Abstract
Objective: To characterize the literature describing the therapeutic use of opioids in the elderly. Data Sources: Two electronic databases, EMBASE and MEDLINE, were searched from years 1990 to September 5, 2018. Relevant reference lists were reviewed. Searches were restricted to English language. Study Selection and Data Extraction: Two reviewers independently screened 827 citations to identify observational studies, population-based cohort studies, retrospective analyses, and control trials looking at the management of persistent pain in patients aged ≥65 years and/or frail patients. Data Synthesis: Thirty-nine articles were included in the systematic review. More specifically, 17 observational studies, 7 population-based cohort studies, 10 retrospective analyses, and 4 controlled trials. The most common etiology of persistent pain was musculoskeletal (50%), and the most often adverse effects reported were central nervous system related (41%) and falls/fractures (39%). Relevance to Patient Care and Clinical Practice: As there is a lack of strong evidence-based recommendations for opioid use in the elderly, this review aims to evaluate opioid use in the elderly and compare their efficacy and safety among this population. Conclusions: Overall, central nervous system adverse effects were most commonly seen in the elderly. However, higher quality evidence is required to further appreciate the dose-related effects on efficacy and safety of opioids in the elderly.
Keywords: opioid, elderly, older adult, persistent pain
Introduction
In 2017, the incidence of persistent pain in a European study conducted in Switzerland was 38.5%, and this number is projected to rise as the average age of the population increases.1,2 In 2009, the American Geriatric Society published now inactive guidelines for management of persistent pain in the elderly that suggested nonopioid pharmacotherapy with acetaminophen as first-line, especially for musculoskeletal pain due to its demonstrated effectiveness and favorable safety profile (evidence level of 1-A).3 The authors also suggest that nonselective nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective inhibitors be considered rarely and with extreme caution as the risks of therapy often outweigh the benefit in the elderly population (evidence level of 1-A), which limits nonopioid analgesic options.3-5 In patients with moderate to severe pain with accompanying functional impairment or diminished quality of life and who have had little or no benefit with nonopioid therapy, guidelines suggest initiating treatment with low doses of opioids, with careful upward titration, while continually monitoring for adverse effects (evidence level of 3a; expert opinion).3 This recommendation provides no specific guidance with regard to specific agent, initial dosing, or key monitoring parameters and reflects a low level of evidence.
When considering analgesia in the elderly, parameters such as expected benefits based on etiology, age-related physiological changes, comorbidities, and potential adverse effects should all be assessed.2,3,6-10 Pharmacokinetic parameters of opioids can vary greatly in the aging population.2,3,6-10 The rate at which certain drugs are absorbed is altered due to a decrease in gastrointestinal transit time and an increase in gastric pH. The distribution of lipophilic drugs is enhanced due to an increase in adipose tissue. Metabolism slows down due to reduced hepatic blood flow and impaired phase I reactions (ie, oxidation, hydroxylation, and dealkylation). Elimination can be altered due to age-related reductions in renal blood flow and glomerular filtration rate.7 Common comorbidities in older adults such as frailty, visual impairment, hearing impairment, cardiovascular disease (ie, hypertension, coronary artery disease, and congestive heart failure), musculoskeletal conditions (ie, arthritis), dementia, stroke, and diabetes mellitus may either increase the risk of side effects (ie, confusion, dizziness, sedation, falls, constipation) from opioid use or attenuate the benefits.3,6
Although opioids are recommended for management of moderate to severe pain in guidelines (evidence level 3-A), there is still a lack of strong evidence-based recommendations for their use in older adults. The objective of this review is to determine which opioids have been studied in older adults and describe their efficacy and safety, if possible.
Methods
Protocol and Registration
A prespecified protocol for our systematic review was created and was registered with PROSPERO, an international database of prospectively registered systematic reviews in health and social care (Registration Number: CRD42018084201).
Eligibility Criteria
We included observational studies, crossover studies, population-based cohort studies, retrospective analyses, case-control studies, nested case-control studies, and randomized control trials in our systematic review. The search was limited to publications from 1970 to September 5, 2018, that were available in English. Studies that included patients ≥65 years old or frail patients who were receiving opioids for the management of persistent pain (defined as pain that continues for greater than 3 months) were eligible for inclusion. Opioids were defined as any of the following: codeine combination products, oxycodone, hydrocodone, hydromorphone, oxymorphone, morphine, methadone, buprenorphine, fentanyl, sufentanil, and tapentadol. Narrative reviews; editorials; studies analyzing acute pain, postoperative pain, and palliative pain; and animal studies were excluded.
Information Sources and Search
A search strategy was developed with assistance from the designated Pharmaceutical Sciences research librarian located at the University of British Columbia. The databases MEDLINE and EMBASE were searched using the following terms: (narcotic analgesic agent/ OR opiate* or opioid or fentanyl or buprenorphine or sufentanil or hydromorphone or morphine or oxycodone or methadone or codeine or hydrocodone or oxymorphone or tapentadol) AND (elder* or senior* or geriatric* or older adult* or frail*) AND (chronic pain/ OR persistent pain or chronic pain) for EMBASE; and (exp Analgesics, Opioid/ OR opiate* or opioid or fentanyl or buprenorphine or hydromorphone or morphine or oxycodone or methadone or codeine) AND (elder* or senior* or geriatric or older adult* or frail*) AND (Chronic Pain/ OR persistent pain or chronic pain) for MEDLINE. Limits applied to both databases included English language and year = “1970–Current (September 5, 2018).” Gray literature was searched using Google Scholar.
Study Selection and Search
Two reviewers (both MJ and HH) independently screened articles. If disagreements occurred, a third reviewer (either KD or GE) resolved any disagreements.
Data Collection Process
The data were extracted by one reviewer (MJ) from all included articles using a prespecified data collection form contained within Excel for Mac 2011, version 14.7.7.
Data Items
Extracted data included information regarding study design, etiology of persistent pain, type of opioid used, dose of opioid, pharmacokinetic parameters assessed, comorbidities, assessment of frailty, adverse effects, drug interactions, hospitalization secondary to opioid toxicity, and overall recommendation for use of opioids. For comorbidities, we were primarily interested in dementia, Parkinson’s disease, history of prior stroke, cardiovascular disease, chronic renal impairment, cirrhosis, and malignancy. For adverse effects, we were interested primarily in respiratory depression, falls, dizziness, confusion, and constipation. The comorbidities and adverse effects were chosen as they were felt to be most relevant to an elderly population and most likely to lead to potential adverse effects.
Risk of Bias in Individual Studies
To assess for bias in individual studies, the Cochrane Risk of Bias tool5 was applied to randomized studies and the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I,11 Table 2) was applied to nonrandomized studies.
Table 2.
Summary of Controlled Trials Included in the Analysis of Opiate Prescribing in the Elderly.
| Author | Design | N | Mean Age | Intervention | Control | Population Characteristics (Incidence %) | Efficacy Outcomes | Adverse Effects (Incidence %) | |
|---|---|---|---|---|---|---|---|---|---|
| Breivik et al47 | PC, DB, RCT | 100 | 62.9 | BTDS 5-20 µg/h | Placebo | Etiology of pain: osteoarthritis (100) | WOMAC; Osteoarthritis | NSS | Dizziness (25) |
| Constipation (24), nausea (37), vomiting (16) | |||||||||
| Pruritus (61) | |||||||||
| Likar et al48 | OL | 30 | 74.3 | BTDS at doses 35, 40, and 50 µg/h | No control | • Etiology of pain: MSK causes (63), neuropathy
(13), cancer (6.5) • Comorbidities: cardiovascular disease (80) |
VAS | NSS | Dizziness (53.3), malaise (30) |
| NRS | NSS | Nausea (40), constipation (30), vomiting (16.7) | |||||||
| Pruritus (20) | |||||||||
| Rauck et al49 | DB, RCT | 156 | 72 | Codeine 30-60 mg/acetaminophen 300-600 mg q 4-6 h prn | Tramadol 50-100 mg po q 4-6 h prn (max: 4000 mg/24 h | Etiology of pain: arthritis (72), back/neck pain (14), neuropathy (7) | Pain intensity score | NSS | Dizziness (4.5) |
| Constipation (9.6), nausea (4.5) | |||||||||
| Kjaersgaard-Andersen et al50 | DB, RCT | 158 | 66 | Codeine 60 mg/paracetamol 1000 mg | Paracetamol 1000 mg po TID | Etiology of pain: arthritis (100) | Pain intensity score | P < .01 for codeine/paracetamol group | Dizziness (3), somnolence (20.3) |
| Constipation (36.1), nausea (32.3), vomiting (14.6) | |||||||||
Abbreviations: PC, placebo controlled; DB, double blind; RCT, randomized control trial; BTDS, buprenorphine transdermal system; WOMAC, Western Ontario and McMaster Universities Arthritis Index; NSS, normal saline solution; OL, open label; MSK, musculoskeletal; VAS, Visual Analog Scale; NRS, Numerical Rating Scale; q 4-6 h, every 4 to 6 hours; prn, as needed; po, by mouth; max, maximum; TID, thrice a day.
Synthesis of Results
Descriptive analysis of the identified studies was completed using information collected from the prespecified outcomes.
Results
Study Selection
Of the 827 non-duplicate articles screened, there were 39 articles that met inclusion criteria (Figure 1).12-50 There were 719 articles excluded after the initial screening of titles and abstracts with an additional 57 articles excluded after full-text review. In total, 39 articles met the eligibility criteria and were included in the systematic review (Figure 1). No articles were found in the gray literature. Overall, 17 observational studies,12-29 7 population-based cohort studies,30-36 10 retrospective analyses,37-46 and 4 controlled trials were included.47-50 The study population sizes ranged from 10 to 800 patients.12-50 Studies were published from 1970 to 2017, with 29 studies published recently from 2010 to 2017. The published journal impact factor ranged from 1.69 to 8.955.12-50
Figure 1.
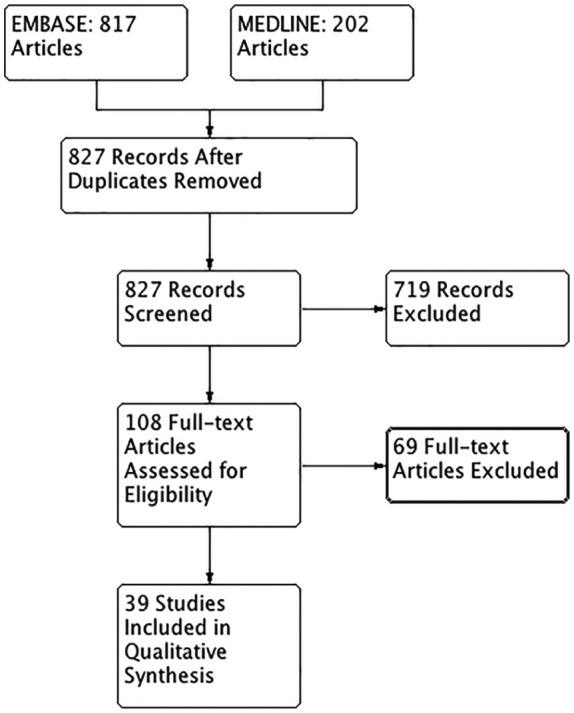
PRISMA flow diagram for the studies included in the analysis of opiate prescribing in the elderly.
Study Characteristics
The range of mean ages across individual studies was 60.5 to 83 years, and 66.6% of participants in the included studies were female.12-50
Summary of Included Studies
Musculoskeletal pain (50%; arthritis, back/hip/knee pain) was the primary etiology of persistent pain in 29 studies.12,16,18,20-23,25-31,33-36,38,39,42,46-50 Other causes of persistent pain identified included peripheral/diabetic neuropathy (24%),12,16,22,31,32,35,36,41,48,49 cancer pain (16%),25,35,48 osteoporosis (11%),21,25,30,33 headache (8%),30,41,46 fibromyalgia (8%),32,36,41 radiculopathy (5%),31,32 postherpetic neuralgia (5%),17,31 gout (3%),41 rheumatoid arthritis (3%),38 ischemic heart disease (3%),38 multiple sclerosis (3%),26 and abdominal pain (3%).46
The most common comorbidities identified across all studies included cardiovascular disease (26%)19,23,25,28,30,33,37,39,43,48 and dementia/cognitive impairment (21%).13,14,18,33,34,37,39,46 Other comorbidities identified included previous stroke (13%),19,25,32,33,37 depression (13%),23,25,26,27,33 renal impairment (8%),28,37,43 Parkinson’s disease (8%),32,33,39 malignancy (8%),19,32,37 frailty (5%),42,45 concomitant substance use (5%),20,33 cirrhosis (3%),37 and asthma/chronic obstructive pulmonary disease (3%).33 Congestive heart failure was considered the primary cause of cardiovascular disease in approximately half of the studies that reported on this outcome.28,33,37,39 In one of the studies, frailty was defined according to the frailty criteria used in the Cardiovascular Health Study: shrinking/sarcopenia, weakness, poor endurance and energy, slowness, and low physical activity level.45 Participants were considered frail if they met 3 or more of the 5 frailty criteria.45 In the other study, participants were categorized as robust, intermediate, or frail using the Study of Osteoporotic Fractures Frailty Index.42
Overall, 28 of the included studies reported on type of opioid assessed.13-19,21-23,25,28-38,44,46-50 The 12 remaining studies were primarily population-based cohort studies and were designed to describe the characteristics of elderly patients receiving opioids for management of persistent pain.12,20,24,26,27,39-43,45 Of the studies that did describe type of opioid used, 13 assessed morphine,13,17,19,25,28,32-34,36-38,44,46 12 assessed oxycodone,13,15,18,25,28,31,32,34,37,38,44,46 11 assessed codeine,13,25,28,33,34,35,38,44,46,49,50 9 assessed fentanyl,13,14,16,25,28,33,34,37,44 9 assessed buprenorphine,22,23,29,30,33,35,44,47,48 6 assessed hydromorphone,13,21,25,34,38,44 5 assessed methadone,13,25,37,38,44 and 1 assessed sufentanil.44 Of the 28 studies that reported on type of opioid used, 16 of these reported on dosing. Dosing regimens of morphine varied across the studies, and included a range of 9 to 120 mg immediate release by mouth over a 24-hour period or 15 mg slow release by mouth once daily.17,19,32,36,37 The study looking at slow release morphine also looked at other long-acting oral formulations of opioids, such as oxycodone 10 mg controlled release by mouth twice daily and compared this with transdermal fentanyl at a dose of 25 µg/h.37 An open-label study evaluating the efficacy and safety of buprenorphine sublingual at a dose of 0.1 mg 3 to 4 times per day over a 14-day period was included in our review.29 In this study, sublingual buprenorphine at this dose was effective in 6 of the 26 patients who completed the study and associated with a higher incidence of nausea, vomiting, and confusion.29 Of the 4 control trials included, 2 looked at buprenorphine transdermal system (BTDS) at doses of 5 to 20 µg/h, and 35, 40, and 50 µg/h, and 2 looked at codeine 30 to 60 mg/acetaminophen 300 mg to 600 mg.47-50 The 2 control trials evaluating BTDS found this agent to be ineffective in reducing arthritic pain and was associated with an increased risk of both dizziness and pruritis.47,48
The most common adverse effects reported in the studies were central nervous system (CNS) related (dizziness, confusion, mental status changes, lethargy, depression, headache, somnolence) and falls/fractures, with the proportion of studies that reported this adverse effect being 41% and 39%, respectively.12,15,18,19,21-23,28-30,34,36,39,40,47-50 In a population-based cohort study assessing the association between opioid dose and fractures, the authors concluded that higher doses of opioids (equivalent to 50 mg equivalent of codeine daily) was associated with a 2-fold increase in risk of fractures.45 Gastrointestinal-related (constipation, N/V) adverse effects contributed to ~15% of adverse effects identified in these studies.12,18,21-23,28-32,34,36,39,47-50
Three of the included studies reported on hospitalizations. In a retrospective analysis conducted by Reid et al in 2010 to describe the characteristics of older adults receiving opioids for chronic noncancer pain, 7 (5%) patients were hospitalized, 5 were hospitalized for altered mental status, 1 for obstipation, and 1 for unintentional overdose.34 In a population-based cohort study looking at the association between fractures and opioid use (codeine in this study), 117 (36.6%) patients were hospitalized for a fracture and 6 (1.9%) died within 2 months of hospitalization for fracture.46 The last study that reported on hospitalizations was a retrospective analysis evaluating the safety of BTDS. In this study, 103 (6%) patients were reported being hospitalized for cardiac failure, chest pain, fall, and transient ischemic attack.30
Summary of Control Trials (Table 1)
Table 1.
Summary of Nonrandomized Studies Included in the Analysis of Opiate Prescribing in the Elderly.
| Author | Study Design | N | Mean Age | Opiate Studied | Population Characteristics (Incidence %) | Adverse Effects (Incidence %) |
|---|---|---|---|---|---|---|
| Kennedy et al12 | Observational | 28 | Not reported | Not reported | • Etiology of pain: back/neck pain (47.1), neuropathy
(23.5), rheumatic disease (23.5) • Comorbidities not reported |
GI upset, sedation; incidence not reported |
| Fain et al13 | Observational | 15 432 | Not reported | • Codeine/acetaminophen • Oxycodone • Hydromorphone • Morphine • Methadone • Fentanyl |
• Etiology of pain not reported • Comorbidities: cognitive impairment—borderline intact (20.4), mild (22.2), moderate (25.8), moderately severe (3.7) |
Not reported |
| Fain et al14 | Observational | 17 052 | Not reported | Fentanyl | • Etiology of pain not reported • Comorbidities: cognitive impairment—mild (19.7), moderate (37.1), moderately severe (10.9) |
Not reported |
| Guerriero, 201615 | Observational | 60 | 81 | Oxycodone/naloxone: • Initial dose: 5/2.5 mg po BID • Mean daily dose of oxycodone at 4 weeks = 14.4 mg ± 4.9 mg • Mean daily dose of oxycodone at 52 weeks = 17.4 mg ± 7.7 mg |
Etiology of pain and comorbidities not reported | • Dizziness (46) |
| Lee et al16 | Observational | 451 | 60.52 | Fentanyl: • Mean dose: 15.5 ± 7.72 |
• Etiology of pain: low back pain (27.7), spinal stenosis
(21.7), arthritis (12.64) • Comorbidities not reported |
Not reported |
| Turner et al17 | Observational | 1311 | 64.5 | Specific opiate not reported. Provided morphine daily
equivalents and categorized into frequency of
use: • Minimal/no use: <5 mg • Intermittent/lower dose use: 5-15 mg • Regular/high-dose use: >15 mg |
Etiology of pain and comorbidities not reported | Not reported |
| Petro et al18 | Observational | 53 | 83 | Oxycodone/naloxone: • Initial dose: 5/2.5 mg po BID • Max: 20/10 mg po BID • Mean daily dose of oxycodone: 10.8 ± 4.9 mg |
Etiology of pain: arthritis (47.2), previous fracture (35.8), arthroplasty (11.3), dementia (mean MMSE score = 18.6 ± 3.0) | Severe constipation (9.4), drowsiness (9.4), nausea (5.7), xerostomia (3.8) |
| Dublin et al19 | Observational | 3434 | Not reported | Provided cumulative morphine in past 10
years: • 0-300 mg = 54% • 330-900 mg = 24.2% • 930-8100 mg = 13.8% • >2730 mg = 79.5% |
• Etiology of pain not reported • Comorbidities: cardiovascular disease (66), malignancy (11), depression (10), previous stroke (6) |
Dementia (23) |
| Enthoven et al20 | Observational | 484 | 66 | Not reported | • Etiology of pain: back pain (100) • Comorbidities: alcohol use disorder (50), nicotine use disorder (18) |
Not reported |
| Ringe et al21 | Observational | 630 | 68 | Hydromorphone: • 4 mg/day = 22.3% • 8 mg/day = 40.2% • 16 mg/day = 7.9% |
• Etiology of pain: osteoarthritis, osteoporosis; incidence
not reported • Comorbidities not reported |
Nausea (19.2), constipation (15.7), dizziness (10.8), fatigue (9.2) |
| Uberall et al22 | Observational | 891 | 72.8 | BTDS: • 5 µg/h = 67.1% • 10 µg/h = 27.3% • 20 µg/h = 5.5% |
• Etiology of pain: back pain (40.5), neuropathy (37.3),
arthritis (12.1) • Comorbidities not reported |
Sleep disturbance (18.2), constipation (8.9), dizziness (1.9) |
| Gianni et al23 | Observational | 93 | 79.7 | BTDS: • 11.7 µg/h = 3.5% • 17.5 µg/h = 11.6% • 35 µg/h = 74.4% • 52.5 µg/h = 9.3% • 70 µg/h = 1.2% |
• Etiology of pain: back pain; incidence not
reported • Comorbidities: depression (26.9), cardiovascular disease (incidence not reported) |
Constipation/nausea (15.7), sleepiness (14.2), pruritus (11.2) |
| Gianni et al24 | Observational | 367 | 78 | Not reported | Etiology of pain and comorbidities not reported | Not reported |
| Park and Lavin25 | Observational | 163 | 72.8 | • Acetaminophen/codeine • Oxycodone • Hydromorphone • Morphine • Methadone • Fentanyl |
• Etiology of pain: arthritis (85.3), back/neck pain (75.5),
cancer pain (23.3), osteoporosis
(19.6) • Comorbidities: type II diabetes (36.2), cardiovascular disease (33.7), depression (22.6), previous stroke (8) |
Not reported |
| Unützer et al26 | Observational | 13 | 72.2 | Not reported | • Etiology of pain: arthritis (100) • Comorbidities: depression (100) |
Not reported |
| Unützer et al27 | Observational | 1801 | 71.6 | Not reported | • Etiology of pain: arthritis (55.6) • Comorbidities: depression (100) |
Not reported |
| Won et al28 | Observational | 4426 | Not reported | • Codeine/acetaminophen • Oxycodone • Morphine • Fentanyl |
• Etiology of pain: arthritis (66.3) • Comorbidities: cardiovascular disease (17.8), chronic renal impairment (2.2) |
Depression (25.4), constipation (10.1), falls (7.6) |
| Nassar et al29 | Observational study | 51 | Not reported | • Sublingual buprenorphine | • Etiology of pain: osteoarthritis (62.7), other
(37.4) • Comorbidities: not reported |
Nausea (15.7), vomiting (9.8), confusion (9.8), dizziness (5.9), drowsiness (3.9), depression (2.0), headache (2.0), hallucinations (2.0), diarrhea (2.0), constipation (2.0), sweating (2.0) |
| Pergolizzi et al30 | Retrospective analysis | 1715 | 72.3 | BTDS | • Etiology of pain: arthritis (61), osteoporosis (16.4),
back pain (14.1) • Comorbidities: cardiovascular disease; hypertension (62.9), dyslipidemia (21) |
Nausea (24), dizziness (16.2), constipation (14.5), somnolence (14.1), headache (12.5), falls (3) |
| Lazzari et al31 | Retrospective Analysis | 186 | 80.7 | Oxycodone/naloxone | • Etiology of pain: radiculopathy (37.1), arthritis (18.3), neuropathy (10.8), postherpetic neuralgia (10.2) | Constipation (35.9) |
| Lee et al32 | Retrospective Analysis | 10 | 75.5 | Morphine: • Initial dose: 1-3 mg TID • Maintenance dose: 5-30 mg/day |
• Etiology of pain: back pain (60), neuropathy (60),
radiculopathy (60), arthritis (20) • Comorbidities: Parkinson’s disease (10), previous stroke (10), malignancy (10) |
Constipation (70) |
| Veal et al33 | Retrospective analysis | 19 581 | 77 | • Codeine/acetaminophen • Oxycodone • Morphine • BTDS • Fentanyl |
• Etiology of pain: musculoskeletal pain
(11.7) • Comorbidities: depression (8.3), cognitive impairment (4.1), cardiovascular disease (2.6), Parkinson’s disease (1.0), previous stroke (1.0) |
Not reported |
| Reid et al34 | Retrospective analysis | 133 | 82 | • Codeine/acetaminophen • Oxycodone • Hydromorphone • Morphine • Fentanyl |
• Etiology of pain: back pain (36), arthritis
(35) • Comorbidities: dementia; incidence not reported |
Constipation (22), mental status changes (16), nausea (10) |
| Gallagher et l35 | Retrospective analysis | 47 282 | Not reported | • Codeine/acetaminophen • Buprenorphine |
• Etiology of pain: arthritis (48.7), back pain (35.3),
cancer pain (16.6) • Comorbidities not reported |
Not reported |
| Raffaeli et al36 | Retrospective Analysis | 32 | 72.3 | Morphine (given intrathecally): • Mean initial dose: 0.41 ± 0.28 mg/day • Mean dose at 48 months: 1.03 ± 0.61 mg/day |
• Etiology of pain: neuropathy (65.6), arthritis
(34.3) • Comorbidities not reported |
Drowsiness (21.9), nausea (21.9), urinary retention (18.8), dizziness (12.5), vomiting (12.5), pruritus (12.5) |
| Rigler et al37 | Retrospective analysis | 766 | Not reported | • Oxycodone CR: 10 mg po BID • Morphine SR: 15 mg po BID • Methadone—no dose provided • Fentanyl 25 µg/h |
• Etiology of pain not reported • Comorbidities: cardiovascular disease (28.5), previous stroke (16.4), dementia (11.6), malignancy (10.4), renal impairment (5.0), cirrhosis (1.4) |
Not reported |
| Solomon et al38 | Retrospective analysis | 1861 | Not reported | • Codeine/acetaminophen • Oxycodone • Hydromorphone • Morphine • Methadone |
• Etiology of pain: rheumatoid arthritis (38.6),
osteoarthritis (33.7), back pain
(27.6) • Comorbidities not reported |
Not reported |
| Won et al39 | Retrospective analysis | 813 | 82.5 | Not reported | • Etiology of pain: musculoskeletal
(87.6) • Comorbidities: dementia (18.5), Parkinson’s disease (7.6) |
Falls/fractures (12.3), sleep disturbance (7.7), constipation (7.6), depression (3.9) |
| Kung et al40 | Retrospective analysis | 193 | 74 | Not reported | Etiology of pain and comorbidities not reported | Depression (9.8) |
| Dobscha et al41 | Population-based cohort | 12 924 | Not reported | Not reported | • Etiology of pain: arthritis, back pain, neuropathy, gout,
headache/migraine, fibromyalgia; incidence not
reported • Comorbidities not reported |
Not reported |
| Krebs et al42 | Population-based cohort | 129 | 74.7 | Not reported | • Etiology of pain: knee pain (65.1), arthritis (61.2), hip
pain (59.7) • Comorbidities: frailty (34.9) |
Not reported |
| Kuo et al43 | Population-based cohort | 800 664 | Not reported | Not reported | • Etiology of pain not reported • Comorbidities: cardiovascular disease—HTN (37.9), renal impairment (4.3) |
Not reported |
| Prunuske et al44 | Population-based cohort | 9 325 603 | Not reported | • Codeine/acetaminophen • Oxycodone • Hydromorphone • Morphine • Methadone • Buprenorphine • Fentanyl • Sufentanil |
Not reported | Not reported |
| Koponen et al45 | Population-based cohort | 605 | 81.9 | Not reported | • Etiology of pain: musculoskeletal pain
(52.9) • Comorbidities: frailty (11.4) |
Not reported |
| Saunders, 201046 | Population-based cohort | 2341 | 72.9 | • Codeine/acetaminophen • Oxycodone • Morphine |
• Etiology of pain: back pain (41.6), pain in extremities
(33.6), arthritis (24.9) • Comorbidities: cognitive impairment—borderline intact (20.4), mild (22.2), moderate (25.8), moderately severe (3.6) |
Fracture (13.7) |
Abbreviations: GI, gastrointestinal; po, by mouth; BID, twice a day; MMSE, Mini-Mental State Exam; BTDS, buprenorphine transdermal patch; TID, thrice a day; CR, controlled release; SR, slow release; HTN, hypertension.
Four controlled trials were included in our review. The 2 most recent studies (2008 and 2010) evaluated BTDS and the other 2 (1990 and 1994) evaluated codeine/acetaminophen.47-50 All of these studies evaluated the respective agents in the management of musculoskeletal pain, with the primary etiology of pain consisting of arthritis.47-50 For efficacy outcomes, each study used standardized scales to measure pain intensity, similar to what is used in regular practice at most sites.47-50 As presented in Table 1, BTDS was not more effective in reduction of pain compared with placebo.47,48 Similarly, codeine-acetaminophen was not more effective than acetaminophen alone but was slightly more effective when compared with tramadol.49,50 As expected, there was an increased incidence of adverse effects with both BTDS and codeine/acetaminophen.47-50
Risk of Bias Across Studies
Of the 4 controlled trials included, only one was considered to be a low risk of bias as per the Cochrane Risk of Bias Assessment Tool10,47 (Table 2). Two of the studies had an unknown risk of bias,49,50 and one was an open-label trial and subsequently had a high risk of bias.48 None of the studies provided much information regarding loss to follow-up and how this was accounted for contributing to the high/unclear risk of bias with regard to outcome data seen in these studies. Last, all of the studies reported on prespecified primary and secondary outcomes, resulting in a low risk of selective reporting of outcome data.
For the nonrandomized studies included, the ROBINS-I tool was applied for assessment of bias.11-46 The majority of these studies had a serious risk of bias (Tables 3 and 4).
Table 3.
Assessment of Risk of Bias of Controlled Trials Using the Cochrane Risk of Bias Tool.5
| Study | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Incomplete Outcome Data | Selective Outcome Data |
|---|---|---|---|---|---|
| Breivik et al47 | Low | Low | Low | Low | Low |
| Likar et al48 | High | High | High | Unknown | Low |
| Rauck et al49 | Unknown | Unknown | Low | Unknown | Low |
| Kjaersgaard-Andersen et al50 | Unknown | Unknown | Low | Low | Low |
Table 4.
Assessment of Risk of Bias in Nonrandomized Studies Using the ROBINS-I Tool.11
| Study | Bias Due to Confounding | Bias in Selection of Participants Into the Study | Bias in Classification of Interventions | Bias Due to Deviations From Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result |
|---|---|---|---|---|---|---|---|
| Kennedy et al12 | Critical | Low | Moderate | Low | Low | Serious | Moderate |
| Pergolizzi et al30 | Moderate | Low | Low | Low | Low | Low | Low |
| Dobscha et al41 | Low | Low | Low | Low | Low | Serious | Low |
| Fain et al13 | No information | Low | Moderate | Low | No information | Low | Low |
| Fain et al14 | Moderate | Low | Low | Low | Low | Serious | Low |
| Guerriero et al15 | Moderate | Low | Low | Low | Low | Serious | Low |
| Krebs et al42 | Serious | Serious | Low | Low | Low | Moderate | Low |
| Kuo et al43 | No information | Low | Low | Moderate | Low | Moderate | Moderate |
| Lazzari et al31 | Serious | Low | Serious | Low | Low | Serious | Serious |
| Lee et al16 | No information | Low | Serious | Low | Serious | Serious | Low |
| Turner et al17 | Serious | Low | Low | Low | Low | Moderate | Low |
| Petro et al18 | Serious | Low | Moderate | Low | Low | Serious | Low |
| Dublin et al19 | Serious | Low | Low | Low | Low | Low | Moderate |
| Lee et al32 | Critical | Low | Low | Low | Low | Low | No information |
| Veal et al33 | Critical | Low | Serious | No information | Low | Low | No information |
| Enthoven et al20 | Serious | Low | Low | Low | Low | Serious | Moderate |
| Prunuske et al44 | No information | Moderate | Low | Low | Low | Low | Low |
| Koponen et al45 | Critical | Low | Serious | No information | Moderate | Serious | Moderate |
| Ringe et al21 | No information | Low | Moderate | No information | Low | Low | Moderate |
| Uberall et al22 | No information | Low | Moderate | Low | Low | Serious | Moderate |
| Gianni et al23 | Serious | Low | Serious | Low | No information | Serious | Moderate |
| Gianni et al24 | No information | Low | Low | No information | Serious | Serious | Serious |
| Park and Lavin25 | Low | Moderate | Low | Moderate | No information | Low | Moderate |
| Reid et al34 | Low | Low | Low | Low | No information | Serious | Low |
| Saunders et al46 | Serious | Low | Low | Low | Low | Serious | Low |
| Gallagher et al7 | Moderate | Moderate | Low | Low | Low | Serious | Low |
| Raffaeli et al36 | Serious | Low | Serious | Moderate | Low | Serious | Serious |
| Unützer et al26 | Serious | Serious | Low | Low | Low | Serious | Low |
| Rigler et al37 | Critical | Serious | Serious | Moderate | Low | Serious | Moderate |
| Soloman et al38 | Low | Low | Low | Moderate | Moderate | Low | Low |
| Won et al39 | Serious | Low | Serious | Low | Low | Serious | Moderate |
| Unützer et al27 | Critical | Low | Low | Low | Low | Serious | Low |
| Won et al28 | Serious | Low | Low | Moderate | Moderate | Low | Moderate |
| Kung et al40 | Low | Low | Low | Low | Low | Serious | Moderate |
| Nassar et aal29 | Moderate | Moderate | Low | Moderate | Low | Serious | Serious |
Abbreviation: ROBINS-I, Risk of Bias in Nonrandomized Studies of Interventions.
Discussion
To our knowledge, this is the first systematic review of studies on opioids use in an elderly population. The majority of evidence we have for opioid use in managing persistent pain in the elderly is for musculoskeletal pain, which is reflected within the current guidelines. A high risk of bias was present in the majority of nonrandomized trials and in all but one of the controlled trials, which precludes drawing any concrete results on efficacy and safety from the observational studies.
Of the included studies, 29 were published in 2010 and onward, with 20 of these published within the past 5 years (2013-2018). Thus, it is clear that this is a topic of interest for many experts, and there will be likely more studies evaluating opioid use in the elderly within the next decade. Given the findings of our systematic review, well-designed clinical trials in the elderly are required in order to inform safe prescribing practices.
Overall, the most common opioids studied are morphine, oxycodone (combination products), and codeine (combination products). The combination of oxycodone/naloxone was found to be both effective and safe for the management of moderate to severe persistent pain in opioid-naïve patients in 2 observational studies and a retrospective analysis included in our review.13,15,18
The most commonly seen adverse effects across the studies were CNS related, specifically dizziness (6.7% to 53.3%), mental status changes (16%), lethargy (9%), depression (9.8% to 25.4%), headache (6.7% to 12.5%), somnolence (2.7% to 20.3%), falls (7.6% to 13.7%), constipation (9.6% to 70%), nausea (0.3% to 40%), and vomiting (1.3% to 16.7%).12,15,18,19,21-23,28-30,34,36,39,40,47-50 Overall, the incidence of respiratory depression was quite low; only one individual was identified as having respiratory depression across the studies.36 In a study that evaluated falls in older patients with persistent pain, the authors were unable to find an association between opioids and falls and concluded that bigger studies need to be done to evaluate this effect.42 Given the elderly are already at a higher risk of both falls and fractures, it would be prudent to only use opioids once non-opioid options have been exhausted and then use the lowest effective dose with close monitoring for CNS side effects and risk of falls.
There are several limitations to our review. First, as the American Geriatric Society still defines aged persons as ≥65 years, we chose this as our parameter. However, as the population continues to age, the results of these studies may no longer reflect our elderly patient population. We were limited to the data that were reported in the studies, and there were no studies that assessed pharmacokinetic parameters or drug interactions, both of which were prespecified outcomes of interest. Only 2 studies included patients with frailty,42,45 so we were unable to evaluate the impact of opioid therapy in this subset of an elderly population. Furthermore, the majority of studies did not evaluate dosing of opioids, making it difficult to provide specific dosing recommendations for the elderly population. There was also a large amount of heterogeneity among the studies included limiting the external generalizability of these results. Last, not all of the included studies reported on efficacy outcomes or safety.
Relevance to Patient Care and Clinical Practice
Central nervous system–related adverse effects and falls remain the most concerning adverse effects and should be monitored closely in susceptible patients. Moving forward, it is imperative for clinicians to continue to consider patient-specific parameters and goals of care when prescribing and dosing opioids in this population and strive to use the lowest effective dose possible.
Conclusions
Ultimately, the low quality of evidence and clinical heterogeneity limits the ability to draw broad conclusions on optimal opioid use in the elderly. As discussed, there is a high prevalence of persistent pain in the elderly population, and as the average age of the population continues to rise, the prevalence of pain will continue to increase. Thus, there is a strong need to continue to further explore and understand opioid use in the elderly population.
Acknowledgments
Authors acknowledge role of Hans Haag in reviewing articles.
Footnotes
Authors’ Note: This work was presented as a poster at the British Columbia Residency Research Night on May 17, 2018 (Vancouver, British Columbia, Canada).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Karen Dahri  https://orcid.org/0000-0001-5653-6056
https://orcid.org/0000-0001-5653-6056
References
- 1. Larsson C, Hansson EE, Sundquist K, Jakobsson U. Chronic pain in older adults: prevalence, incidence, and risk factors. Scand J Rheumatol. 2017;46:317-325. doi: 10.1080/03009742.2016.1218543 [DOI] [PubMed] [Google Scholar]
- 2. Kaye AD, Baluch A, Scott JT. Pain management in the elderly population: a review. Ochsner J. 2010;10:179-187. [PMC free article] [PubMed] [Google Scholar]
- 3. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331-1346. doi: 10.1111/j.1532-5415.2009.02376.x [DOI] [PubMed] [Google Scholar]
- 4. Davis MP, Srivastava M. Demographics, assessment and management of pain in the elderly. Drugs Aging. 2003;20:23-57. doi: 10.2165/00002512-200320010-00003 [DOI] [PubMed] [Google Scholar]
- 5. Higgins JPT, Altman DG, Gøtzsche PCet al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmader KE, Baron R, Haanpää MLet al. Treatment considerations for elderly and frail patients with neuropathic pain. Mayo Clin Proc. 2010;85(3 suppl):S26-S32. doi: 10.4065/mcp.2009.0646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effect. Clin Interv Aging. 2008;3:273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barber JB, Gibson SJ. Treatment of chronic non-malignant pain in the elderly: safety considerations. Drug Saf. 2009;32:457-474. doi: 10.2165/00002018-200932060-00003 [DOI] [PubMed] [Google Scholar]
- 9. Gallagher R. Methadone: an effective, safe drug of first choice for pain management in frail older adults. Pain Med. 2009;10:319-326. doi: 10.1111/j.1526-4637.2008.00551.x [DOI] [PubMed] [Google Scholar]
- 10. Zhang W, Moskowitz RW, Nuki Get al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137-162. doi: 10.1016/j.joca.2007.12.013 [DOI] [PubMed] [Google Scholar]
- 11. Sterne JAC, Hernán MA, Reeves BCet al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kennedy MC, Cousins G, Henman MC. Analgesic use by ageing and elderly patients with chronic non-malignant pain: a qualitative study. Int J Clin Pharm. 2017;39:798-807. doi: 10.1007/s11096-017-0466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fain KM, Alexander GC, Dore DD, Segal JB, Zullo AR, Castillo-Salgado C. Frequency and predictors of analgesic prescribing in US nursing home residents with persistent pain. J Am Geriatr Soc. 2017;65:286-293. doi: 10.1111/jgs.14512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fain KM, Castillo-Salgado C, Dore DD, Segal CB, Zullo AR, Alexander GC. Inappropriate fentanyl prescribing among nursing home residents in the United States. J Am Med Dir Assoc. 2017;18:138-144. doi: 10.1016/j.jamda.2016.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guerriero F, Roberto A, Greco MT, Sgarlata C, Rollone M, Corli O. Long-term efficacy and safety of oxycodone-naloxone prolonged release in geriatric patients with moderate-to-severe chronic noncancer pain: a 52-week open-label extension phase study. Drug Des Devel Ther. 2016;10:1515-1523. doi: 10.2147/DDDT.S106025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee J, Yoon JS, Lee JHet al. Clinical usefulness of long-term application of fentanyl matrix in chronic non-cancer pain: improvement of pain and physical and emotional functions. Clin Orthop Surg. 2016;8:465-474. doi: 10.4055/cios.2016.8.4.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turner JA, Shortreed SM, Saunders KW, LeResche L, Von Korff M. Association of levels of opioid use with pain and activity interference among patients initiating chronic opioid therapy: a longitudinal study. Pain. 2016;157:849-857. doi: 10.1097/j.pain.0000000000000452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petro E, Ruffini E, Cappuccio Met al. Low-dose oral prolonged-release oxycodone/naloxone for chronic pain in elderly patients with cognitive impairment: an efficacy-tolerability pilot study. Neuropsychiatr Dis Treat. 2016;12:559-569. doi: 10.2147/NDT.S98511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dublin S, Walker RL, Gray SLet al. Prescription opioids and risk of dementia or cognitive decline: a prospective cohort study. J Am Geriatr Soc. 2015;63:1519-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Enthoven WTM, Scheele J, Bierma-Zeinstra SMAet al. Analgesic use in older adults with back pain: the BACE study. Pain Med. 2014;15:1704-1714. doi: 10.1111/pme.12515 [DOI] [PubMed] [Google Scholar]
- 21. Ringe JD, Schäfer S, Wimmer AM, Giesecke T. Use of OROS® hydromorphone in the treatment of osteoarthritis and osteoporosis: a pooled analysis of three non-interventional studies focusing on different starting doses. Wien Klin Wochenschr. 2012;124:25-31. doi: 10.1007/s00508-011-0076-y [DOI] [PubMed] [Google Scholar]
- 22. Uberall MA, Müller-Schwefe GHH. Low-dose 7-day transdermal buprenorphine in daily clinical practice—perceptions of elderly patients with moderate non-malignant chronic pain. Curr Med Res Opin. 2012;28:1585-1595. doi: 10.1185/03007995.2012.731387 [DOI] [PubMed] [Google Scholar]
- 23. Gianni W, Madaio AR, Ceci Met al. Transdermal buprenorphine for the treatment of chronic noncancer pain in the oldest old. J Pain Symptom Manage. 2011;41:707-714. [DOI] [PubMed] [Google Scholar]
- 24. Gianni W, Madaio RA, Di Cioccio Let al. Prevalence of pain in elderly hospitalized patients. Arch Gerontol Geriatr. 2010;51:273-276. doi: 10.1016/j.jpainsymman.2010.06.015 [DOI] [PubMed] [Google Scholar]
- 25. Park J, Lavin R. Risk factors associated with opioid medication misuse in community-dwelling older adults with chronic pain. Clin J Pain. 2010;26:647-655. [DOI] [PubMed] [Google Scholar]
- 26. Unützer J, Hantke M, Powers Det al. Care management for depression and osteoarthritis pain in older primary care patients: a pilot study. Int J Geriatr Psychiatry. 2008;23:1166-1171. doi: 10.1002/gps.2048 [DOI] [PubMed] [Google Scholar]
- 27. Unützer J, Ferrell B, Lin EHB, Marmon T. Pharmacotherapy of pain in depressed older adults. J Am Geriatr Soc. 2004;52:1916-1922. doi: 10.1111/j.1532-5415.2004.52519.x [DOI] [PubMed] [Google Scholar]
- 28. Won AB, Lapane KL, Vallow S, Schein J, Morris JN, Lipsitz LA. Persistent nonmalignant pain and analgesic prescribing patterns in elderly nursing home residents. J Am Geriatr Soc. 2004;52:867-874. [DOI] [PubMed] [Google Scholar]
- 29. Nassar MA, McLeavy MA, Knox J. An open study of sub-lingual buprenorphine in the treatment of chronic pain in the elderly. Curr Med Res Opin. 1986;10:251-255. doi: 10.1185/03007998609110446 [DOI] [PubMed] [Google Scholar]
- 30. Pergolizzi JV, Raffa RB, Marcum Z, Colucci S, Ripa SR. Safety of buprenorphine transdermal system in the management of pain in older adults. Postgrad Med. 2017;129:92-101. doi: 10.1080/00325481.2017.1270699 [DOI] [PubMed] [Google Scholar]
- 31. Lazzari M, Marcassa C, Natoli Set al. Switching to low-dose oral prolonged-release oxycodone/naloxone from WHO-Step I drugs in elderly patients with chronic pain at high risk of early opioid discontinuation. Clin Interv Aging. 2016;11:641-649. doi: 10.2147/CIA.S105821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee J, Lakha SF, Mailis A. Efficacy of low-dose oral liquid morphine for elderly patients with chronic non-cancer pain: retrospective chart review. Drugs Real World Outcomes. 2015;2:369-376. doi: 10.1007/s40801-015-0048-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veal FC, Bereznicki LRE, Thompson AJ, Peterson GM. Use of opioid analgesics in older Australians. Pain Med. 2015;16:1519-1527. doi: 10.1111/pme.12720 [DOI] [PubMed] [Google Scholar]
- 34. Reid MC, Henderson CR, Jr, Papaleontiou Met al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Med. 2010;11:1063-1071. doi: 10.1111/j.1526-4637.2010.00883.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gallagher AM, Leighton-Scott J, van Staa TP. Utilization characteristics and treatment persistence in patients prescribed low-dose buprenorphine patches in primary care in the United Kingdom: a retrospective cohort study. Clin Ther. 2009;31:1707-1715. doi: 10.1016/j.clinthera.2009.08.022 [DOI] [PubMed] [Google Scholar]
- 36. Raffaeli W, Righetti D, Caminiti Aet al. Implantable intrathecal pumps for the treatment of noncancer chronic pain in elderly population: drug dose and clinical efficacy. Neuromodulation. 2008;11:33-39. doi: 10.1111/j.1525-1403.2007.00140.x [DOI] [PubMed] [Google Scholar]
- 37. Rigler SK, Shireman TI, Kallenbach L. Predictors of long-acting opioid use and oral versus transdermal route among older Medicaid beneficiaries. Am J Geriatr Pharmacother. 2007;5:91-99. doi: 10.1016/j.amjopharm.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 38. Solomon DH, Avorn J, Wang PSet al. Prescription opioid use among older adults with arthritis or low back pain. Arthritis Rheum. 2006;55:35-41. doi: 10.1002/art.21697 [DOI] [PubMed] [Google Scholar]
- 39. Won A, Lapane KL, Vallow S, Schein J, Morris JN, Lipsitz LA. Long-term effects of analgesics in a population of elderly nursing home residents with persistent nonmalignant pain. J Gerontol A Biol Sci Med Sci. 2006;61:165-169. doi: 10.1093/gerona/61.2.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kung F, Gibson SJ, Helme RD. Factors associated with analgesic and psychotropic medications use by community-dwelling older people with chronic pain. Aust N Z J Public Health. 1999;23:471-474. doi: 10.1111/j.1467-842X.1999.tb01301.x [DOI] [PubMed] [Google Scholar]
- 41. Dobscha SK, Lovejoy TI, Morasco BJet al. Predictors of improvements in pain intensity in a national cohort of older veterans with chronic pain. J Pain. 2016;17:824-835. doi: 10.1016/j.jpain.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krebs EE, Paudel M, Taylor BCet al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group. Association of opioids with falls, fractures, and physical performance among older men with persistent musculoskeletal pain. J Gen Intern Med. 2016;31:463-469. doi: 10.1007/s11606-015-3579-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuo Y, Raji MA, Chen N, Hasan H, Goodwin JS. Trends in opioid prescriptions among Part D Medicare recipients from 2007 to 2012. Am J Med. 2016;129:221.e21-221.e30. doi: 10.1016/j.amjmed.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prunuske JP, St Hill CA, Hager KDet al. Opioid prescribing patterns for non-malignant chronic pain for rural versus non-rural US adults: a population-based study using 2010 NAMCS data. BMC Health Serv Res. 2014;14:563. doi: 10.1186/s12913-014-0563-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koponen MPH, Bell JS, Karttunen NM, Nykänen IA, Desplenter FAM, Hartikainen SA. Analgesic use and frailty among community-dwelling older people: a population-based study. Drugs Aging. 2013;30:129-136. doi: 10.1007/s40266-012-0046-8 [DOI] [PubMed] [Google Scholar]
- 46. Saunders KW, Dunn KM, Merrill JOet al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25:310-315. doi: 10.1007/s11606-009-1218-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Breivik H, Ljosaa TM, Stengaard-Pedersen Ket al. A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of a low-dose 7-day buprenorphine transdermal patch in osteoarthritis patients naïve to potent opioids. Scand J Pain. 2010;1:122-141. doi: 10.1016/j.sjpain.2010.05.035 [DOI] [PubMed] [Google Scholar]
- 48. Likar R, Vadlau E, Breschan C, Kager I, Korak-Leiter M, Ziervogel G. Comparable analgesic efficacy of transdermal buprenorphine in patients over and under 65 years of age. Clin J Pain. 2008;24:536-543. [DOI] [PubMed] [Google Scholar]
- 49. Rauck RL, Ruoff GE, McMillen JI. Comparison of tramadol and acetaminophen with codeine for long-term pain management in elderly patients. Curr Ther Res Clin Exp. 1994;55:1417-1431. doi: 10.1016/S0011-393X(05)80748-9 [DOI] [Google Scholar]
- 50. Kjaersgaard-Andersen P, Nafei A, Skov Oet al. Codeine plus paracetamol versus paracetamol in longer-term treatment of chronic pain due to osteoarthritis of the hip. A randomised, double-blind, multi-centre study. Pain. 1990;43:309-318. doi: 10.1016/0304-3959(90)90028-C [DOI] [PubMed] [Google Scholar]


