Abstract
Epithelial integrity and barrier function are maintained during cytokinesis in vertebrate epithelial tissues. The changes in localization and the roles of tricellular tight junction molecule lipolysis-stimulated lipoprotein receptor (LSR) during cytokinesis are not well known, although new tricellular tight junctions form at the flank of the midbody during cytokinesis. In this study, we investigated the changes in localization and the role of LSR at the midbody and centrosome during cytokinesis using human endometrial carcinoma cell line Sawano, comparing the tricellular tight junction molecule tricellulin; bicellular tight junction molecules occludin, claudin-7, zonula occludens-1, and cingulin; and the epithelial polarized related molecules apoptosis-stimulating of p53 protein 2, PAR3, and yes-associated protein. During cytokinesis induced by treatment with taxol, the epithelial barrier was maintained and the tricellular tight junction molecules LSR and tricellulin were concentrated at the flank of the acetylated tubulin–positive midbody and in γ-tubulin-positive centrosomes with the dynein adaptor Hook2, whereas the other molecules were localized there as well. All the molecules disappeared by knockdown using small interfering RNAs. Furthermore, by the knockdown of Hook2, the epithelial barrier was maintained and most of the molecules disappeared from the centrosome. These findings suggest that LSR may play crucial roles not only in barrier function but also in cytokinesis.
Keywords: ASPP2, bicellular tight junctions, centrosome, cytokinesis, Hook2, LSR, midbody, tricellular tight junctions, tricellulin, YAP
Introduction
In vertebrate tissues, epithelial cells maintain tissue homeostasis via cell–cell junctions that are essential for maintaining the epithelial barrier function of epithelial cells via adherens junctions (AJs) and tight junctions (TJs).1 TJs, which consist of claudin (CLDN)-mediated bicellular TJs (bTJs) and tricellular TJs (tTJs), seal the paracellular spaces between adjacent cells and form a selective barrier.2 tTJs form at the convergence of bTJs where three epithelial cells meet in polarized epithelia.3,4 Lipolysis-stimulated lipoprotein receptor (LSR) is a novel molecular constituent of tricellular contacts localized in most epithelial tissues. It has a barrier function and recruits tricellulin (TRIC), which was the first tTJ molecule discovered.3,5 LSR is also involved in the invasion and migration of cancer cells via yes-associated protein (YAP).6
Epithelial integrity and barrier function are maintained during cytokinesis in vertebrate epithelial tissues.7,8 During cytokinesis, an actomyosin-based contractile ring is formed at the cell equator and generates force to physically separate one cell into two daughter cells.9,10 The midbody is the final cellular link between the two daughter cells destined to be separated. AJ and bTJ molecules are concentrated in the midbody during cell division.11,12 Furthermore, de novo tTJs appear at the flank of the midbody during cytokinesis.13 YAP, which plays an important role in the Hippo pathway to control cell size, is also concentrated in the midbody to help cytokinesis.14
Centrosomes work as the microtubule-organizing center and contribute to chromosome separation during mitosis. They are composed of two centrioles and pericentriolar material and include at least 70 proteins in interphase, many of which contain coiled-coil domains.15 During mitosis, the centrosomes ensure equal segregation of the chromosomes. Failure to execute this process accurately is deleterious to the cell and may result in altered chromosome number and cancer.16 The bTJ molecule occludin (OCLN) localizes to the centrosomes and modifies mitotic entry.17
The dynein adaptor Hook2 is a member of the Hook protein family and functions as a linker protein that binds to microtubules and organelles.18 Hook2 is localized in the centrosome and contributes to centrosomal function and aggresome formation.19,20 It mediates the assembly of the dynein–dynactin complex and regulates mitotic progression and cytokinesis.21 It also interacts with the epithelial cell polarity molecules PAR3 and PAR6α and controls centrosome orientation during polarized cell migration.22 However, its interactions with bTJ and tTJ molecules remain unknown.
In this study, we focused on the midbody and centrosome during cell division and investigated the changes in the localization and the role of LSR during cytokinesis using human endometrial carcinoma cell line Sawano, comparing the tTJ molecule TRIC; bTJ molecules OCLN, CLDN-7, zonula occludens-1 (ZO-1), and cingulin; epithelial polarized related molecules apoptosis-stimulating of p53 protein 2 (ASPP2) and PAR3; and the Hippo pathway molecule YAP. During cytokinesis induced by treatment with Taxol, the epithelial barrier was maintained and the tTJ molecules LSR and TRIC were concentrated on the flank of the acetylated tubulin–positive midbody and in γ-tubulin-positive centrosomes with Hook2.
Materials and Methods
Ethics Statement
The protocol for human study was reviewed and approved by the ethics committee of the Sapporo Medical University School of Medicine. Written informed consent was obtained from each patient who participated in the investigation. All experiments were carried out in accordance with the approved guidelines and the Declaration of Helsinki.
Antibodies and Reagents
Rabbit polyclonal anti-TRIC, anti-CLDN-7, anti-OCLN, anti-ZO-1, and anti-cingulin antibodies were obtained from Zymed Laboratories (San Francisco, CA). Rabbit polyclonal anti-LSR and anti-Hook2 antibodies were from Novus Biologicals (Littleton, CO). A mouse monoclonal anti-LSR antibody was from Abnova (Taipei, Taiwan). A rabbit polyclonal anti-PAR3 was from Millipore Co. (Bedford, UK). A rabbit polyclonal anti–phosphorylated YAP (pYAP) antibody was from Cell Signaling Technology (Danvers, MA). Mouse monoclonal anti–acetylated tubulin (T7451), anti-ASPP2 (DX54.10), and rabbit polyclonal anti-γ-tubulin antibodies were from Sigma-Aldrich (St. Louis, MO). A mouse monoclonal anti-γ-tubulin antibody was from GeneTex, Inc. (Irvine, CA). A rabbit polyclonal anti-ASPP2 antibody was from Bioss (Woburn, MA). Alexa 488 (green)–conjugated anti-rabbit IgG and Alexa 594 (red)–conjugated anti-mouse IgG antibodies and Alexa 594 (red)–conjugated phalloidin were from Molecular Probes, Inc. (Eugene, OR). Taxol (paclitaxel) was obtained from Sigma-Aldrich (St. Louis). A horseradish peroxidase (HRP)-conjugated polyclonal goat anti-rabbit IgG was from Dako A/S (Glostrup, Denmark). The ECL Western blotting system was from GE Healthcare UK, Ltd. (Buckinghamshire, UK).
Cell Line Culture and Treatment
The human endometrial cancer cell line Sawano (RCB1152) was purchased from RIKEN BioResource Center (Tsukuba, Japan). Sawano cells were maintained in minimum essential medium (Sigma-Aldrich; Merck Millipore, Burlington, MA) supplemented with 10% dialyzed fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA). The medium contained 100 U/ml penicillin, 100 µg/ml streptomycin, and 50 µg/ml amphotericin B. The cells were plated on 35- and 60-mm culture dishes, which were coated with rat tail collagen (500 µg dried tendon/ml in 0.1% acetic acid), and incubated in a humidified 5% CO2 incubator at 37C. They were treated with 100 ng/ml Taxol.
Small Interfering (siRNA) Experiment
siRNA duplex oligonucleotides against LSR and TRIC were synthesized by Thermo Fisher Scientific. siRNA duplex oligonucleotides against ASPP2 and YAP were synthesized by Santa Cruz Biotechnology (Dallas, TX). siRNA duplex oligonucleotides against PAR3 and Hook2 were synthesized by IDT (Tokyo, Japan). The sequences were as follows: siRNA of LSR (sense: 5′-CCCACGCAACCCAUCGUCAUCUGGA-3′; antisense: 5′-UCCAGAUGACGAUGGGUUGCGUGGG-3′), siRNA of TRIC (sense: 5′-GACAGACAAAGAGACUCAGAAGUUA-3′; antisense: 5′-UAACUUCUGAGUCUCUUUGUCUGUC-3′), siRNA of ASPP2-A (sc37457, sense: 5′- CUAGAAGCCUUACGAAAGAtt-3′; antisense: 5′-UCUUUCGUAAGGCUUCUAGtt-3′), siRNA of ASPP2-B (sc37457, sense: 5′-CCAUCUCUGUCCCAUCAUAtt-3′; antisense: 5′-UAUGAUGGGACAGAGAUGGtt-3′), siRNA of ASPP2-C (sc37457, sense: 5′-CAAGAAUCCUGUCUAGUAAtt-3′; antisense: 5′-UUACUAGACAGGAUUCUUGtt-3′), siRNA of PAR3 (sense: 5′-CCAAGCCAUGCGUACACCCAUCATT-3′; antisense: 5′-AAUGAUGGGUGUACGCAUGGCUUGGCG-3′), siRNA of YAP-A (sc38637, sense: 5′-CCACCAAGCUAGAUAAAGAtt-3′; antisense: 5′-UCUUUAUCUAGCUUGGUGGtt-3′), siRNA of YAP-B (sc38637, sense: 5′-GCAUGAGACAAUUUCCAUAtt-3′; antisense: 5′-UAUGGAAAUUGUCUCAUGCtt-3′), siRNA of YAP-C (sc38637, sense: 5′-GGGUGUGCCUAUCAUAACAtt-3′; antisense: 5′-UGUUAUGAUAGGCACACCCtt-3′), siRNA of Hook2-M1 (sense: 5′-GGAGACUCUGAUUUUAUAUUUGAGA-3′; antisense: 5′-UCUCAAAUAUAAAAUCAGAGUCUCCUG-3′), siRNA of Hook2-M2 (sense: 5′-GGACCACAUCCAGAGAAUCAUGACG-3′; antisense: 5′-CGUCAUGAUUCUCUGGAUGUGGUCCUG-3′), and siRNA of Hook2-M3 (sense: 5′-GGUCAGCAAUCUGAAGAUGGUGUTA-3′; antisense: 5′-UAAGACCAUCUUCAGAUUGCUGACCUU-3′). At 24 hr after plating, the Sawano cells were transfected with 100 nM siRNAs of LSR, TRIC, ASPP2 (A, B, C), PAR3, YAP (A, B, C), and Hook2 (M1, M2, M3) using Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA) for 48 hr. A scrambled siRNA sequence (BLOCK-iT Alexa Fluor Fluorescent; Invitrogen) was used as a control siRNA. Some cells were transfected with 100 nM siRNA of LSR, TRIC, ASPP2, PAR3, YAP, and Hook2 with or without 100 ng/ml Taxol.
Western Blot Analysis
The Sawano cells were scraped from a 35-mm dish containing 300 μl of buffer (1 mM NaHCO3 and 2 mM phenylmethylsulfonyl fluoride), collected in microcentrifuge tubes, and then sonicated for 10 sec. The protein concentrations of the samples were determined using a BCA protein assay reagent kit (Pierce Chemical Co.; Rockford, IL). Aliquots of 15 μl of protein/lane for each sample were separated by electrophoresis in 5% to 20% SDS polyacrylamide gels (Wako; Osaka, Japan) and electrophoretically transferred to a nitrocellulose membrane (Immobilon; Millipore Co.). The membrane was saturated for 30 min at room temperature with a blocking buffer (25 mM Tris, pH 8.0, 125 mM NaCl, 0.1% Tween 20, and 4% skim milk) and incubated with anti-LSR, anti-TRIC, anti-ASPP2, anti-PAR3, and anti-actin antibodies (1:1000) at room temperature overnight. Then it was incubated with HRP-conjugated anti-mouse and anti-rabbit IgG antibodies at room temperature for 1 hr. The immunoreactive bands were detected using an ECL Western blotting system.
Immunoprecipitation
The Sawano cells in 60-mm dishes were washed with PBS, and 300 µl of NP-40 lysis buffer (50 mM Tris-HCl, 2% NP-40, 0.25 mM sodium deoxycholate, 150 mM NaCl, 2 mM EGTA, 0.1 mM Na3VO4, 10 mM NaF, 2 mM PMSF) was added to the dishes. The cells were scraped off, collected in microcentrifuge tubes, and then sonicated for 10 sec. Cell lysates were incubated with protein A-sepharose CL-4B (Pharmacia LKB Biotechnology; Uppsala, Sweden) for 1 hr at 4C and then clarified by centrifugation at 15,000 g for 10 min. The supernatants were incubated with the polyclonal anti-Hook2 antibody, anti-pYAP antibody, anti-TRIC antibody, anti-LSR antibody, anti-PAR3 antibody, or anti-ASPP2 antibody bound to protein A-Sepharose CL-4B overnight at 4C. After incubation, immunoprecipitates were washed extensively with the same lysis buffer and subjected to Western blot analysis with anti-Hook2, anti-LSR, anti-TRIC, anti-ASPP2, anti-PAR3, and anti-γ-tubulin antibodies.
Immunocytochemistry
The Sawano cells in 35-mm glass-coated wells (Iwaki; Chiba, Japan) were fixed with cold acetone and ethanol (1:1) at −20C for 10 min. After rinsing in PBS, the cells were incubated with anti-Hook2, anti-LSR, anti-TRIC, anti-OCLN, anti-CLDN-7, anti-ZO-1, anti-cingulin, anti-pYAP, anti-ASPP2, anti–acetylated tubulin, and anti-γ-tubulin antibodies (1:100) and Alexa 594-phalloidin (1:200) overnight at 4C. Alexa Fluor 488 (green)–conjugated anti-rabbit IgG and Alexa Fluor 592 (red)–conjugated anti-mouse IgG (Invitrogen) were used as secondary antibodies. The specimens were examined and photographed with an Olympus IX 71 inverted microscope (Olympus Co.; Tokyo, Japan) and a confocal laser scanning microscope (LSM510; Carl Zeiss, Jena, Germany).
Transmission Electron Microscopy (TEM) and Immunotransmission Electron Microscopy (IM-TEM) Analysis
The preembedding labeling method was used. The cells were fixed with 1% paraformaldehyde in 3% sucrose/0.1 M PBS (pH 7.4) at 4C for 2 hr and were permeabilized with 0.2% Triton X-100. They were treated at 4C for 30 min with a blocking buffer (4% skim milk in 0.1 M PBS) and were then incubated with anti-LSR, TRIC, PAR3, and pYAP antibodies (1:100) overnight at 4C. After incubation, the specimens were washed five times with PBS for 5 min each and incubated with secondary antibody–conjugated 12-nm colloidal gold (1:50; Jackson Immuno Research Laboratories, West Grove, PA). After five washes with PBS for 5 min each, the specimens were fixed with 2.5% glutaraldehyde in PBS overnight at room temperature, dehydrated through a graded ethanol series, embedded in epoxy resin, and cut into ultrathin sections with a Sorvall MT6000 ultramicrotome (DuPont/Sorvall; New Castle, DE). The ultrathin sections were stained with uranyl acetate and lead citrate and examined with a transmission electron microscope (H7500; Hitachi, Tokyo, Japan).
Cell Cycle Assay
The cell cycle distribution analysis was performed using a Muse Cell Cycle Assay Kit (Merck Millipore) according to the manufacturer’s instructions. The Sawano cells in 35-mm dishes were trypsinized, centrifuged, washed with PBS, and fixed in 70% ethanol for over 2 hr. After that, the cells were centrifuged, washed with PBS, dissolved in PBS, and mixed with the Muse Cell Cycle reagent (Merck Millipore). The samples were analyzed with a Muse Cell Analyzer (Merck Millipore).
Measurement of Transepithelial Electrical Resistance (TEER)
The Sawano cells were cultured to confluence in the inner chambers of 12-mm Transwell inserts with 0.4-µm pore-size filters (Corning Life Sciences; Tewksbury, MA). TEER was measured using an EVOM voltameter with an ENDOHM-12 electrode chamber (World Precision Instruments; Sarasota, FL) on a heating plate (Fine; Tokyo, Japan) adjusted to 37C. The values are expressed in standard units of ohms per square centimeter and presented as mean ± SD. For calculation, the resistance of blank filters was subtracted from that of filters covered with cells.
Data Analysis
Signals were quantified using Scion Image Beta 4.02 Win (Scion Co.; Frederick, MA). Each set of results shown is representative of at least three separate experiments. Results are presented as mean ± SEM. Differences between groups were tested by ANOVA followed by a post hoc test and an unpaired two-tailed Student’s t-test and considered to be significant when p<0.05.
Results
Localization of TJ Molecules at the Membranes, Cell Cycle, and Epithelial Barrier During Cytokinesis Induced by Taxol in Sawano Cells
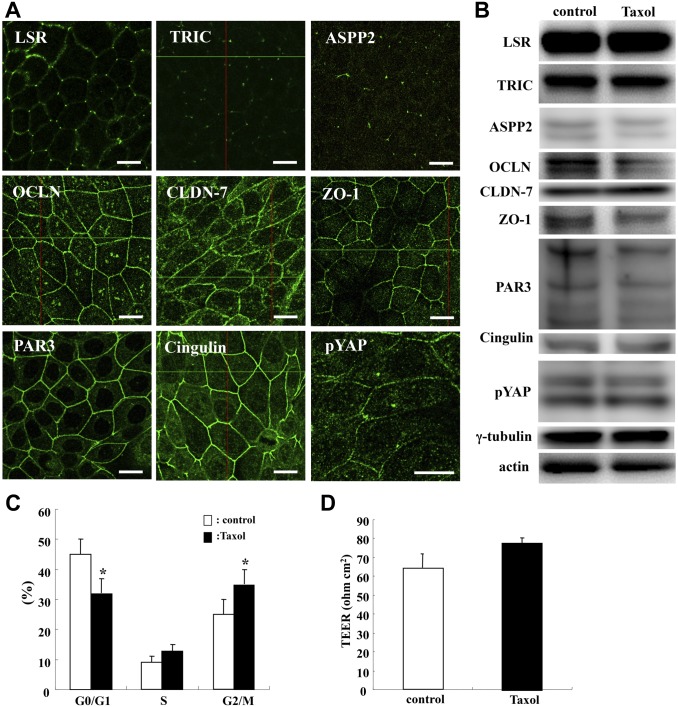
To investigate the localization of TJ molecules at the membranes, cell cycle, and epithelial barrier during cytokinesis, Sawano cells were treated with 100 ng/ml Taxol for 24 hr and subjected to immunocytochemistry and Western blotting analysis of TJ molecules LSR, TRIC, OCLN, CLDN-7, ZO-1, ASPP2, PAR3, cingulin, and pYAP; cell cycle assay; and epithelial barrier function assay. In both control and Taxol-treated cells, tTJ molecules LSR and TRIC were localized at the tricellular membranes as was ASPP2, and bTJ molecules OCLN, CLDN-7, ZO-1, PAR3, and cingulin were localized at the bicellular membranes as was pYAP (Fig. 1A). There was no change in the localization and expression of TJ molecules ASPP2 and pYAP caused by Taxol (Fig. 1A and B, Supplemental Fig. 1). In Taxol treatment, a decrease in the number of cells in the G0/G1 phase and an increase in the number of cells in the G2/M phase were observed compared with the control (Fig. 1C). There was no significant change in the epithelial barrier function induced by Taxol (Fig. 1D).
Figure 1.
Effects of Taxol in Sawano cells. (A) Images of immunocytochemical staining for LSR, TR, ASPP2, OCLN, CLDN-7, ZO-1, PAR3, cingulin, and pYAP in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Bars: 10 μm. (B) Western blot analysis of LSR, TRIC, ASPP2, OCLN, CLDN-7, ZO-1, PAR3, cingulin, pYAP, and γ-tubulin in Sawano cells treated with 100 ng/ml Taxol for 24 hr. (C) Bar graph of cell cycle assay in Sawano cells treated with 100 ng/ml Taxol for 24 hr. (D) Bar graph of TEER values representing barrier function of Sawano cells treated with 100 ng/ml Taxol for 24 hr. Abbreviations: LSR, lipolysis-stimulated lipoprotein receptor; TRIC, tricellulin; ASPP2, apoptosis-stimulating of p53 protein 2; OCLN, occludin; CLDN-7, claudin-7; ZO-1, zonula occludens-1; TEER, transepithelial electrical resistance.
TJ Molecules ASPP2 and pYAP Are Concentrated at or in the Midbody During Cytokinesis by Taxol in Sawano Cells
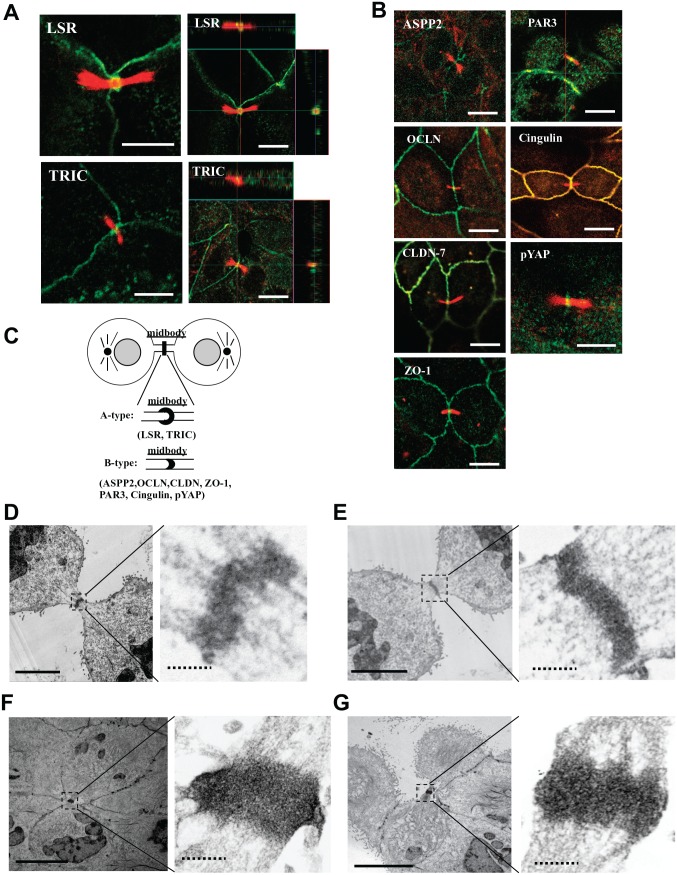
To investigate whether the TJ molecules ASPP2 and YAP localized in the midbody during cytokinesis, Sawano cells were treated with 100 ng/ml Taxol and subjected to immunocytochemistry for LSR, TRIC, ASPP2, OCLN, CLDN-7, ZO-1, PAR3, cingulin, and acetylated tubulin and TEM analysis of LSR, TRIC, PAR3, and pYAP (Figs. 2 and 4). We examined more than six midbodies in immunocytochemistry for all antibodies. LSR and TRIC were concentrated at the acetylated tubulin–positive midbody, seemingly surrounding it (A type, Fig. 2A and C). OCLN, cingulin, CLDN-7, ZO-1, ASPP2, PAR3, ZO-1, and pYAP were concentrated in the midbody (B type, Fig. 2B). In TEM analysis of Taxol-treated cells, the gold particles of LSR, TRIC, PAR3, and pYAP were observed in regions of the midbody with high electron density (Fig. 2D–G).
Figure 2.
Localization of tight junction molecules ASPP2 and pYAP in the midbody during cytokinesis induced by Taxol in Sawano cells. (A) Immunocytochemical staining for LSR (green) and TRIC (green) with acetylated tubulin (red) in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Bars: 5 μm. (B) Immunocytochemical staining for ASPP2 (green), PAR3 (green), OCLN (green), cingulin (green), CLDN-7 (green), pYAP (green), and ZO-1 (green) with acetylated tubulin (red) in the midbody in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Bars: 5 μm. (C) Schematic image of localization of tight junction molecules ASPP2 and pYAP in the midbody. (D) Immunoelectron microscopic staining for LSR in Sawano cells. Bar lines: 5 μm, dotted bar lines: 1 μm. (E) Immunoelectron microscopic staining for TRIC in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Bar lines: 5 μm, dotted bar lines: 1 μm. (F) Immunoelectron microscopic staining for PAR3 in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Bar lines: 5 μm, dotted bar lines: 1 μm. (G) Immunoelectron microscopic staining for pYAP in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Abbreviations: ASPP2, apoptosis-stimulating of p53 protein 2; pYAP, phosphorylated YAP; LSR, lipolysis-stimulated lipoprotein receptor; TRIC, tricellulin; OCLN, occludin; CLDN-7, claudin-7; ZO-1, zonula occludens-1. Bar lines: 5 μm, dotted bar lines: 1 μm.
Figure 4.
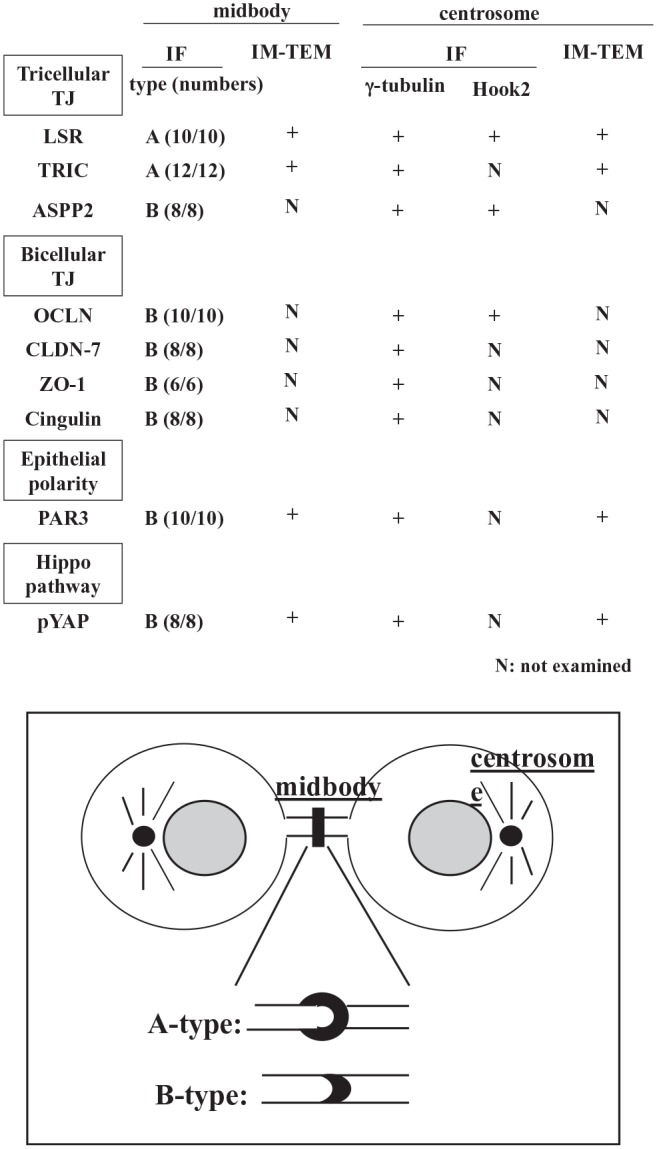
Summary of localization of tight junction molecules ASPP2 and pYAP in the midbody and centrosome. Abbreviations: ASPP2, apoptosis-stimulating of p53 protein 2; pYAP, phosphorylated yes-associated protein; IF, immunofluorescence staining; IM-TEM, immunotransmission electron microscopy; N, not examined; LSR, lipolysis-stimulated lipoprotein receptor; TRIC, tricellulin; OCLN, occludin; CLDN-7, claudin-7; ZO-1, zonula occludens-1.
TJ Molecules ASPP2 and pYAP Are Localized in the Centrosome During Cytokinesis Induced by Taxol in Sawano Cells
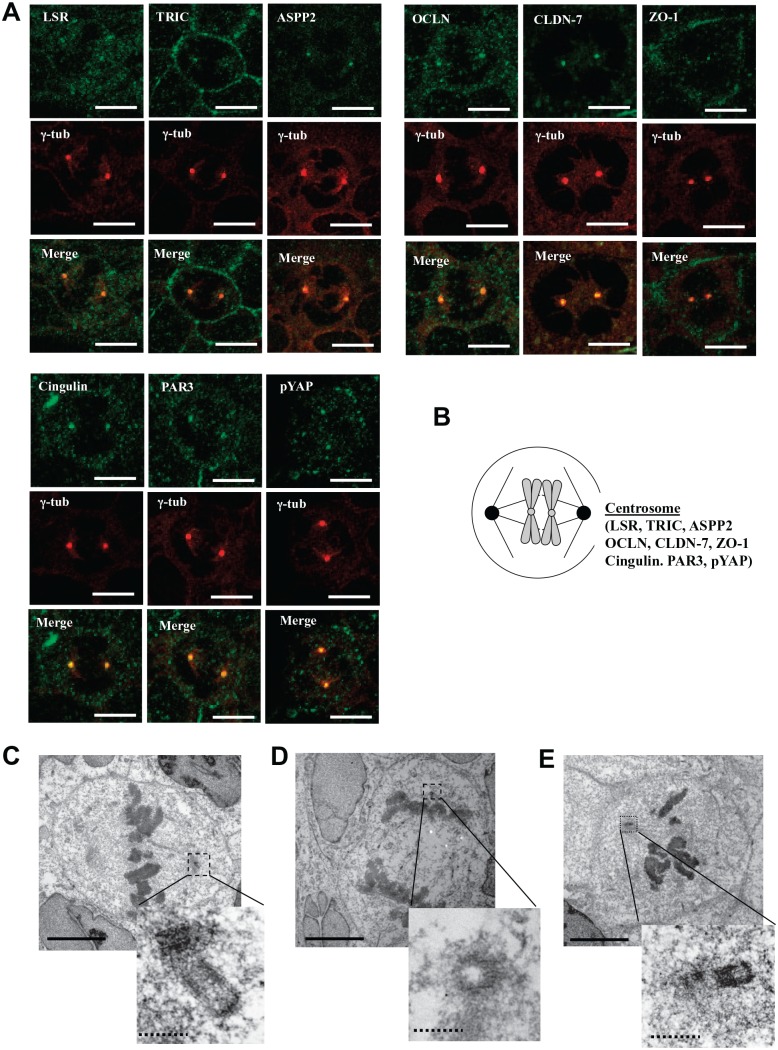
To investigate whether the TJ molecules ASPP2 and YAP localized in the centrosomes during cytokinesis, we treated the Sawano cells with 100 ng/ml Taxol and conducted immunocytochemistry for LSR, TRIC, ASPP2, PAR3, OCLN, cingulin, CLDN-7, ZO-1, and pYAP with γ-tubulin and TEM analysis of LSR, TRIC, PAR3, and pYAP (Figs. 3 and 4). All TJ molecules ASPP2 and pYAP were localized in the centrosomes during cytokinesis induced by Taxol in the Sawano cells (Fig. 3A and B). In TEM analysis of Taxol-treated cells, the gold particles of LSR, TRIC, and pYAP were observed in regions of the centrosome with high electron density (Fig. 3C–E).
Figure 3.
Localization of tight junction molecules ASPP2 and pYAP in centrosome during cytokinesis induced by Taxol in Sawano cells. (A) Immunocytochemical staining for LSR, TRIC, ASPP2, OCLN, CLDN-7, ZO-1, cingulin, PAR3, and pYAP with γ-tubulin (centrosome) in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Bars: 5 μm. (B) Schematic image of localization of tight junction molecules ASPP2 and pYAP in centrosome. (C) Immunoelectron microscopic staining for LSR in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Bar lines: 5 μm, dotted bar lines: 1 μm. (D) Immunoelectron microscopic staining for TRIC in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Bar lines: 5 μm, dotted bar lines: 1 μm. (E) Immunoelectron microscopic staining for pYAP in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Abbreviations: ASPP2, apoptosis-stimulating of p53 protein 2; pYAP, phosphorylated yes-associated protein; LSR, lipolysis-stimulated lipoprotein receptor; TRIC, tricellulin; OCLN, occludin; CLDN-7, claudin-7; ZO-1, zonula occludens-1. Bar lines: 5 μm, dotted bar lines: 1 μm.
Knockdown of TJ Molecules ASPP2 and pYAP Does Not Affect the Morphology and Localization of the Midbody and Centrosome During Cytokinesis Induced by Taxol in Sawano Cells
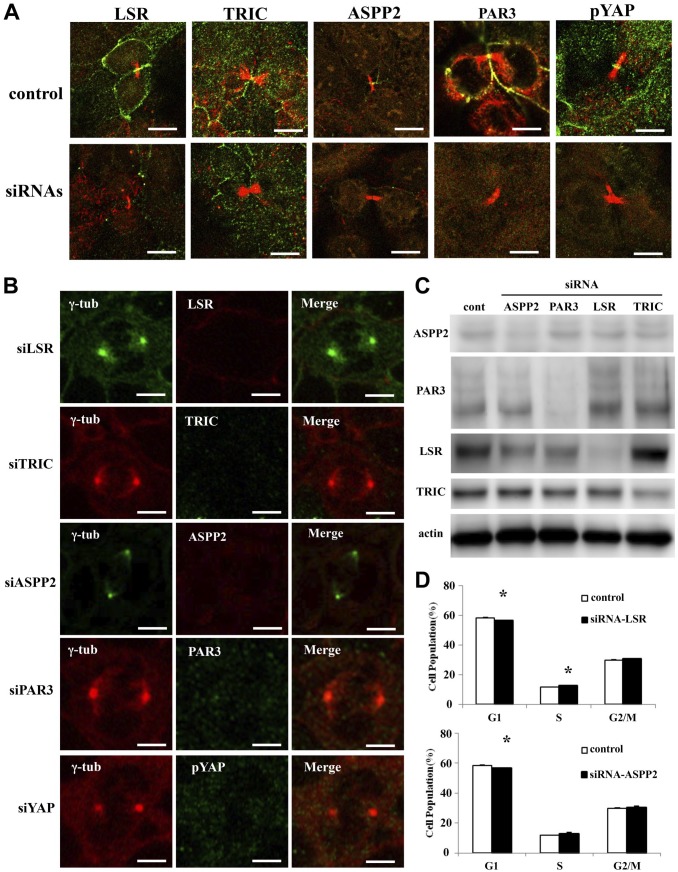
To investigate whether the knockdown of TJ molecules ASPP2 and pYAP affected the morphology and localization of the midbody and centrosome during cytokinesis, Sawano cells were transfected with siRNAs of LSR, TRIC, ASPP2, PAR3, and pYAP before treatment with 100 ng/ml Taxol and examined by Western blotting and immunocytochemistry for LSR, TRIC, ASPP2, PAR3, and pYAP. In Western blot analysis, knockdown of LSR, TRIC, ASPP2, and PAR3 by the siRNAs was confirmed (Fig. 5C, Supplemental Fig. 1). With knockdown of LSR, TRIC, ASPP2, and PAR3, their loss was observed in the midbody and centrosome but it did not affect the morphology and localization of the midbody and centrosome during cytokinesis (Fig. 5A and B)
Figure 5.
Effects of knockdown of LSR, TRIC, ASPP2, PAR3, and YAP in Sawano cells. (A) Immunocytochemical staining for LSR (green), TRIC (green), ASPP2 (green), PAR3 (green), and pYAP (green) with acetylated tubulin (red) in Sawano cells treated with 100 ng/ml Taxol after transfection with siRNAs of LSR, TRIC, ASPP2, PAR3, and pYAP. Bars: 5 μm. (B) Immunocytochemical staining for LSR, TRIC, ASPP2, PAR3, and pYAP with γ-tubulin in Sawano cells treated with 100 ng/ml Taxol after transfection with siRNAs of LSR, TRIC, ASPP2, PAR3, and pYAP. Bars: 5 μm. (C) Bar graph of cell cycle assay in Sawano cells transfected with siRNA of LSR. (D) Bar graph of cell cycle assay in Sawano cells transfected with siRNA of ASPP2. Abbreviations: LSR, lipolysis-stimulated lipoprotein receptor; TRIC, tricellulin; ASPP2, apoptosis-stimulating of p53 protein 2; pYAP, phosphorylated yes-associated protein; OCLN, occludin; CLDN-7, claudin-7; ZO-1, zonula occludens-1; siRNA, small interfering RNA.
Knockdown of LSR and ASPP2 Affects Cell Cycle in Sawano Cells
To investigate whether the knockdown of TJ molecules ASPP2 and pYAP affected the cell cycle, Sawano cells were transfected with siRNAs of LSR, TRIC, ASPP2, PAR3, and pYAP. With transfection of siRNA-LSR, a decrease in the number of cells in the G0/G1 phase and an increase in the number of cells in the S phase were observed (Fig. 5D). After transfection of siRNA-ASPP2, a decrease in the number of cells in the G0/G1 phase was observed (Fig. 5D). In this study, knockdown of PAR3 and pYAP did not affect the cell cycle.
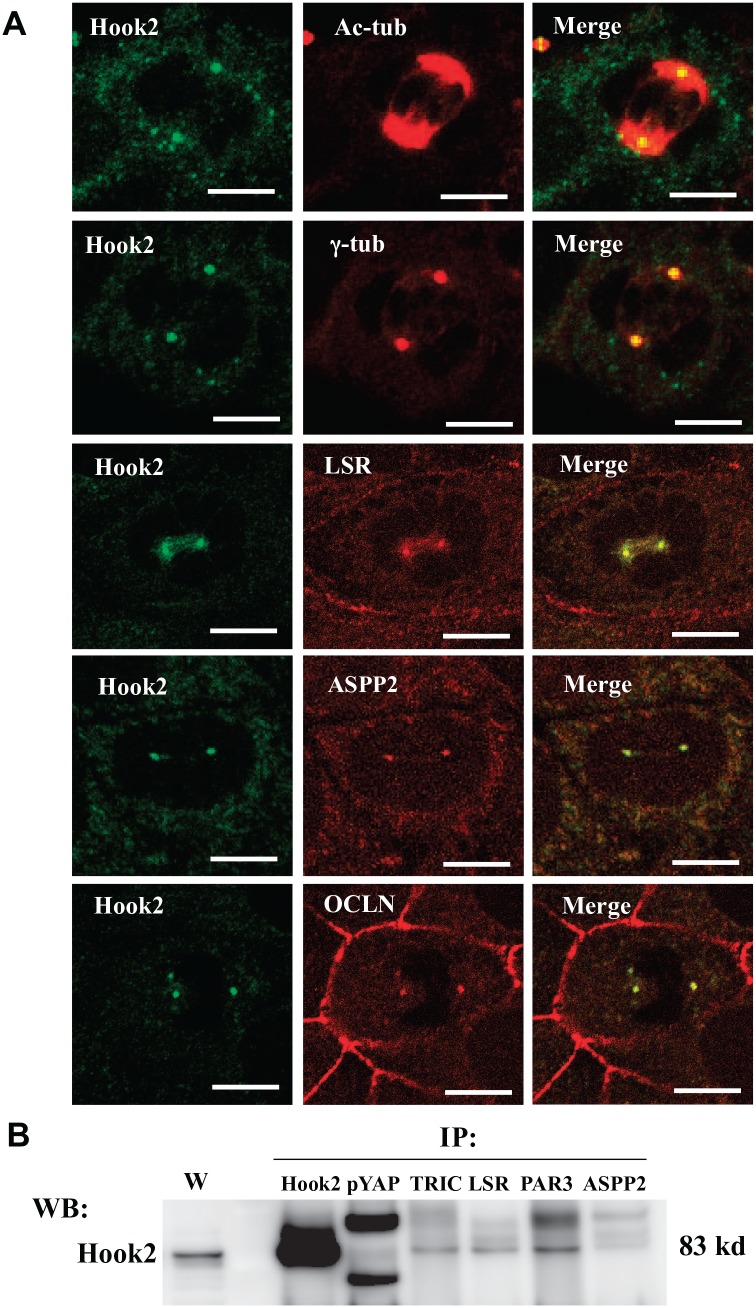
TJ Molecules ASPP2 and pYAP Are Colocalized With Hook2 in the Centrosome During Cytokinesis in Sawano Cells
To investigate whether the TJ molecules ASPP2 and YAP contributed to Hook2 in the centrosome during cytokinesis, we treated the cells with 100 ng/ml Taxol and conducted immunocytochemistry for LSR, TRIC, ASPP2, PAR3, OCLN, cingulin, CLDN-7, ZO-1, pYAP, γ-tubulin, and Hook2. Hook2 was colocalized with γ-tubulin in centrosomes, and LSR, TRIC, ASPP2 and OCLN were colocalized with Hook2 in centrosomes (Fig. 6A). Furthermore, when using an anti-Hook2 antibody, immunoprecipitates with pYAP, TRIC, LSR, PAR3, and ASPP2 were detected (Fig. 6B, Supplemental Fig. 1).
Figure 6.
Tight junction molecules ASPP2 and pYAP are colocalized with Hook2 in centrosomes during cytokinesis in Sawano cells. (A) Immunocytochemical staining for acetylated tubulin, γ-tubulin, LSR, ASPP2, and OCLN with Hook2 in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Bars: 5 μm. (B) Western blotting for pYAP, TRIC, LSR, PAR3, and ASPP2 in immunoprecipitates with anti-Hook2 antibody in Sawano cells treated with 100 ng/ml Taxol for 24 hr. Abbreviations: ASPP2, apoptosis-stimulating of p53 protein 2; pYAP, phosphorylated yes-associated protein; LSR, lipolysis-stimulated lipoprotein receptor; OCLN, occludin; TRIC, tricellulin.
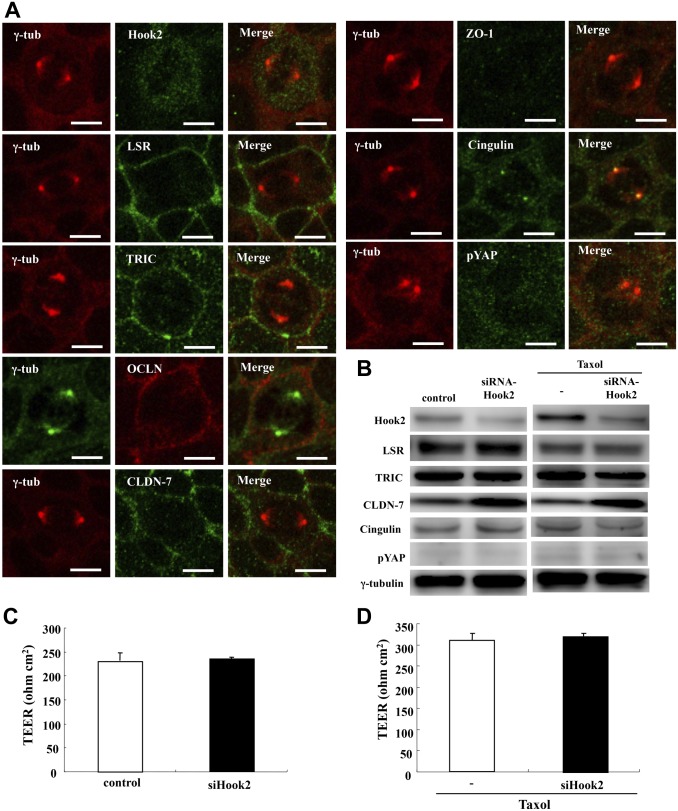
TJ Molecules ASPP2 and pYAP Disappear With Knockdown of Hook2 in the Centrosome During Cytokinesis in Sawano Cells
To investigate whether knockdown of Hook2 affected the localization and expression of TJ molecules ASPP2 and pYAP in centrosomes and the epithelial barrier during cytokinesis, Sawano cells were transfected with siRNAs of Hook2 before treatment with 100 ng/ml Taxol and examined by Western blotting and immunocytochemistry for LSR, TRIC, ASPP2, PAR3, and pYAP and epithelial barrier analysis. With knockdown of Hook2, LSR, TRIC, OCLN, CLDN-7, ZO-1, and pYAP, they disappeared from the centrosome as indicated by γ-tubulin, but even after knockdown of cingulin, it was present in the centrosome (Fig. 7A). There was no change in the expression of LSR, TRIC, ASPP2, PAR3, pYAP, or the epithelial barrier function with or without Taxol (Fig. 7B–D, Supplemental Fig. 1).
Figure 7.
Effects of knockdown of Hook2 in centrosomes during cytokinesis induced by Taxol in Sawano cells. (A) Immunocytochemical staining for Hook2, LSR, TRIC, OCLN, CLDN-7, ZO-1, cingulin, and pYAP with γ-tubulin in Sawano cells treated with 100 ng/ml Taxol after transfection with siRNA of Hook2. Bars: 5 μm. (B) Western blot analysis of Hook2, LSR, TRIC, CLDN-7, cingulin, pYAP, and γ-tubulin in Sawano cells treated with or without 100 ng/ml Taxol after transfection with siRNA of Hook2. (C) Graph of TEER values representing barrier function of Sawano cells transfected with siRNA of Hook2. (D) Graph of TEER values representing barrier function of Sawano cells treated with 100 ng/ml Taxol after transfection with siRNA of Hook2. Abbreviations: LSR, lipolysis-stimulated lipoprotein receptor; TRIC, tricellulin; OCLN, occludin; CLDN-7, claudin-7; ZO-1, zonula occludens-1; pYAP, phosphorylated yes-associated protein; TEER, transepithelial electrical resistance; siRNA, small interfering RNA.
Discussion
In this study, we first found that tTJ molecules LSR and TRIC were concentrated at the flank of the midbody and localized in the centrosome with the binding protein Hook2 during cytokinesis in vertebrate epithelial cells.
AJ and bTJ molecules are concentrated in the midbody during cytokinesis.11,12 YAP is concentrated at the midbody to help cytokinesis.13 The polarity scaffold protein PATJ binds and colocalizes with YAP at the cytokinesis midbody.13 In this study, during cytokinesis induced by Taxol in Sawano cells, the epithelial barrier was maintained and the tTJ molecules LSR and TRIC were concentrated at the acetylated tubulin–positive midbody, seemingly surrounding it (type A in Fig. 2C), whereas the bTJ molecules OCLN, CLDN-7, ZO-1, and cingulin; epithelial polarized related molecules ASPP2 and PAR3; and the Hippo pathway molecule YAP were also localized in the midbody (type B in Fig. 2C). All the molecules, including LSR, disappeared from the midbody during cytokinesis induced by knockdown using siRNAs. Recently, it was reported that new tTJs formed at the flank of the midbody during cytokinesis.13 Accordingly, it was thought that the tTJ molecules LSR and TRIC, but not the bTJ molecules, were concentrated at the flank of the midbody.
When we investigated normal human epithelial cells during cytokinesis induced by Taxol in normal human nasal epithelial cells, LSR, TRIC, and YAP were also concentrated at the midbody (Supplemental Method, Supplemental Fig. 2). These results indicated that changes in the localization of tTJ molecules could be observed not only in a cancer cell line but also in normal cells.
On the other hand, ASPP2 binds and colocalizes with PAR3 to act as a key regulator of cell polarity and YAP activity.23–25 Loss of LSR contributes to YAP and promotes the malignancy of cancer cells.6 In this study, nevertheless, ASPP2 was localized at tricellular contacts similar to LSR and TRIC in non-treated Sawano cells (Fig. 1) and in the midbody during cytokinesis induced by Taxol (type B in Fig. 2C). Furthermore, knockdown of ASPP2 slightly affected the cell cycle as did knockdown of LSR. It is possible that LSR and ASPP2 may contribute to cytokinesis via YAP.
The bTJ molecule OCLN localizes to centrosomes and modifies mitotic entry.17 Hook2 is localized in centrosomes, binds directly to centriolin/CEP110, and contributes to centrosomal function and aggresome formation.19 It interacts with the epithelial cell polarity molecules PAR3 and PAR6α and controls centrosome orientation during polarized cell migration.22 In this study, all molecules including LSR were colocalized with Hook2, which bound to all the molecules in the centrosome during cytokinesis. After knockdown of Hook2, the epithelial barrier was maintained and most molecules expected for cingulin disappeared from the centrosome during cytokinesis. These results suggested that Hook2 interacted with not only the epithelial cell polarity molecules but also bTJ and tTJ molecules and might control centrosome orientation.
In conclusion, the tTJ molecule LSR, like TRIC, was concentrated in de novo tricellular contacts forming on the flank of the midbody and localized in the centrosome with the binding protein Hook2 during cytokinesis in vertebrate epithelial cells. Tricellular junction molecules may play crucial roles not only in barrier function but also in cytokinesis. The localization and the roles of cell–cell junction molecules at the midbody and in the centrosome during cytokinesis remain less known for vertebrates than for invertebrates. As the specific roles of tTJs remained unclear during cytokinesis in our study, more studies using vertebrate tissues will be necessary to elucidate the cell division of not only normal cells but also those of diseases, including cancer.
Supplemental Material
Supplemental material, 2019-00123R1_Production_Supplemental_Figures_online_supp for Localization of Tricellular Tight Junction Molecule LSR at Midbody and Centrosome During Cytokinesis in Human Epithelial Cells by Takumi Konno, Takayuki Kohno, Shin Kikuchi, Hiroshi Shimada, Seiro Satohisa, Kenichi Takano, Tsuyoshi Saito and Takashi Kojima in Journal of Histochemistry & Cytochemistry
Footnotes
Competing Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: TKonno, TKohno, KT, and TKojima designed and coordinated the study and wrote the main manuscript text. TKonno, SK, and HS analyzed the data. SS and TS contributed reagents/materials. All authors reviewed the manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour and Welfare of Japan.
Contributor Information
Takumi Konno, Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Takayuki Kohno, Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Shin Kikuchi, Department of Anatomy, Sapporo Medical University School of Medicine, Sapporo, Japan.
Hiroshi Shimada, Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan; Department of Obstetrics and Gynecology, Sapporo Medical University School of Medicine, Sapporo, Japan.
Seiro Satohisa, Department of Obstetrics and Gynecology, Sapporo Medical University School of Medicine, Sapporo, Japan.
Kenichi Takano, Department of Otolaryngology, Sapporo Medical University School of Medicine, Sapporo, Japan.
Tsuyoshi Saito, Department of Obstetrics and Gynecology, Sapporo Medical University School of Medicine, Sapporo, Japan.
Takashi Kojima, Department of Cell Science, Research Institute for Frontier Medicine, Sapporo Medical University School of Medicine, Sapporo, Japan.
Literature Cited
- 1. Campbell HK, Maiers JL, DeMali KA. Interplay between tight junctions & adherens junctions. Exp Cell Res. 2017;358(1):39–44. doi: 10.1016/j.yexcr.2017.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 2013;340(6137):1185–9. doi: 10.1126/science.1235249. [DOI] [PubMed] [Google Scholar]
- 3. Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171(6):939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Furuse M, Izumi Y, Oda Y, Higashi T, Iwamoto N. Molecular organization of tricellular tight junctions. Tissue Barriers. 2014;2:e28960. doi: 10.4161/tisb.28960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, Nishi E, Furuse M. LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci. 2011;124;(Pt 4):548–55. doi: 10.1242/jcs.072058. [DOI] [PubMed] [Google Scholar]
- 6. Shimada H, Abe S, Kohno T, Satohisa S, Konno T, Takahashi S, Hatakeyama T, Arimoto C, Kakuki T, Kaneko Y, Takano K, Saito T, Kojima T. Loss of tricellular tight junction protein LSR promotes cell invasion and migration via upregulation of TEAD1/AREG in human endometrial cancer. Sci Rep. 2017;7:37049. doi: 10.1038/srep37049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jinguji Y, Ishikawa H. Electron microscopic observations on the maintenance of the tight junction during cell division in the epithelium of the mouse small intestine. Cell Struct Funct. 1992;17(1):27–37. [DOI] [PubMed] [Google Scholar]
- 8. Baker J, Garrod D. Epithelial cells retain junctions during mitosis. J Cell Sci. 1993;104;(Pt 2):415–425. [DOI] [PubMed] [Google Scholar]
- 9. Fededa JP, Gerlich DW. Molecular control of animal cell cytokinesis. Nat Cell Biol. 2012;14(5):440–447. doi: 10.1038/ncb2482. [DOI] [PubMed] [Google Scholar]
- 10. Green RA, Paluch E, Oegema K. Cytokinesis in animal cells. Annu Rev Cell Dev Biol. 2012;28:29–58. doi: 10.1146/annurev-cellbio-101011-155718. [DOI] [PubMed] [Google Scholar]
- 11. Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, Balda MS. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol. 2006;26(6):2387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kojima T, Kokai Y, Chiba H, Osanai M, Kuwahara K, Mori M, Mochizuki Y, Sawada N. Occludin and claudin-1 concentrate in the midbody of immortalized mouse hepatocytes during cell division. J Histochem Cytochem. 2001;49(3):333–40. [DOI] [PubMed] [Google Scholar]
- 13. Higashi T, Arnold TR, Stephenson RE, Dinshaw KM, Miller AL. Maintenance of the epithelial barrier and remodeling of cell-cell junctions during cytokinesis. Curr Biol. 2016;26(14):1829–42. doi: 10.1016/j.cub.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bui DA, Lee W, White AE, Harper JW, Schackmann RC, Overholtzer M, Selfors LM, Brugge JS. Cytokinesis involves a nontranscriptional function of the Hippo pathway effector YAP. Sci Signal. 2016;9(417):ra23. doi: 10.1126/scisignal.aaa9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426(6966):570–4. [DOI] [PubMed] [Google Scholar]
- 16. Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139(4):663–78. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 17. Runkle EA, Sundstrom JM, Runkle KB, Liu X, Antonetti DA. Occludin localizes to centrosomes and modifies mitotic entry. J Biol Chem. 2011;286(35):30847–58. doi: 10.1074/jbc.M111.262857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krämer H, Phistry M. Mutations in the Drosophila hook gene inhibit endocytosis of the boss transmembrane ligand into multivesicular bodies. J Cell Biol. 1996;133(6):1205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Szebenyi G, Hall B, Yu R, Hashim AI, Krämer H. Hook2 localizes to the centrosome, binds directly to centriolin/CEP110 and contributes to centrosomal function. Traffic. 2007;8(1):32–46. [DOI] [PubMed] [Google Scholar]
- 20. Szebenyi G, Wigley WC, Hall B, Didier A, Yu M, Thomas P, Krämer H. Hook2 contributes to aggresome formation. BMC Cell Biol. 2007;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dwivedi D, Kumari A, Rathi S, Mylavarapu SVS, Sharma M. The dynein adaptor Hook2 plays essential roles in mitotic progression and cytokinesis. J Cell Biol. 2019;218(3):871–94. doi: 10.1083/jcb.201804183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pallesi-Pocachard E, Bazellieres E, Viallat-Lieutaud A, Delgrossi MH, Barthelemy-Requin M, Le Bivic A, Massey-Harroche D. Hook2, a microtubule-binding protein, interacts with Par6α and controls centrosome orientation during polarized cell migration. Sci Rep. 2016;6:33259. doi: 10.1038/srep33259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cong W, Hirose T, Harita Y, Yamashita A, Mizuno K, Hirano H, Ohno S. ASPP2 regulates epithelial cell polarity through the PAR complex. Curr Biol. 2010;20(15):1408–1414. doi: 10.1016/j.cub.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Bu F, Royer C, Serres S, Larkin JR, Soto MS, Sibson NR, Salter V, Fritzsche F, Turnquist C, Koch S, Zak J, Zhong S, Wu G, Liang A, Olofsen PA, Moch H, Hancock DC, Downward J, Goldin RD, Zhao J, Tong X, Guo Y, Lu X. ASPP2 controls epithelial plasticity and inhibits metastasis through β-catenin-dependent regulation of ZEB1. Nat Cell Biol. 2014;16(11):1092–1104. doi: 10.1038/ncb3050. [DOI] [PubMed] [Google Scholar]
- 25. Royer C, Koch S, Qin X, Zak J, Buti L, Dudziec E, Zhong S, Ratnayaka I, Srinivas S, Lu X. ASPP2 links the apical lateral polarity complex to the regulation of YAP activity in epithelial cells. PLoS ONE. 2014;9(10):e111384. doi: 10.1371/journal.pone.0111384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 2019-00123R1_Production_Supplemental_Figures_online_supp for Localization of Tricellular Tight Junction Molecule LSR at Midbody and Centrosome During Cytokinesis in Human Epithelial Cells by Takumi Konno, Takayuki Kohno, Shin Kikuchi, Hiroshi Shimada, Seiro Satohisa, Kenichi Takano, Tsuyoshi Saito and Takashi Kojima in Journal of Histochemistry & Cytochemistry