Abstract
Increasing evidence has indicated that PDZ binding kinase (PBK) promotes proliferation, invasion, and therapeutic resistance in a variety of cancer types. However, the physiological function and therapy-resistant role of PBK in GBM remain underexplored. In this study, PBK was identified as one of the most therapy-resistant genes with significantly elevated expression level in GBM. Moreover, the high expression level of PBK was essential for GBM tumorigenesis and radio-resistance both in vitro and in vivo. Clinically, aberrant activation of PBK was correlated with poor clinical prognosis. In addition, inhibition of PBK dramatically enhanced the efficacy of radiation therapy in GBM cells. Mechanically, PBK-dependent transcriptional regulation of CCNB2 was critical for tumorigenesis and radio-resistance in GBM cells. Collectively, PBK promotes tumorigenesis and radio-resistance in GBM and may serve as a novel therapeutic target for GBM treatment.
Introduction
Glioblastoma (GBM) is one of the most malignant and incurable primary brain tumors in the adult central nervous system with a median survival of 15 months [1]. Currently, the prognosis of GBM patients is still dismal despite the application of standard treatment protocol including the radiotherapy plus concomitant and adjuvant temozolomide after maximum tumor resection [2,3]. Accumulating evidence has indicated that the aberrant activation of multiple human kinases is one of the major causes of tumor recurrence [[4], [5], [6]]. Therefore, it is urgent to identify the key kinase involved in therapeutic resistance and its underlying mechanism which may be helpful for the identification of novel therapeutic target for GBM.
PDZ binding kinase (PBK) is a novel serine–threonine kinase related to the mitogen-activated protein kinase (MAPK) family and functions as an important nexus for multiple oncogenic signaling pathways including p38, extracellular signal-regulated kinase 1/2 (ERK1/2), and FAK/Src-MMP signaling [7,8]. It has been reported that PBK plays a major role in mitotic phosphorylation, regulation of DNA damage repair, tumorigenesis, and therapeutic resistance through various protein phosphorylation reactions [9,10]. Increasing evidence has shown that excessive activation of PBK contributes to tumor proliferation, invasion, as well as treatment failure in a variety of tumor types [[11], [12], [13]]. Recent studies demonstrated that overexpression of PBK was correlated with poor prognosis of hepatocellular carcinoma and promoted migration and invasion of tumor cells through ETV4-uPAR signaling pathway [14]. Furthermore, PBK overexpression was found to be associated with recurrence of aggressive prostate cancer by stabilization of the androgen receptor [15]. In addition, another recent study has shown that the PBK inhibitor could inhibit multiple PBK downstream signaling pathways, disrupt colorectal carcinoma (CRC) cell mitosis, and affect cell cycle, indicating that PBK inhibitor may serve as an effective anti-CRC oncogenic target [8]. However, the physiological function and therapy-resistant role of PBK in GBM remain underexplored.
In this study, PBK was identified as one of the most therapy-resistant genes with significantly elevated expression level in GBM samples. Moreover, the high expression level of PBK was essential for GBM tumorigenesis and radio-resistance both in vitro and in vivo. Clinically, PBK was highly enriched in high-grade glioma compared with low-grade glioma according to tissue microarray analysis of 82 glioma samples. Aberrant activation of PBK was correlated with poor clinical prognosis. In addition, silencing or inhibition of PBK dramatically enhanced the efficacy of radiation therapy in GBM cells. Mechanically, PBK-dependent transcriptional regulation of Cyclin B2 (CCNB2) was critical for tumorigenesis and radio-resistance in GBM cells. Altogether, PBK may serve as a novel therapeutic target for GBM treatment.
Materials and Methods
Ethics
The usage of tumor samples and experimental animals (nude mice) involved in this study were approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University (Xi'an, Shaanxi, China 710061; Approval No. 2016-021). All the necessary consent forms were signed for using clinical tumor samples.
In Vitro Cell Culture
All the GBM cell lines (U87, U138, U251, U373) and normal human astrocytes involved in this study were provided by the Translational Medicine Center of the First Affiliated Hospital of Xi'an Jiaotong University. GBM cells were cultured in Dulbecco's modified Eagle's medium-nutrient mixture F12 (DMEM/F12; Gibco, 10565042) supplemented with 10% fetal bovine serum (FBS; Gibco, 16140071) at 37 °C. The medium was refreshed every 3 days.
Irradiation Assay
For in vitro irradiation, U87 and U251 GBM cells were plated into 6-well plates at a density of 5 × 106 cells per well and then cultured with DMEM/F12 at 37 °C for 48 hours. Next, GBM cells were exposed to 12 Gy X-ray. These cells treated with radiation were used for in vitro assays. For in vivo irradiation, mice implanted with GBM cells received radiation treatment (12 Gy) at the first day of the in vivo experiment and then were fed under the same circumstances with the mice without radiation treatment.
RNA Isolation and Quantitative PCR
RNeasy min kit (QIAGEN) was used for RNA isolation according to the manufacturer's instructions. The RNA concentration was detected by Nanodrip 2000 (Thermo Scientific) and cDNA was synthesized by using iScript reverse transcription supermix of reverse transcription–quantitative polymerase chain reaction (RT-qPCR; Bio-Rad). Furthermore, cDNA was analyzed by RT-qPCR and GAPDH was selected as an internal control. Quantitative PCR was performed as previously described [16]. The primers involved in this study were shown as follows: PBK forward, TAGGAGTCTCTCTACCACTGGA and reverse, TCCCACAAAGTAAGGCCAAAG; CCNB2 forward, TGCTCTGCAAAATCGAGGACA and reverse, GCCAATCCACTAGGATGGCA; CCND1 forward, GCTGCGAAGTGGAAACCATC and reverse, CCTCCTTCTGCACACATTTGAA; GAPDH forward, GGAGCGAGATCCCTCCAAAAT and reverse GGCTGTTGTCATACTTCTCATGG.
Western Blotting
Sample lysates containing protease inhibitor cocktail (Sigma Aldrich) were used for Western blotting analysis. Equal amounts of protein lysates were loaded onto the wells of 12% precast SDS-PAGE gel and then transferred to a PVDF membrane (Invitrogen). The membrane was treated with the target antibodies overnight at 4 °C cold room after incubation of 1 hour with 5% skimmed milk. Amersham ECL Western Blot System (GE Healthcare Life Sciences) was used to visualize the protein expression level of each sample. β-Actin was served as the loading control. The following antibodies were used in the present study: anti-PBK primary antibody (Abcam, ab75987; Rabbit); β-Actin antibody (Abcam, ab8227; Rabbit); IgG antibody (Abcam, ab171870; Rabbit; negative control); anti-rabbit secondary antibody (Abcam, ab150077; Goat, horseradish peroxidase-conjugated).
Lentivirus Infection
The pGFP-shPBK lentivirus particles were purchased from ORIGene Technologies Incorporation (RC203295L2V). U87 cells (5 × 104) were cultured in 6-well plates for 24 hours and incubated with lentivirus for another 24 hours according to the manufacturer's protocol. Transfection efficiency was evaluated by RT-qPCR and Western blotting.
In Vitro Cell Cloning Assay
U87 cells were seeded in 6-well plates (1 × 103 cells per well) and cultured for 14 days. Each well was performed by triplicate. Then, 1% paraformaldehyde (Chemical Book, 30525-89-4) was used for cell fixation for 30 min. Furthermore, cells were stained with crystal violet (Chemical Book, CB0331) for 10 min and cell clones were counted under an inverted fluorescence microscope (Olympus IX71).
In Vitro Cell Proliferation Assay
AlamarBlue assay was used to evaluate cell proliferation ability according to the manufacturer's protocol. U87 cells were seeded in 24-well plates at a density of 1 × 103 cells per well and incubated with 10% alamarBlue reagent (Invitrogen, DAL1025) for 8 hours. Then the fluorescence intensity of each well was read for evaluation of cell proliferation.
Luciferase Assay
CCNB2 3′ UTR Lenti-reporter-Luciferase virus was purchased from Applied Biological Materials Incorporation (MV-m03396). U87 cells were transfected with CCNB2 promoter-luciferase reporter lentivirus or empty vector. After 5-day cell culture, these cells were further infected with either shPBK lentivirus or nontarget virus for another 5 days. The Bright-Glo™ Luciferase Assay system (Promega Corporation, Madison, WI, USA) was applied to test the luciferase activity of each sample, and Renilla luciferase activity was used as the result normalization.
Intracranial Xenograft Tumor Models
Female nude mice aged 6 weeks were selected for the establishment of intracranial xenograft tumor models (10 mice per group). U87 cells of each group (1 × 105/cells in 5 μl of PBS) were implanted into the brains of the immunocompromised mice after proper anesthesia. Mice were sacrificed by an overdose of ketamine and xylazine once neuropathological symptoms were identified during daily behavior observation, including unsteady gait, leg paralysis, arched back, and 15% weight loss.
Immunohistochemistry and Expression Quantification
The mouse brain slices were incubated with primary antibodies overnight at 4 °C cold room and washed with cold PBS. The HRP-conjugated secondary antibody was used for another 1 hour incubation at room temperature. DAB substrate kit (Vector) was selected for the detection of immunosignals. Hematoxylin or Hoechst was used for nuclei counterstain. Slices without incubation of primary antibody were served as the negative control. The following antibodies were used in this study: anti-PBK primary antibody (Abcam, ab75987; Rabbit); IgG (Abcam, ab171870; Rabbit; negative control); goat anti-rabbit secondary antibody (Abcam, ab150077; Goat, horseradish peroxidase-conjugated). The expression of PBK was quantified by German immunohistochemical scoring (GIS). According to the GIS system, immunoreactivity score equals positive cell score multiplied by staining intensity score. As for positive cell score system: 0, negative; 1, <10% positive; 2, 11%–50% positive; 3, 51%–80% positive; 4, >80% positive. As for staining intensity score system: 0, negative; 1, weakly positive; 2, moderately positive; 3, strongly positive. Over five points of immunoreactivity score were considered as high expression. Conversely, less than five points of immunoreactivity score was identified as low expression.
Immunocytochemistry
Immunofluorescence (immunocytochemistry) was performed according to the manufacturer's protocol. Briefly, GBM cells were seeded on the coverslip in 24-well plates at a density of 1 × 104/mL. Cells were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature and permeabilized with 0.5% Triton x-100 in PBS for 10 min. Then cells were incubated with primary antibody at 200-time dilution (1:200) in PBS for 1 hour at 37 °C. Next, cells were further incubated with fluorochrome-conjugated secondary antibody at 1000-time dilution (1:1000) in PBS for 1 hour at 37 °C in dark. Cells were incubated with 1 ml Hoechst 33342 solution and mounted with a drop of mounting medium. The fluorescence signals were captured by an inverted fluorescence microscope. The following antibodies were used in this study: anti-PBK primary antibody (Abcam, ab226923; Rabbit); Anti-rabbit Alexa Fluor® 488-conjugated secondary antibody (Abcam, ab150077; Goat); IgG antibody (Abcam, ab171870; Rabbit; negative control); Hoechst 33342 solution (Thermo Scientific, 62249).
Gene Expression Analysis
The gene expression data of 538 human kinome-wide screening were extracted from two GEO datasets (GSE67089, Mao et al. [16]; GSE7696, Murat et al. [17]). The expression of the kinase-encoding genes was analyzed by hierarchical biclustering using Cluster 3.0 software. Average linkage and Euclidean distance were used as the clustering method and the similarity metric. Fold changes were used for comparisons of different gene expressions.
Spearman Correlation Analysis
Gene expression datasets of TCGA GBM and low-grade glioma were extracted from official TCGA website (cancergenome.nih.gov/). Spearman correlation analysis was performed for genetic correlation analysis. The r coefficient varies between 1 and -1. The strong positive or negative correlation of genes is identified while the r is either close to 1 or -1. F-test was used for the calculation of the statistical significance.
Statistical Analysis
All the statistical analysis was performed by using SPSS 22.0 software (IBM, Armonk, NY, USA). Data were presented as mean ± standard deviation with three replicates. The student's t-test and one-way ANOVA were used for data analysis of paired or multiple groups. The log-rank test was used for Kaplan–Meier survival analysis. Multivariate Cox stepwise regression was selected for further multivariate survival analysis. P < 0.05 was considered as statistically significant differences.
Results
Pbk was Highly Expressed in GBM Cells
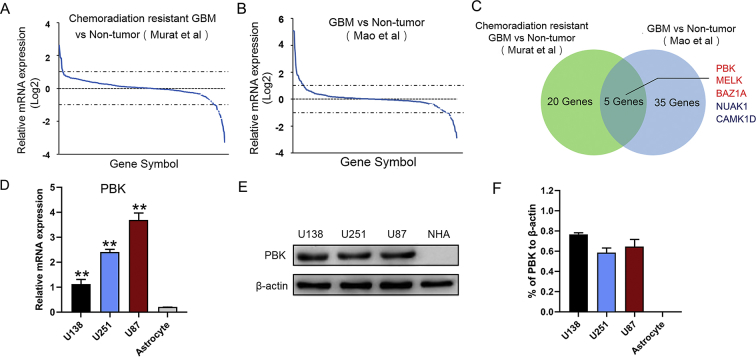
It is well known that activation of multiple protein kinases plays a major role in tumor recurrence and treatment failure [[18], [19], [20]]. To explore the molecular mechanism of GBM tumorigenesis and therapeutic resistance, we compared the expression of 538 human protein kinase encoding genes in previously published DNA microarray databases related to World Health Organization grade and chemoradiation resistance (Tables S1 and S2) [16,17,21]. Differentially expressed kinase encoding genes of two-fold increase or decrease were extracted from both databases (Figure 1A and B). Five kinase encoding genes were shown to be overlapped in these datasets including PBK, MELK, BAZ1A, NUAK1, and CAMK1D (Figure 1C). It appeared that PBK, MELK, and BAZ1A were upregulated genes in GBM, whereas NUAK1 and CAMK1D were downregulated in these two datasets. Moreover, the activation of PBK was found to be closely involved in tumorigenesis and progression in a wide variety of tumor types [8,12,22,23]. Thus, this study focused on the further characterization of PBK in GBM. In addition, the expression of PBK was validated by RT-qPCR and Western blotting. The results showed that PBK expression was highly enriched in GBM cell lines compared with astrocyte cells (Figure 1D–F; Figure S1).
Figure 1.
PBK was highly expressed in GBM cells. (A) Kinome-wide screening of 538 human kinase-encoding genes in chemoradiation resistant GBM cells compared with nontumor (Murat's dataset [17]). (B) Kinome-wide screening of 538 human kinase-encoding genes in GBM cells compared with nontumor (Mao's dataset [16]). (C) Venn diagram identified PBK as one of the most differentially expressed kinase-encoding genes in these two databases. (D) PBK was highly enriched in GBM cell lines compared with astrocyte according to RT-qPCR analysis. (E and F) The Western blotting analysis showed the elevated expression levels of PBK in GBM cell lines compared with normal human astrocytes (NHA). β-Actin served as the control. **P < 0.01.
PBK Promoted GBM Tumorigenesis Both In Vitro and In Vivo
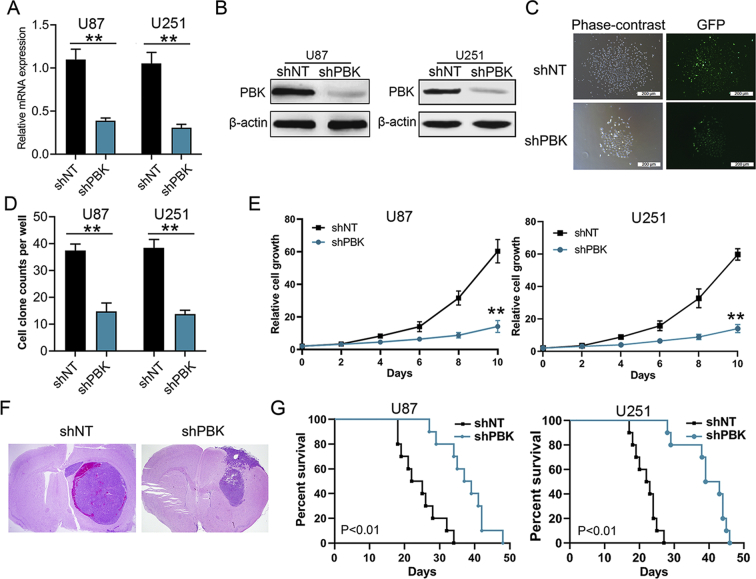
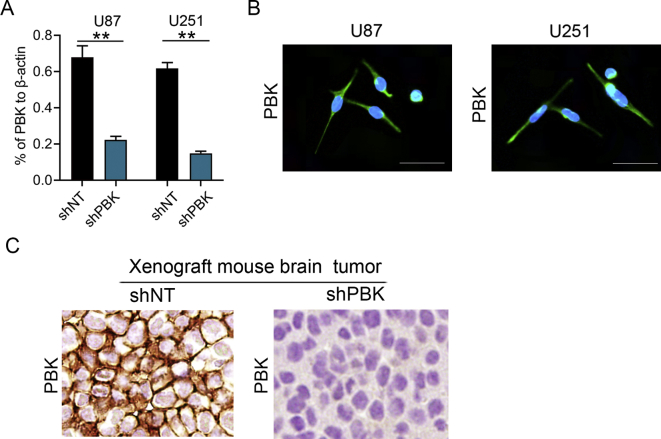
To explore the influence of PBK on GBM tumorigenesis, U87 and U251 GBM cells were selected for further in vitro and in vivo studies. The endogenous PBK expression was silenced by infection of pGFP-shPBK lentivirus, and nontargeting lentivirus was used as control. Furthermore, RT-qPCR and Western blotting were performed for validation of the lentivirus efficiency. The results showed that both mRNA and protein expression levels of PBK were significantly decreased in shPBK U87 and U251 cells (Figure 2A and B; Figure S2A). Immunocytochemistry staining analysis showed that the PBK localized in both cytosolic and nuclear areas in U87 and U251 GBM cell lines (Figure S2B). The colony formation assay and cell growth assay were performed to examine the ability of tumor proliferation in vitro. We found that the colony formation ability was significantly suppressed and the cell proliferation rate was remarkably inhibited by knockdown of PBK expression (Figure 2C–E). Then the functional role of PBK in GBM tumorigenesis was further evaluated by intracranial xenograft mouse models. As shown in Figure 2F, smaller tumor volume was found in xenograft mouse models implanted with shPBK GBM cells compared with control. The expression of PBK was also significantly inhibited in shPBK mouse tumor compared with control (Figure S2C). In addition, the xenograft mice could survive much longer by silencing the PBK expression in GBM cells compared with control (U87 group median survival, 38 d vs 23.5 d; U251 group median survival, 39.3d vs 21.6 d; Figure 2G). These results indicated that PBK could be a functional kinase which promotes GBM tumorigenesis both in vitro and in vivo.
Figure 2.
PBK promoted GBM tumorigenesis both in vitro and in vivo. (A) The mRNA expression level of PBK in GBM cells after transfection with shRNA against PBK (shPBK) or nontargeting control (shNT). (B) The protein expression level of PBK in GBM cells after transfection with shPBK or shNT. β-Actin served as the control. (C) Representative phase-contrast and fluorescence images of cell cloning assay in GBM cells transfected with shPBK and shNT. (D) Cell cloning assay showed reduced cell cloning numbers after knock-down of PBK in GBM cells. (E) The cell proliferation rate was remarkably inhibited by knockdown of PBK expression in GBM cells. (F) Representative H&E staining of brain sections of intracranial xenograft mice. (G) Kaplan–Meier analysis showed longer survival of xenograft mice implanted with shPBK GBM cells compared with control. **P < 0.01.
Overexpression of PBK Indicated Poor Prognosis in Glioma Patients
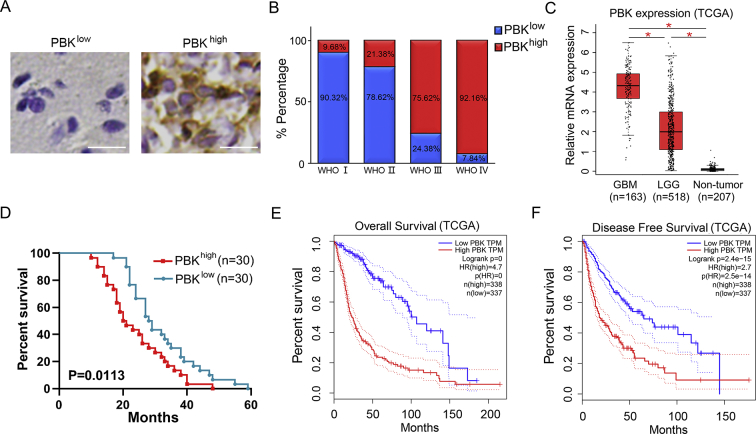
To investigate the clinical relevance of PBK expression, we performed immunohistochemistry staining for PBK expression in 82 glioma samples derived from surgical resection during 2010–2018 at the Department of Neurosurgery of the First Affiliated Hospital of Xi'an Jiaotong University (Table S3). The results showed that PBK localized in both cytosolic and nuclear areas of glioma cells and was highly expressed in high-grade glioma samples according to their GIS (Figure 3A and B). Overexpression of PBK accounted for 92.16% and 75.62% in GBM and grade Ⅲ glioma separately compared with that in low-grade glioma samples (Figure 3B). Furthermore, PBK expression was highly enriched in GBM compared with low-grade glioma and nontumor according to the analysis of the TCGA database (Figure 3C). In addition, it was observed that glioma patients with lower PBK expression survived much longer compared with those with higher expression (median survival, 28.5 months vs 20.5 months; Figure 3D). Similar results were found by analyzing the overall and disease-free survivals according to PBK expression levels in TCGA glioma database (Figure 3E and F). Then, we performed multivariate Cox stepwise regression analysis with age, KPS score, the extent of resection, and PBK expression in our patient cohort. It appeared that extent of tumor resection and PBK expression were the independent prognostic factors for glioma in our cohort (median survival, 27.96 months; Table S5). Taken together, PBK might serve as a clinical prognostic biomarker in glioma.
Figure 3.
Overexpression of PBK indicates poor prognosis in glioma patients. (A) Representative IHC staining images of PBK in GBM and low-grade glioma samples. The white bar represents 20 μm. (B) PBK was overexpressed in high-grade glioma samples compared with low-grade glioma samples. (C) TCGA database analysis showed PBK was highly enriched in GBM compared with low-grade glioma (LGG) and nontumor. (D) Kaplan–Meier analysis for PBK expression in glioma patient samples. (E) Kaplan–Meier analysis of the TCGA database showed a shorter overall survival of patients with elevated PBK levels. (F) Kaplan–Meier analysis of the TCGA database showed a shorter disease-free survival of patients with elevated PBK levels. *P < 0.05.
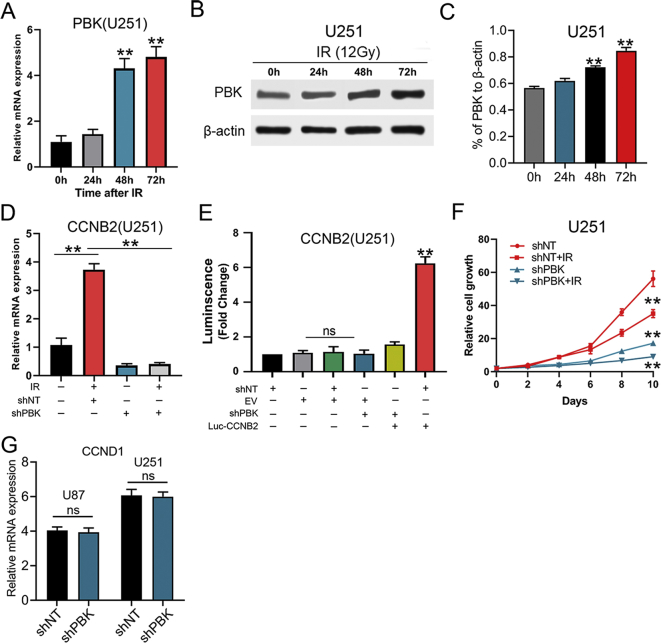
PBK was Essential for Radio-Resistance by Transcriptional Regulation of CCNB2 in GBM cells
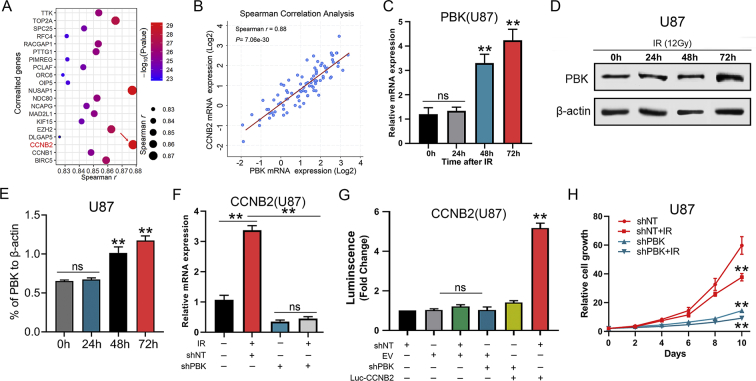
We further analyzed the most correlated genes with PBK in the TCGA GBM database to explore the potential transcriptional regulation involved with PBK (Table S4). The results indicated that CCNB2 was the most correlated genes with PBK among the top 20 genes (Spearman r = 0.88, P < 0.01; Figure 4A and B). Moreover, it was noticed that both mRNA and protein levels of PBK increased gradually with the extension of irradiation time (Figure 4C–E; Figures S3, A–C). Similarly, the CCNB2 expression levels were significantly elevated by irradiation of 12 Gy. Furthermore, this expression elevation effect of CCNB2 could be compromised by preliminary RNA interference of PBK which indicated CCNB2 might serve as a downstream target of PBK (Figure 4F; Figure S3D). This result was further confirmed by luciferase assay showing that PBK was essential for transcriptional activation of CCNB2 promoter (Figure 4G; Figure S3E). In addition, evidence from in vitro cell growth assay showed that GBM cells growth was dramatically suppressed by silencing PBK, and radio-sensitivity of U87 and U251 cells was also increased after knock-down of PBK (Figure 4H; Figure S3F). However, PBK did not regulate CCND1 at mRNA level in our GBM cells (Figure S3G). In conclusion, PBK was essential for radio-resistance by transcriptional regulation of CCNB2 in GBM cells.
Figure 4.
PBK is essential for radio-resistance by transcriptional regulation of CCNB2 in GBM cells. (A) CCNB2 was identified as the most correlated genes to PBK according to Spearman correlation analysis of the TCGA database. (B) CCNB2 showed a strong correlation to PBK at mRNA expression level (Spearman r = 0.88; P < 0.01). (C) The mRNA expression levels of PBK in U87 cells exposed to radiation at different time points (12 Gy). (D and E) Western blotting analysis of PBK in U87 cell postradiation (12 Gy). (F) The RT-qPCR analysis showed the upregulated CCNB2 effect of irradiation on U87 cells could be compromised by preliminary RNA interference of PBK. (G) Luciferase assay indicated transfection of shPBK resulted in a remarkable decrease of transcription activity of CCNB2 promoter in U87 cells. (H) Cell proliferation assay showed GBM cells growth was dramatically suppressed by silencing PBK, and radio-sensitivity of U87 cells was also increased after knock-down of PBK. **P < 0.01. ns represents no significance.
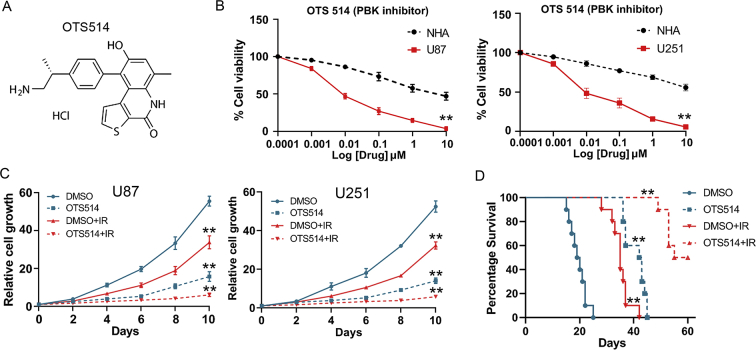
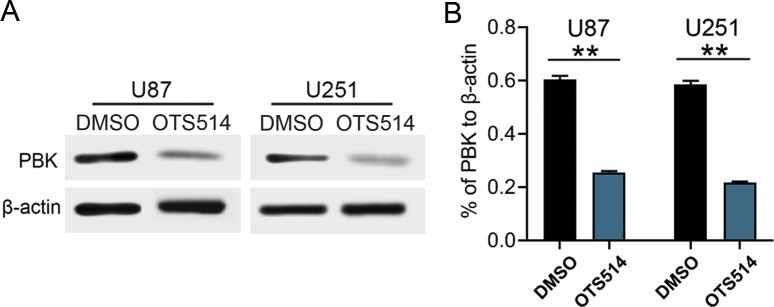
The PBK Inhibitor Reduced Tumorigenesis and Radio-Resistance of GBM Cells
As shown in Figure 2, PBK was essential for GBM tumorigenesis both in vitro and in vivo. It is necessary to test the effect of PBK inhibitor on GBM treatment. OTS514, as shown in Figure 5A, was reported to be an effective PBK inhibitor in ovarian cancer and lung cancer [24,25]. Therefore, we examined the effect of OTS514 on the growth and tumorigenesis in our GBM cells. First, OTS514 significantly inhibited PBK expression in U87 and U251 GBM cells (Figure S4, A and B). Second, GBM cell viability was dramatically and dose-dependently inhibited by OTS514 (Figure 5B). Third, OTS514 (0.01 μM) and irradiation showed significant synergistic effects on the suppression of GBM cell growth in vitro (Figure 5C). Fourth, the in vivo effect of OTS514 with the combination of irradiation was evaluated by intracranial xenograft mouse models. The tail vein injection of OTS514 (20 mg/kg/day) started at day 5 after U87 cell implantation and continued for the following seven days. Consistently, OTS514 treatment significantly prolonged the survival time of intracranial xenograft mice with or without irradiation compared with control (Figure 5D). Collectively, these data suggested that OTS514 might act as an effective PBK inhibitor, which could reduce tumorigenesis and radio-resistance of GBM cells.
Figure 5.
The PBK inhibitor reduces tumorigenesis and radio-resistance of GBM cells. (A) Chemical structure of PBK inhibitor OTS514. (B) Cell viability analysis of GBM cells and normal human astrocytes (NHA) treated with OTS514. (C) OTS514 (0.01 μM) and irradiation showed significant synergistic effects on the suppression of GBM cell growth in vitro. (D) OTS514 (20 mg/kg/day) treatment significantly prolonged the survival time of U87 implanted mice with or without irradiation compared with control. **P < 0.01.
Discussion
Increasing evidence has indicated that aberrant activation of pathogenetic kinases is strongly connected to the tumorigenesis, therapeutic resistance, and tumor recurrence of a variety of cancer types [[26], [27], [28]]. Multiple kinase inhibitors have shown promising results of reducing both cell proliferation and tumor volume of xenograft mouse models [6,8,29]. PBK is a novel serine–threonine kinase closely related to the MAPK family. Previous studies have demonstrated that activation of PBK is involved in multiple protein phosphorylation reactions including mitotic regulation, mediation of tumorigenesis, and therapeutic resistance in breast cancer, lung cancer, and colon cancer [12,13,23]. A recent study identified PBK as a novel enriched gene in peritumoral brain zone of GBM and showed the strong involvement of PBK in GBM aggressiveness including radio-chemoresistance [30]. In this study, we identified PBK as the most therapy-resistant related genes in GBM samples. Furthermore, the elevated expression level of PBK was closely related to aggressive GBM tumorigenesis and radio-resistance, as well as poor clinical outcomes. OTS514, a PBK inhibitor, significantly reduced the GBM cell proliferation and radio-resistance both in vitro and in vivo. Our findings were consistent with Kruthika's research. Furthermore, we explored the potential mechanism of PBK in GBM radio-resistance.
Mechanically, PBK was reported as a nexus for multiple oncogenic signaling pathways including c-Jun p38, p53, ERK1/2, ETV4-uPAR, and c-Jun N-terminal kinase 1, 2, and 3 (JNK1/2/3) signaling pathways [8,14,31]. Yang et al. found that elevated expression level of PBK could activate the promoter of uPAR expression and enhance the binding of ETV4 to uPAR promoter in hepatocellular carcinoma [14]. PBK knockdown resulted in mitochondrial dysfunction, activation of mitochondrial apoptotic pathway and downregulated expression of CDC2 and cyclin B in acute myeloid leukemia [10]. Furthermore, silencing of PBK increased apoptosis and G2/M arrest in colorectal carcinoma cells, while overexpression of PBK promoted tumor cell proliferation through suppression of p53 function as well as cell cycle–related genes [31]. In this study, we primarily demonstrated that PBK was essential for tumorigenesis and radio-resistance in GBM. However, the downstream target of PBK in GBM remains unclear.
To explore the potential regulatory mechanism of PBK in GBM, we performed Spearman correlation analysis between PBK and 18962 genes in the TCGA database and found CCNB2 was the most correlated gene with PBK. CCNB2, a member of the B-type cyclin family, was reported as an important regulator of cell cycle which might cause G2/M arrest [32]. In addition, CCNB2 was found to be highly expressed and correlated with poor clinical prognosis in multiple cancer types including breast cancer, lung cancer, prostate cancer, and hepatocellular carcinoma [[32], [33], [34], [35]]. Furthermore, inhibition of CCNB2 resulted in reduced cell proliferation, migration, and therapeutic resistance through the regulation of aberrant cell cycle [33,34]. However, the upstream regulator and potential role of CCNB2 involved in GBM radiotherapy resistance remain unknown. In this study, we found that PBK was essential for activation of CCNB2 promoter in GBM cells. Furthermore, inhibition of PBK contributed to downregulation of CCNB2 and suppression of GBM proliferation and radio-resistance both in vitro and in vivo. However, the direct binding factor of CCNB2 promoter area is still unclear. RT-qPCR showed PBK did not regulate CCND1 at mRNA level indicating PBK does not regulate cyclin expression in general. Therefore, further experiments including co-immunoprecipitation and chromatin immunoprecipitation sequencing need to be performed to better understand the detailed mechanism of the PBK-CCNB2 signaling pathway in GBM.
Conclusions
PBK-dependent transcriptional regulation of CCNB2 promotes tumorigenesis and radio-resistance in GBM. PBK may serve as a novel therapeutic target for GBM.
Acknowledgments
The authors would like to thank Dr. Wang Jia and all the other members of the Neurosurgery Department of The First Affiliated Hospital of Xi'an Jiaotong University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2019.09.011.
Author's contributions
PM: design and perform the experiments, analyze the data, financial support, and manuscript writing
GB: design the experiments and analyze the data
YCW: perform the experiments and collect the data
CWD: collect the data and statistical analysis
XY: perform the experiments and collect the data
XYG: analyze the data and statistical analysis
RCL: analyze the data and manuscript writing
MDW: design the experiments and manuscript writing.
Conflict of Interest
The authors declare no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81602207) and Special Foundation for “Class A Project” of The First Affiliated Hospital of Xi'an Jiaotong University.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
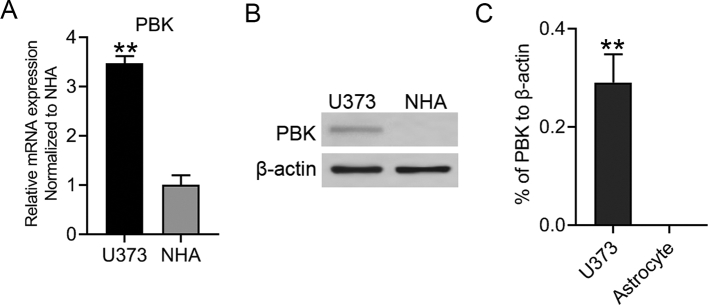
Figure S1.
(A) RT-qPCR analysis between U373 GBM cells and normal human astrocytes (NHA). (B and C) The Western blotting analysis showed an elevated expression level of PBK in U373 cells compared with normal human astrocytes (NHA). β-actin served as the control. **P<0.01.
Figure S2.
(A) The protein expression level of PBK in GBM cells after transfection with shPBK or shNT. β-actin served as the control. (B) Representative fluorescence images of PBK in U87 and U251 cells. The white bar represents 10 μm. (C) Representative IHC staining of PBK in intra-cranial xenograft mouse implanted with GBM cells.**P<0.01.
Figure S3.
(A) The mRNA expression levels of PBK in U251 cells exposed to radiation at different time points (12 Gy). (B and C) Western blotting analysis of PBK in U251 cell post-radiation (12 Gy). (D) The RT-qPCR analysis showed the upregulated CCNB2 effect of irradiation on U251 cells could be compromised by preliminary RNA interference of PBK. (E) Luciferase assay indicated transfection of shPBK resulted in a remarkable decrease of transcription activity of CCNB2 promoter in U251 cells. (F) Cell proliferation assay showed GBM cells growth were dramatically suppressed by silencing PBK, and radio-sensitivity of U251 cells was also increased after knock-down of PBK. (G) The RT-qPCR analysis showed PBK did not regulate CCND1 expression at mRNA level in GBM cells. **P<0.01. ns represents no significance.
Figure S4.
(A and B) OTS514 (0.01 μM) inhibited the PBK expression at the protein level in GBM cells. **P<0.01.
References
- 1.Lee J.H., Lee J.E., Kahng J.Y., Kim S.H., Park J.S., Yoon S.J., Um J.Y., Kim W.K., Lee J.K., Park J. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560(7717):243–247. doi: 10.1038/s41586-018-0389-3. [DOI] [PubMed] [Google Scholar]
- 2.Brandes A.A., Tosoni A., Franceschi E., Sotti G., Frezza G., Amista P., Morandi L., Spagnolli F., Ermani M. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009;27(8):1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 3.Alexander B.M., Cloughesy T.F. Adult Glioblastoma. J Clin Oncol. 2017;35(21):2402–2409. doi: 10.1200/JCO.2017.73.0119. [DOI] [PubMed] [Google Scholar]
- 4.Wei W., Shin Y.S., Xue M., Matsutani T., Masui K., Yang H., Ikegami S., Gu Y., Herrmann K., Johnson D. Single-cell phosphoproteomics resolves adaptive signaling dynamics and informs targeted combination therapy in glioblastoma. Cancer Cell. 2016;29(4):563–573. doi: 10.1016/j.ccell.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vehlow A., Cordes N. DDR1 (discoidin domain receptor tyrosine kinase 1) drives glioblastoma therapy resistance by modulating autophagy. Autophagy. 2019;15(8):1487–1488. doi: 10.1080/15548627.2019.1618540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha D., Wakimoto H., Peters C.W., Antoszczyk S.J., Rabkin S.D., Martuza R.L. Combinatorial effects of VEGFR kinase inhibitor axitinib and oncolytic virotherapy in mouse and human glioblastoma stem-like cell models. Clin Cancer Res. 2018;24(14):3409–3422. doi: 10.1158/1078-0432.CCR-17-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudet S., Branton D., Lue R.A. Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl Acad Sci U S A. 2000;97(10):5167–5172. doi: 10.1073/pnas.090102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao T., Hu Q., Hu X., Lei Q., Feng Z., Yu X., Peng C., Song X., He H., Xu Y. Novel selective TOPK inhibitor SKLB-C05 inhibits colorectal carcinoma growth and metastasis. Cancer Lett. 2019;445:11–23. doi: 10.1016/j.canlet.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Stauffer S., Zeng Y., Zhou J., Chen X., Chen Y., Dong J. CDK1-mediated mitotic phosphorylation of PBK is involved in cytokinesis and inhibits its oncogenic activity. Cell Signal. 2017;39:74–83. doi: 10.1016/j.cellsig.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Liu H., Cao H., Song B., Zhang W., Zhang W. PBK/TOPK mediates promyelocyte proliferation via Nrf2-regulated cell cycle progression and apoptosis. Oncol Rep. 2015;34(6):3288–3296. doi: 10.3892/or.2015.4308. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa C., Senba M., Mori N. Mitotic kinase PBK/TOPK as a therapeutic target for adult T cell leukemia/lymphoma. Int J Oncol. 2018;53(2):801–814. doi: 10.3892/ijo.2018.4427. [DOI] [PubMed] [Google Scholar]
- 12.Dou X., Wei J., Sun A., Shao G., Childress C., Yang W., Lin Q. PBK/TOPK mediates geranylgeranylation signaling for breast cancer cell proliferation. Cancer Cell Int. 2015;15:27. doi: 10.1186/s12935-015-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J., Yuan D., Xing T., Su H., Zhang S., Wen J., Bai Q., Dang D. Ginsenoside Rh2 inhibiting HCT116 colon cancer cell proliferation through blocking PDZ-binding kinase/T-LAK cell-originated protein kinase. J Ginseng Res. 2016;40(4):400–408. doi: 10.1016/j.jgr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Q.X., Zhong S., He L., Jia X.J., Tang H., Cheng S.T., Ren J.H., Yu H.B., Zhou L., Zhou H.Z. PBK overexpression promotes metastasis of hepatocellular carcinoma via activating ETV4-uPAR signaling pathway. Cancer Lett. 2019;452:90–102. doi: 10.1016/j.canlet.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Warren A.Y., Massie C.E., Watt K., Luko K., Orafidiya F., Selth L.A., Mohammed H., Chohan B.S., Menon S., Baridi A. A reciprocal feedback between the PDZ binding kinase and androgen receptor drives prostate cancer. Oncogene. 2019;38(7):1136–1150. doi: 10.1038/s41388-018-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao P., Joshi K., Li J., Kim S.H., Li P., Santana-Santos L., Luthra S., Chandran U.R., Benos P.V., Smith L. Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A. 2013;110(21):8644–8649. doi: 10.1073/pnas.1221478110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murat A., Migliavacca E., Gorlia T., Lambiv W.L., Shay T., Hamou M.F., de Tribolet N., Regli L., Wick W., Kouwenhoven M.C. Stem cell-related "self-renewal" signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26(18):3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 18.Dorand R.D., Nthale J., Myers J.T., Barkauskas D.S., Avril S., Chirieleison S.M., Pareek T.K., Abbott D.W., Stearns D.S., Letterio J.J. Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science. 2016;353(6297):399–403. doi: 10.1126/science.aae0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo J., Chakraborty A.A., Liu P., Gan W., Zheng X., Inuzuka H., Wang B., Zhang J., Zhang L., Yuan M. pVHL suppresses kinase activity of Akt in a proline-hydroxylation-dependent manner. Science. 2016;353(6302):929–932. doi: 10.1126/science.aad5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruscetti M., Leibold J., Bott M.J., Fennell M., Kulick A., Salgado N.R., Chen C.C., Ho Y.J., Sanchez-Rivera F.J., Feucht J. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science. 2018;362(6421):1416–1422. doi: 10.1126/science.aas9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 22.Joel M., Mughal A.A., Grieg Z., Murrell W., Palmero S., Mikkelsen B., Fjerdingstad H.B., Sandberg C.J., Behnan J., Glover J.C. Targeting PBK/TOPK decreases growth and survival of glioma initiating cells in vitro and attenuates tumor growth in vivo. Mol Cancer. 2015;14:121. doi: 10.1186/s12943-015-0398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih M.C., Chen J.Y., Wu Y.C., Jan Y.H., Yang B.M., Lu P.J., Cheng H.C., Huang M.S., Yang C.J., Hsiao M. TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer. Oncogene. 2012;31(19):2389–2400. doi: 10.1038/onc.2011.419. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda Y., Park J.H., Miyamoto T., Takamatsu N., Kato T., Iwasa A., Okabe S., Imai Y., Fujiwara K., Nakamura Y. T-LAK cell-originated protein kinase (TOPK) as a prognostic factor and a potential therapeutic target in ovarian cancer. Clin Cancer Res. 2016;22(24):6110–6117. doi: 10.1158/1078-0432.CCR-16-0207. [DOI] [PubMed] [Google Scholar]
- 25.Park J.H., Inoue H., Kato T., Zewde M., Miyamoto T., Matsuo Y., Salgia R., Nakamura Y. TOPK (T-LAK cell-originated protein kinase) inhibitor exhibits growth suppressive effect on small cell lung cancer. Cancer Sci. 2017;108(3):488–496. doi: 10.1111/cas.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boichuk S., Galembikova A., Dunaev P., Valeeva E., Shagimardanova E., Gusev O., Khaiboullina S. A novel receptor tyrosine kinase switch promotes gastrointestinal stromal tumor drug resistance. Molecules. 2017;22(12) doi: 10.3390/molecules22122152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Shaurova T., Shoemaker S., Petkovich M., Hershberger P.A., Wu Y. Tumor-targeted nanoparticles deliver a vitamin D-based drug payload for the treatment of EGFR tyrosine kinase inhibitor-resistant lung cancer. Mol Pharm. 2018;15(8):3216–3226. doi: 10.1021/acs.molpharmaceut.8b00307. [DOI] [PubMed] [Google Scholar]
- 28.Wang L., Leite de Oliveira R., Huijberts S., Bosdriesz E., Pencheva N., Brunen D., Bosma A., Song J.Y., Zevenhoven J., Los-de Vries G.T. An acquired vulnerability of drug-resistant melanoma with therapeutic potential. Cell. 2018;173(6):1413–1425. doi: 10.1016/j.cell.2018.04.012. e1414. [DOI] [PubMed] [Google Scholar]
- 29.Gramza A.W., Corless C.L., Heinrich M.C. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15(24):7510–7518. doi: 10.1158/1078-0432.CCR-09-0190. [DOI] [PubMed] [Google Scholar]
- 30.Kruthika B.S., Jain R., Arivazhagan A., Bharath R.D., Yasha T.C., Kondaiah P., Santosh V. Transcriptome profiling reveals PDZ binding kinase as a novel biomarker in peritumoral brain zone of glioblastoma. J Neurooncol. 2019;141(2):315–325. doi: 10.1007/s11060-018-03051-5. [DOI] [PubMed] [Google Scholar]
- 31.Hu F., Gartenhaus R.B., Eichberg D., Liu Z., Fang H.B., Rapoport A.P. PBK/TOPK interacts with the DBD domain of tumor suppressor p53 and modulates expression of transcriptional targets including p21. Oncogene. 2010;29(40):5464–5474. doi: 10.1038/onc.2010.275. [DOI] [PubMed] [Google Scholar]
- 32.Qian X., Song X., He Y., Yang Z., Sun T., Wang J., Zhu G., Xing W., You C. CCNB2 overexpression is a poor prognostic biomarker in Chinese NSCLC patients. Biomed Pharmacother. 2015;74:222–227. doi: 10.1016/j.biopha.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Li R., Jiang X., Zhang Y., Wang S., Chen X., Yu X., Ma J., Huang X. Cyclin B2 overexpression in human hepatocellular carcinoma is associated with poor prognosis. Arch Med Res. 2019;50(1):10–17. doi: 10.1016/j.arcmed.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Horning A.M., Wang Y., Lin C.K., Louie A.D., Jadhav R.R., Hung C.N., Wang C.M., Lin C.L., Kirma N.B., Liss M.A. Single-cell RNA-seq reveals a subpopulation of prostate cancer cells with enhanced cell-cycle-related transcription and attenuated androgen response. Cancer Res. 2018;78(4):853–864. doi: 10.1158/0008-5472.CAN-17-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shubbar E., Kovacs A., Hajizadeh S., Parris T.Z., Nemes S., Gunnarsdottir K., Einbeigi Z., Karlsson P., Helou K. Elevated cyclin B2 expression in invasive breast carcinoma is associated with unfavorable clinical outcome. BMC Cancer. 2013;13:1. doi: 10.1186/1471-2407-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.