Abstract
Bone metastasis (BM) in cancer remains a critical issue because of its associated clinical and biological complications. Moreover, BM can alter the quality of life and survival rate of cancer patients. Growing evidence suggests that bones are a fertile ground for the development of metastasis through a “vicious circle” of bone resorption/formation and tumor growth. This review aims to outline the current major issues in the diagnosis and management of BM in the most common types of osteotropic cancers and describe the mechanisms and effects of BM. First, we discuss the incidence of BM through the following questions: Are we witnessing an increase in incidence, and are we now better equipped with modern imaging techniques? Is the advent of efficient bone resorption inhibitors affecting the bigger picture of BM management? Second, we discuss the potential effects of cancer progression and well-prescribed drugs, such as multitarget tyrosine kinase inhibitors, inhibitors of the mammalian target of rapamycin, and immune checkpoint inhibitors, on BM. Finally, we examine the duality of the effects of some therapies that may help in cancer treatment but may also contribute to further BM.
Introduction
Bone metastasis (BM) can occur during the course of the disease in many solid tumors. BM typically occurs in breast, lung, prostate, thyroid, and renal carcinoma, and the bone is the third most prevalent site of metastasis after the liver and lung [1,2]. However, this category of “osteotropic” cancers is now largely out-of-date, and BM is commonly identified in many other solid tumors, such as melanoma, gastrointestinal tumors, and head and neck cancers [3]. The management of BM has also gained much interest among clinicians, because BM has major functional effects and can affect the quality of life of patients. BM may cause complications such as pain, nerve root or spine cord compression, vertebral or peripheral fractures, hypercalcemia, and bone marrow infiltration that leads to cytopenia [2,4,5]. These events also affect patient survival rates [6].
Many focal treatments that use bone resorption inhibitors (BRIs) can prevent or reduce the occurrence of BM complications. The detection of BM has also improved with the advent of imaging techniques with increased sensitivity and specificity in detecting bone lesions [7].
The results of fundamental scientific studies have also led to major breakthroughs in understanding the mechanisms underlying BM development. Bone tissue provides a good environment for metastatic tumor cells and is rich in hematopoietic cells, bone cells, and growth factors and other molecules involved in bone-homing and the growth, dormancy, and resistance of tumor cells. This bone microenvironment is also an active and fertile ground for the development of BM [8].
Systemic anticancer treatments such as chemotherapy, targeted therapy, and immune checkpoint therapy can increase the survival rate of patients. However, the mechanisms of action of these therapies also affect the bone microenvironment, and these effects may result in the development of a fertile ground for certain tumor cells, the proliferation of which can lead to BM development.
This review aims to outline the current state of knowledge on BM, particularly its epidemiology, diagnosis, treatment, and underlying molecular mechanisms.
Epidemiology of Bone Metastasis
We examined the epidemiology of BM in the most common types of osteotropic cancers, such as prostate, breast, lung, and renal cell carcinoma, using the results of recent major phase III clinical trials and meta-analyses that have been published over the past 10 years (Table 1). Most of the phase III trials did not consider the BM incidence rate, but among those studies that did, we found an increase in the detection of BM during cancer progression.
Table 1.
Updated Data of BM Rates from Clinical Trials and Meta-analysis on Most Common Osteotropic Cancers.
| Primary Site | Patients and Trial Characteristics | Line of Treatment | BM Rate | Bone Event (SRE, SSE) | Study Ref |
|---|---|---|---|---|---|
| BREAST | Randomized phase 2 study. Comparing palbociclib + letrozole vs letrozole alone. N = 165 patients, postmenopausal women with advanced estrogen receptor-positive and HER2-negative breast cancer who had not received any systemic treatment for their advanced disease. |
Line 1 | 20% palbociclib + letrozole vs. 15% letrozole (bone only) | NS | PALOMA-1 Finn, 2015 [15] |
| Randomized phase 3 study. Comparing palbociclib + letrozole or placebo + letrozole. N = 666 patients, postmenopausal women with ER-positive, HER2-negative breast cancer, who had not had prior treatment for advanced disease. |
Line 1 | 23.2% palbociclib-letrozole vs. 21.6% letrozole (bone only) | NS | PALOMA-2 Finn, 2016 [16] |
|
| Randomized phase 3 study. Comparing pertuzumab, trastuzumab, and docetaxel with placebo, trastuzumab, and docetaxel. N = 808 patients, metastatic breast cancer who had not received previous chemotherapy or anti-HER2 therapy for their metastatic disease to receive the pertuzumab combination or the placebo combination. |
Line 1 | 12% “nonvisceral” | NS | CLEOPATRA Swain, 2015 [12] |
|
| Randomized phase 3 study. Comparing, fulvestrant plus palbociclib to fulvestrant plus placebo. N = 521 patients, women aged 18 years or older with hormone-receptor-positive, HER2-negative metastatic breast cancer that had progressed on previous endocrine therapy. |
L2 | 22% fulvestrant + palbociclib vs. 21% fulvestrant + placebo (bone only) | NS | PALOMA-3 Cristofanilli, 2016 [17] |
|
| Randomized phase 3 study. Comparing everolimus and exemestane versus exemestane and placebo. N = 724 patients, with hormone-receptor-positive advanced breast cancer who had recurrence or progression while receiving previous therapy with a nonsteroidal aromatase inhibitor in the adjuvant setting or to treat advanced disease (or both). |
L2 | 76% | NS | BOLERO-2 Baselga, 2012 [18] |
|
| Randomized phase 3 study. Comparing either trastuzumab emtansine or capecitabine and lapatinib. N = 991 patients, women aged 18 years or older with HER2-positive unresectable, locally advanced or metastatic breast cancer previously treated with trastuzumab and a taxane. |
>L2 | 32% “nonvisceral” | NS | EMILIA Diéras V, 2017 [13] |
|
| Randomized phase 3 trial. Comparing olaparib to standard therapy with single-agent chemotherapy of the physician's choice (capecitabine, eribulin, or vinorelbine). N = 302 patients, with a germline BRCA mutation and human epidermal growth factor receptor type 2 (HER2)-negative metastatic breast cancer who had received no more than two previous chemotherapy regimens for metastatic disease. |
>L2 | 7.8% olaparib vs. 6.2% placebo (bone only) | NS | OLYMPIAD Robson, 2017 [19] |
|
| Randomized phase 3 study. Comparing eribulin mesylate to Treatment physician choice. N = 762 patients, women with locally recurrent or metastatic breast cancer who had received between two and five previous chemotherapy regimens (two or more for advanced disease), including an anthracycline and a taxane, unless contraindicated. |
>L2 | 60% | NS | EMBRACE Cortes, 2011 [14] |
|
| PROSTATE | Randomized phase 3 study. Comparing androgen deprivation therapy (ADT) plus docetaxel to ADT alone. N = 192 patients. |
Castration-naïve | 81% | NS | GETUG 15 Gravis G, 2013 [20] |
| Randomized phase 3 study. Comparing standard of care only (SOC-only; control), standard of care plus zoledronic acid (SOC + ZA), standard of care plus docetaxel (SOC + Doc), or standard of care with both zoledronic acid and docetaxel (SOC + ZA + Doc). N = 2962 patients. It recruits men with high-risk, locally advanced, metastatic, or recurrent prostate cancer who are starting first-line long-term hormone therapy. |
Castration-naïve | 54% | NS | STAMPEDE James MD, 2016 [21] |
|
| Randomized phase 3 study. Comparing ADT plus docetaxel to ADT alone. N = 790 patients. |
Castration-naïve | NS | NS | CHAARTED Sweeney, 2015 [22] |
|
| Randomized phase 3 trial. Comparing ADT plus abiraterone acetate plus prednisone (abiraterone group) to ADT plus dual placebos (the placebo group). N = 1199 patients. |
Castration-naïve | 97% abiraterone group vs. 98% placebo group | 15% bone pain and 2% spinal cord compression | LATITUDE Fizazi, 2017 [23] |
|
| Randomized phase 3 trial. Comparing abiraterone acetate plus prednisone (abiraterone group) to placebo plus prednisone (placebo group). N = 1088 asymptomatic or mildly symptomatic patients with chemotherapy-naive prostate cancer. |
Castration-résistant | NS | NS | COU-AA-302 Ryan, 2015 [24] |
|
| Randomized phase 3 trial. Comparing cabazitaxel 20 mg/m2 plus daily prednisone to 25 mg/m2 plus daily prednisone. N = 1168 patients. |
Castration-resistant | 88.7% arm C20 vs. 88.9 arm C25 | NS | FIRSTANA Oudard, 2017 [25] |
|
| Randomized phase 3 study. Comparing prednisone + abiraterone acetate to placebo. N = 1195 patients, with metastatic castration-resistant prostate cancer progressing after docetaxel. |
Castration-resistant | 90% in both arms | 29% (abiraterone) vs. 33% (placebo) | COU-AA-301 Fizazi, Lancet, 2012 [26] |
|
| Randomized phase 3 study. Comparing oral enzalutamide to placebo. N = 1199 men with castration-resistant prostate cancer after docetaxel. |
Castration-resistant | 92% included 38% of patients with >20 lesions | NS | AFFIRM Sher HI, NEJM, 2012 [27] |
|
| Randomized phase 3 study. Comparing mitoxantrone to cabazitaxel 25 mg/m2. N = 755 patients, men with metastatic castration-resistant prostate cancer who had received previous hormone therapy, but whose disease had progressed during or after treatment with a docetaxel-containing regimen. |
Castration-resistant | 83.6% (87% mitoxantrone vs. 80% cabazitaxel) | 45% | TROPIC de Bono, 2010 [28] | |
| LUNG | Randomized phase 3 study. Comparing oral erlotinib 150 mg per day or 3-week cycles of standard intravenous chemotherapy of cisplatin plus docetaxel or gemcitabine. Carboplatin with docetaxel with gemcitabine was allowed in patients unable to have cisplatin. N = 174 patients with NSCLC and EGFR mutations with no history of chemotherapy for metastatic disease. |
NSCLC Line 1 | 33% | NS | EURTAC Rosell, 2012 [36] |
| Randomized phase 3 study. Comparing gefitinib or carboplatin-paclitaxel. N = 230 patients with metastatic, NSCLC and EGFR mutations who had not previously received chemotherapy to receive gefitinib or carboplatin-paclitaxel. |
Line 1 | NS | NS | North-East Japan Study Group Maemondo, 2010 [31,32] |
|
| Randomized phase 3 study. Comparing oral crizotinib to intravenous chemotherapy pemetrexed, plus either cisplatin, or carboplatin. N = 343 patients with advanced ALK-positive nonsquamous NSCLC who had received no previous systemic treatment for advanced disease. |
Line 1 | NS | NS | PROFILE1014 Solomon, 2014 [33] |
|
| Randomized phase 3 study. Comparing alectinib to crizotinib. N = 207 patients, ALK inhibitor-naive with ALK-positive NSCLC, who were chemotherapy-naive or had received one previous chemotherapy regimen, in Japan. |
Line 1 | NS | NS | J-ALEX Hida, 2017 [34] |
|
| Randomized phase 3 study. Comparing osimertinib to a standard EGFR-TKI. N = 556 patients with previously untreated, EGFR mutation-positive advanced NSCLC. |
Line 1 | NS | NS | FLAURA Soria, 2018 [35] |
|
| Randomized phase 3 study. Comparing oral crizotinib to intravenous chemotherapy with either pemetrexed or docetaxel. N = 347 patients with locally advanced or metastatic ALK-positive lung cancer who had received one prior platinum-based regimen. |
>Line 1 | NS | NS |
NCT00932451 Shaw, 2013 [37] |
|
| Randomized phase 3 study. Comparing two schemes of maintenance treatment with bevacizumab and bevacizumab plus pemetrexed treatment, respectively, after an induction treatment with bevacizumab, cisplatin, and pemetrexed. N = 376 patients with advanced nonsquamous NSCLC received first line. |
>Line 1 | NS | NS | AVAPERL Barlesi, 2013 [38] |
|
| Randomized phase 3 study. Comparing maintenance pemetrexed plus best supportive care or with placebo plus BSC. N = 1022 patients with advanced non-squamous NSCLC with no previous systemic chemotherapy for lung cancer. 939 participated in the induction phase. Of these, 539 patients were randomly assigned. |
>Line 1 | NS | NS | PARAMOUNT Paz-Arés, 2012 [39] |
|
Comparing nivolumab to docetaxel. N = 272 patients, with stage IIIB or IV squamous-cell NSCLC who had disease recurrence after one prior platinum-containing regimen were eligible. |
>Line 1 Immune-checkpoint trials |
NS | NS | CheckMate 017 Brahmer, 2015 [40] |
|
Patients with NSCLC that had progressed during or after platinum-based doublet chemotherapy to receive nivolumab or docetaxel. Of 582 randomized patients, 287 were treated with nivolumab and 268 were treated with docetaxel. |
CheckMate 057 Borghaei, 2015 [41] |
||||
Patients with previously treated NSCLC with PD-L1 expression on at least 1% of tumor cells were randomly assigned (1:1:1) to receive pembrolizumab 2 mg/kg, pembrolizumab 10 mg/kg, or docetaxel 75 mg/m(2) every 3 weeks. N = 1034 patients: 345 allocated to pembrolizumab 2 mg/kg, 346 allocated to pembrolizumab 10 mg/kg, and 343 allocated to docetaxel we enrolled. |
KEYNOTE-010 Herbst, 2016 [42] |
||||
N = 1225 patients recruited who had squamous or nonsquamous NSCLC, who had received one to two previous cytotoxic chemotherapy regimens (one or more platinum-based combination therapies) for stage IIIB or IV NSCLC. 425 patients were randomly assigned to receive atezolizumab, and 425 patients were assigned to receive docetaxel. |
OAK Rittmeyer, 2017 [43] |
||||
| KIDNEY | Randomized phase 3 study. Comparing the efficacy and safety of pazopanib and sunitinib as first-line therapy. N = 1110 patients with clear-cell, metastatic renal cell carcinoma, in a 1:1 ratio, to receive a continuous dose of pazopanib (557 patients) or sunitinib (553 patients). |
Line 1 | 20% pazopanib vs. 15% sunitinib | NS | COMPARZ Motzer, 2013 [46] |
| Double-blind cross-over study evaluated patient preference for pazopanib or sunitinib and the influence of health-related quality of life (HRQoL) and safety factors on their stated preference. N = 169 patients with metastatic RCC were randomly assigned to pazopanib then sunitinib or the reverse sequence. |
Line 2 | NS | NS | PISCES Escudier, 2014 [49] |
|
| Randomized phase 3 study. Comparing axitinib with sorafenib as second-line therapy in patients with metastatic renal cell cancer. N = 723 patients, with clear-cell carcinoma who progressed despite first-line therapy containing sunitinib, bevacizumab plus interferon-alfa, temsirolimus, or cytokines. |
Line 2 | 18% | NS | AXIS Rini, 2011 [50] |
|
| Randomized phase 3 study. Comparing nivolumab with everolimus in patients with RCC who had received previous treatment. N = 821 patients with advanced clear-cell renal cell carcinoma for which they had received previous treatment with one or two regimens of antiangiogenic therapy |
Line 2 | 18% | NS | Nivolumab vs. Everolimus Motzer, 2015 [51] |
|
| Randomized phase 3 study. Comparing the efficacy and safety of cabozantinib versus the mTOR inhibitor everolimus N = 658 patients with advanced renal cell carcinoma who progressed after previous VEGFR tyrosine-kinase inhibitor treatment. |
Line 3 | 23% Cabozantinib vs. 20% everolimus | NS | METEOR TK Choueiri, 2016 [47] |
|
| Randomized phase 3 study. Comparing dovitinib with sorafenib as a third-line targeted therapy in metastatic RCC. N = 570 patients with clear-cell metastatic RCC who received one prior VEGF-targeted therapy and one prior mTOR inhibitor. |
Line 3 | 35% dovitinib vs 42% sorafenib | NS | GOLD RCC Motzer, 2014 [48] |
BM, bone metastasis; EGFR, epidermal growth factor receptor; mTOR, mammalian target of rapamycin; NS, not specified; NSCLC, non–small-cell lung cancer; RCC, renal cell carcinoma; SRE, skeletal-related event; SSE, symptomatic skeletal event; VEGF, vascular endothelial growth factor.
Breast Cancer
A meta-analysis and review of literature was carried out by Body et al., in 2017 to estimate the proportion of breast cancer patients who later developed BM [9]. Their study collected data from >175,000 patients included in clinical trials. Based on the data, 12% of patients with stage I–III breast cancer who had undergone surgery developed BM. Their study also showed that the incidence of BM in patients who developed metastases during follow-up was 55%. This was lower than the rate reported in an autopsy series [10], in which 58% of patients with metastatic breast cancer from the outset had BM [10]. An even higher value of 73% was reported in a monocentric series of 264 patients after considering those treated across various treatment lines [11].
Recent chemotherapy trials, such as the CLEOPATRA trial [12] of first-line treatment and the EMILIA trial of second-line treatment, stratified patients according to the presence of visceral and nonvisceral disease (i.e., presence of BM) [13]. In the EMBRACE trial for eribulin as third-line treatment for metastatic breast cancer, 60% of patients had BM [14].
Recent trials of hormone therapy and anti-cyclin-dependent kinases—PALOMA-1, -2, and -3—stratified patients according to the presence of “bone-only disease,” and the incidence of BM was around 20% at baseline [[15], [16], [17]]. In the BOLERO-2 study, which aimed to evaluate the potential of using mammalian target of rapamycin (mTOR) inhibitors in combination with exemestane in a subsequent line after unsuccessful first-line hormone therapy with aromatase inhibitors, the BM incidence rate at the start of the study was higher, at 76% [18]. In contrast, a recent trial of poly(ADP-ribose) polymerase inhibitors in patients with BRCA mutations (the OlympiAD trial) showed a low incidence rate of exclusive BM after at least two lines of treatment, but the patients were “triple-negative” patients with high rates of visceral metastases [19].
Prostate Cancer
The BM incidence rate is particularly high in patients with metastatic prostate cancer. However, relevant data from all recent trials are not available.
The GETUG-AFU 15 trial found an 81% incidence rate of BM in patients with metastatic castration-naive prostate cancer [20], whereas the STAMPEDE trial found a rate of 54% [21]; however, the proportion of BM patients was not indicated in the CHAARTED trial [22]. More recently, in a similar population, the phase III LATITUDE trial on the efficacy of abiraterone in combination with first-line hormone therapy found BM in >97% of patients [23].
In patients with metastatic castration-resistant prostate cancer (mCRPC), the BM incidence rate is well described and appears to be an important focus in new-generation hormone therapy trials [[24], [25], [26], [27], [28]].
Lung Cancer
The BM incidence rate is also high in lung cancer (20–40%), with BM being increasingly detected owing to recent progress in imaging technology [29]. BM is diagnosed early in 80% of cases but can also occur at a later stage during follow-up [29].
In non–small-cell lung cancer (NSCLC), adenocarcinoma is the histological type most commonly associated with the onset of BM [30]. However, most first-line clinical trials have not documented the BM incidence rate. These trials include those testing epidermal growth factor receptor (EGFR) inhibitors such as gefitinib [31,32], anaplastic lymphoma kinase inhibitors such as crizotinib [33] or alectinib [34], and—more recently—third-generation EGFR inhibitors such as osimertinib [35]. In contrast, the incidence rate of brain metastasis was more commonly documented in such trials. Only the EURTAC trial, a randomized phase III study comparing erlotinib with standard platinum-based chemotherapy as the first-line treatment in EGFR-mutant NSCLC, reported that 33% of patients had BM at baseline [36]. Trials testing EGFR inhibitors in advanced NSCLC also did not specify BM incidence rates, nor did trials testing crizotinib in advanced lung cancers [37] or maintenance chemotherapy trials such as the AVAPERL trial for bevacizumab [38] or the PARAMOUNT trial for pemetrexed [39].
Regarding immune checkpoint inhibitors, the following four recently published randomized phase III trials also did not document BM incidence rates: CheckMate 017 [40], CheckMate 057 [41], KEYNOTE-010 [42], and OAK [43].
Small-cell lung cancer is also associated with BM incidence, although data are limited. In one study, the diagnostic rate of BM was 26.7% in a retrospective cohort of 363 patients in Greece [44].
Renal Cell Carcinoma
The bone is the most common metastatic site in renal cell carcinoma [45]. Recent trials have found an increase in the diagnostic rate of BM between the first and third lines of treatment in renal cell carcinoma. The value started at 17.4% in patients with BM at baseline [46], increasing to 22.4% in the second line of treatment in the METEOR trial [47], and reached 38.5% in the third line of treatment in a trial comparing dovitinib with sorafenib in patients pretreated with targeted sunitinib antiangiogenic therapy [48].
Improvement in the Detection and Management of BM
Although it appears that clinical trials to date indicate that BM incidence increases during cancer progression, this may in part be due to the increased sensitivity of imaging techniques in detecting bone lesions. This increased sensitivity along with the increased use of BRIs and a more systematic search for the clinical consequences of BM has made it possible to refine clinical procedures for improving patient treatment.
Awareness of the Clinical Consequences of BM
Skeletal-related events (SREs) include the occurrence of pain, need for radiotherapy, hypercalcemia, pathological fracture, and spinal cord or nerve root compression [2]. In clinical studies, SREs are relevant methodological criteria for assessing the effects of BRIs. Meanwhile, symptomatic skeletal events (SSEs) include a symptomatic fracture, need for surgery or bone radiotherapy for symptoms, and symptomatic spinal compression [52]. SSEs require careful detection because when they present as clinical consequences of BM, they can mask the diagnosis of asymptomatic vertebral fractures that can lead to musculoskeletal disorders and increase the risk of new fractures and falls. SSEs and SREs are common in BM during cancer progression and can impair the patients' quality of life [2,4,5]. The major complications of BM typically occur early during cancer progression—every 3–6 months on average if the bone disease is not treated with BRIs [2]. These complications cluster around periods of tumor progression, becoming more frequent as the disease progresses and treatment options decrease. More than 80% of BM are located in the axial skeleton; the vertebrae, ribs, and hips are therefore the sites most frequently affected during SREs [4]. The percentage of patients who experience SREs varies depending on the cancer type. In a previous breast cancer series, SREs were present in 68% of patients in general [53], 49% of patients with metastatic prostate cancer [54], and 48% of patients with lung cancer [55]. The median number of SREs per patient per year was reported to be 3.7 for breast cancer [53], 1.47 for metastatic prostate cancer [54], and 2.71 for lung cancer and other solid tumors [55].
Regarding the most recent data for prostate cancer, in the COU-AA-301 trial, approximately 90% of patients in each arm had BM at baseline and showed similar pain scores [26]. In new-generation hormone therapy trials of abiraterone, the incidence of SREs was 29% in patients receiving the drug, higher than the incidence of 33% observed in the placebo group, and the time to onset of SREs was significantly longer among those receiving the drug (median, 25.0 vs. 20.3 months; p = 0.0001) [28]. In chemotherapy trials such as the TROPIC trial, which evaluated the effects of cabazitaxel after previous treatment with docetaxel, the BM incidence rate was 83.6% and the SRE rate was 45% [28].
For breast cancer, recent trials have not evaluated the occurrence of SREs. Bone pain was observed in < 10% of cases, as a side effect of treatment, and was not considered as SREs or SSEs.
In a study on small-cell lung cancer, the prognosis of patients with an early onset of BM was worse than that of those with a late onset (p = 0.015). However, the SRE rate was similar in both groups: 20.8% in those with an early onset and 20.6% in those with a late onset [44]. In a meta-analysis, the median survival time of patients with BM and subsequent SREs was 7 months [56].
Evolution of Imaging Techniques
The rate of BM detection has improved considerably due to the improvement in imaging techniques. Bone tissue is not included in the Response Evaluation Criteria in Solid Tumors, as bone evaluation is difficult because of the varying forms that bone metastatic disease can take. Each imaging technique has its own proposed criteria for evaluating BM; bone condensation of lytic lesions in computed tomography (CT), fat colonization of bone lesions in magnetic resonance imaging (MRI), and decreased standardized uptake values in metabolic imaging can all be considered good markers of changes in bone. However, the type of BM can differ for the same type of cancer. In breast cancer, for example, BM can be lytic or mixed. In addition, while BM may appear to be heterogeneous at baseline, each lesion can progress differently during treatment. There are also no guidelines and consensual criteria for the morphological evaluation of BM burden, neither at diagnosis nor at evaluation during treatment. Currently, there is no single reliable imaging technique that can detect and evaluate all types of BM in all types of cancer [57].
In prostate cancer, choline positron emission tomography (choline-PET), MRI, and prostate-specific membrane antigen–based positron emission tomography (PSMA-PET) allow better detection of all metastases, especially BM [7,58]. In a meta-analysis on advanced prostate cancer, Shen et al. showed that MRI (97%) was superior to choline-PET (91%) and bone scan (79%) in terms of sensitivity of detection. MRI showed a specificity of detection of 95%; choline-PET, 99%; and bone scan, 82% [59]. With other cancers, irrespective of whether they were osteotropic, the use of combined imaging techniques led to the same results [59]. In another study of 123 patients who experienced a relapse in prostate cancer, PSMA-PET showed a higher detection rate than choline-PET/CT for bone lesions (98% vs. 64%), although ultimately there were mismatches for both [60]. Nevertheless, the evaluation of bone tumor response in imaging remains a topic of discussion in prostate cancer, because no single imaging technique is consistently superior in detecting bone metastatic disease across all tumor types and clinical scenarios [61].
Efficacy of Bone Resorption Inhibitors
BRIs have been used to limit the clinical consequences of BM and currently form part of the clinical routine [62]. Denosumab has been found to be noninferior to zoledronic acid (ZA) in delaying time to the first on-study SRE (hazard ratio [HR], 0.84; 95% confidence interval [CI], 0.71–0.98; p = 0.0007) in patients with advanced cancer and BM (excluding breast and prostate cancer) or myeloma [63].
An ad hoc analysis of the outcomes of a previous study showed that denosumab significantly delayed the time to the first on-study SRE (HR, 0.81; 95% CI, 0.68–0.96) and the time to the first and subsequent SREs (rate ratio, 0.85; 95% CI, 0.72–1.00), compared with ZA, in a subgroup of 1597 patients with solid tumors (excluding those with multiple myelomas) [64].
For prostate cancer, in a trial by Fizazi et al., denosumab showed an 18% better median time to onset of the first bone event than did ZA, suggesting its use for solid tumors [65]. Denosumab also reduced the risk of complications of either SREs or SSEs in mCRPC [66].
For breast cancer, denosumab was superior to ZA in delaying the time to the first on-study SRE (HR, 0.82; 95% CI, 0.71–0.95; p = 0.01) and the time to the first and subsequent on-study SREs (rate ratio, 0.77; 95% CI, 0.66–0.89; p = 0.001) [67].
The use of BRIs in current anticancer treatments has been shown to cause bone remodeling and may also affect survival [68]. In an exploratory analysis of Henry et al.'s trial, denosumab was found to be associated with better overall survival in metastatic lung cancer than was ZA [69].
Occurrence of Bone Metastasis during Cancer Follow-Up
In US registries (Oncology Services Comprehensive Electronic Records: 569,000 patients, 52 cancer centers), the cumulative incidence of BM, including that for all solid tumors and stages, was 2.9% at 30 days, 4.8% at 1 year, 5.6% at 2 years, 6.9% at 5 years, and 8.4% at 10 years. The incidence varied significantly by tumor type, with the highest incidence of BM in prostate cancer (18–29%), followed by lung (10.4–12.9%), kidney (5.8–9.9%), and breast cancer (3.4–8.1%) [70]. A review by Hernandez et al. summarizes the BM incidence rates during cancer progression [70].
A previous study revealed that in breast cancer with a longer follow-up, the proportion of patients developing BM increased over time due to the natural course of the disease and prolonged survival [71]. As mentioned above, Body et al. confirmed that among patients who developed metastatic disease during follow-up, 55% had BM [9]. The exclusive bone diseases in breast cancer were predominantly of the luminal A phenotypes, with strong hormone receptor overexpression, luminal B/HER2−, and luminal B/HER2+ features. The difference in terms of exclusive bone disease was significant in triple-negative and exclusive HER2+ breast cancer [71]. Significant differences in exclusive bone disease according to age at diagnosis (between <65 years and >65 years, in favor of older subjects) and initial lymph node status were also found. A retrospective study by Gerratana et al. supports the hypothesis that in addition to holding a potential organotropism, the immunophenotype (luminal A, B, HER2+ or triple negative) is the main driver of outcome [72].
Another explanation for the increase in BM incidence during follow-up could be that the bone itself contributes to metastatic phenomena through bone homing or, on the contrary, the bone itself could be involved in mechanisms of resistance to cancer therapies.
Appearance of Further Bone Metastasis
Interactions between the Bone Microenvironment and Primary Tumor
The hypothesis of a bone-induced “vicious circle” has been used to describe how tumor cells interact with the bone microenvironment to cause destruction of the bone, which in itself creates a fertile ground for tumor growth [61].
Tumor cells destroy the virtuous cycle of bone remodeling by stimulating osteoclast differentiation and activity through secretion of interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNFα), monocyte colony-stimulating factor (M-CSF), and vascular endothelial growth factor (VEGF). Thus, the destruction of the bone matrix allows the release of transforming growth factor (TGF)-β, insulin-like growth factor, fibroblast growth factor, bone metalloproteinases, and platelet-derived growth factor (PDGF), which are pro-tumor growth factors. Bone metastatic cells also influence osteoblast activity by stimulating the production of the parathormone-related protein, IL-1, IL-6, prostaglandin E2, TNFα, endothelin-1, bone metalloproteinase, and various growth factors. In turn, osteoblasts secrete factors that stimulate osteoclastogenesis, including the receptor activator of nuclear factor kappa-B ligand (RANKL), M-CSF, and IL-6 [73]. The overexpression of RANKL then results in the increased formation, activation, and survival of osteoclasts, thereby improving bone resorption [74].
Eventually, newly formed mineralized bone matrix and osteoblasts become entrapped and differentiated into osteocytes, the most abundant cells of bone tissue. Osteocytes modulate bone turnover by regulating osteoblasts and their functions through the secretion of RANKL, sclerostin, and Dickkopf-related protein 1 [61].
Another major player in this vicious circle is the MET receptor. One of the first studies on BM in prostate cancer that investigated the role of MET and its main ligand, the hepatocyte growth factor (HGF), was the Knudsen study, which showed a significant increase in MET expression in BM and an inverse correlation between MET and androgen receptor expression. The authors of the study analyzed 90 prostatectomy specimens and found that MET was expressed in half of the primary cancers and in all metastases [75]. Another study by Grano et al. described the expression and roles of HGF/MET binding in osteoblasts and osteoclasts. The activation of MET increased the intracellular calcium levels and promoted Src phosphorylation in osteoclasts, whereas the activation of MET induced cell cycle progression in osteoblasts. This finding suggests that the HGF ligand is a coupling factor for osteoblasts and osteoclasts [76].
Finally, tumor-derived exosomes were recently shown to prepare a favorable microenvironment at future metastasis sites and to mediate nonrandom patterns of metastasis [77].
Role of Anticancer Therapies
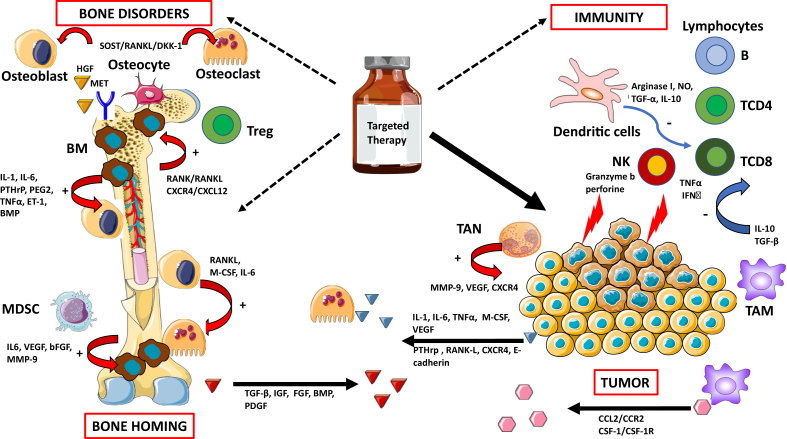
Conventional cytotoxic chemotherapy has a limited effect on bone tissue, while targeted therapies could have a direct effect on bone remodeling [63]. This raises the question of a possible bone-induced resistance against targeted therapies or, in contrast, further BM induced by targeted therapies via the reinduction of the “vicious cycle” (Figure 1).
Figure 1.
Targeted therapies and their potential effects on bone remodeling and suggested contribution to bone carcinogenesis and resistance to treatment. Targeted therapies have improved the outcomes of patients with osteotropic cancers, but the reactivation of different pathways can induce bone disorders and contribute to resistance to treatment. This remodeling of the tumor, bone microenvironment, and immunity may participate in clonal selection of metastases, both in the bone—by reactivating a “vicious circle” promoting bone homing—and in other organs. bFGF: basic fibroblast growth factor, BMP: bone marrow protein, DKK-1: Dickkopf-related protein 1, ET-1: endothelin-1, HGF: hepatocyte growth factor, IGF: insulin growth factor, IL: interleukin, IFNγ: interferon-gamma, M-CSF: monocyte-colony stimulating factor, MDSC: myeloid-derived suppressor cells, MMP-9: matrix metalloproteinase 9, NO: nitric oxide, NK: natural killer cell, PEG2: prostaglandin E2, PDGF: platelet-derived growth factor, PTHrP: parathormone-related protein, RANK: receptor activator of nuclear factor kappa-B, RANKL: receptor activator of nuclear factor kappa-B ligand, SOST: sclerostin, TAM: tumor-associated macrophages, TAN: tumor-associated neutrophils, TGF: transforming growth factor, TNFα: tumor necrosis factor-alpha, Treg: regulatory T cells, VEGF: vascular endothelial growth factor. Arrows in red (+): activation process. Arrows in blue (−): inhibition process.
Multitarget Tyrosine Kinase Inhibitors (TKIs). TKIs prescribed in melanoma (vemurafenib and dabrafenib) and NSCLC (erlotinib and crizotinib) cause the production of a complex “secretome” that activates multiple pathways in cancerous cells sensitive to the drugs used [78].
These TKIs also have proven antitumor efficacy in BM. In a retrospective series of NSCLC, treatment with EGFR-TKIs was an independent contributor to the occurrence of SRE throughout the course of the disease. There were fewer SREs in patients receiving EGFR-TKIs such as erlotinib or gefitinib than in patients treated with conventional chemotherapy without bisphosphonates [79].
Multitarget TKIs seem to have significant effects on bone remodeling, and the effects could differ depending on the molecules and doses used [80]. The most equivocal clinical manifestations of this bone remodeling are the osteosclerotic changes found on CT scans that show renewed ossification [81]. In patients with BM who were benefiting from gefitinib, the elevation of bone alkaline phosphatase levels or levels of bone resorption markers such as C-terminal telopeptide of type I collagen (ICTP) and the phosphatase alkaline/ICTP ratio demonstrates the bone resorption inhibition and antitumor effects of gefitinib [82].
For cabozantinib, a TKI that acts against VEGFR2, AXL, and MET, the serum total alkaline phosphatase and ICTP levels were reduced by ≥50% in 57% of evaluable patients [83]. Cabozantinib was also tested in mCRPC patients in a phase III trial (COMET-1) [84]. Despite a negative outcome for overall survival, a better response was noted on bone scans in the cabozantinib arm (42% vs. 3% with prednisone); levels of bone biomarkers such as bone-specific alkaline phosphatase, N-terminal cross-linked telopeptides of type I collagen, and C-terminal cross-linked telopeptides of type I collagen decreased, suggesting an active effect on BM [87]. One of the most important targets of cabozantinib is the MET receptor. Through MET/HGF binding, CD14+ monocytes differentiate into mature osteoclasts. In addition, the activation of MET can be triggered by osteoblasts and lead to the migration of tumor cells from the blood to the bone [85].
Collectively, the inhibition of c-MET and VEGFR2 in osteoblasts reduces the expression of RANKL and M-CSF and is associated with decreased tumor-induced osteolysis [86], suggesting that MET and VEGFR2 may be promising therapeutic targets in BM. In prostate cancer, the inhibition of VEGFR2 and MET in endothelial cells and their direct effects on osteoblasts are responsible for cabozantinib-induced tumor inhibition [87].
Some TKIs also exhibit dual effects on the bone. Imatinib, a multitarget TKI against C-KIT and PDGFR, among others, can inhibit the proliferation of osteoblasts but also activate them through the inhibition of PDGFRβ [88]. One of the options going forward could be a drug dosage based on Gobin et al.'s work on osteosarcoma cells, which showed that low doses of imatinib mesylate increase in vitro mineralization, in contrast to high doses, which decrease mineralization [89] (Figure 1).
mTOR Inhibitors. Deregulation of the PI3K/AKT/mTOR pathway is involved in both physiological and carcinological osteoclastogenesis. mTOR is an antiapoptotic target acting downstream of M-CSF, RANKL, and TNFα, which are essential for the differentiation, survival, and activity of osteoclasts [90] (Figure 1).
The inhibition of the mTOR pathway leads to increased osteoprotegerin expression and apoptosis of osteoclasts, which could also induce osteoblastogenesis. In an in vitro study of murine and human breast cancer lines, everolimus showed an osteoprotective effect [91]. Among clinical trials on breast cancer, the BOLERO-2 trial evaluated everolimus in combination with exemestane after failure of a first-line hormone therapy with aromatase inhibitors. After 18 months, the incidence of bone progression, including the appearance of a new BM or progression of preexisting BM, had reduced. Survival without bone progression was also significantly higher in the subset of patients with baseline BM in the combined exemestane-everolimus arm. Interestingly, bone remodeling was inhibited at 6 and 12 weeks in patients treated with everolimus and exemestane, independent of the presence of BM and bisphosphonate use at baseline [18]. All these data indicate the osteoprotective effect of everolimus.
Immune Checkpoint Inhibitors (ICI). An interplay exists between bone tissue and the immune system. In particular, immune cells within the bone marrow are found near osteoclasts and osteoblasts [92]. Osteoimmune regulation, therefore, could be a determinant of metastatic growth in the bone microenvironment.
The numerous niches in the bone microenvironment, with high levels of immune cells, may have an effect on the progression of primary tumors to BM [93,94]. Natural killer cells kill tumor cells by apoptosis, mediated by granzyme B and perforin. Cytotoxic T-CD8+ cells release TNFα and interferon (IFN)-γ to eliminate cancerous cells [95,96].
Deregulation of IFN type I is an essential mediator of bone tumor progression. In fact, the loss of IFN type I signaling in metastatic lesions results in a decrease in the regulation of immune cell activation, with inhibition of innate immune priming of cytotoxic cells, and an increase in angiogenesis and bone degradation, leading to ineffective bone homing of circulating immune cells (immune exclusion), all of which facilitate metastatic progression [97].
Regulatory T cells promote bone cancerization via CXCR4/CXCL12 signaling [98] or the RANK/RANKL axis [99,100]. Tumor-associated macrophages promote the tropism of tumor cells to bone through CCL2/CCR2 or CSF-1/CSF-1R signaling [101]. At the same time, these macrophages secrete high levels of IL-10 and TGF-β to reduce the activation of CD4+ and CD8+ T cells [102].
TGF-β increases osteolytic and osteoblastic activity, which destabilizes bone remodeling while simultaneously stimulating the growth of tumor cells. This, in turn, promotes immunosuppression, which is enhanced by the direct immune modulation of TGF-β in plastic populations, such as neutrophils. Therapeutic blockade of TGF-β has been effective in reducing bone tumor burden by promoting antitumor immune activity and inhibiting bone destruction [97].
Dendritic cells suppress the cytotoxic capacity of CD8+ T cells by producing arginase I, nitric oxide, TGF-α, and IL-10, which inhibit the immune response and thus promote tumor progression [103].
Myeloid-derived suppressor cells release chemokines such as IL-6, VEGF, basic fibroblast growth factor, and matrix metalloproteinase 9 and promote BM progression [92]. Tumor-associated neutrophils can also release CXCR4, VEGF, and matrix metalloproteinase 9 for this purpose [104]. Tumor cells also release other factors such as RANKL, E-cadherin, CXCR4, and parathormone-related protein, which promote bone homing and the occurrence of osteolytic bone lesions [92] (Figure 1).
Bone Resorption Inhibitors. Modification of the microenvironmental secretome by the combined effects of multitarget TKIs and BRIs can affect the bone, and this needs to be better evaluated in clinical trials. The association between immune checkpoints and denosumab seems to have been the most studied topic recently, because RANKL is not only involved in osteoclastogenesis but also has immunological effects [105].
The results of two retrospective observational studies in patients with melanoma and lung cancer with BM, treated with anti-PD1 or anti-CTLA, showed better survival and a better response rate in favor of the association of ICI and BRI, without an increase in toxicities [106,107]. Nevertheless, some interactions between BRIs and targeted antiangiogenic therapies create a breeding ground for complications, such as osteonecrosis of the jaw, which can affect prognosis [108].
Conclusions and Perspectives
BM is now being considered more seriously in clinical practice because of the improvement in imaging techniques and availability of efficient BRIs and other anticancer treatments. Moreover, targeted antitumor therapies provide a better survival rate for patients but may also induce further BM during the follow-up period. In fact, while combating disease progression, these targeted therapies might activate specific pathways that can lead to an imbalance in the bone microenvironment and cause metastasis to spread further. Altogether, these concerns illustrate that the bone has a major role in carcinogenesis because it is involved in both pro- and antitumor metastatic processes and resistance to treatment. Consequently, several axes of exploration need to be developed further:
-
-
First, the evaluation of bone tumor burden needs to be standardized through the elaboration of bone imaging criteria associated with clinical and biological markers. This will allow effective determination of metastatic bone disease burden at diagnosis and better evaluation of the response of BM to treatment.
-
-
Second, the effects of targeted therapies on the bone need to be assessed during clinical trials to determine what can be expected in terms of BM progression and SREs.
-
-
Third, the optimal use of BRIs (such as time to initiation, pattern of administration, and time to discontinuation) must be determined in the context of the global strategy of bone disease treatment, including bone focal treatment and systemic treatment.
-
-
Finally, knowledge to better understand the effects of targeted therapies on the bone microenvironment and the factors involved in both pro- and antitumor metastatic processes and resistance to treatment must be refined.
Author Contributions
Martine Duterque-Coquillaud and Marie-Hélène Vieillard conceived the idea for the review. Anthony Turpin and Marie-Hélène Vieillard searched the literature. Anthony Turpin and Martine Duterque-Coquillaud wrote the manuscript. Anthony Turpin generated the figure. All authors reviewed and approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest Statement
The authors declare no competing interests.
Acknowledgments
We apologize to authors whose publications could not be included in this review due to limited space. We would like to thank Editage (www.editage.com) and Séverine Marchant (Centre Oscar Lambret) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2019.10.012.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Roodman G.D. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 2.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Fornetti J., Welm A.L., Stewart S.A. Understanding the Bone in Cancer Metastasis. J Bone Miner Res. 2018;33:2099–2113. doi: 10.1002/jbmr.3618. [DOI] [PubMed] [Google Scholar]
- 4.Selvaggi G., Scagliotti G.V. Management of bone metastases in cancer: a review. Crit Rev Oncol Hematol. 2005;56:365–378. doi: 10.1016/j.critrevonc.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Coleman R.E. Bisphosphonates: clinical experience. Oncologist. 2004;9(Suppl 4):14–27. doi: 10.1634/theoncologist.9-90004-14. [DOI] [PubMed] [Google Scholar]
- 6.McDougall J.A., Bansal A., Goulart B.H.L., McCune J.S., Karnopp A., Fedorenko C., Greenlee S., Valderrama A., Sullivan S.D., Ramsey S.D. The Clinical and Economic Impacts of Skeletal-Related Events Among Medicare Enrollees With Prostate Cancer Metastatic to Bone. Oncologist. 2016;21:320–326. doi: 10.1634/theoncologist.2015-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecouvet F.E., Oprea-Lager D.E., Liu Y., Ost P., Bidaut L., Collette L., Deroose C.M., Goffin K., Herrmann K., Hoekstra O.S. Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: a consensus recommendation from the EORTC Imaging Group. Lancet Oncol. 2018;19:e534–e545. doi: 10.1016/S1470-2045(18)30571-0. [DOI] [PubMed] [Google Scholar]
- 8.Massagué J., Obenauf A.C. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Body J.-J., Quinn G., Talbot S., Booth E., Demonty G., Taylor A., Amelio J. Systematic review and meta-analysis on the proportion of patients with breast cancer who develop bone metastases. Crit Rev Oncol/Hematol. 2017;115:67–80. doi: 10.1016/j.critrevonc.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Coleman R.E., Rubens R.D. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuchuk I., Hutton B., Moretto P., Ng T., Addison C.L., Clemons M. Incidence, consequences and treatment of bone metastases in breast cancer patients-Experience from a single cancer centre. J Bone Oncol. 2013;2:137–144. doi: 10.1016/j.jbo.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swain S.M., Baselga J., Kim S.-B., Ro J., Semiglazov V., Campone M., Ciruelos E., Ferrero J.-M., Schneeweiss A., Heeson S. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diéras V., Miles D., Verma S., Pegram M., Welslau M., Baselga J., Krop I.E., Blackwell K., Hoersch S., Xu J. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cortes J., O’Shaughnessy J., Loesch D., Blum J.L., Vahdat L.T., Petrakova K., Chollet P., Manikas A., Diéras V., Delozier T. Twelves, EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician’s Choice Versus E7389) investigators, Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 15.Finn R.S., Crown J.P., Lang I., Boer K., Bondarenko I.M., Kulyk S.O., Ettl J., Patel R., Pinter T., Schmidt M. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 16.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.-A., Gelmon K., Harbeck N., Lipatov O.N., Walshe J.M., Moulder S. Palbociclib and Letrozole in Advanced Breast Cancer. New Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 17.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.-A., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 18.Baselga J., Campone M., Piccart M., Burris H.A., Rugo H.S., Sahmoud T., Noguchi S., Gnant M., Pritchard K.I., Lebrun F. Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robson M., Im S.-A., Senkus E., Xu B., Domchek S.M., Masuda N., Delaloge S., Li W., Tung N., Armstrong A. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 20.Gravis G., Fizazi K., Joly F., Oudard S., Priou F., Esterni B., Latorzeff I., Delva R., Krakowski I., Laguerre B. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:149–158. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 21.James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R., Ritchie A.W.S., Parker C.C., Russell J.M., Attard G. STAMPEDE investigators, Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweeney C.J., Chen Y.-H., Carducci M., Liu G., Jarrard D.F., Eisenberger M., Wong Y.-N., Hahn N., Kohli M., Cooney M.M. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fizazi K., Tran N., Fein L., Matsubara N., Rodriguez-Antolin A., Alekseev B.Y., Özgüroğlu M., Ye D., Feyerabend S., Protheroe A. LATITUDE Investigators, Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 24.Ryan C.J., Smith M.R., Fizazi K., Saad F., Mulders P.F.A., Sternberg C.N., Miller K., Logothetis C.J., Shore N.D., Small E.J. COU-AA-302 Investigators, Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 25.Oudard S., Fizazi K., Sengeløv L., Daugaard G., Saad F., Hansen S., Hjälm-Eriksson M., Jassem J., Thiery-Vuillemin A., Caffo O. Cabazitaxel Versus Docetaxel As First-Line Therapy for Patients With Metastatic Castration-Resistant Prostate Cancer: A Randomized Phase III Trial-FIRSTANA. J Clin Oncol. 2017;35:3189–3197. doi: 10.1200/JCO.2016.72.1068. [DOI] [PubMed] [Google Scholar]
- 26.Fizazi K., Scher H.I., Molina A., Logothetis C.J., Chi K.N., Jones R.J., Staffurth J.N., North S., Vogelzang N.J., Saad F. COU-AA-301 Investigators, Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13:983–992. doi: 10.1016/S1470-2045(12)70379-0. [DOI] [PubMed] [Google Scholar]
- 27.Scher H.I., Fizazi K., Saad F., Taplin M.-E., Sternberg C.N., Miller K., de Wit R., Mulders P., Chi K.N., Shore N.D. AFFIRM Investigators, Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 28.de Bono J.S., Oudard S., Ozguroglu M., Hansen S., Machiels J.-P., Kocak I., Gravis G., Bodrogi I., Mackenzie M.J., Shen L. TROPIC Investigators, Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 29.Kuchuk M., Addison C.L., Clemons M., Kuchuk I., Wheatley-Price P. Incidence and consequences of bone metastases in lung cancer patients. J Bone Oncol. 2013;2:22–29. doi: 10.1016/j.jbo.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H., Zhang Y., Zhu H., Yu J. Risk factors for bone metastasis in completely resected non-small-cell lung cancer. Future Oncol. 2017;13:695–704. doi: 10.2217/fon-2016-0237. [DOI] [PubMed] [Google Scholar]
- 31.Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., Gemma A., Harada M., Yoshizawa H., Kinoshita I. North-East Japan Study Group, Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 32.Mok T.S., Wu Y.-L., Thongprasert S., Yang C.-H., Chu D.-T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 33.Solomon B.J., Mok T., Kim D.-W., Wu Y.-L., Nakagawa K., Mekhail T., Felip E., Cappuzzo F., Paolini J., Usari T. PROFILE 1014 Investigators, First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 34.Hida T., Nokihara H., Kondo M., Kim Y.H., Azuma K., Seto T., Takiguchi Y., Nishio M., Yoshioka H., Imamura F. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39. doi: 10.1016/S0140-6736(17)30565-2. [DOI] [PubMed] [Google Scholar]
- 35.Soria J.-C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T. FLAURA Investigators, Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 36.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica, Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 37.Shaw A.T., Kim D.-W., Nakagawa K., Seto T., Crinó L., Ahn M.-J., De Pas T., Besse B., Solomon B.J., Blackhall F. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 38.Barlesi F., Scherpereel A., Rittmeyer A., Pazzola A., Ferrer Tur N., Kim J.-H., Ahn M.-J., Aerts J.G.J.V., Gorbunova V., Vikström A. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089) J Clin Oncol. 2013;31:3004–3011. doi: 10.1200/JCO.2012.42.3749. [DOI] [PubMed] [Google Scholar]
- 39.Paz-Ares L., de Marinis F., Dediu M., Thomas M., Pujol J.-L., Bidoli P., Molinier O., Sahoo T.P., Laack E., Reck M. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13:247–255. doi: 10.1016/S1470-2045(12)70063-3. [DOI] [PubMed] [Google Scholar]
- 40.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borghaei H., Paz-Ares L., Horn L., Spigel D.R., Steins M., Ready N.E., Chow L.Q., Vokes E.E., Felip E., Holgado E. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herbst R.S., Baas P., Kim D.-W., Felip E., Pérez-Gracia J.L., Han J.-Y., Molina J., Kim J.-H., Arvis C.D., Ahn M.-J. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 43.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C. OAK Study Group, Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charpidou A., Tsagouli S., Gkiozos I., Grapsa D., Moutsos M., Kiagia M., Syrigos K. Bone metastases in patients with small cell lung carcinoma: rate of development, early versus late onset, modality of treatment, and their impact on survival. A single-institution retrospective cohort study. Clin Exp Metastasis. 2016;33:453–460. doi: 10.1007/s10585-016-9789-7. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi M., Sun M., Jeldres C., Shariat S.F., Trinh Q.-D., Briganti A., Tian Z., Schmitges J., Graefen M., Perrotte P. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23:973–980. doi: 10.1093/annonc/mdr362. [DOI] [PubMed] [Google Scholar]
- 46.Motzer R.J., Hutson T.E., Cella D., Reeves J., Hawkins R., Guo J., Nathan P., Staehler M., de Souza P., Merchan J.R. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 47.Choueiri T.K., Escudier B., Powles T., Tannir N.M., Mainwaring P.N., Rini B.I., Hammers H.J., Donskov F., Roth B.J., Peltola K. METEOR investigators, Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17:917–927. doi: 10.1016/S1470-2045(16)30107-3. [DOI] [PubMed] [Google Scholar]
- 48.Motzer R.J., Porta C., Vogelzang N.J., Sternberg C.N., Szczylik C., Zolnierek J., Kollmannsberger C., Rha S.Y., Bjarnason G.A., Melichar B. Dovitinib versus sorafenib for third-line targeted treatment of patients with metastatic renal cell carcinoma: an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:286–296. doi: 10.1016/S1470-2045(14)70030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escudier B., Porta C., Bono P., Powles T., Eisen T., Sternberg C.N., Gschwend J.E., De Giorgi U., Parikh O., Hawkins R. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32:1412–1418. doi: 10.1200/JCO.2013.50.8267. [DOI] [PubMed] [Google Scholar]
- 50.Rini B.I., Escudier B., Tomczak P., Kaprin A., Szczylik C., Hutson T.E., Michaelson M.D., Gorbunova V.A., Gore M.E., Rusakov I.G. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 51.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R. CheckMate 025 Investigators, Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hussain A., Lee R.J., Graff J.N., Halabi S. The evolution and understanding of skeletal complication endpoints in clinical trials of tumors with metastasis to the bone. Crit Rev Oncol Hematol. 2019;139:108–116. doi: 10.1016/j.critrevonc.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipton A., Theriault R.L., Hortobagyi G.N., Simeone J., Knight R.D., Mellars K., Reitsma D.J., Heffernan M., Seaman J.J. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. doi: 10.1002/(sici)1097-0142(20000301)88:5<1082::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 54.Saad F., Gleason D.M., Murray R., Tchekmedyian S., Venner P., Lacombe L., Chin J.L., Vinholes J.J., Goas J.A., Zheng M. Zoledronic Acid Prostate Cancer Study Group, Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–882. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 55.Rosen L.S., Gordon D., Tchekmedyian N.S., Yanagihara R., Hirsh V., Krzakowski M., Pawlicki M., De Souza P., Zheng M., Urbanowitz G. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 56.Yong M., Jensen A.Ö., Jacobsen J.B., Nørgaard M., Fryzek J.P., Sørensen H.T. Survival in breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999-2007) Breast Cancer Res Treat. 2011;129:495–503. doi: 10.1007/s10549-011-1475-5. [DOI] [PubMed] [Google Scholar]
- 57.O’Sullivan G.J. Imaging of bone metastasis: An update. WJR. 2015;7:202. doi: 10.4329/wjr.v7.i8.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Oronzo S., Coleman R., Brown J., Silvestris F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J Bone Oncol. 2019;15 doi: 10.1016/j.jbo.2018.10.004. 004–004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen G., Deng H., Hu S., Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014;43:1503–1513. doi: 10.1007/s00256-014-1903-9. [DOI] [PubMed] [Google Scholar]
- 60.Schwenck J., Rempp H., Reischl G., Kruck S., Stenzl A., Nikolaou K., Pfannenberg C., la Fougère C. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. doi: 10.1007/s00259-016-3490-6. [DOI] [PubMed] [Google Scholar]
- 61.Dewulf J., Vangestel C., Verhoeven Y., van Dam P., Elvas F., van den Wyngaert T., Clézardin P. Bone metastases in the era of targeted treatments: insights from molecular biology. Q J Nucl Med Mol Imaging. 2019;63(2):98–111. doi: 10.23736/S1824-4785.19.03203-5. [DOI] [PubMed] [Google Scholar]
- 62.Liede A., Wade S., Lethen J., Hernandez R.K., Warner D., Abernethy A.P., Finelli A. An Observational Study of Concomitant Use of Emerging Therapies and Denosumab or Zoledronic Acid in Prostate Cancer. Clin Ther. 2018;40:536–549. doi: 10.1016/j.clinthera.2017.12.015. e3. [DOI] [PubMed] [Google Scholar]
- 63.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., Scagliotti G.V., Sleeboom H., Spencer A., Vadhan-Raj S. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 64.Henry D., Vadhan-Raj S., Hirsh V., von Moos R., Hungria V., Costa L., Woll P.J., Scagliotti G., Smith G., Feng A. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22:679–687. doi: 10.1007/s00520-013-2022-1. [DOI] [PubMed] [Google Scholar]
- 65.Fizazi K., Carducci M., Smith M., Damião R., Brown J., Karsh L., Milecki P., Shore N., Rader M., Wang H. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. The Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith M.R., Coleman R.E., Klotz L., Pittman K., Milecki P., Ng S., Chi K.N., Balakumaran A., Wei R., Wang H. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26:368–374. doi: 10.1093/annonc/mdu519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stopeck A.T., Lipton A., Body J.-J., Steger G.G., Tonkin K., de Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 68.Coleman R., Gnant M., Morgan G., Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104:1059–1067. doi: 10.1093/jnci/djs263. [DOI] [PubMed] [Google Scholar]
- 69.Scagliotti G.V., Hirsh V., Siena S., Henry D.H., Woll P.J., Manegold C., Solal-Celigny P., Rodriguez G., Krzakowski M., Mehta N.D. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol. 2012;7:1823–1829. doi: 10.1097/JTO.0b013e31826aec2b. [DOI] [PubMed] [Google Scholar]
- 70.Hernandez R.K., Wade S.W., Reich A., Pirolli M., Liede A., Lyman G.H. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18:44. doi: 10.1186/s12885-017-3922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diessner J., Wischnewsky M., Stüber T., Stein R., Krockenberger M., Häusler S., Janni W., Kreienberg R., Blettner M., Schwentner L. Evaluation of clinical parameters influencing the development of bone metastasis in breast cancer. BMC Cancer. 2016;16 doi: 10.1186/s12885-016-2345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerratana L., Fanotto V., Bonotto M., Bolzonello S., Minisini A.M., Fasola G., Puglisi F. Pattern of metastasis and outcome in patients with breast cancer. Clin Exp Metastasis. 2015;32:125–133. doi: 10.1007/s10585-015-9697-2. [DOI] [PubMed] [Google Scholar]
- 73.Maurizi A., Rucci N. The Osteoclast in Bone Metastasis: Player and Target. Cancers (Basel) 2018;10 doi: 10.3390/cancers10070218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen S.-C., Kuo P.-L. Bone Metastasis from Renal Cell Carcinoma. Int J Mol Sci. 2016;17:987. doi: 10.3390/ijms17060987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knudsen B.S., Gmyrek G.A., Inra J., Scherr D.S., Vaughan E.D., Nanus D.M., Kattan M.W., Gerald W.L., Vande Woude G.F. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60:1113–1117. doi: 10.1016/s0090-4295(02)01954-4. [DOI] [PubMed] [Google Scholar]
- 76.Grano M., Galimi F., Zambonin G., Colucci S., Cottone E., Zallone A.Z., Comoglio P.M. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci U.S.A. 1996;93:7644–7648. doi: 10.1073/pnas.93.15.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoshino A., Costa-Silva B., Shen T.-L., Rodrigues G., Hashimoto A., Tesic Mark M., Molina H., Kohsaka S., Di Giannatale A., Ceder S. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Obenauf A.C., Zou Y., Ji A.L., Vanharanta S., Shu W., Shi H., Kong X., Bosenberg M.C., Wiesner T., Rosen N. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature. 2015;520:368–372. doi: 10.1038/nature14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun J.-M., Ahn J.S., Lee S., Kim J.A., Lee J., Park Y.H., Park H.C., Ahn M.-J., Ahn Y.C., Park K. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer. 2011;71:89–93. doi: 10.1016/j.lungcan.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Ségaliny A.I., Tellez-Gabriel M., Heymann M.-F., Heymann D. Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers. J Bone Oncol. 2015;4:1–12. doi: 10.1016/j.jbo.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamashita Y., Aoki T., Hanagiri T., Yoshii C., Mukae H., Uramoto H., Korogi Y. Osteosclerotic lesions in patients treated with gefitinib for lung adenocarcinomas: a sign of favorable therapeutic response. Skeletal Radiol. 2012;41:409–414. doi: 10.1007/s00256-011-1253-9. [DOI] [PubMed] [Google Scholar]
- 82.Okano Y., Nishio M. [Efficacy of gefitinib in treatment of lung cancer patients with bone metastasis] Clin Calcium. 2008;18:527–533. [PubMed] [Google Scholar]
- 83.Smith D.C., Smith M.R., Sweeney C., Elfiky A.A., Logothetis C., Corn P.G., Vogelzang N.J., Small E.J., Harzstark A.L., Gordon M.S. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31:412–419. doi: 10.1200/JCO.2012.45.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith M., De Bono J., Sternberg C., Le Moulec S., Oudard S., De Giorgi U., Krainer M., Bergman A., Hoelzer W., De Wit R. Phase III Study of Cabozantinib in Previously Treated Metastatic Castration-Resistant Prostate Cancer: COMET-1. J Clin Oncol. 2016;34:3005–3013. doi: 10.1200/JCO.2015.65.5597. [DOI] [PubMed] [Google Scholar]
- 85.Di Nunno V., Cimadamore A., Santoni M., Scarpelli M., Fiorentino M., Ciccarese C., Iacovelli R., Cheng L., Lopez-Beltran A., Massari F. Biological issues with cabozantinib in bone metastatic renal cell carcinoma and castration-resistant prostate cancer. Future Oncol. 2018;14:2559–2564. doi: 10.2217/fon-2018-0158. [DOI] [PubMed] [Google Scholar]
- 86.Lee C., Whang Y.M., Campbell P., Mulcrone P.L., Elefteriou F., Cho S.W., Park S.I. Dual targeting c-met and VEGFR2 in osteoblasts suppresses growth and osteolysis of prostate cancer bone metastasis. Cancer Lett. 2018;414:205–213. doi: 10.1016/j.canlet.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 87.Varkaris A., Corn P.G., Parikh N.U., Efstathiou E., Song J.H., Lee Y.-C., Aparicio A., Hoang A.G., Gaur S., Thorpe L. Integrating Murine and Clinical Trials with Cabozantinib to Understand Roles of MET and VEGFR2 as Targets for Growth Inhibition of Prostate Cancer. Clin Can Res. 2016;22:107–121. doi: 10.1158/1078-0432.CCR-15-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vandyke K., Fitter S., Dewar A.L., Hughes T.P., Zannettino A.C.W. Dysregulation of bone remodeling by imatinib mesylate. Blood. 2010;115:766–774. doi: 10.1182/blood-2009-08-237404. [DOI] [PubMed] [Google Scholar]
- 89.Gobin B., Moriceau G., Ory B., Charrier C., Brion R., Blanchard F., Redini F., Heymann D. Imatinib mesylate exerts anti-proliferative effects on osteosarcoma cells and inhibits the tumour growth in immunocompetent murine models. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0090795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sousa S., Clézardin P. Bone-Targeted Therapies in Cancer-Induced Bone Disease. Calcified Tissue Int. 2018;102:227–250. doi: 10.1007/s00223-017-0353-5. [DOI] [PubMed] [Google Scholar]
- 91.Browne A.J., Kubasch M.L., Göbel A., Hadji P., Chen D., Rauner M., Stölzel F., Hofbauer L.C., Rachner T.D. Concurrent antitumor and bone-protective effects of everolimus in osteotropic breast cancer. Breast Cancer Res. 2017;19:92. doi: 10.1186/s13058-017-0885-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiang L., Gilkes D.M. The Contribution of the Immune System in Bone Metastasis Pathogenesis. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20040999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gilkes D.M. Implications of Hypoxia in Breast Cancer Metastasis to Bone. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17101669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Futakuchi M., Fukamachi K., Suzui M. Heterogeneity of tumor cells in the bone microenvironment: Mechanisms and therapeutic targets for bone metastasis of prostate or breast cancer. Adv Drug Del Rev. 2016;99:206–211. doi: 10.1016/j.addr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 95.Philippe C., Philippe B., Fouqueray B., Perez J., Lebret M., Baud L. Protection from tumor necrosis factor-mediated cytolysis by platelets. Am J Pathol. 1993;143:1713–1723. [PMC free article] [PubMed] [Google Scholar]
- 96.Palumbo J.S., Talmage K.E., Massari J.V., La Jeunesse C.M., Flick M.J., Kombrinck K.W., Jirousková M., Degen J.L. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 97.Owen K.L., Parker B.S. Beyond the vicious cycle: The role of innate osteoimmunity, automimicry and tumor-inherent changes in dictating bone metastasis. Mol Immunol. 2017;110:57–68. doi: 10.1016/j.molimm.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 98.Zou L., Barnett B., Safah H., Larussa V.F., Evdemon-Hogan M., Mottram P., Wei S., David O., Curiel T.J., Zou W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004;64:8451–8455. doi: 10.1158/0008-5472.CAN-04-1987. [DOI] [PubMed] [Google Scholar]
- 99.Tan W., Zhang W., Strasner A., Grivennikov S., Cheng J.Q., Hoffman R.M., Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakashima T. [Bone metastasis and RANKL] Clin Calcium. 2014;24:1201–1208. [PubMed] [Google Scholar]
- 101.Ries C.H., Cannarile M.A., Hoves S., Benz J., Wartha K., Runza V., Rey-Giraud F., Pradel L.P., Feuerhake F., Klaman I. Targeting Tumor-Associated Macrophages with Anti-CSF-1R Antibody Reveals a Strategy for Cancer Therapy. Cancer Cell. 2014;25:846–859. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 102.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 103.Capietto A.-H., Faccio R. Immune regulation of bone metastasis. Bonekey Rep. 2014;3:600. doi: 10.1038/bonekey.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schernberg A., Blanchard P., Chargari C., Deutsch E. Neutrophils, a candidate biomarker and target for radiation therapy? Acta Oncol. 2017;56:1522–1530. doi: 10.1080/0284186X.2017.1348623. [DOI] [PubMed] [Google Scholar]
- 105.van Dam P.A., Verhoeven Y., Trinh X.B., Wouters A., Lardon F., Prenen H., Smits E., Baldewijns M., Lammens M. RANK/RANKL signaling inhibition may improve the effectiveness of checkpoint blockade in cancer treatment. Crit Rev Oncol Hematol. 2019;133:85–91. doi: 10.1016/j.critrevonc.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 106.Liede A., Hernandez R.K., Wade S.W., Bo R., Nussbaum N.C., Ahern E., Dougall W.C., Smyth M.J. An observational study of concomitant immunotherapies and denosumab in patients with advanced melanoma or lung cancer. Oncoimmunology. 2018;7 doi: 10.1080/2162402X.2018.1480301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Angela Y., Haferkamp S., Weishaupt C., Ugurel S., Becker J.C., Oberndörfer F., Alar V., Satzger I., Gutzmer R. Combination of denosumab and immune checkpoint inhibition: experience in 29 patients with metastatic melanoma and bone metastases. Cancer Immunol Immunother. 2019;68(7):1187–1194. doi: 10.1007/s00262-019-02353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smidt-Hansen T., Folkmar T.B., Fode K., Agerbaek M., Donskov F. Combination of zoledronic Acid and targeted therapy is active but may induce osteonecrosis of the jaw in patients with metastatic renal cell carcinoma. J Oral Maxillofac Surg. 2013;71:1532–1540. doi: 10.1016/j.joms.2013.03.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.