Abstract
Matrix metalloproteinase-1 (MMP-1) is a predominant collagenase enzyme that cleaves collagen fibers, contributing to skin wrinkling. Matrix metalloproteinase-1 inhibitors of herbal origin may provide an earnest probability to offer a novel curative approach against MMP-1-mediated collagenolysis, prompted by ultraviolet (UV)-induced overexpression of MMP-1. In this in silico study, we have explored the MMP-1 inhibitory potential of selected terpenoids from Dalbergia sissoo extracts. Two triterpenoids (lupeol and betulin), 1 diterpenoid (phytol), and 1 ester derivative of lupeol (lupeol acetate) were studied along with a reference inhibitor (doxycycline) using molecular docking approach. Non covalent interaction between the target ligands was found. Lupeol was found interacting with amino acid (AA) residues in the catalytic domain of MMP-1 with 3 hydrogen bonds (H-bond) formation, phytol with 1 and doxycycline with 2 H-bonds, whereas betulin and lupeol acetate were not able to form any H-bond with the AA residues in the catalytic site of the target protein. However, hydrophobic interaction between these ligands and protein was evident with select residues. The binding affinity of lupeol was highest (binding free energy, ΔG = −8.24 kcal/mol), which was greater than reference drug, doxycycline (ΔG = −8.05 kcal/mol). Lupeol acetate and phytol displayed a ΔG value of −7.12 and −7.06 kcal/mol, respectively, whereas betulin holds less binding affinity for the target receptor (ΔG = −4.66 kcal/mol). In silico pharmacokinetic studies demonstrated drug-like properties of the ligand compounds. This study shows that hydroxyl groups present in the ligands play a substantial role in establishing protein ligand interaction via hydrogen bonding.
Keywords: Terpenoids, Dalbergia sissoo, MMP-1, skin wrinkling, molecular docking, non covalent interaction
Introduction
Photoaging, as contrast to chronological aging (age-dependent aging), is caused due to extrinsic factors like ultraviolet radiations (UVRs), mainly short wavelength ultraviolet rays (UV-B) and is distinguished by excessive wrinkling of skin, changes in pigmentation and collagen tissue degradation.1,2 Breakdown of collagen due to UV-B exposure causes discernible changes in skin structure, resulting in wrinkling. Collagen is the major constituent of the extracellular matrix (ECM) including elastin, proteoglycans, fibronectin, and type-I collagen. Disruption and degradation of these proteins by UV-B progresses the skin aging.3 Matrix metalloproteinases (MMPs) are endopeptidases containing Zn2+ at their catalytic (CAT) site, which can degrade all kinds of ECM proteins. Matrix metalloproteinases expression is induced by accumulated reactive oxygen species (ROS) which are built up by UVRs. The increased buildup of ROS stimulates various signal transduction pathways like mitogen-activated protein kinase (MAPK) pathway by activating growth factor receptors and cell surface cytokines. Ultraviolet radiation (eg, UV-A and UV-B) elicits the activation of transcription factors like activator protein-1 (AP-1) via ROS, in turn, triggering induction of expression of MMPs, including MMP-1 (an interstitial collagenase that hydrolyzes the collagen), which degrade type-I and type-III collagen.4,5 Other MMPs like MMP-2, -3, and -9 further take part and cause damage to skin connective tissues by collagenase or gelatinase activity, consequently forming skin wrinkles.6,7
Structural features of all MMPs include 2 zinc ions (Zn2+), 1 positioned in the active site, known to carry out the CAT process, and the other is related to structural functions akin to the calcium ions (Ca2+) present in these enzymes. The active site Zn2+ binds to 3 histidine residues generally and to a molecule of water when the enzyme is in active state. Depending on the nature of the enzyme, the structural Zn2+ binds to 3 different histidine residues and to a fourth different amino acid (AA) residue, other than histidine, for example, glutamic acid in case of matrilysin.8 Along with these 2 molecules of Zn2+ ions in the CAT domain, there are present 3 Ca2+ ions of which 2 Ca2+ ions are placed near the CAT cleft and the third Ca2+ ion is present near the hemopexin-like (HPX) domain. These Ca2+ ions play an important role in imparting structural and conformational stability to the enzyme.9 Binding specificity of the MMPs is determined by the S1′ subsite or S1′ specificity pocket, which is the most conspicuous pocket present in the CAT domains of MMPs. The S1′ specificity pocket is defined by the AA residue at position 218 and the loop (S1′ specificity loop) present at the backside of the pocket.10 The nature of AA residue at the position 218 confers the inhibitor specificity among MMPs while S1′ specificity loop is responsible for the overall conformation of the S1′ pocket. Matrix metalloproteinases have generally 2 types of S1′ specificity pockets, which can be summarized as the large, open pocket as professed in case of MMP-2,11 -3, -8, -9,12 and -13 and the small, clasped pocket as observed in the instance of MMP-1 and -7.13 Like all MMPs, a full-length MMP-1 consists of an N-terminal pro-domain (propeptide) destined to maintain dormancy, a CAT domain (160 AA residues) contains CAT Zn2+, a linker region (16 AA residues) followed by the C-terminal HPX domain, which is a 4-bladed (bI-bIV) propeller structure. The CAT domain of MMP-1 is comprised of a highly twisted 5-stranded β-sheet (4 parallel and 1 antiparallel β-strands), which is spanned by 3 α-helices.14
In addition to the natural, endogenous inhibitors like tissue inhibitors of metalloproteinases (TIMPs), there are synthetic and exogenous inhibitors of MMPs which are being selectively developed for many diseases like arthritis, periodontal disease, and cancer.8 As MMP-1 is the major collagenolytic enzyme responsible for collagen degradation in photodamaged skin,15 its inhibition through synthetic or exogenous inhibitors, which can be of herbal origin as well, can subdue UV-induced degradation of collagen. Computational techniques allow exploration of structural organization, conformation, mode of interaction with respective inhibitors and physicochemical properties of particular targets involved in progression of various diseases and help save time and cost in further experimental explorations. Various molecular modeling techniques have been developed in recent past for virtual drug screening, which include molecular dynamics simulation, molecular docking, target prediction, pharmacophore modeling, homology modeling, and quantum mechanics.16 Molecular docking is one of the most commonly used molecular modeling method to study potential inhibitors against a number of target protein/receptor involved in the development of many diseases.17 Molecular docking technique gives analytical insights on energetics of binding, intermolecular interactions of a ligand-protein complex to evaluate the stability of the complex and conformational change assessment during the process of docking.18 Matrix metalloproteinase-1 is the most studied collagenolytic protease in the MMP family. Molecular dynamics simulation studies on X-ray crystallographic structure of MMP-1 in complex with a collagen model triple helical peptide (THP) to reveal important atomistic, structural, and conformational details of MMP-1 have been accomplished.19,20 In a recent report, conformational dynamics of MMP-1. THP complex have been studied to explain the early stages of collagenolysis, where the interaction between MMP-1 and THP was modeled through molecular docking.21
Dalbergia sissoo Roxb. (Fabaceae) is a common timber wood and medicinal plant, being used in folk medicine to cure many diseases including skin ailments. Previous studies showed cosmetic property of D. sissoo with respect to sunscreening activity.22 In this work, we have made an in silico attempt (via MMP-1 inhibition) by molecular docking study to screen the anti-wrinkling activity of D. sissoo extract components, particularly 2 triterpenoids (lupeol and betulin) and a diterpenoid (phytol) characterized by gas chromatography and mass spectrometry (GC-MS) previously.22 Furthermore, lupeol acetate (ester derivative of lupeol) was also docked to compare the binding affinity of both lupeol and lupeol acetate toward MMP-1. Doxycycline, a nonselective MMP inhibitor was taken as a reference drug to compare the binding efficacy of ligand-protein interaction. Selection of doxycycline as a reference was made on the basis of its status as an “only MMP inhibitor” approved by the U.S. Food and Drug Administration.23
Methodology
Description of ligands
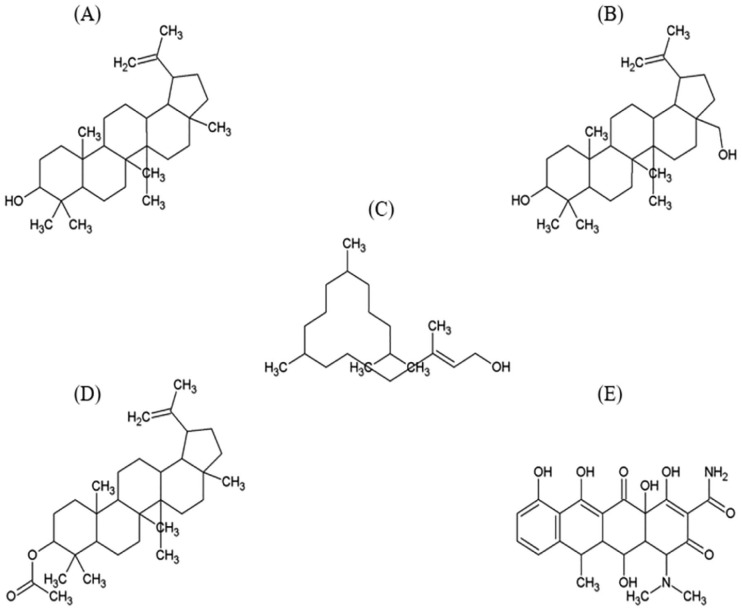
Lupeol is a pentacyclic lupane-type triterpenoid, in which the hydrogen is substituted by a hydroxyl group at 3 beta position, having molecular formula C30H50O and molecular weight 426.729 g/mol. Figure 1A illustrates the structure of lupeol.24
Figure 1.
Structure of ligand compounds (A) Lupeol, (B) Betulin, (C) Phytol, (D) Lupeol acetate, and (E) Reference inhibitor Doxycycline.
Betulin is also a pentacyclic lupane-type triterpenoid, which too has a hydroxyl group at 3 beta position (like in lupeol) and also a 28-hydroxymethyl substituent and a double bond at 20(29) position. Its molecular formula is C30H50O2, molecular weight 442.728 g/mol. The structure of betulin is depicted in Figure 1B.25
Phytol is a linear diterpenoid, which is used in the preparation of vitamin E and K synthetically and is a constituent of chlorophyll. Its molecular formula is C20H40O and molecular weight is 296.539 g/mol.26 Structural illustration of phytol is presented in Figure 1C.
Lupeol acetate, Figure 1D is an ester derivative of lupeol with molecular formula C32H52O2 and molecular weight 468.766 g/mol.27
Doxycycline is an antibiotic that inhibits MMPs activation and expression nonselectively,28 having molecular formula C22H24N2O8 and molecular mass 444.44 g/mol.29 Structure of doxycycline is shown in Figure 1E.
Physicochemical properties of the target protein (human MMP-1)
Physicochemical properties of human MMP-1 were predicted using ProtParam (https://web.expasy.org/protparam/) with UniProt ID P03956. ProtParam is a protein analysis tool, which computes various physicochemical parameters like molecular weight, composition of AA, atomic composition, theoretical isoelectric point (pI), extinction coefficient, expected half-life, aliphatic index, instability index, and grand average of hydropathicity (GRAVY) of a protein.30 Full-length MMP-1 protein (PDB ID: 2CLT) is depicted in Figure 2A.
Figure 2.
Ribbon representation of human MMP-1 full-length protein (PDB ID: 2CLT), catalytic domain of MMP-1 (PDB ID: 3SHI) (A) dimeric assembly of 2CLT (chain A represented by magenta color and chain B by light blue color), (B) trimeric assembly of 3SHI (chain A represented by red, chain G by magenta, and chain M by cyan color).
MMP-1 indicates matrix metalloproteinase-1.
Ligands preparation
Chemical structures of all the ligands were obtained from Pubchem database at National Institute of Health (https://www.ncbi.nlm.nih.gov/pccompound). Structure of ligands was drawn using ACD/Chemsketch (www.acdlabs.com) and Open Babel version 2.3.2 was used to convert the files in .pdb format.
Retrieval of target protein and structure optimization
Crystal structure of the CAT domain of human MMP-1 (PDB ID: 3SHI) was recouped from Protein Data Bank (PDB) (https://www.rcsb.org) as shown in Figure 2B. The structure of 3SHI is presented as trimeric assembly, where the 3 chains (A, G, and M) are present in different orientations.31 Direct retrieval of CAT domain of the protein was done considering the fact that it is essential for interaction with specific inhibitors and tissue inhibitors of MMP-1 (TIMP-1).32 All heteroatoms (i.e. nonreceptor atoms such as water, which is used in cocrystallization of protein structure) were removed except Ca and Zn atoms. Energy minimization was done with Swiss-PdbViewer version 4.1.0 using default parameters.
Molecular docking study
In order to assess the binding affinity of the ligands toward the CAT domain of human MMP-1 (PDB ID: 3SHI), molecular docking was carried out using AutoDock, version 4.2 (Scripps Research Institute, USA) following a previously reported protocol.33 AutoDockTools (ADT), version 1.5.6 (Scripps Research Institute, USA) were used for analysis of docking results. AutoDock utilizes the Lamarckian genetic algorithm (LGA), which enables arbitrary modifications in the structural parameters of the ligands to identify the most energy favorable ligand-protein binding conformations. The ligands were kept in flexible form and protein in the rigid form throughout the procedure. Polar hydrogens were added to protein during the structure optimization, and Kollman charges were assigned prior to the docking. Docked structures were visualized using Chimera UCSF 1.10.2 (http://www.cgl.ucsf.edu/chimera). Doxycycline, a broad spectrum MMP inhibitor, was also docked with 3SHI to compare the results.
Ligands evaluation
Ligands were assessed by absorption, distribution, metabolism, and excretion (ADME) parameters. Pharmacokinetic properties and drug likeness of the ligands were predicted, and physicochemical descriptors were computed using SwissADME program (www.swissadme.ch) developed by the Molecular Modeling Group of the Swiss Institute of Bioinformatics.34 Lipinski’s rule of five was applied for the ligands to be proposed as drugs.35 Other filters were also checked for the drug likeness of the ligands. Ligand efficiency (LE) was calculated using the formula LE = ΔG/N, where, ΔG is the minimum binding energy, and N is the number of nonhydrogen atoms.36 Oral bioavailability and skin permeability were also assessed to discover the suitability of the ligands to be used in topically applied formulations.
Results
Physicochemical properties of target protein (Human MMP-1)
Human MMP-1 exhibited hydrophilic nature with a GRAVY value of −0.572, stable protein with an instability index of 35.46 which is lower than 40 (a value greater than 40 predicts that protein may be unstable). More properties have been listed in Table 1.
Table 1.
Physicochemical properties of human MMP-1.
| Sl no. | Physicochemical property | Value |
|---|---|---|
| 1 | Number of amino acids | 469 |
| 2 | Amino acid composition | Negatively charged residues (Asp + Glu): 58
Positively charged residues (Arg + Lys): 54 |
| 3 | Molecular weight | 54006.90 Da |
| 4 | Theoretical isoelectric point (pI) | 6.47 |
| 5 | Total number of atoms | 7492 |
| 6 | Chemical formula | C2458H3666N656O699S13 |
| 7 | Extinction coefficients | 76 905a and 76 780b M−1 cm−1 |
| 8 | Instability index | 35.46 |
| 9 | Aliphatic index | 65.27 |
| 10 | Estimated half-life | 30 hr in mammalian reticulocytes, in vitro, >20 hr in yeast, in vivo, >10 hr in Escherichia coli, in vivo, considering that M (Met) is N-terminal residue |
| 11 | Grand average of hydropathicity (GRAVY)c | −0.572 |
Abbreviation: MMP-1, matrix metalloproteinase-1.
At 0.1% absorbance (OD = 1.424) in water at 280 nm supposing all pairs of cysteine residues form cystines.
At 0.1% absorbance (OD = 1.424) in water at 280 nm considering all cysteine residues are reduced.
GRAVY score is the sum of hydropathy values of all the amino acids divided by the number of residues in the sequence. Hydropathy is a property that has a strong feeling for water including both attraction and repulsion, ie, hydophilicity and hydrophobicity, respectively.37 A GRAVY score >0, signifies the hydrophobic nature of protein and <0 (negative value), indicates hydrophilicity.38
Molecular docking
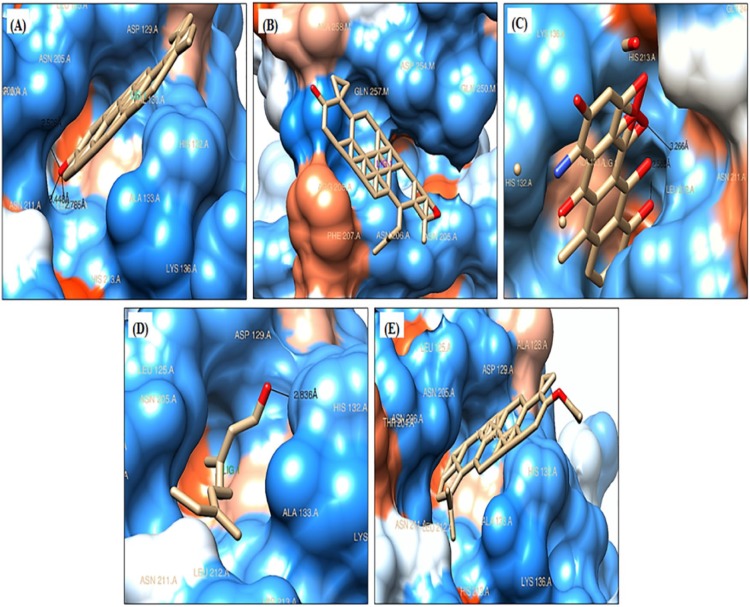
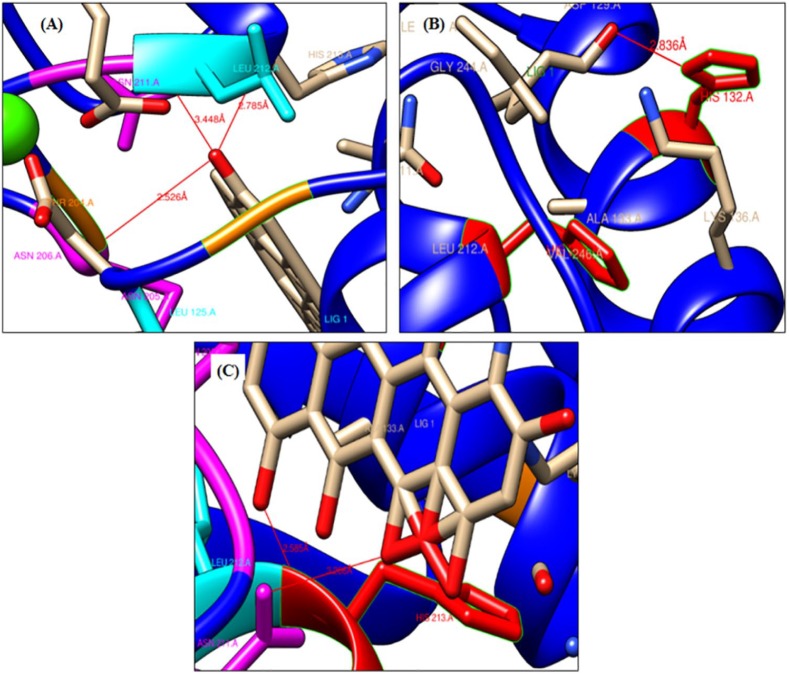
The test ligands and doxycycline, a reference inhibitor were docked into the CAT domain of human MMP-1 (PDB ID: 3SHI). The docked pose of all the ligand-3SHI complexes have been shown in Figure 3. The docking study revealed that lupeol interacted with 3SHI through the formation of H-bonds with Thr204 residue within 2.526 Å distance, Asn211 residue within the distance of 3.44 Å, and with Leu212 residue within 2.785 Å distance (Figure 4A). Betulin does not form any H-bond with the AA residues of 3SHI, Figure 3B. Rather, it interacts via hydrophobic contacts described in the discussion section. Phytol binds with His132 residue within the distance of 2.836 Å with minimum binding energy (ΔG) of −7.06 kcal/mol (Figure 4B). While, acetate derivative of lupeol (lupeol acetate) was unable to form H-bond with 3SHI (Figure 3E), but hydrophobic interaction was evident. Doxycycline, the reference inhibitor, was found to bind at Asn211 (3.266 Å) and His213 (2.585 Å) residues with ΔG of −8.05 kcal/mol (Figure 4C), which was lower than that of lupeol (−8.24 kcal/mol). Asn211 was the common AA residue, which interacts with lupeol and doxycycline. Total intermolecular energy of docking and inhibition constants for ligands-3SHI and doxycline-3SHI complex are summarized in Table 2.
Figure 3.
Poses of ligands docked into catalytic domain of MMP-1 (PDB ID: 3SHI): (A) lupeol, (B) betulin, (C) doxycycline, (D) phytol, and (E) lupeol acetate.
MMP-1 indicates matrix metalloproteinase-1.
Figure 4.
A closer and ribbon representation of hydrogen bonding between 3SHI and ligands: (A) lupeol interacts with 3 H-bonds (shown by red line), (B) phytol with 1 H-bond, and (C) doxycycline with 2 H-bonds.
Table 2.
Minimum binding energy (ΔG) of test ligands and doxycycline (standard) and parameters of molecular interaction with target protein (catalytic domain of human MMP-1, PDB ID: 3SHI).
| Sl no. | Compound | Pubchem ID | ΔG (kcal/mol) | Final intermolecular energy (kcal/mol) | VdW + HB + DE (kcal/mol) | Inhibition constant (Ki) (µM) | Residues involved in H-bonding |
|---|---|---|---|---|---|---|---|
| 1 | Lupeol | 259846 | −8.24 | −9.13 | −9.10 | 0.91 | Thr204, Asn211, Leu212 |
| 2 | Doxycycline | 54671203 | −8.05 | −8.65 | −9.04 | 1.26 | Asn211, His213 |
| 3 | Lupeol acetate | 92157 | −7.12 | −8.31 | −8.37 | 6.06 | – |
| 4 | Phytol | 5280435 | −7.06 | −9.51 | −9.53 | 6.65 | His132 |
| 5 | Betulin | 72326 | −4.66 | −5.56 | −5.65 | 382.49 | – |
Abbreviations: DE, desolvation energy; HB, hydrogen bond; VdW, Van der Waals.
Hydrophobic interactions and hydrogen bond analysis
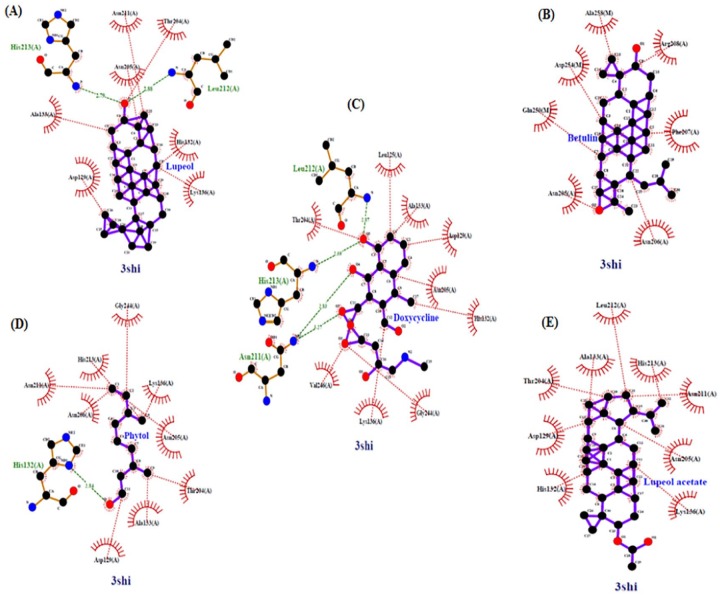
Two-dimensional representation diagrams of protein-ligand complexes were generated using LigPlot+, version 2.139 from the output file (.pdb) obtained by molecular docking. Explanatory and diagrammatic illustration of hydrophobic interactions as well as intermolecular hydrogen bonding is presented that occurred within the protein-inhibitor complexes. Amino acid residues that interact directly with the inhibitors involved residues like Asp129, Ala133, Lys136, Thr204, and Asn205, which were shared residues among protein-inhibitor complexes (Figure 5).
Figure 5.
Hydrogen bonding and hydrophobic interactions between 3SHI and ligands: (A) lupeol, (B) betulin, (C) doxycycline, (D) phytol, and (D) lupeol acetate.
Ligands evaluation
Absorption, distribution, metabolism, and excretion assessment showed the drug likeness property of all the test ligands along with the reference compound (doxycycline) as per Lipinski’s rule of five. This rule states that an orally active candidate drug must not violate >1 criteria proposed in the rule, which are: (1) a molecular weight <500, (2) H-bond acceptors <10, (3) H-bond donors <5, and (4) Log P (lipophilicity) value <5. Other important measures like topological surface area (TPSA), number of rotatable bonds and molar refractivity were also computed. A TPSA value less than 140 Å2, number of rotatable bonds fewer than 10, and molar refractivity between 40 and 130 are required for a compound to be good candidate drug.40 Skin permeation coefficient of the ligands was also recorded from the computed descriptors in SwissADME. All the parameters are presented in Table 3. Other filters like Ghose, Veber, and Egen filters were also assessed by SwissADME to determine the lead likeness of the ligands. Lupeol, betulin, and lupeol acetate violated 3 parameters of Ghose filter, where these ligands displayed higher values of log P, molar refractivity, and number of atoms than the upper qualifying range which are 5.6 for log P, 130 for molar refractivity and 70 for the number of atoms. Phytol exceeded log P value and doxycycline could not reach even the lower range of log P value (−0.4). No violations observed by lupeol, betulin, and lupeol acetate when veber filter was applied, whereas phytol and doxycycline expressed a violation that was more than 10 rotatable bonds and TPSA greater than 140 Å2, respectively. Egen filter criteria were violated by 1 parameter in case of all ligands where doxycycline presented TPSA greater than 131.6 Å2 (threshold value) and other ligands expressed log P value greater than 5.88 (threshold value). Drug scores, mutagenicity and tumorigenicity were determined by OSIRIS Property Explorer developed by Thomas Sander (https://www.organic-chemistry.org/prog/peo/). Highest drug score was reported in case of reference inhibitor (doxycycline = 0.49) followed by phytol (0.21), betulin (0.15), lupeol (0.13), and lupeol acetate (0.11). No mutagenic and tumorigenic effects were observed in case of all the ligands. As far as oral bioavailability of the ligands is concerned, all the terpenoids were found less suitable to be proposed as oral drug because they violated at least 2 criteria, lipophilicity and insolubility in case of lupeol (Figure 6A); betulin (Figure 6B); and lupeol acetate (Figure 6D) and lipophilicity and flexibility as observed in oral bioavailability radar of phytol (Figure 6C). The reference compound doxycycline showed violation of only 1 criterion, that is, polarity (Figure 6E), and its skin permeation coefficient (−8.63 cm/s) was also noticeably less than selected terpenoids. The order of skin permeation ability (Log Kp value) of the ligands was as follows: lupeol acetate (−1.74 cm/s) >lupeol (−1.90 cm/s) >phytol (−2.29 cm/s) >betulin (−3.12 cm/s) >doxycycline (−8.63 cm/s), and a small Log Kp value represents less cutaneous permeability.41
Table 3.
Parameters for drug likeness using SwissADME (Lipinski/Pfizer filter).
| S. No. | Compound | M (<500) | HBA (<10) | HBD (<5) | MR (40-130) | (MLogP) (<4.15) | TPSA (Å2) | Log Kp (cm/s) | Drug likeness |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lupeol | 426.72 | 1 | 1 | 135.14 | 6.92 | 20.23 | −1.90 | Yes |
| 2 | Betulin | 442.72 | 2 | 2 | 136.30 | 6.00 | 40.46 | −3.12 | Yes |
| 3 | Lupeol acetate | 468.75 | 2 | 0 | 144.88 | 7.08 | 26.30 | −1.74 | Yes |
| 4 | Phytol | 296.53 | 1 | 1 | 98.94 | 5.25 | 20.23 | −2.29 | Yes |
| 5 | Doxycycline | 444.43 | 9 | 6 | 110.91 | −2.08 | 181.62 | −8.63 | Yes |
Abbreviations: HBA, hydrogen bond acceptor; HBD, hydrogen bond donor; Log Kp, skin permeation coefficient; M, molecular mass; MLogP, lipophilicity; MR, molar refractivity; TPSA, topological surface area.
Figure 6.
Oral bioavailability radars of the ligands constructed using canonical SMILES of the ligands in SwissADME. Colored zone is the suitable physicochemical space for oral bioavailability.
FLEX indicates flexibility; INSATU, insaturation; INSOLU, insolubility; LIPO, lipophilicity.
Ligand efficiency values indicate that phytol is the most efficient ligand having an LE value of 0.336 kcal/mol/nonhydrogen atoms, followed by lupeol (0.265 kcal/mol/nonhydrogen atoms), doxycycline (0.251 kcal/mol/nonhydrogen atoms), lupeol acetate (0.209 kcal/mol/nonhydrogen atoms), and betulin (0.145 kcal/mol/nonhydrogen atoms).
Discussion
Prolonged exposure to solar UV rays can affect the human skin in various ways and may cause skin aging, resulting from the excessive buildup of ROS. Photoaging of skin is linked with induction of MMPs expression and repression of collagen synthesis. Matrix metalloproteinase-1 is a major collagenolytic enzyme, which gets induced by UV irradiation and is accountable for collagen degradation in severely photodamaged skin,15,42 and it is the most widely studied collagenolytic MMP.19 Matrix metalloproteinase-1 induction happens following the UV irradiation that stimulates the expression of c-Jun/c-Fos (components of the AP-1 heterodimer complex) which in turn, induces AP-1 activity, consequently upregulating the MMP-1 and downregulating the synthesis of type-I collagen. Therefore, increased expression of MMP-1 causes degradation of collagen, thereby decreasing the suppleness of the skin.3 Inhibiting the activity of MMP-1 can be a useful strategy to prevent the skin photoaging. Bioactive natural products have been attracting the interest of researchers from cosmetic industry due to their implications in rectifying skin-related problems like wrinkling because of their antioxidative and antiphotoaging properties. In an earlier study, terpenoid-rich methanolic extracts from D. sissoo above ground parts have been shown to possess photoprotective properties.22 In this study, we attempted to extend our research to explore the anti-wrinkling property of the selected terpenoids, in silico.
Selected compounds have been explored for their binding affinity with MMP-1 CAT domain using molecular docking approach. AutoDock has been proven an effective tool in predicting bound orientation and binding energy of the small molecule ligands with macromolecular targets, in a quick and precise way. It implements a grid-based procedure that facilitates a prompt estimation of binding energy of test conformations where target protein or macromolecule is embedded in the grid.33 Molecular docking run revealed minimum binding energy (ΔG) of lupeol (−8.24 kcal/mol) to be lower than the reference compound doxycycline (−8.05 kcal/mol) followed by lupeol acetate (−7.12 kcal/mol), phytol (−7.06 kcal/mol), and betulin (−4.66 kcal/mol) toward the target protein. Lower ΔG indicates a good binding affinity of ligands with target protein. Calculated inhibition constant (Ki) was also lowest for lupeol (0.92 µM), which was again lower than doxycycline (1.26 µM), suggesting a strong binding property of lupeol toward the target protein. Based on Ki values, betulin (382.49 µM) turns out to be the weakest binder of MMP-1. Even though, lupeol acetate displayed ΔG (−7.12 kcal/mol) and Ki values (6.06 µM) equivalent to phytol (ΔG = −7.06 kcal/mol and Ki = 6.65 µM), it could not form H-bonds with AA residues in the CAT domain. This could be due to the absence of the hydroxyl group which was replaced by an ester moiety at the C-3 position, which reduced the binding affinity as evidenced in the case of lupeol acetate. In a recent study, it was shown that the replacement of OH group of lupeol at C-3 position by a ketone group diminished the inhibitory potential of the resulting compound lupenone toward β-site amyloid precursor protein cleaving enzyme 1 (BACE1).43 No H-bond formation was observed in the case of betulin also, despite containing 2 OH groups, 1 at C-3 position and the other at C-17 position. Conformation of the OH group at C-3 position, as well as the free movement of the OH group plays an important role in imparting interaction strength toward target entity.44 Hence, it can be concluded that the inhibition potential of the drug candidates depends on the ability of the ligand to form H-bonds with the target molecule. Furthermore, H-bonds increase the drug target affinities by optimizing the hydrophobic interactions and determining the protein conformation. H-bonds also play an important role in keeping the inhibitor in a steady-state inside the binding cavity of the target protein.45 In addition, hydrophobic contacts are important contributors along with hydrogen bonding in stabilizing energetically preferred ligands, in conformationally open protein structures.46 In this study, lupeol and phytol were found to interact with the chain A in the CAT domain of MMP-1 through H-bonds. Lupeol and the reference compound doxycycline shared 1 AA residue (Asn211 of chain A) involved in hydrogen bonding. Apart from this, lupeol formed an extra H-bond with Thr204 residue. All the hydrophobic contacts and hydrogen bonding between 3SHI and ligands (inhibitors) were analyzed using the program LigPlot+. Chimera was also used to find H-bonds at the interface of the protein-ligand complex and returned the same results, except in 2 cases where 1 H-bond was additional in 3SHI-doxycycline complex (Leu212 of chain A, Figure 5C), and Thr204 was replaced by His213 in forming the H-bond in the chain A, in the case of 3SHI-lupeol complex, as shown in LigPlot+ diagram (Figure 5A). Both the software returned the same results for phytol (H-bond between His132 residue of chain A and oxygen atom of OH group, Figure 5D), betulin (no H-bond, Figure 5B), and lupeol acetate (no H-bond, Figure 5E). It is expected that slight changes can arise concerning the orientation and the situation of ligands at the active site of the target protein when using different softwares.46 This can be due to the dynamic nature of intermolecular contacts at the protein-ligand contact edge that are weak in nature and be swapped for another type of bond, which is dependent on the chemical environment around the target-ligand connection.46 As far as hydrophobic interactions are concerned, all the ligands were found to interact with hydrophobic residues of the chain A in the CAT site of the MMP-1, except for betulin which formed hydrophobic contacts with the residues of chain M also, that included the residues Gln250, Asp254, and Ala258 and chain A residues involved in this interaction were Arg208, Phe207, Asn205, and Asn206. Lupeol exhibited 7 hydrophobic contacts with the chain A (Asp129, Ala133, Lys136, Thr204, Asn205, Asn211, and His132). Phytol showed 9 hydrophobic contacts with chain A, 5 residues in common with lupeol (Asp129, Ala133, Lys136, Thr204, Asn205, Asn211, His213, Asn206, and Gly244). Lupeol acetate also displayed 9 hydrophobic contacts with the chain A of 3SHI, which includes the same residues as in phytol except for Asn206 and Gly244, which were switched by His132 and Leu212. The reference drug doxycycline revealed 9 hydrophobic contacts with the same chain, 5 residues (underlined) shared with lupeol and phytol (Asp129, Ala133, Thr204, Asn205, Lys136, His132, Gly244, Val246, and Leu125). These results, taken together, indicate that lupeol is the strongest inhibitor of MMP-1 followed by doxycycline and phytol as they form H-bonds with the target protein. Moreover, based on oral bioavailability radars and Log Kp values of the ligands, doxycycline is the most orally bioavailable drug, and lupeol acetate is more skin permeable, and lupeol stands second in terms of skin permeability. But the overall results including molecular interactions indicate that lupeol can be recommended as an antiwrinkle agent. In addition, in vitro antiaging effect of lupeol has been reported recently using aged dermal fibroblast model that confirmed MMP-1 inhibitory activity of lupeol but no indication was stated for lupeol to be used as topical antiaging agent.47 Phytol has been used widely as cosmetic and fragrance ingredient.48 A recent study indicated that phytol prevented oxidative stress induced aging of HaCaT keratinocytes that suggest the incorporation of phytol in cosmetic formulations to inhibit cellular senescence.49 However, no MMP inhibitory activity of phytol is reported until date. Our findings support the in vitro demonstration of the lupeol and phytol as an anti-aging agent as well as provide insight into the interaction mechanism of the above said ligands and MMP-1. However, further refinement studies after molecular docking through molecular dynamic simulation are required to provide further insights into the flexibility and dynamics of the 3SHI-ligand complex and collagenolytic mechanism.
Conclusion
Molecular docking analysis of the selected terpenoids showed that lupeol and phytol were able to bind with the AA residues in the CAT domain of MMP-1, a major collagen-degrading MMP, with hydrogen bonding. Among the interacting compounds, lupeol displayed an increased binding affinity than reference drug, doxycycline. While, lupeol acetate and phytol exhibited almost equal significant binding energy. In silico pharmacokinetics and ADME analysis indicated these compounds to be potent inhibitory drugs for MMP-1 and potentially safe to be developed into active pharmaceutical drug against MMP-1 and among these tested compounds, lupeol is the most active in terms of docking score and hydrogen bonding. Furthermore, these findings support the MMP inhibitory activity of lupeol in vitro and provide an insight into the binding mode of lupeol to the MMP-1. Hence, these can be recommended to be incorporated in the topical formulations to prevent the wrinkling of skin caused due to the degradation of collagen by MMP-1. However, further studies are required to substantiate the MMP-1 inhibitory property of these compounds in vitro.
Acknowledgments
The authors acknowledge Guru Gobind Singh Indraprastha University, New Delhi, for providing the Indraprastha Research Fellowship (IPRF) and Short Term Research Fellowship (STRF) to S.Y. for the duration of 2011 to 2017. The authors thank Ratnika Sharma for her help in docking study and providing the docking software suite.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Faculty Research Grant Scheme (FRGS)/GGSIPU(2016-17) to PG.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: P.G. conceived the idea of the study. S.Y. and P.G. devised the design of the study. S.Y. carried out all the work, analyzed the results, and wrote the paper. Both the authors read and approved the final version of the manuscript.
ORCID iDs: Shagufta Yasmeen  https://orcid.org/0000-0002-9751-7289
https://orcid.org/0000-0002-9751-7289
Promila Gupta  https://orcid.org/0000-0001-6097-8850
https://orcid.org/0000-0001-6097-8850
References
- 1. Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16-29. [DOI] [PubMed] [Google Scholar]
- 2. Rittie L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5:a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jung H-Y, Shin J-C, Park S-M, Kim N-R, Kwak W, Choi B-H. Pinus densiflora extract protects human skin fibroblasts against UVB-induced photoaging by inhibiting the expression of MMPs and increasing type I procollagen expression. Toxicol Rep. 2014;1:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiang H-M, Chan S-Y, Chu Y, Wen K-C. Fisetin ameliorated photodamage by suppressing the mitogen-activated protein kinase/matrix metalloproteinase pathway and nuclear factor-κB pathways. J Agric Food Chem. 2015;63:4551-4560. [DOI] [PubMed] [Google Scholar]
- 5. Poulalhon N, Farge D, Roos N, et al. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent? J Biol Chem. 2006;281:33045-33052. [DOI] [PubMed] [Google Scholar]
- 6. Rittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002;1:705-720. [DOI] [PubMed] [Google Scholar]
- 7. Uitto J. The role of elastin and collagen in cutaneous aging: intrinsic aging versus photoexposure. J Drugs Dermatol. 2008;7:s12-s16. [PubMed] [Google Scholar]
- 8. de AndradeLeite SR. Inhibitors of human collagenase, MMP1. Eclética Química. 2009;34:87-102. [Google Scholar]
- 9. Voit-Ostricki L, Lovas S, Watts C. Conformation and domain movement analysis of human matrix metalloproteinase-2: role of associated Zn2+ and Ca2+ ions. Int J Mol Sci. 2019;20:E4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stams T, Spurlino JC, Smith DL, et al. Structure of human neutrophil collagenase reveals large S1′ specificity pocket. Nat Struct Biol. 1994;1:119-123. [DOI] [PubMed] [Google Scholar]
- 11. Esser CK, Bugianesi RL, Caldwell CG, et al. Inhibition of stromelysin-1 (MMP-3) by P1′-biphenylylethyl carboxyalkyl dipeptides. J Med Chem. 1997;40: 1026-1040. [DOI] [PubMed] [Google Scholar]
- 12. Wahl RC, Pulvino TA, Mathiowetz AM, et al. Hydroxamate inhibitors of human gelatinase B (92 kDa). Bioorg Med Chem Lett. 1995;5:349-352. [Google Scholar]
- 13. Welch AR, Holman CM, Huber M, Brenner MC, Browner MF, Van Wart HE. Understanding the P1′ specificity of the matrix metalloproteinases: effect of S1′ pocket mutations in matrilysin and stromelysin-1. Biochemistry. 1996;35: 10103-10109. [DOI] [PubMed] [Google Scholar]
- 14. Iyer S, Visse R, Nagase H, Acharya KR. Crystal structure of an active form of human MMP-1. J Mol Biol. 2006;362:78-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brennan M, Bhatti H, Nerusu KC, et al. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem Photobiol. 2003;78:43-48. [DOI] [PubMed] [Google Scholar]
- 16. Ferreira LG, Oliva G, Andricopulo AD. Target-based molecular modeling strategies for schistosomiasis drug discovery. Future Med Chem. 2015;7:753-764. [DOI] [PubMed] [Google Scholar]
- 17. Konc J, Lesnik S, Janezic D. Modeling enzyme-ligand binding in drug discovery. J Cheminform. 2015;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prada-Gracia D, Huerta-Yepez S, Moreno-Vargas LM. Application of computational methods for anticancer drug discovery, design, and optimization. Bol Med Hosp Infant Mex. 2016;73:411-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh W, Fields GB, Christov CZ, Karabencheva-Christova TG. Importance of the linker region in matrix metalloproteinase-1 domain interactions. RSC Adv. 2016;6:23223-23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singh W, Fields GB, Christov CZ, Karabencheva-Christova TG. Effects of mutations on structure-function relationships of matrix metalloproteinase-1. Int J Mol Sci. 2016;17:E1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Karabencheva-Christova TG, Christov CZ, Fields GB. Conformational dynamics of matrix metalloproteinase-1. Triple-helical peptide complexes. J Phys Chem B. 2018;122:5316-5326. [DOI] [PubMed] [Google Scholar]
- 22. Yasmeen S, Gupta P. In vitro demonstration of Dalbergia sissoo (Indian rosewood) methanolic extracts as potential agents for sunscreening and DNA nick prevention. Int J Pharm Pharmceut Sci. 2016;8:175-181. [Google Scholar]
- 23. Palasuk J, Windsor LJ, Platt JA, Lvov Y, Geraldeli S, Bottino MC. Doxycycline-loaded nanotube-modified adhesives inhibit MMP in a dose-dependent fashion. Clin Oral Investig. 2018;22:1243-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. https://pubchem.ncbi.nlm.nih.gov/compound/259846
- 25. https://pubchem.ncbi.nlm.nih.gov/compound/72326
- 26. https://pubchem.ncbi.nlm.nih.gov/compound/5280435
- 27. https://pubchem.ncbi.nlm.nih.gov/compound/92157
- 28. Jung J-J, Razavian M, Kim H-Y, et al. Matrix metalloproteinase inhibitor, doxycycline and progression of calcific aortic valve disease in hyperlipidemic mice. Sci Rep. 2016;6:32659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. https://pubchem.ncbi.nlm.nih.gov/compound/54671203
- 30. Gasteiger E, Hoogland C, Gattiker A, et al. Protein identification and analysis tools on the ExPASy server. In: Walker JM, ed. The Proteomics Protocols Handbook. Totowa, NJ: Humana Press; 2005:571-607. [Google Scholar]
- 31. Bertini I, Calderone V, Cerofolini L, et al. The catalytic domain of MMP-1 studied through tagged lanthanides. FEBS Lett. 2012;586:557-567. [DOI] [PubMed] [Google Scholar]
- 32. Vallon R, Muller R, Moosmayer D, Gerlach E, Angel P. The catalytic domain of activated collagenase I (MMP-1) is absolutely required for interaction with its specific inhibitor, tissue inhibitor of metalloproteinases-1 (TIMP-1). Eur J Biochem. 1997;244:81-88. [DOI] [PubMed] [Google Scholar]
- 33. Morris GM, Huey R, Lindstrom W, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009; 30:2785-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1:337-341. [DOI] [PubMed] [Google Scholar]
- 36. Abad-Zapatero C. Ligand efficiency indices for effective drug discovery. Expert Opin Drug Discov. 2007;2:469-488. [DOI] [PubMed] [Google Scholar]
- 37. Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105-132. [DOI] [PubMed] [Google Scholar]
- 38. Huang H-J, Chen W-Y, Wu J-H. Total protein extraction for metaproteomics analysis of methane producing biofilm: the effects of detergents. Int J Mol Sci. 2014;15:10169-10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778-2786. [DOI] [PubMed] [Google Scholar]
- 40. Veber DF, Johnson SR, Cheng H-Y, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem. 2002;45:2615-2623. [DOI] [PubMed] [Google Scholar]
- 41. Geinoz S. Assessment of Drug Permeation: Theory, Methods and Applications to Skin and Bacteria [PhD thesis]. Lausanne, Switzerland: Université de Lausanne; 2002. [Google Scholar]
- 42. Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17:E868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Koirala P, Seong SH, Jung HA, Choi JS. Comparative molecular docking studies of lupeol and lupenone isolated from Pueraria lobata that inhibits BACE1: probable remedies for Alzheimer’s disease. Asian Pac J Trop Med. 2017;10:1117-1122. [DOI] [PubMed] [Google Scholar]
- 44. Zhang C, Wang X, Cui J, et al. Synthetic analogues of betulinic acid as potent inhibitors of PS1/BACE1 interaction to reduce Aβ generation. Chinese J Chem. 2017;35:103-112. [Google Scholar]
- 45. Ntombela T, Fakhar Z, Ibeji CU, et al. Molecular insight on the non-covalent interactions between carbapenems and L,D-transpeptidase 2 from Mycobacterium tuberculosis: ONIOM study. J Comput Aided Mol Des. 2018;32:687-701. [DOI] [PubMed] [Google Scholar]
- 46. Patil R, Das S, Stanley A, Yadav L, Sudhakar A, Varma AK. Optimized hydrophobic interactions and hydrogen bonding at the target-ligand interface leads the pathways of drug-designing. PLoS ONE. 2010;5:e12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park YM, Park SN. Inhibitory effect of lupeol on MMPs expression using aged fibroblast through repeated UVA irradiation. Photochem Photobiol. 2019;95:587-594. [DOI] [PubMed] [Google Scholar]
- 48. McGinty D, Letizia CS, Api AM. Fragrance material review on phytol. Food Chem Toxicol. 2010;48:S59-S63. [DOI] [PubMed] [Google Scholar]
- 49. Jeong SH. Inhibitory effect of phytol on cellular senescence. Biomed Dermatology. 2018;2:13. [Google Scholar]