Abstract
Basal cell carcinoma is driven by the aberrant activation of hedgehog signaling. DEAD (Asp-Glu-Ala-Asp) box protein 5 is frequently overexpressed in human cancer cells and associated with the tumor growth and invasion. The purpose of this study was to investigate the role of DEAD (Asp-Glu-Ala-Asp) box protein 5 in the growth, migration, and invasion of basal cell carcinoma. The role of DEAD (Asp-Glu-Ala-Asp) box protein 5 was detected by quantitative real-time polymerase chain reaction, Western blot, and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling assay in basal cell carcinoma cells. The associations between JAK2/STAT3 pathway and DEAD (Asp-Glu-Ala-Asp) box protein 5 were analyzed in basal cell carcinoma cells. Results showed that DEAD (Asp-Glu-Ala-Asp) box protein 5 is overexpressed in basal cell carcinoma cells. DEAD (Asp-Glu-Ala-Asp) box protein 5 knockdown inhibited the migration and invasion of basal cell carcinoma cells. DEAD (Asp-Glu-Ala-Asp) box protein 5 knockdown increased the apoptosis of basal cell carcinoma cells induced by tunicamycin. Results found that DEAD (Asp-Glu-Ala-Asp) box protein 5 knockdown increased JAK2 and STAT3 expression in basal cell carcinoma cells. JAK2 inhibitor decreased STAT3 expression and abolished the inhibitory effects of DEAD (Asp-Glu-Ala-Asp) box protein 5 silencing on migration and invasion in basal cell carcinoma cells. In conclusion, these results indicate that DEAD (Asp-Glu-Ala-Asp) box protein 5 is a potential target for inhibiting basal cell carcinoma cells growth, migration, and invasion by downregulating JAK2/STAT3 pathway.
Keywords: DDX5, BCC, migration, invasion, JAK2, STAT3
Introduction
Basal cell carcinoma (BCC) is one of the most common type of human skin cancers.1 Studies have found that BCC is characterized by BRCA2, BRCA1-associated protein-1 gene and P53 gene mutations, and aberrant expression of hedgehog signaling pathway.2-4 Previous study reports that the pathologic differential diagnosis of BCC includes cutaneous mixed sweat gland tumor of the skin, myoepithelioma, and myoepithelial carcinoma.5 Basal cell carcinoma is associated with exposure to radiation in various populations, such as atomic bomb survivors, radiologists, and interventional cardiologists.6-8 Metastatic BCC is exceedingly uncommon, with a poorly defined natural history, and its incidence, risk factors, patterns of spread, prognosis, and potential treatment options are not well understood.9
DNA replication plays an important process in BCC cells growth and invasion that can be exploited in cancer therapy.10 DEAD (Asp-Glu-Ala-Asp) box protein 5 (DDX5) is a prototypical member of the DEAD/H-box protein family and involves in ontogenesis and spermatogenesis in several human malignancies.11 DEAD (Asp-Glu-Ala-Asp) box protein 5 is also an ATP-dependent RNA helicase, which is first identified as a potential target for the treatment of breast cancer.12 Study has showed that DDX5 locus is frequently amplified in breast cancer and it can regulate DNA replication, which is required for cell proliferation in a subset of breast cancer cells.13 In addition, findings show that DDX5 plays an important role in the proliferation and tumorigenesis of non-small-cell lung cancer cells by activating the β-catenin signaling pathway, suggesting DDX5 may serve as a novel prognostic marker and potential therapeutic target in the treatment of non-small-cell lung cancer.14 Furthermore, knockdown of DDX5 inhibits the proliferation and tumorigenesis in esophageal cancer, and it may be a novel potential therapeutic target for the prevention and treatment of esophageal cancer.15 However, the expression and biological role of DDX5 in BCC remains largely unknown.
In this study, we examined the role of DDX5 in regulating BCC cell growth and invasion and explored its possible molecular mechanism. Report has showed that inhibition of JAK/STAT3 pathway involves in angiogenic activity in BCC cell16 and a study indicates that DDX5 expression is associated with tumor cells growth.17 In addition, overexpression of STAT3 inhibited BCCs tumor progression in the inflammatory microenvironment.18 Furthermore, activation of phosphorylated STAT3 increases viability of BCC cells and promote BCC development.19 Therefore, we assumed that DDX5 regulated the growth and invasion of BCC cells through JAK/STAT3 signaling pathway.
Materials and Methods
Ethical Statement
This study was approved by the Ethical Committee of Tongren Hospital, Shanghai Jiao Tong University School of Medicine (Shanghai, China). All patients were required to write informed consent.
Clinical Tissues
Twenty patients with micronodular BCC were recruited in this study. A total of 20 micronodular BCC tissues and matched adjacent nontumor tissues (10 cm far away from BCC tissues) were collected from Shanghai Jiao Tong University School of Medicine (Shanghai, China) during August 2014 to July 2018. Tumor tissues and adjacent nontumor tissues were obtained from patients, who did not receive chemotherapy, radiotherapy, or other treatments before tumor resection. The BCC tissues and adjacent nontumor tissues were stored at −70°C for further analysis.
Cells Culture
Basal cell carcinoma cells and normal basal cells were obtained from BCC tissues and matched adjacent nontumor tissues, respectively. Primary BCC cell line was established as described previously.20 All cells were cultured and maintained in Dulbecco modified Eagle medium (DMEM, Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum (Merck KGaA), 100 U/mL penicillin, 100 µg/mL streptomycin, sodium pyruvate, and L-glutamine at 37°C with 5% CO2.
Small Interfering RNA Transfection
BCC cells (1 × 105 cells/well) were seeded in 6-well for 24 hours at 37°C. The medium was removed and Opti-MEM (Invitrogen, Thermo Fisher Scientific, Waltham, Massachusetts) was added and kept for 24 hours at 37°C. The small interfering RNA (siRNA) sequences corresponding to the gene were designed and synthesized by Genepharma (Shanghai GenePharma Co, Ltd, Shanghai, China). The siRNA sequences against DDX5 (siR-DDX5): sense, 5′-AUCGAUUACGACACUAUCATT-3′; antisense, 5′-CCAUACGAUCCGUCGUCUUCG-3′; siRNA-vector: sense, 5′-CUCGUCUCAUUGATGACAGTT-3′; antisense, 5′-CGAUUAGCUUACGUAGC-3′. The siRNAs (100 pmol) were transfected into BCC cells using 5 µL of lipofectamine RNAiMax reagent (Invitrogen, Thermo Fisher Scientific) according to manufacturer’s instruction. The efficacy of transfection in BCC cells was confirmed using quantitative real-time polymerase chain reaction (qRT-PCR; Supplementary Material Figure 1). After 72 hours of transfection, cells were treated with Jak2 inhibitor (JAK2IR; 1 mg/mL, 420099, Sigma-Aldrich, St. Gallen, Switzerland) or STAT3 inhibitor (STAT3IR; 1 mg/mL, 573125, Sigma-Aldrich, St. Gallen, Switzerland) with phosphate-buffered saline (PBS) as control for 12 hours at 37°C for further analysis.
Quantitative Real-Time PCR
Total RNA was extracted from the BCC or normal basal cells using RNeasy Mini kit (Qiagen Sciences, Inc, Gaithersburg, Maryland) according to the manufacturer’s protocol. The relative messenger RNA (mRNA) expression was determined by qRT-PCR. The RNA integrity number was approximately 2.12. A total of 1 μg RNA was reverse transcribed into complementary DNA (cDNA) using cDNA Synthesis Kit (Product code: 11117831001, Roche, Shanghai, China). The cDNA (1 μg) was subjected to qRT-PCR using an FastStart Universal SYBR Green Master (4913850001, Roche) and a LightCycler 480 Real-Time PCR system (Roche). All the primers were synthesized by Invitrogen (Invitrogen, Thermo Fisher Scientific, Table 1). Polymerase chain reaction thermocycling conditions were 45 amplification cycles, denaturation at 95°C, primer annealing at 64°C with touchdown to 56°C, and applicant extension at 72°C. Relative levels of mRNA expression were calculated using the 2−ΔΔCq method21 and results were expressed as a fold change relative to β-actin control.
Table I.
Primers for qRT-PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Caspase -3 | CATGATTAGCAAGTTACAGTGATGC | CACAGTCTTAAGTGGGGGGA |
| Caspase-9 | CGTTCTATGGTTACCGACATGACG | GTTCCATAGTCATTGAGCATTGTG |
| Bcl-xl | GCTGGTGGTTGACTTTCTCTCC | GGCTTCAGTCCTGTTCTCTTCG |
| Bcl-2 | GATGAAGTACATCCATTATAAGCTGTCACA | ‘-GCGCTCAGCCCTGTGCCACCTGTGGTCCAC |
| DDX5 | GCCGGGACCGAGGGTTTGGT | CTTGTGCTGTGCGCCTAGCCA |
| JAK2 | TTTACTTACTCTCGTCTCCACAGTC | TTTACTTACTCTCGTCTCCACAGTA |
| STAT3 | AGCTGAGCGTGTGTGACAGT | ACCCATGGGATTACACTTGG |
| β-Actin | CGGAGTCAACGGATTTGGTC | AGCCTTCTCCATGGTCGTGA |
Abbreviations: DDX5, DEAD (Asp-Glu-Ala-Asp) box protein 5; qRT-PCR, quantitative real-time polymerase chain reaction.
Western Blotting
Basal cell carcinoma or normal basal cells were homogenized in lysate buffer containing protease inhibitor (Sigma-Aldrich, St. Gallen, Switzerland) and centrifuged at 8000 × g at 4°C for 10 minutes. Protein concentration was measured by a bicinchoninic acid protein assay kit (Thermo Scientific, Pittsburgh, Pennsylvania). Subsequently, protein samples (40 µg) were loaded and separated using 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, as described previously.22 Subsequently, proteins were subsequently blotted on a nitrocellulose membrane and hybridized using rabbit antihuman primary antibodies: DDX5 (1:2000, ab21696, Abcam, Cambridge, UK), Claudin3 (1:1000, ab15102, Abcam), MTA3 (1:1000, ab87275, Abcam), Caspase-3 (1:1000, ab238440, Abcam, Cambridge, UK), Caspase-9 (1:1000, ab32539, Abcam, Cambridge, UK), Bcl-2 (1:1000, ab32124, Abcam, Cambridge, UK), Bcl-xl (1:1000, ab32370, Abcam, Cambridge, UK), and β-actin (1:1000, ab8226, Abcam, Cambridge, UK) after blocking in 5% bovine serum albumin (Sigma-Aldrich) for 1 hour at 37°C. Membranes were then incubated with horseradish peroxidase (HRP)-conjugated goat antirabbit immunoglobulin G (IgG) monoclonal antibody (mAb; PV-6001, ZSGB-BIO, Beijing, China) secondary antibodies for 24 hours at 4°C. The membrane was also washed with TBST for 3 times and protein bands were detected by an enhanced chemiluminescence detection system, and the band intensities were analyzed by ImageJ software 1.2.
Cell Migration and Invasion analysis
Basal cell carcinoma cells were transfected with siR-DDX5 and/or treated with JAK2IR (1 mg/mL, 420099, Sigma-Aldrich, St. Gallen, Switzerland) or STAT3IR (1 mg/mL, 573125, Sigma-Aldrich, St. Gallen, Switzerland); 1 × 104 /well concentration of the BCC cells with 150 μL serum free DMEM were added into the upper chamber with the noncoated membrane. Matrigel-uncoated and -coated migration inserts (8 µm pore size; Millipore, Bedford, MA, USA) were used to evaluate cell migration and invasion. After 24 hours incubation, BCC cells were fixed in 4% paraformaldehyde for 10 minutes at 37°C. Cells were washed with PBS 3 times and stained with 0.1% crystal violet dye (Sigma-Aldrich, St. Gallen, Switzerland) for 15 minutes at 37°C. The cells were removed with a cotton swab and counted at 3 randomly selected views using a light microscope (Olympus BX51, Olympus; Tokyo, Japan).
Immunohistochemistry Analysis
Basal cell carcinoma tissues and matched adjacent nontumor tissues were fixed in 4% paraformaldehyde overnight and then embedded in paraffin wax; 4 µm BCC tissue sections were deparaffinized in xylene, rehydrated through graded ethanols, followed by blocking of endogenous peroxidase activity in 3% hydrogen peroxide for 10 minutes at room temperature and analyzed for DDX-5 expression. Tumor sections were incubated with specific primary antibodies for DDX5 (1:2000, ab21696, Abcam) for 12 hours at 4°C. Tumor tissues were then incubated with HRP-conjugated goat anti-rabbit IgG mAb (1:5000, dilution, PV-6001, ZSGB-BIO). A Ventana Benchmark automated staining system was used for purpose protein expression in tumor tissues (Olympus BX51, Olympus). The staining results were semiquantitatively evaluated by the multiply of staining intensity and the percentage of positive staining cells (magnifications: ×400).
Terminal Deoxynucleotidyl Transferase-Mediated Deoxyuridine Triphosphate Nick-End Labeling Assay
The treated BCC cells (1 × 106) were treated with tunicamycin (1 μg/mL) for 4 hours at 37°C and fixed with 10% paraformaldehyde for 10 minutes at room temperature. Cells were washed with PBS and apoptosis of BCC cells was analyzed using terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay kit (DeadEnd Colorimetric Tunel System, Promega, Madison, Wisconsin) according to the manufacturer’s instructions. Cells were immersed in 50 μL TUNEL reaction fluid in a humid environment at 37°C for 1 hour. After washing with PBS 3 times, cells were incubated with 4′,6-diamidino-2-phenylindole at 37°C for 30 minutes. Finally, samples were washed with PBS 3 times and then captured with a ZEISS LSM 510 confocal microscope at 488 nm. The apoptosis rate was calculated by using the software of Developer XD 1.2 (Definiens AG, Munich, Germany).
Statistical Analysis
Data are expressed as the mean ± standard deviation and performed at least 3 independent replicates. All data were analyzed by SPSS software 19.0 (SPSS, Inc, Chicago, Illinois). Data were analyzed using Student t test or one-way analysis of variance followed by Tukey multiple comparison post hoc tests; *P < .05 and **P < .01 were considered statistically significant.
Results
Expression of DDX5 in BCC Tissues and BCC Cell Lines
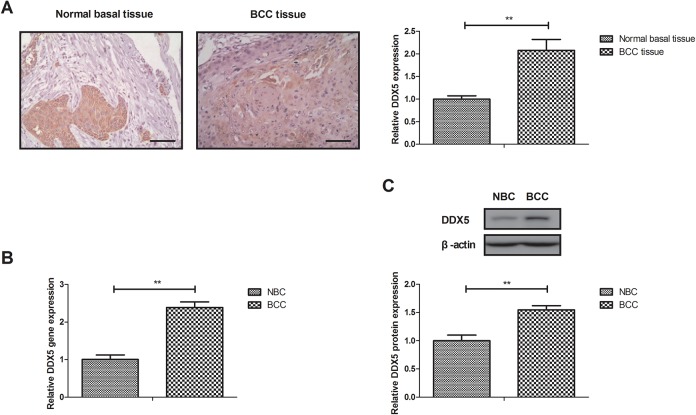
Expression of DDX5 was investigated in BCC tissues and BCC cell lines. Results showed that DDX5 expression was approximately 2.08-fold in BCC tissue samples compared with normal basal tissue (Figure 1A, P < .01). As shown in Figure 1B and C, BCC cell line expressed higher level of DDX5 gene (2.39-fold, BCC vs normal basal cell line [NBC]; P < .01) and protein (1.55-fold, BCC vs NBC; P < .01) expression level than NBC. These results suggest that DDX5 expression is upregulated in BCC tissues and cell lines.
Figure 1.
Expression of DDX5 between BCC tissues and BCC cell lines. A, Expression of DDX5 in BCC tissue samples and normal basal tissue. DDX5 gene (B) and protein (C) expression in BCC cells and normal basal cell lines. Mamplification, ×40. **P < .01 vs normal basal tissue or cells. BCC indicates basal cell carcinoma; DDX5, DEAD (Asp-Glu-Ala-Asp) box protein 5.
DDX5 Knockdown Inhibits BCC Cell Line Migration and Invasion
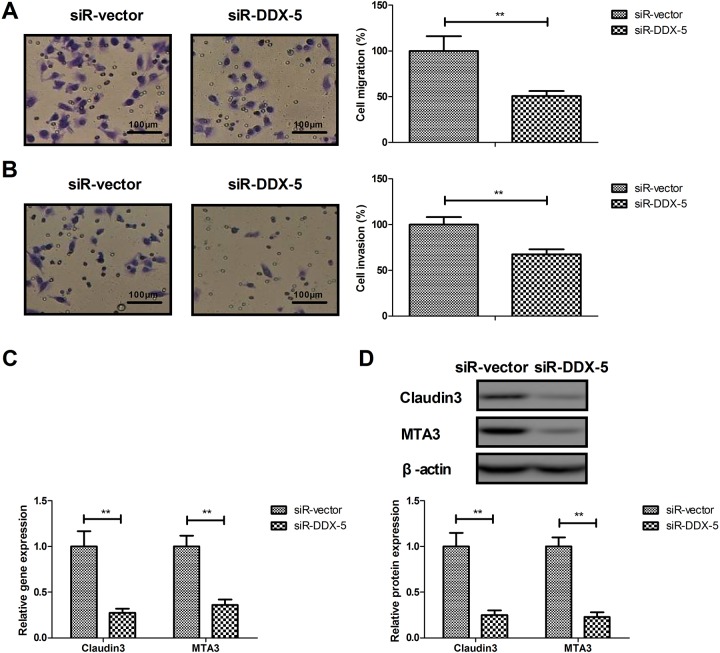
The effects of DDX5 on migration and invasion of BCC cells were analyzed in this study. DEAD (Asp-Glu-Ala-Asp) box protein 5 knockdown (siR-DDX5) inhibited the migration and invasion of BCC cells (Figure 2A and B, P < .01 vs siR vector). Results observed that knockdown of DDX5 inhibited the expression of Claudin3 and MTA3 in BCC cells (Figure 2C and D, P < .01). These results suggest that DDX5 involves in BCC cell line migration and invasion.
Figure 2.
Effects of DDX5 knockdown on BCC cell line migration and invasion. Effects of DDX5 knockdown on migration (A) and invasion (B) of BCC cells. Effects of DDX5 knockdown on gene (C) and protein (D) expression of Claudin3 and MTA3 in BCC cells. **P < .01 vs siR vector. BCC indicates basal cell carcinoma; DDX5, DEAD (Asp-Glu-Ala-Asp) box protein 5.
DDX5 Knockdown Promotes BCC Cells Apoptosis
The effects of DDX5 knockdown on BCC cells apoptosis were analyzed in this study. As shown in Figure 3A, DDX5 knockdown increased the apoptosis of BCC cells compared to control (P < .01 vs siR vector). Gene and protein expression of the active fragment for caspase-3 and caspase-9 was increased by DDX5 knockdown in BCC cells compared to the cells transfected by siR vector (Figure 3B and C, P < .01 vs siR vector). DEAD (Asp-Glu-Ala-Asp) box protein 5 knockdown decreased Bcl-2 and Bcl-xl expression in BCC cells (Figure 3D and E, P < .01 vs siR vector). These results suggest that DDX5 knockdown promotes BCC cells apoptosis.
Figure 3.
Effects of DDX5 knockdown on BCC cells apoptosis. A, DDX5 knockdown increases the apoptosis of BCC cells. Effects of DDX5 knockdown on gene (B) and protein (C) expression of pro caspase-3 and cleaved caspase-3 and caspase-9 in BCC cells. Effects of DDX5 knockdown on gene (D) and protein (E) expression level of Bcl-2 and Bcl-xl in BCC cells. **P < .01 vs siR vector. BCC indicates basal cell carcinoma; DDX5, DEAD (Asp-Glu-Ala-Asp) box protein 5.
DDX5 Knockdown Regulates BCC Cells Metastasis via JAK2/STAT3 Pathway
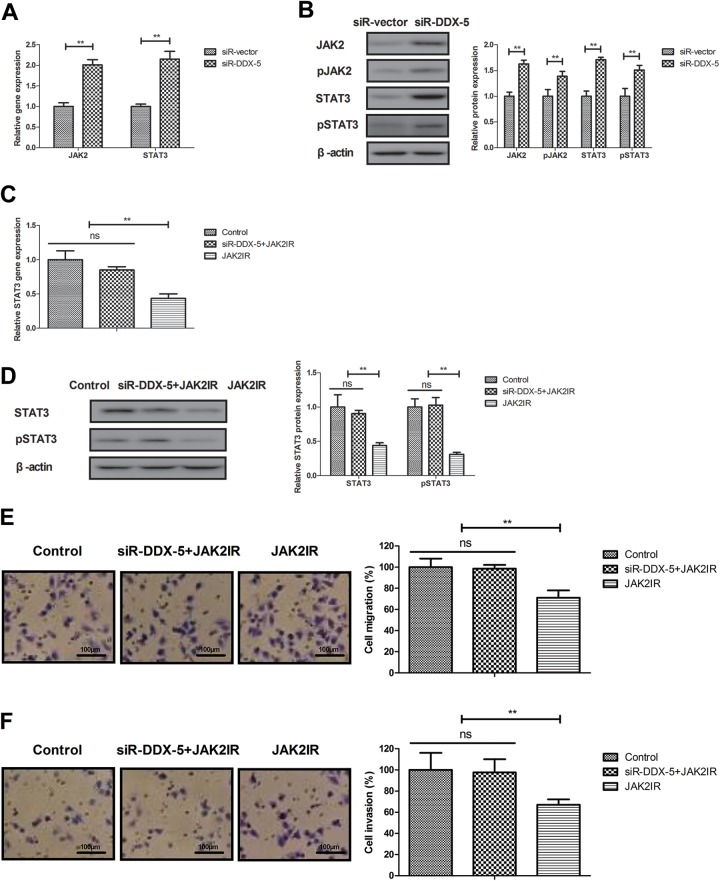
The potential mechanism mediated by DEAD (Asp-Glu-Ala-Asp) box protein 5 was analyzed in BCC cells. Results found that DDX5 knockdown increased JAK2 and STAT3 expression in BCC cells (Figure 4A and B, P < .01 vs siR vector). However, JAK2IR decreased STAT3 expression and abolished the inhibitory effects of DDX5 knockdown (siR-DDX5 + JAK2IR) on migration and invasion in BCC cells (Figure 4C-F, P < .01 vs control or siR-DDX5 + JAK2IR). These results suggest that DDX5 knockdown downregulates JAK2/STAT3 pathway in BCC cells.
Figure 4.
DEAD (Asp-Glu-Ala-Asp) box protein 5 knockdown regulates BCC cells metastasis via JAK2/STAT3 pathway. Effects of DDX5 knockdown on gene (A) and protein (B) expression of JAK2 and STAT3 expression in BCC cells. Effects of JAK2IR on DDX5 knockdown-decreased STAT3 gene (C) and protein (D) expression in BCC cells. Effects of JAK2IR on DDX5 knockdown-decreased migration (E) and invasion (F) in BCC cells. **P < .01 vs control or siR-DDX5 + JAK2IR. BCC indicates basal cell carcinoma; DDX5, DEAD (Asp-Glu-Ala-Asp) box protein 5; JAK2IR, JAK2 inhibitor.
DDX5 Knockdown Regulates BCC Cells Apoptosis via JAK2/STAT3 Pathway
The potential mechanism of apoptosis mediated by DDX5 was analyzed in BCC cells. The results showed that JAK2IR increased apoptosis and abolished the proapoptotic effects of DDX5 knockdown in BCC cells (Figure 5A, P < .01, vs control or siR-DDX5 + JAK2IR). Also, STAT3IR abolished the proapoptotic effects of DDX5 knockdown in BCC cells (Figure 5B, P < .01, vs control or siR-DDX5 + STAT3IR). Results demonstrated that JAK2IR canceled DDX5 knockdown-regulated caspase-3, caspase-9, Bcl-2, and Bcl-xl expression in BCC cells (Figure 5C-D; P < .01 vs control or siR-DDX5 + JAK2IR, P < .01 vs control or siR-DDX5 + STAT3IR, respectively). These results suggest that DDX5 knockdown regulates BCC cells apoptosis via JAK2/STAT3 pathway.
Figure 5.
DEAD (Asp-Glu-Ala-Asp) box protein 5 knockdown regulates BCC cells apoptosis via JAK2/STAT3 pathway. Effects of JAK2 inhibitor (A) or STAT3 inhibitor (B) DDX5 knockdown-increased apoptosis of BCC cells. Effects of JAK2 inhibitor on DDX5 knockdown-regulated caspase-3 and caspase-9 (C), Bcl-2 and Bcl-xl (D) expression in BCC cells. **P < .01 vs control or siR-DDX5 + JAK2IR. BCC indicates basal cell carcinoma; DDX5, DEAD (Asp-Glu-Ala-Asp) box protein 5; JAK2IR, JAK2 inhibitor.
Discussion
Previous reports have indicated that DDX5 expression is overexpressed in human cancer tissues compared with normal tissues including breast cancer, gastric cancer, and prostate cancer.13,23,24 Evidence indicates that inhibition of JAK2/STAT3 signaling pathway induces cancer cells apoptosis and decreases migration of cancer cell, such as ovarian cancer, colorectal cancer, and gastric cancer.25-27 In this study, we analyzed DDX5 expression in BCC tissue and BCC cell line. This study also explored the possible mechanism of apoptosis and invasion mediated by DDX5 in BCC cells. Results in this study indicate that DDX5 is overexpressed in BCC tissues and BCC cell lines. Findings in this study suggest that DDX5 induces BCC cells apoptosis, inhibits migration and invasion through inhibition of the JAK2/STAT3 signal pathway.
Claudins are key cells adhesion molecules, which are associated with cancer development.28 Study has found that MTA3 can regulate malignant progression of human cancer through various signaling pathways.29-31 Here, we reported that DDX5 knockdown inhibited migration and invasion of BCC cells. However, we will further investigate the expression of DDX5 in the more aggressive BCC subtypes based on their morphologies in our future work.
Increasing apoptotic sensitivity plays a crucial role in the treatment of human cancer.32-34 In this study, we showed that DDX5 knockdown increased the apoptosis of BCC cells induced by tunicamycin. Study has found that inducing mitochondria-dependent cleavage of caspase-9 and -3 or decreasing in Bcl-2 and Bcl-xl can promote human BCC apoptosis.35 Our data demonstrated that DDX5 knockdown induced caspase-9 and -3 expression and decreased Bcl-2 and Bcl-xl through interfering with JAK2/STAT3 signaling pathway in BCC cells.
A study has found that under in vitro experimental condition, activation of JAK/STAT3 signaling pathway induces BCC cells invasion.16 Activating STAT3 signaling pathway in BCC contributes tumor growth and tumor progression in the inflammatory microenvironment for BCC cells.18 Findings in this study reported that JAK2IR decreased STAT3 expression and abolished the regulatory effects of DDX5 knockdown on apoptosis, migration, and invasion of BCC cells. Findings also found that JAK2 and STAT3IR abolished DDX5 knockdown-regulated caspase-3, caspase-9, Bcl-2, and Bcl-xl gene and protein expression in BCC cells. However, other regulatory factors involved in the progression of BCC cells growth and invasion need to be further studied in our future investigation.
In conclusion, we have shown that DDX5 knockdown increased tunicamycin-induced apoptosis in BCC cells and suppressed migration and invasion of BCC cells. Our findings suggest that the mechanism of DDX5 may involve modulation of the JAK2/STAT3 signaling pathway in BCC cells. However, antitumor mechanisms of DDX5 need to be clarified further, and the experimental cancer mice performed in the future.
Supplemental Material
Supplementary_material for DDX5 Silencing Suppresses the Migration of Basal cell Carcinoma Cells by Downregulating JAK2/STAT3 Pathway by Zhe Quan, Bei-bei Zhang, Fang Yin, Jiru Du, Yuan-ting Zhi, Jin Xu and Ningjing Song in Technology in Cancer Research & Treatment
Abbreviations
- BCC
basal cell carcinoma
- cDNA
complementary DNA
- DDX5
DEAD (Asp-Glu-Ala-Asp) box protein 5
- DMEM
Dulbecco modified Eagle medium
- HRP
horseradish peroxidase
- IgG
immunoglobulin G
- JAK2IR
JAK2 inhibitor
- mRNA
messenger RNA
- mAb
monoclonal antibodies
- NBC
normal basal cell line
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative real-time polymerase chain reaction
- siRNA
small interfering RNA
- STAT3IR
STAT3 inhibitor
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling
Footnotes
Authors’ Note: The analyzed data sets generated during the study are available from the corresponding author on reasonable request. This study was approved by the Ethic Committee of Shanghai Jiao Tong University School of Medicine. The approval number given by the Ethics Committee is 2017-020-01.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Project supported by Shanghai Tongren Hospital (TRYJ201508) and Changning district health and family planning commission (20164Y005).
ORCID iD: Quan Zhe  https://orcid.org/0000-0002-6584-5296
https://orcid.org/0000-0002-6584-5296
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kallam AR, Satyanarayana MA, Aryasomayajula S, Krishna BA. Basal cell carcinoma developing from trichoepithelioma: review of three cases. J Clin Diagn Res. 2016;10(3):PD17–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kuang S, Li H, Feng J, Xu S, Le Y. Correlation of BRCA2 gene mutation and prognosis as well as variant genes in invasive urothelial carcinoma of the bladder. Cancer Biomark. 2019;25(2):203–212. [DOI] [PubMed] [Google Scholar]
- 3. Wadt KA, Aoude LG, Johansson P, et al. A recurrent germline BAP1 mutation and extension of the BAP1 tumor predisposition spectrum to include basal cell carcinoma. Clin Genet. 2015;88(3):267–272. [DOI] [PubMed] [Google Scholar]
- 4. Wang YM, Huang YS, Ma ZH, et al. Frequency and features of TP53 mutation in 30 Chinese patients with sporadic basal cell carcinoma. Clin Exp Dermatol. 2014;39(7):829–834. [DOI] [PubMed] [Google Scholar]
- 5. Cohen PR. Basal cell carcinoma with myoepithelial differentiation: case report and literature review. Cureus. 2018;10(1):e2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ericson MB, Wennberg AM, Larko O. Review of photodynamic therapy in actinic keratosis and basal cell carcinoma. Ther Clin Risk Manag. 2008;4(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- 7. Ionescu DN, Arida M, Jukic DM. Metastatic basal cell carcinoma: four case reports, review of literature, and immunohistochemical evaluation. Arch Pathol Lab Med. 2006;130(1):45–51. [DOI] [PubMed] [Google Scholar]
- 8. Shi Y, Jia R, Fan X. Ocular basal cell carcinoma: a brief literature review of clinical diagnosis and treatment. Onco Targets Ther. 2017;10:2483–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verkouteren JAC, Ramdas KHR, Wakkee M, Nijsten T. Epidemiology of basal cell carcinoma: scholarly review. Br J Dermatol. 2017;177(2):359–372. [DOI] [PubMed] [Google Scholar]
- 10. Munday JS, French A, Thomson N. Detection of DNA sequences from a novel papillomavirus in a feline basal cell carcinoma. Vet Dermatol. 2017;28(2):236–e60. [DOI] [PubMed] [Google Scholar]
- 11. Fang DA, Wang Q, Wang J, He L, Liu LH, Wang Y. A novel DDX5 gene in the freshwater crayfish Cherax quadricarinatus is highly expressed during ontogenesis and spermatogenesis. Genet Mol Res. 2011;10(4):3963–3975. [DOI] [PubMed] [Google Scholar]
- 12. Mazurek A, Luo W, Krasnitz A, Hicks J, Powers RS, Stillman B. DDX5 regulates DNA replication and is required for cell proliferation in a subset of breast cancer cells. Cancer Discov. 2012; 2(9): 812–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Huang J, Hu Z. RNA helicase DDX5 regulates microRNA expression and contributes to cytoskeletal reorganization in basal breast cancer cells. Mol Cell Proteomics. 2012;11(2):M111, 011932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z, Luo Z, Zhou L, Li X, Jiang T, Fu E. DDX5 promotes proliferation and tumorigenesis of non-small-cell lung cancer cells by activating beta-catenin signaling pathway. Cancer Sci. 2015;106(10):1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma Z, Feng J, Guo Y, et al. Knockdown of DDX5 Inhibits the proliferation and tumorigenesis in esophageal cancer. Oncol Res. 2017;25(6):887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jee SH, Chu CY, Chiu HC, et al. Interleukin-6 induced basic fibroblast growth factor-dependent angiogenesis in basal cell carcinoma cell line via JAK/STAT3 and PI3-kinase/Akt pathways. J Invest Dermatol. 2004. Dec;123(6):1169–1175. [DOI] [PubMed] [Google Scholar]
- 17. Tago K, Funakoshi-Tago M, Itoh H, et al. Arf tumor suppressor disrupts the oncogenic positive feedback loop including c-Myc and DDX5. Oncogene. 2015;34(3):314–322. [DOI] [PubMed] [Google Scholar]
- 18. Jia J, Shi Y, Yan B, et al. LGR5 expression is controlled by IKKα in basal cell carcinoma through activating STAT3 signaling pathway. Oncotarget. 2016;7(19):27280–27294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brennan-Crispi DM, Overmiller AM, Tamayo-Orrego L, et al. Overexpression of desmoglein 2 in a mouse model of Gorlin syndrome enhances spontaneous basal cell carcinoma formation through STAT3-mediated Gli1 expression. J Invest Dermatol. 2019;139(2):300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Svobodova M, Raudenska M, Gumulec J, et al. Establishment of oral squamous cell carcinoma cell line and magnetic bead-based isolation and characterization of its CD90/CD44 subpopulations. Oncotarget. 2017;8(39):66254–66269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 22. Wai-Hoe L, Wing-Seng L, Ismail Z, Lay-Harn G. SDS-PAGE-Based quantitative assay for screening of kidney stone disease. Biol Proced Online. 2009;11:145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du C, Li DQ, Li N, et al. DDX5 promotes gastric cancer cell proliferation in vitro and in vivo through mTOR signaling pathway. Sci Rep. 2017;7:42876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taniguchi T, Iizumi Y, Watanabe M, et al. Resveratrol directly targets DDX5 resulting in suppression of the mTORC1 pathway in prostate cancer. Cell Death Dis. 2016;7(5):e2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du W, Hong J, Wang YC, et al. Inhibition of JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via mitochondrial pathway. J Cell Mol Med. 2012;16(8):1878–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jo M, Park MH, Kollipara PS, et al. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258(1):72–81. [DOI] [PubMed] [Google Scholar]
- 27. Judd LM, Menheniott TR, Ling H, et al. Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo. PLoS One. 2014; 9(5):e95993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katayama A, Handa T, Komatsu K, et al. Expression patterns of claudins in patients with triple-negative breast cancer are associated with nodal metastasis and worse outcome. Pathol Int. 2017;67(8):404–413. [DOI] [PubMed] [Google Scholar]
- 29. Jiao T, Li Y, Gao T, et al. MTA3 regulates malignant progression of colorectal cancer through Wnt signaling pathway. Tumour Biol. 2017;39(3):1010428317695027. [DOI] [PubMed] [Google Scholar]
- 30. Li H, Sun L, Xu Y, et al. Overexpression of MTA3 correlates with tumor progression in non-small cell lung cancer. PloS One. 2013;8(6):e66679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujita N, Jaye DL, Kajita M, Geigerman C, Moreno CS, Wade PA. MTA3, a Mi-2/NuRD complex subunit, regulates an invasive growth pathway in breast cancer. Cell. 2003;113(2):207–219. [DOI] [PubMed] [Google Scholar]
- 32. Gleave ME, Zellweger T, Chi K, et al. Targeting anti-apoptotic genes upregulated by androgen withdrawal using antisense oligonucleotides to enhance androgen- and chemo-sensitivity in prostate cancer. Invest New Drugs. 2002;20(2):145–158. [DOI] [PubMed] [Google Scholar]
- 33. Burow ME, Weldon CB, Chiang TC, et al. Differences in protein kinase C and estrogen receptor alpha, beta expression and signaling correlate with apoptotic sensitivity of MCF-7 breast cancer cell variants. Int J Oncol. 2000;16(6):1179–1187. [DOI] [PubMed] [Google Scholar]
- 34. Marcelli M, Marani M, Li X, et al. Heterogeneous apoptotic responses of prostate cancer cell lines identify an association between sensitivity to staurosporine-induced apoptosis, expression of Bcl-2 family members, and caspase activation. Prostate. 2000;42(4):260–273. [DOI] [PubMed] [Google Scholar]
- 35. Wu CS, Chen GS, Lin PY, et al. Tazarotene induces apoptosis in human basal cell carcinoma via activation of caspase-8/t-Bid and the reactive oxygen species-dependent mitochondrial pathway. DNA Cell Biol. 2014; 33(10): 652–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_material for DDX5 Silencing Suppresses the Migration of Basal cell Carcinoma Cells by Downregulating JAK2/STAT3 Pathway by Zhe Quan, Bei-bei Zhang, Fang Yin, Jiru Du, Yuan-ting Zhi, Jin Xu and Ningjing Song in Technology in Cancer Research & Treatment