Abstract
Background and purpose
To assess anatomic changes during intensity modulated radiotherapy (IMRT) for nasopharyngeal carcinoma (NPC) and to determine its dosimetric impact.
Patients and methods
Twenty patients treated with IMRT for NPC were enrolled in this study. A second CT was performed at 38 Gy. Manual contouring of the macroscopic tumor volumes (GTV) and the planning target volumes (PTV) were done on the second CT. We recorded the volumes of the different structures, D98 %, the conformity, and the homogeneity indexes for each PTV. Volume percent changes were calculated.
Results
We observed a significant reduction in tumor volumes (58.56 % for the GTV N and 29.52 % for the GTV T). It was accompanied by a significant decrease in the D98 % for the 3 PTV (1.4 Gy for PTV H, p = 0.007; 0.3 Gy for PTV I, p = 0.03 and 1.15 Gy for PTV L, p = 0 0.0066). In addition, we observed a significant reduction in the conformity index in the order of 0.02 (p = 0.001) and 0.01 (p = 0.007) for PTV H and PTV I, respectively. The conformity variation was not significant for PTV L. Moreover, results showed a significant increase of the homogeneity index for PTV H (+ 0.03, p = 0.04) and PTV L (+ 0.04, p = 0.01).
Conclusion
Tumor volume reduction during the IMRT of NPC was accompanied by deterioration of the dosimetric coverage for the different target volumes. It is essential that a careful adaptation of the treatment plan be considered during therapy for selected patients.
Keywords: Nasopharyngeal carcinoma, Intensity modulated radiotherapy, Anatomic changes, Dose distribution.
Abreviations: NPC, Nasopharyngeal Carcinoma; IMRT, Intensity Modulated Radiation Therapy; OAR, Organs At Risk; CTint, Initial CT; GTV, Gross Tumor Volume; CTV, Clinical Target Volume; PTV, Planning Target Volume; CTV (H), High Risk Clinical Target Volume; CTV (I), Intermediate Risk Clinical Target Volume; CTV (L), Low Risk Clinical Target Volume; PTV (H), High Risk Planning Target Volume; PTV (I), Intermediate Risk Planning Target Volume; PTV (L), Low Risk Planning Target Volume; CTmi, Mid-treatment CT; Vi, Initial Volume; Vm, Mid-treatment Volume; ID, Dice Index; CI, Conformity Indice; HI, Homogeneity Indice
1. Introduction
Radiotherapy is the standard treatment for nasopharyngeal carcinoma (NPC). Recently, several types of research have been developed to improve the therapeutic results through the combination of systemic treatment[1], [2], [3], [4] and the use of new radiotherapy techniques.
The use of Intensity Modulated Radiation Therapy (IMRT) has not only significantly reduced side effects and improved quality of life but also improved disease control and overall survival.5,6 Thus, it is considered the standard technique for NPC radiotherapy. IMRT is a promising technique for this site because of its capacity to provide high-dose conformity to irregularly concave tumor volumes and to spare multiple, proximal organs at risk, including, especially, the nerve structures and parotid glands. The fundamental characteristic of this new radiotherapy technique is the possibility to generate a high dose gradient between different target volumes and organs at risk (OAR).
The particularity of NPC compared to other head and neck cancers is its proximity to critical risk organs whose dysfunction can cause invalid toxicity, especially for the brainstem, spinal cord, and ocular structures. It is also characterized by a large node volume repressing the parotid glands.
During the 7 weeks of radiotherapy for NPC, patient anatomy changes can be observed.7 These changes may concern tumor volume, particularly nodes, as well as specific OAR. This variation is mainly due to weight loss and tumor response. It may influence the dose distribution to target volume and OAR.
Adaptive radiotherapy, which is a new concept under development, makes it possible to take into account and integrate these patient-specific changes that could occur during treatment.8
To assess the magnitude of this problem, we prospectively measured the anatomic variability of irradiated target volumes during NPC IMRT and studied their impact on the accuracy of dose delivery.
2. Patients and methods
2.1. Patients characteristics
Between September 2016 and March 2018, twenty patients treated with IMRT for non-metastatic NPC in our institution were enrolled in this prospective study, including 14 men (70 %) and 6 women (30 %).
According to the TNM 8th edition, tumors were classified as T0 in 1 case (5 %), T1 in 8 cases (40 %), T2 in 2 cases (10 %), T3 in 1 case (5 %) and T4 in 8 cases (40 %). They belonged to the N0 group in 1 case (5 %), N1 in 3 cases (15 %), N2 in 13 cases (65 %) and N3 in 3 cases (20 %).
All patients received neoadjuvant chemotherapy with 3 cycles of TPF (Cisplatin 75 mg/m²: day 1, Docetaxel 75 mg / m²: day 1 and 5FU 750 mg / m²: day 1 to day 5) administered every 21 days and concomitant chemoradiotherapy with weekly Cisplatin 40 mg/m2.
2.2. First treatment planning
The initially planned dosimetry was performed according to the protocol adopted in our institution. Two dosimetric CTs were made under the same conditions for all patients: the first before the neoadjuvant chemotherapy and the other after the 3dTPF cycle. After the fusion of the two scanners, the delineation was performed on CT after chemotherapy, and it is considered as the initial or reference CT (CTint). Target volumes were defined according to the ICRU reports 50, 62, and 83,9,10 including gross tumor volumes (GTV), clinical target volumes (CTV), and planning target volumes (PTV).
The GTV T consisted of the residual volume after neoadjuvant chemotherapy, including the entire nasopharyngeal mucosa. The high risk CTV T (H) included a 3 mm margin around the GTV in all directions except posterior, one near the brainstem, where it was reduced to 1 mm. The intermediate risk CTV T (I) was defined as the CTV (H) plus the region for potential microscopic disease, and the initial tumor volume before chemotherapy. The PTVs T (H and I) were generated by adding a 3 mm margin around the CTVs (H and I).
For the node volume, the GTV N corresponds to the persistent macroscopic node after chemotherapy. The CTV N (H) is obtained by a 5 mm margin around the GTV N excluding the bone, and the prophylactic or low-risk CTV N (L) included bilateral II, III, IVa, Vabc and VIIab sectors. The Ib node area was included only if involved. The delineation of the different CTV N (L) was done according to the consensus published by Grégoire.11 A 3 mm margin was added to obtain the PTVs N to the CTVs N (H and L) after adjustment to the external contour. Finally, three PTVs were obtained with three dose levels prescribed according to Table 1.
Table 1.
Planning target volumes definition.
| Definition | Dose (daily fraction) | |
|---|---|---|
| PTV H | PTV T (H) + PTV N (H) | 69.96 Gy (2.12) |
| PTV I | PTV (H) + PTV T (I) | 60 Gy (1.81) |
| PTV L | PTV (I) + PTV N (L) | 54 Gy (1.63) |
PTV : planning target volume, H : high, I : intermediate, L : low.
The delineation of the OARs was made by referring to the experts' recommendations and published contouring atlas.[12], [13], [14]
IMRT planning was performed using the inverse planning software on the Varian 13 eclipse treatment planning system. The technique used was the integrated boost (SIB). The 3 PTVs (H, I, L) received respectively 69.96 Gy,60 Gy, and 54 Gy in 33 fractions. We treated all patient using seven static IMRT fields (0°, 51°, 102°, 153°, 204°, 255° and 306°) with a 6 MV photon energy from the Varian iX linear electron accelerator. Inverse planning involves defining dose constraints by setting decreasing priority ranging from PTVs to OARs from the most to the least critical. Repetitive iterations are done for this purpose until obtaining the best dosimetric compromise.
2.3. Second treatment planning
For each patient, a second dosimetric CT was performed at a dose of 38 Gy and then was fused with the initial planning scan. Manual contouring of the macroscopic tumor volumes (GTV T and N) and the planning target volumes (PTV H, I and L) were done in the second CT according to the same recommendations as initially. In order to minimize the inter-observer variations, the contouring of the second computed tomography (CTmi) was completed by the same observer.
A new structure “volume of interest” was created on the two scanners (CT int and CT mi). It included the entire volume of the patient from the skull vault to the inferior border of the clavicle.
The initial treatment plan was transferred to the CTmi respecting the anatomic position of the planning treatment isocenter. The recalculation of the dose distribution has been fulfilled keeping the same unit monitor number.
2.4. Data collection
We collected the following data for each patient and each structure:
-
-
The initial volume (Vi) and the mid-treatment volume (Vm) from which the relative difference was calculated according to the formula(.
-
-
The difference of the isocenter position between the first and second CT in the 3 spaces axes: the X-axis (or latero-medial), the Y-axis (or anteroposterior) and the Z-axis (or craniocaudal).
-
-
The Dice index (ID) or Dice Similarity coefficient : ID = 2x()
Secondly, we recorded for each PTV from the two CT: the maximum dose (Dmax), the minimum dose (D98 %), the dose received by 95 % of the volume (D95 %) and the dose received by 2 % of the volume (D2 %). The Conformity and homogeneity indices (CI, HI) were calculated according to the two equations, respectively, IC = ; IH =
We also measured the patient's weight at the beginning, at mid-treatment, and we calculated the percentage of weight loss.
Statistical data analysis was done on the SPSS 20 software. The normality of the quantitative variables was verified by the Shapiro-Wilk test. For the normal distribution variables, the matched-paired t-test was cured for the mean comparison and the Pearson test for the correlations. For variables with non-normal distribution, the Wilcoxon test for mean comparison and the Spearman test for correlations were used. We compared the means according to the T and N stages with the ANOVA test. p value <0.05 was considered as statisticaly significant.
3. Results
3.1. Volumetric variations
Significant reductions in tumor volumes were found between the two scans. The median was -7.4 cc [−28.1 ; −0.5] for the GTV T (p <10−³) and −7.9 cc [−117.9 ; -1.1] for the GTV N (p <10-³). The value of the median decrease in the volume of interest patient was −947.5 cc (−10.54 %). This volume decrease was significantly correlated with weight loss (median of 3.3 Kg, p <10−³). The correlation coefficient was 0.67.
For PTVs, the median reduction was −52.6 cc, −36 cc and −37.6 cc for PTV H, PTV I and PTV L respectively, p <10−³ (Table 2).
Table 2.
Target volumes changes.
| Structure | Volume variation (cc) |
Percentage (%) |
||
|---|---|---|---|---|
| Median [extremes] | Mean ± SD | Median [extremes] | Mean ± SD | |
| GTV T | −7.4 [−28.1 ; −0.5] |
−8.9 ± 6.2 | −30 [−59.6 ; −3.6] |
−29.5 ± 15.3 |
| GTV N | −7.9 [−117.9 ; −1.1] |
−16.2 ± 27.3 | −56.2% [−81.4 ; −34] |
−58.2 ± 12.5 |
| PTV H | −52.6 [−334.5 ; −10] |
−70.9 ± 70.1 | −34.4 [−76.2 ; −7.6] |
−34.2 ± 14.6 |
| PTV I | −36 [−323.8 ; −13.1] |
−60.1 ± 70.6 | −13 [−58.9 ; 5.5] |
−16.9 ± 12.2 |
| PTV L | −37.6 [−303.9 ; −7.6] |
−79.7 ± 83.9 | −6.24 [−33.1 ; 1.7] |
−11.6 ± 10 |
GTV : Gross Tumor Volume, PTV : Planning Target Volume, T : Tumor, N : Node, H : High, I : Intermediate, L : Low, SD : Standard Derivation.
There was no relationship between decreasing tumor volumes and weight loss. However, we observed a significant correlation between the reduction of GTV N, PTV I and N stage (p = 0.01 and 0.04, respectively) (Table 3).
Table 3.
Isocenter movements of target volumes (mm).
| Structure | X axe |
Y axe |
Z axe |
|||
|---|---|---|---|---|---|---|
| Median [extremes] | Mean ± SD | Median [extremes] | Mean ± SD | Median [extremes] | Mean ± SD | |
| GTV T | +0.2 [−1 ; 3] |
+0.3 ± 1 | +1.1 [-0.6 ; 4.5] | +1.3 ± 1.1 | +0.8 [-5.4 ; 3,2] |
+0.5 ± 2,1 |
| GTV N | +1.8 [-12 ; 18] |
+1.5 ± 8.5 | −0.9 [-5.6 ; 1.2] |
−0.2 ± 4.3 | +2 [-13 ; 19] |
+2.4 ± 6.4 |
| PTV H | +0.7 [-10 ; 9.9] |
+0.4 ± 5.7 | −0.7 [−3.5 ; 8.1] |
+0.6 ± 3 | +3.8 [-3.9 ; 9.5] |
+5.7 ± 8.6 |
| PTV I | −0.2 [−16 ; 9.6] |
−0.3 ± 5.5 | −2.2 [−6.1 ; 8.8] |
−1.9 ± 2.9 | +6.8 [-21 ; 21] |
+6.2 ± 8.1 |
| PTV L | −0,3 [−7.1 ; 1.7] |
−0.5 ± 1.9 | −0.1 [−3.6 ; 5.1] |
+0.1 ± 2.2 | +2.3 [-16 ; 13] |
+2.18 ± 7 |
GTV : Gross tumor volume, PTV : Planning Target Volume, T : Tumor, N : Node, H : high, I : Intermediate, L : Low, SD : Standard Derivation.
3.2. Geometric variations
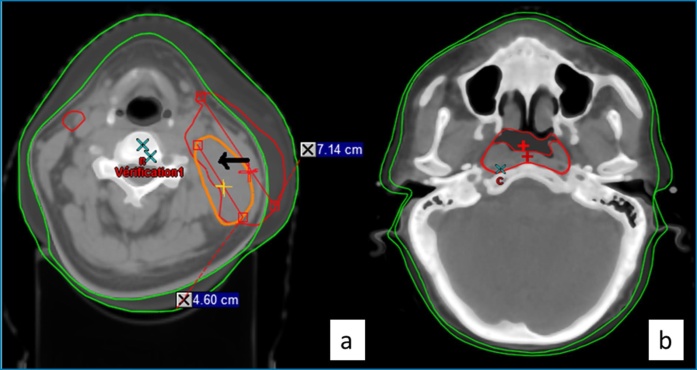
At 38 Gy, a supra-millimeter migration of the GTV N isocentre was found in the X-axis (Fig. 1a). The median shifting was 1.8 mm [−12; 18.7]. In the Y-axis, the variation concerned the GTV T and the PTV I with a median of movement of +1.1 mm [−0.6; 4.5] (Fig. 1b) and −2.2 mm [−6.1; 8.8], respectively.
Fig. 1.
Geometric variation of the target volume.
1a: Nodal Gross Tumor Volume isocenter shift on the X-axis (initial isocenter in red (dark line), mid-treatment in orange (white line)
1b: Tumoral Gross Tumor Volume isocenter shift on the Y-axis (initial isocenter in light red (light), mid-treatment in drak red (dark)))
The Dice Index was low for GTV N with a median of 0.51 [0.14 ; 0.71]. It was 0.79 [0.57 ; 0.93] and 0.75 [0.39 ; 0.95] for GTV T and PTV H, respectively. For PTV I, this index was 0.91 [0.57 ; 0.95] and for PTV L, it was 0.88 [0.69 ; 0.99].
There was no correlation between the positional variations of target volumes and weight loss or T and N stage.
3.3. Dosimetric variations
Compared to the initially planned dosimetry, a mean addition of 0.76 ± 1.2 Gy was observed at mid-treatment for the D max (1.08 % of the prescribed dose). This increase was statistically significant (p = 0.009).
This behavior was accompanied by a significant decrease in the D98 % for the 3 PTVs, with a value of 1.4 ± 2.1 Gy for PTV H (p = 0.007), 0.3 ± 1 Gy for PTV I(p = 0.03) and 1.15 ± 1.6 Gy (0 0.0066) for PTV L.
In addition, we observed a significant reduction in the conformity index of 0.02 ± 0.03 (p = 0.001) and0.01 ± 0.01(p = 0.007) for PTV H and PTV I, respectively. The conformity variation was not statistically significant for PTV L (−0.004 ± 0.009, p = 0.07).
Moreover, results showed a significant increase in the homogeneity index for PTV H (+0.03 ± 0.14, p = 0.04) and PTV L (+ 0.04 ± 0.06, p = 0.01). However, no significant difference was observed for PTV I (+ 0.02 ± 0.1, p = 0.1) (Table 4).
Table 4.
Dosimetric variations between planned CT scan and mid-treatment CT scan.
| Volume | D98 % (Gy) |
CI |
HI |
|||
|---|---|---|---|---|---|---|
| Median [extremes] | Mean ± SD | Median [extremes] | Mean ± SD | Median [extremes] | Mean ± SD | |
| PTV H | −0.3 [−5.7 ; 1.1] |
−1.4 ± 2.1 | −0.02 [−0.1 ; 0.01] |
−0.02 ± 0.03 | 0.04 [−0.4 ; 0.4] |
0.03 ± 0.1 |
| PTV I | −0.005 [-2.1 ; 1.1] |
−0.3 ± 1 | −0.005 [−0.07 ; 0.01] |
−0.01 ± 0.01 | 0.01 [−0.04 ; 1.1] |
0.02 ± 0.1 |
| PTV L | −1 [−5.1 ; 0.9] |
−1.1 ± 1.6 | 0 [−0.04 ; 0] |
−0.004 ± 0.009 | 0.04 [−0.4 ; 0.18] |
0.04 ± 0.06 |
PTV : Planning Target Volume, CI : Conformity Index, HI : Homogeneity Index, H : High, I : Intermediate, L : Low, CT : Computed Tomography, D98 % : Dose received by 98 % of the volume, SD : Standard Derivation.
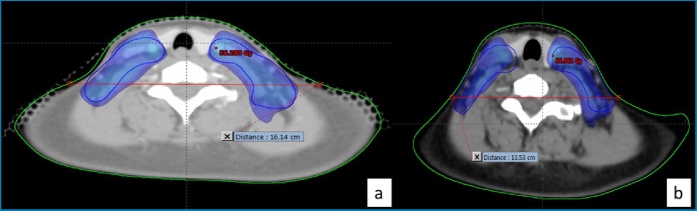
There was no relationship between the change in the target volume coverage and the T stage. However, the homogeneity deterioration of the PTV L was correlated with the N stage (p = 0.022). Indeed, the variation was -0.14 for N0, +0.055 for N1, +0.06 for N2 and +0.025 for N3 (Fig. 2).
Fig. 2.
Deterioration of Low Risk Planning Target Volume (blue) at the lower part of the neck.
2a: Initially planned dosimetry : 51.3 Gy dose distribution ; Neck Diameter : 16.14 cm
2b: Visualization of the initial dosimetry on the mid-treatment CT : 51.3 Gy dose distribution ; Neck Diameter : 13.53 cm
4. Discussion
New high precision radiotherapy techniques are characterized by better conformity to target volumes while sparing OAR. These new techniques have recently incorporated anatomical variations of the patient during treatment and have tried to adapt to them.
For NPC, a significant anatomic change during the course of radiation therapy was observed. This is due to a primary tumor, involved lymph nodesreponse, and weight loss. The dose coverage to target volumes and/or typical structures may change significantly during the course of IMRT, especially toward the later part of the treatment. Anatomic changes could compromise the dose coverage to the tumor volumes and potentially reduce local control of the diseases. However, the planning CT images are obtained before the start of radiotherapy, and replanning CT is not a common practice for NPC.
In this prospective study, the target volume changes and their dosimetric impact were measured and compared between the planned and at middle treatment for NPC patients during IMRT.
A significant reduction in target volumes was found at 38 Gy, with a more considerable decrease for lymph node volume in the order of 58.56 % versus 29.52 % for GTV T. The synergistic effect of radiotherapy and chemotherapy on the reduction of tumor volume has been well established.15 However, the degree of the response depends on individual radiosensitivity and delivered doses.
These anatomic changes observed in our study were mostly consistent with the results of other published studies. Wang et al. reported an average GTV N reduction of 40.2 % at 38 Gy with concomitant radiochemotherapy.16
In a retrospective series17 of 33 patients treated for NPC, the decrease in GTV T volume was estimated at 4.14 cc after 20 Gy and 32.51 cc after 40 Gy.
We also found a significant decrease in the volume of the 3 PTVs. The most considerable reduction was in PTV H with −34.4 %, followed by PTV I (-16.96 %), and PTV L (-11. 64 %). Our results were similar to those of Cheng et al.,18 who observed a significant lowering in all target volumes for 19 NPC patients. This decrease was 9.3 % for the GTV T and16.2 % for the GTV N. It was 11.12 %, 8.89 %, and 0.8 %, respectively, for PTV high, medium and low risk for tumor volume (T) and 12.41 %, 9.9 % and 5.6 % for node volume (N).
In addition to the volumetric target volume modifications, positional changes were found in our study with a migration of the GTV T isocenter on the anteroposterior axis (Y) of a mean of 1.3 mm and the GTN N of 1.5 mm on the X-axis and 2.4 mm on the Z axis, suggesting the tumor response. Recently, Tan et al. have reported the same results19 with an average migration of 1.5–2.5 mm for the GTV at the first week to reach from 5.2 mm to 6.2 mm at the last week.
The current study revealed significant anatomic and positional changes in tumor and node volumes, which induced changes of dose distribution in IMRT for NPC. We observed a decrease in the D98 % of the 3 PTVs, with a mean of −1.82 Gy for the PTV H, -0.46 Gy for the PTV I and -1.49 Gy for the PTV L. These dose reductions were accompanied by a deterioration in both the conformity index (−0.004 to −0.02), and the homogeneity index (+0.02 to +0.04).
The decrease in the lateral diameter of treatment volume, especially in the lower part of the neck during radiotherapy for head and neck tumors, is a well-known event. Its causes include patient weight loss resulting from radiation side effects and reduction of nodal masses.
Some authors suggested that changes in the irradiated volume diameters lead to changes in dose distribution and may cause a loss in the coverage of target volumes.20 Indeed, their study demonstrated a significant worsening of the target volume coverage related to geometry changes during the course of IMRT. Hansen et al.21 found a significant diminution in target volume doses in a retrospective study of 13 patients. This decrease affected 92 % of patients. It ranged from 0.8 to 6.3 Gy (p = 0.02) for PTV H and 0.2–7.4 Gy (p = 0.003) for PTV I, which is consistent with our results.
In addition, we found an increase in the maximal dose of 0.76 Gy on average, an equivalent to 1.08 % in the prescribed dose. It is observed for75 % of cases. The rise of the high dose was reported by many authors (16–1922). Cheng et al. observed a significant augmentation of the D95 % by a mean of 0.3 Gy for the lymph nodes and 0.1 Gy for the tumor volume. For PTVs, this rise was not significant.18 This finding was similar to the report of Wei Wang et al.22 who also demonstrated an increase in D95 %. It was 4.91 % for CTV and 8.19 % for GTV N. In their study, 28 patients were treated for NPC with a repeated CT scan at the 25th fraction. On the other hand, there was no significant change in the target volume coverage during radiotherapy for head and neck cancer.16
In our study, all patients underwent a second verification CT, in the middle of treatment, for measuring the anatomic changes and a dosimetric comparison with the initial dosimetric plan. But no patients were treated using replanning. Therefore, the clinical implication of replanning after 18 fractions of NPC IMRT with concurrent chemotherapy with regard to ameliorating dose coverage and tumor control was unknown and cannot be accomplished without an extensive prospective study.
5. Conclusion
Based on our study data and literature results, it would not be reasonable to propose adaptive radiotherapy for all patients treated for nasopharyngeal carcinoma but rather for a subgroup of patients who would benefit.
This approach should be discussed for selected patients who have large tumors and/or lymph node, with significant weight loss and tumor volume shrinkage. For these patients, a new planning treatment adapted to anatomic changes could improve the target volumes coverage and reduce doses to the organs at risk. Other investigations are also necessary to improve the contribution of this approach and to verify the criteria for the choice of candidates for replanning. This validation should necessarily involve prospective studies and randomized trials showing the impact of this approach in terms of toxicities and oncological findings.
Financial disclosure statement
No funding has been received for the completion of this research
Conflicts of interest
None.
References
- 1.Baujat B., Audry H., Bourhis J. Chemotherapy as an adjunct to radiotherapy in locally advanced nasopharyngeal carcinoma. Cochrane Database Syst Rev. 2006;18(4) doi: 10.1002/14651858.CD004329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanchard P., Lee A., Marguet S. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi: 10.1016/S1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L., Zhao C., Ghimire B. The role of concurrent chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma among endemic population: A meta-analysis of the phase III randomized trials. BMC Cancer. 2010;10(1) doi: 10.1186/1471-2407-10-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langendijk J.A., Leemans Ch R., Buter J., Berkhof J., Slotman B.J. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: A meta-analysis of the published literature. J Clin Oncol. 2004;22(22):4604–4612. doi: 10.1200/JCO.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 5.Zhang M.-X., Li J., Shen G.-P. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: A 10-year experience with a large cohort and long follow-up. Eur J Cancer. 2015;51(17):2587–2595. doi: 10.1016/j.ejca.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Peng G., Wang T., Yang K. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. Conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–293. doi: 10.1016/j.radonc.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Barker J.L., Garden A.S., Ang K.K. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int JRadiat OncolBiolPhys. 2004;59(4):960–970. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Yan D. Adaptive radiotherapy: Merging principle into clinical practice. Semin Radiat Oncol. 2010;20(2):79–83. doi: 10.1016/j.semradonc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Chavaudra J., Bridier A. Définition des volumes en radiothérapie externe : Rapports ICRU 50 et 62. CancerRadiother. 2001;5(5):472–478. doi: 10.1016/s1278-3218(01)00117-2. [DOI] [PubMed] [Google Scholar]
- 10.International Commission on Radiation Units and Measurements (ICRU) https://icru.org/testing/reports/prescribing-recording-and-reporting-intensity-modulated-photon-beam-therapy-imrt-icru-report-83. [PubMed]
- 11.Grégoire V., Ang K., Budach W. Delineation of the neck node levels for head and neck tumors: A 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol. 2014;110(1):172–181. doi: 10.1016/j.radonc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Sun Y., Yu X-L Luo W., Lee A.W., Wee J.T.S., Lee N. Recommendation for a contouring method and atlas of organs at risk in nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Radiother Oncol. 2014;110(3):390–397. doi: 10.1016/j.radonc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Brouwer C.L., Steenbakkers R.J., Bourhis J. CT-based delineation of organs at risk in the head and neck region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG consensus guidelines. Radiother Oncol. 2015;117(1):83–90. doi: 10.1016/j.radonc.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 14.Noël G., Antoni D., Barillot I., Chauvet B. Délinéation des organes à risque et contraintes dosimétriques. CancerRadiother. 2016;20:S36–S60. doi: 10.1016/j.canrad.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 15.O’Sullivan B. Nasopharynx Cancer: Therapeutic value of chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(2 Suppl):S118–21. doi: 10.1016/j.ijrobp.2007.04.085. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Lu J., Xiong X. Anatomic and dosimetric changes during the treatment course of intensity-modulated radiotherapy for locally advanced nasopharyngeal carcinoma. Med Dosim. 2010;35(2):151–157. doi: 10.1016/j.meddos.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L., Wan Q., Zhou Y., Deng X., Xie C., Wu S. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2011;98(1):23–27. doi: 10.1016/j.radonc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Cheng H.C.Y., Wu V.W.C., Ngan R.K.C. A prospective study on volumetric and dosimetric changes during intensity-modulated radiotherapy for nasopharyngeal carcinoma patients. RadiotherOncol. 2012;104(3):317–323. doi: 10.1016/j.radonc.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Tan W., Ye J., Xu R. The tumor shape changes of nasopharyngeal cancer during chemoradiotherapy: The estimated margin to cover the geometrical variation. Quant Imaging Med Surg. 2016;6(2):115–124. doi: 10.21037/qims.2016.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senkus-Konefka E., Naczk E., Borowska I., Badzio A., Jassem J. Changes in lateral dimensions of irradiated volume and their impact on the accuracy of dose delivery during radiotherapy for head and neck cancer. RadiotherOncol. 2006;79(3):304–309. doi: 10.1016/j.radonc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Hansen EK, Bucci MK, Quivey JM, Weinberg V, Xia P. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat OncolBiolPhys. [DOI] [PubMed]
- 22.Wang W., Yang H., Hu W. Clinical study of the necessity of replanning before the 25th fraction during the course of intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J of Radiat OncolBiolPhys. 2010;77(2):617–621. doi: 10.1016/j.ijrobp.2009.08.036. [DOI] [PubMed] [Google Scholar]