containing 10-mL 2% agar, 5 mM KPO4, 1 mM CaCl2, and 1 mM MgSO4 as previously described.14,20,24 Chemotaxis assays were conducted as described previously.14 Briefly, 1 μL of urine was added at 2 spots on one end of the assay plates, and 0.5 μL of 1 M sodium azide was added at 2 spots on both ends of the plates. As the diluent of urine samples, we used water, and we confirmed that animals showed no chemotaxis behaviour to 1 µL of water. Thus, no diluent was put on the opposite side of the plate. Animals were collected, washed 3 times with chemotaxis buffer (0.05% gelatine, 5 mM KPO4, 1 mM CaCl2, and 1 mM MgSO4), and transferred to the centre of the plate to move freely for 30 min. Approximately 50 to 100 synchronized young adults were used per plate. The chemotaxis index was calculated as the number of animals in the region near the urine samples minus the animals in the region without the samples divided by the total number of animals.14,24 The format of an agar plate to count animals is described in Supplementary Figure S4. The average chemotaxis indices of more than 10 assay plates were determined. Hirotsu et al14 reported that the chemotaxis index is positive in urine samples from patients with diluted by 10- to 1000-fold and is not observed in urine samples from healthy volunteers. Therefore, running behaviour against 10- to 1000-fold diluted urine was investigated; positive peaks were considered as positive results, and a lack of chemotaxis was considered as a negative result. In this study, the result of N-NOSE was obtained using 10-fold diluted urine samples. We performed 2 sets of chemotaxis assay, in which the one was for preoperative urine samples, and the other was for postoperative samples.
Statistical analyses
Receiver operating characteristic analysis was performed based on logistic regression using SPSS, version 25 (IBM Co, Armonk, NY, USA). A value of P < .05 was considered significant. In ROC analysis, chemotaxis indices listed in Supplementary Table S2 were used, in which the preoperative chemotaxis indices were considered as cancer samples, and postoperative indices were considered as healthy samples. In ROC analysis, the asymptotic significance (null hypothesis: AUC = 0.5, α level = 0.05) was obtained, which indicates the reliability of the AUC (ie, whether the value of AUC is significantly different from the value of 0.5 AUC).
Supplemental Material
Supplemental material, Revised_SupplementaryInformation_4_xyz2778816687648 for Behavioural Response Alteration in Caenorhabditis elegans to Urine After Surgical Removal of Cancer: Nematode-NOSE (N-NOSE) for Postoperative Evaluation by Hirotake Kusumoto, Kotaro Tashiro, Syunji Shimaoka, Koichiro Tsukasa, Yukiko Baba, Saori Furukawa, Junichiro Furukawa, Toyokuni Suenaga, Masaki Kitazono, Sadao Tanaka, Toru Niihara, Takaaki Hirotsu and Takayuki Uozumi in Biomarkers in Cancer
Acknowledgments
The authors appreciate the support of the Division of Clinical Laboratory and Clinical Application of Nanpuh Hospital.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
When the study was started, the author belonged to the affiliation of “4” and “5”, and in the end of the experiments, the author belonged to the affiliation of “4” and “6”
Author Contributions: H.K., T.H., and T.U. designed the conception of the study. T.H. and T.U. made the experimental design of the N-NOSE test. T.N., J.F., S.F., Y.B., K.T., S.S., K.T., and M.K. collected urine and serum samples from the participants. T.N., T.S., and S.T. evaluated clinical data. H.K., T.Y., T.U., and T.H. interpreted the results. H.K., T.U., and T.H. made and modified the manuscript.
Data Availability: The data that support the findings of this study are available from the corresponding author, upon reasonable request.
ORCID iD: Takayuki Uozumi  https://orcid.org/0000-0002-6107-3452
https://orcid.org/0000-0002-6107-3452
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Jagust P, de Luxán-Delgado B, Parejo-Alonso B, Sancho P. Metabolism-based therapeutic strategies targeting cancer stem cells. Front Pharmacol. 2019;10:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ouyang M, Liao T, Lu Y, et al. Laparoscopic versus open surgery in lateral lymph node dissection for advanced rectal cancer: a meta-analysis. Gastroenterol Res Pract. 2019;2019:7689082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Socha J, Pietrzak L, Zawadzka A, Paciorkiewicz A, Krupa A, Bujko K. A systematic review and meta-analysis of pT2 rectal cancer spread and recurrence pattern: implications for target design in radiation therapy for organ preservation. Radiother Oncol. 2019;133:20-27. [DOI] [PubMed] [Google Scholar]
- 4. Dehal A, Patel S, Kim S, Shapera E, Hussain F. Cutaneous metastasis of rectal cancer: a case report and literature review. Perm J. 2016;20:74-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim SH, Song B, Kim BW, et al. Predictive value of [18F]FDG PET/CT for lymph node metastasis in rectal cancer. Sci Rep. 2019;9:4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pang R, Law WL, Chu AC, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603-615. [DOI] [PubMed] [Google Scholar]
- 7. Cysouw MCF, Kramer GM, Schoonmade LJ, Boellaard R, de Vet HCW, Hoekstra OS. Impact of partial-volume correction in oncological PET studies: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2017;44:2105-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Cutsem E, Verheul HM, Flamen P, et al. Imaging in colorectal cancer: progress and challenges for the clinicians. Cancers (Basel). 2016;8:E81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin JT. Screening of gastric cancer: who, when, and how. Clin Gastroenterol Hepatol. 2014;12:135-138. [DOI] [PubMed] [Google Scholar]
- 10. Nicholson BD, Shinkins B, Pathiraja I, et al. Blood CEA levels for detecting recurrent colorectal cancer. Cochrane Database Syst Rev. 2015;2015:CD011134. doi: 10.1002/14651858.cd011134.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang LN, OuYang PY, Xiao WW, et al. Elevated CA19-9 as the most significant prognostic factor in locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Medicine. 2015;94:e1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carpelan-Holmström M, Louhimo J, Stenman UH, Alfthan H, Haglund C. CEA, CA 19-9 and CA 72-4 improve the diagnostic accuracy in gastrointestinal cancers. Anticancer Res 2002; 22:2311-2316. [PubMed] [Google Scholar]
- 13. Łukaszewicz-Zaja̧c M, Mroczko B, Gryko M, Kȩdra B, Szmitkowski M. Comparison between clinical significance of serum proinflammatory proteins (IL-6 and CRP) and classic tumor markers (CEA and CA 19-9) in gastric cancer. Clin Exp Med. 2011;11:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirotsu T, Sonoda H, Uozumi T, et al. A highly accurate inclusive cancer screening test using Caenorhabditis elegans scent detection. PLoS ONE. 2015;10:e0118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours – Revised RECIST guideline (version 1.1). Japanese J Cancer Chemother. 2009;45:228-247. [DOI] [PubMed] [Google Scholar]
- 16. Grayson K, Gregory E, Khan G, Guinn BA. Urine biomarkers for the early detection of ovarian cancer – are we there yet? Biomark Cancer. 2019;11:1179299X1983097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shahid M, Lee MY, Yeon A, et al. Menthol, a unique urinary volatile compound, is associated with chronic inflammation in interstitial cystitis. Sci Rep. 2018;8:10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shirasu M, Touhara K. The scent of disease: volatile organic compounds of the human body related to disease and disorder. J Biochem. 2011;150:257-266. [DOI] [PubMed] [Google Scholar]
- 19. Ferrari E, Wittig A, Basilico F, et al. Urinary proteomics profiles are useful for detection of cancer biomarkers and changes induced by therapeutic procedures. Molecules. 2019;24:E794. doi: 10.3390/molecules24040794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515-527. [DOI] [PubMed] [Google Scholar]
- 21. Larsch J, Flavell SW, Liu Q, Gordus A, Albrecht DR, Bargmann CI. A circuit for gradient climbing in C. elegans chemotaxis. Cell Rep. 2015;12:1748-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taniguchi G, Uozumi T, Kiriyama K, Kamizaki T, Hirotsu T. Screening of odor-receptor pairs in Caenorhabditis elegans reveals different receptors for high and low odor concentrations. Sci Signal. 2014;7:ra39. [DOI] [PubMed] [Google Scholar]
- 23. Uozumi T, Hirotsu T, Yoshida K, et al. Temporally-regulated quick activation and inactivation of Ras is important for olfactory behaviour. Sci Rep. 2012;2:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yoshida K, Hirotsu T, Tagawa T, et al. Odour concentration-dependent olfactory preference change in C. elegans. Nat Commun. 2012;3:711-739. [DOI] [PubMed] [Google Scholar]
- 25. Yu P, Zhou M, Qu J, et al. The dynamic monitoring of CEA in response to chemotherapy and prognosis of mCRC patients. BMC Cancer. 2018;18:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahlquist DA. Universal cancer screening: revolutionary, rational, and realizable. NPJ Precis Oncol. 2018;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruhen O, Meehan K. Tumor-derived extracellular vesicles as a novel source of protein biomarkers for cancer diagnosis and monitoring. Proteomics. 2018;19:e1800155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Revised_SupplementaryInformation_4_xyz2778816687648 for Behavioural Response Alteration in Caenorhabditis elegans to Urine After Surgical Removal of Cancer: Nematode-NOSE (N-NOSE) for Postoperative Evaluation by Hirotake Kusumoto, Kotaro Tashiro, Syunji Shimaoka, Koichiro Tsukasa, Yukiko Baba, Saori Furukawa, Junichiro Furukawa, Toyokuni Suenaga, Masaki Kitazono, Sadao Tanaka, Toru Niihara, Takaaki Hirotsu and Takayuki Uozumi in Biomarkers in Cancer