Abstract
Background
Healthcare decisions made on the basis of insufficient evidence may potentially have ineffective or even harmful consequences. The proportion of older ages (over 65 years) in randomized controlled trials (RCTs) for severe asthma is not enough to establish whether anti-IL-5/IL-5R therapies are equally effective in the elderly as in younger subjects.
Methods
In order to assess the relationship between age and the efficacy of anti-IL-5 monoclonal antibodies (mABs) with respect to the risk of exacerbations and changes in FEV1, a meta-regression analysis via random-effect method was carried out by plotting the effect estimates (outcome variables) resulting from the pairwise meta-analysis with the age of asthmatic subjects (explanatory variable). A comprehensive literature search was performed for pivotal RCTs on the effects of anti-IL-5/IL-5R in severe asthma, with the following keywords: “asthma and mepolizumab”, “asthma and reslizumab” and “asthma and benralizumab”. The study was restricted to “clinical trials”, “age over 65” and “humans”. Data were checked for age, exacerbation rates, changes from baseline in FEV1, and blood eosinophil (Eos) count. Secondary outcomes included inhaled and oral medication use, clinical scores and quality of life.
Results
A total of 10 studies were analysed. Age did not modulate the efficacy of anti-IL-5/IL-5R treatment against the risk of exacerbation neither in the overall population (coefficient −0.007, P = 0.89), nor in patients with high blood Eos count (coefficient 0.075, P = 0.30). The blood Eos level drove the efficacy of anti-IL-5/IL-5R mABs against the risk of exacerbation regardless of age (coefficient −0.27, P < 0.001). Age did not significantly affect the efficacy of anti-IL-5/IL-5R mABs with respect to the change in FEV1 (coefficient −7.15, P = 0.190); however, in high Eos subjects this improvement tended to be less evident in the more advanced age ranges (coefficient −15.18, P = 0.087). In addition, anti-IL-5/IL-5R mABs reduced ACQ score (P < 0.001 vs. placebo), SGRQ score (P < 0.001 vs. placebo), Total Asthma Symptom Score (P < 0.05 vs. placebo), and the use of oral glucocorticoids (P < 0.001 vs. placebo).
Conclusions
Age does not negatively affect the efficacy of anti-IL-5/IL-5R mABs. These findings support the use of anti-IL-5/IL-5R mABs in asthmatics of different age ranges.
Keywords: Severe asthma, Age, Anti-IL5, Therapy, Eosinophils
Abbreviations: yrs, years; RCTs, Randomized Controlled Trials; mABs, monoclonal antibodies; Eos, eosinophils
Introduction
Asthma is a common chronic inflammatory disease that affects more than 300 million people worldwide, with an estimated 10% suffering from the severe, and often uncontrolled, forms of the disease,.1,2 It has been estimated that the prevalence of asthma in older populations does not differ from that of younger populations.3 The importance of recognizing asthma as a disease that also occurs in the older populations is justified by the fact that the mortality rate is higher in these subjects.4 Patients with severe asthma need high-dose inhaled corticosteroids (ICS) and long acting β2-agonists (LABA) and, despite this treatment, they may remain symptomatic.5 This increases the risk of serious and frequent exacerbations, hospital admissions, and results in high healthcare costs.6,7 Severe asthma includes several phenotypes that may have different responses to treatment. Among them, the eosinophilic phenotype depicts a condition of propensity to exacerbations and predisposition to symptom instability with decreased lung function8,9; for this reason, most of the new biological treatments have targeted the eosinophilic inflammation.
Interleukin (IL)-5 is the main mediator of the inflammatory cascade in eosinophilic asthma. IL-5 exerts its effects by binding specifically to the alpha chain of the IL-5 receptor (IL-5R), and acts by controlling eosinophil development, maturation and activation in the bone marrow, as well as subsequent mobilization and survival. It has been widely demonstrated that anti-IL-5 treatments, which cause a reduction of eosinophilia, are effective in patients with severe asthma and uncontrolled symptoms. By inhibiting the inflammatory pathways involved in the activation of eosinophils, which have a prominent role in the type 2 inflammatory response, these drugs offer new additional therapies toward a broader population of patients with severe asthma, who are not responsive or not completely controlled with standard treatment. It is therefore fundamental to establish who will benefit from these approaches.
Although asthma is often considered a disease of younger people, the high prevalence of asthma in the community indicates that older individuals also suffer from the disease. Asthma in the most advanced ages seems to represent a specific phenotype characterized by more severe, but often less perceived, airway obstruction, a mixed-type of airway inflammation and frequent comorbidities. Optimal management of asthma in older populations has always received poor attention, probably because of the complexity of this disease. This condition is characterized by an overall worsening of quality of life, and asthma-related mortality in subjects over 65 years old is increasing.4,10 The GINA guidelines clearly underline that asthma treatment in the older populations is complicated by several factors, such as increased number of comorbidities and their associated symptoms and treatment, together with a reduced coordination when using the inhaler especially caused by declining sensory perception.5 For these reasons, the pharmacological treatment of asthma in older people needs to be carefully and properly chosen. The older population is susceptible to medication side effects and is also more likely to be affected by drugs interactions.
The most recent studies on the efficacy of biological drugs have gradually considered as eligible a population consisting of participants over 65 years of age. Nevertheless, it is impossible to ascertain in individual studies whether, and to what extent, the response to biological treatment is affected by ageing. Indeed, the proportion of older ages in randomized controlled trials (RCTs) is not sufficient to allow the evaluation of the efficacy and safety of anti-IL-5/IL-5R therapies in aged participants. Data on efficacy of asthma medications in the most advanced ages are limited because these patients are often excluded from clinical studies.11
The rationale of this investigation is that the efficacy of anti-IL-5/IL-5R monoclonal antibodies (mABs) may be influenced by the age of subjects enrolled in RCTs, and the meta-analysis approach was chosen to increase precision of the estimated intervention effect and test the role of potentially modifying factors. To this aim, we explored whether in RCTs including also older subjects (≥65 yrs of age) age might represent an independent factor influencing the impact of anti-IL-5/IL-5R mABs on the risk of asthma exacerbations and change in FEV1. As secondary outcomes, we also explored the effect of age on inhaled and oral medication use, clinical scores and quality of life.
Methods
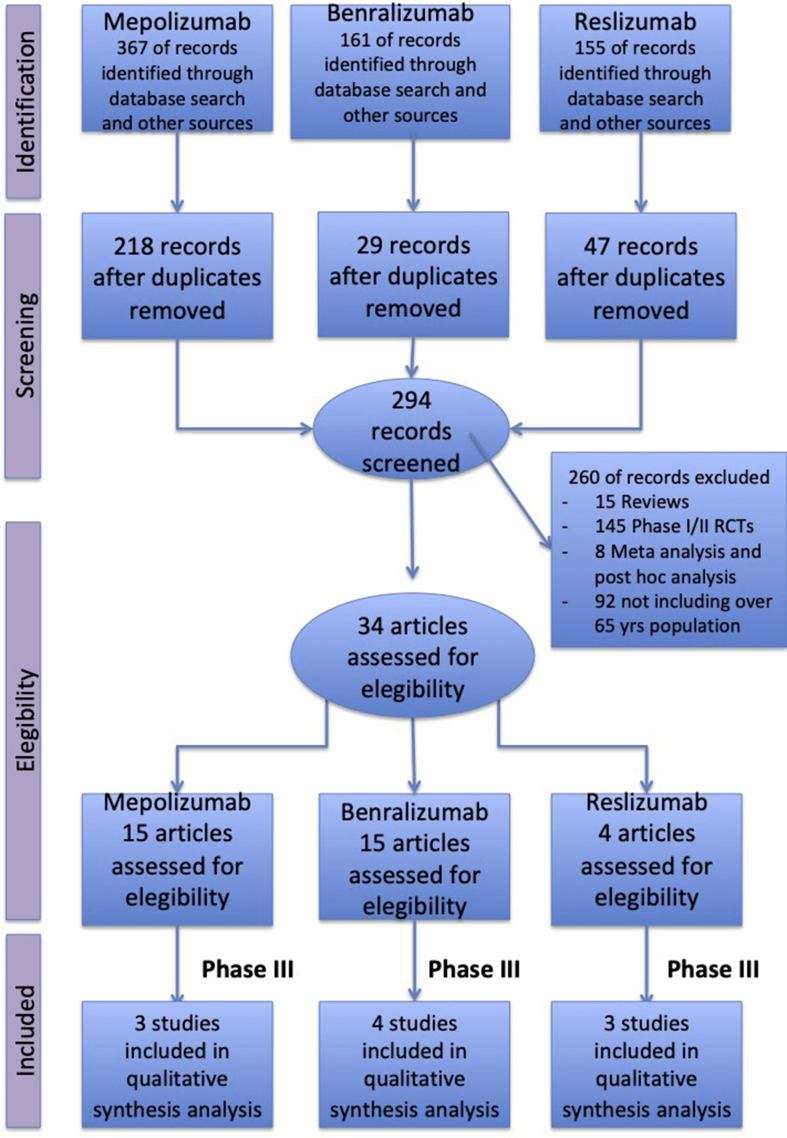
The quantitative synthesis was performed in agreement with the PRISMA-P,12 with the flow diagram reported in Fig. 1, and satisfied all the recommended items reported by the PRISMA-P checklist.12 A comprehensive literature search was performed in Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, Scopus, Web of Science, ClinicalTrials.gov and EU Clinical Trials Register databases for pivotal RCTs written in English and concerning the effect of anti-IL-5 mABs in severe asthma. In order to analyze high-quality data consultative from the scientific community, we did not contact companies to access their grey literature. Two authors (SP, AB) independently screened titles and abstracts of all the potential studies identified in the search and agreed to the selected ones, which met the criteria of including subjects over 65 years of age, and the dosages of the licenced drug. RCTs in which subjects over 65 years of age had been excluded were not included in this quantitative synthesis in order to avoid any potential publication bias related to the restriction of the investigated population to non-elderly patients. The PICO (Patient problem, Intervention, Comparison, and Outcome) framework was used to develop the literature search strategy, as previously described.12 Namely, the "Patient problem” included subject affected by severe asthma, as defined by the GINA document5; the “Intervention” regarded the administration of anti-IL-5 mABs; the “Comparison” was performed with regard to placebo; the “Outcomes” were the risk of exacerbation and change in FEV1. Exacerbations are responsible for high morbidity, mortality and healthcare costs related to asthma, and are defined by the use of short courses of systemic corticosteroids, with or without visits to the emergency department. Lung function, specifically low pre-bronchodilator forced expiratory flow in 1 s (FEV1) is the most commonly reported lung function measure in RCTs, and is an independent predictor of asthma exacerbations.
Fig. 1.
PRISMA flow diagram for the identification of studies included in the meta-regression analysis concerning the relationship between age and the impact of anti-IL-5/IL-5R mABs the risk of asthma exacerbation and change in FEV1. See Methods for details
We included RCTs comparing anti-IL-5/IL-5R therapy with placebo, in addition to current standard of care for asthma (ICS, with or without a second controller such as a long-acting β2 agonist [LABA]). We also included studies that initiated a reduction in standard asthma management as part of the steroid-sparing protocol. In particular, we performed a literature search using MEDLINE for the years 2014–2017 including keywords such as “asthma and mepolizumab”, “asthma and reslizumab” and “asthma and benralizumab”. The study was restricted to “clinical trials”, “age over 65” and “humans”. We excluded meta-analysis, systematic reviews, post hoc analysis and phase 1 and/or 2 studies. Data from the studies were extracted and checked for study age, exacerbation rates, change from baseline in FEV1, and blood eosinophil (Eos) count. Data were extracted in agreement with DECiMAL recommendations.13 The endpoints of this study were the relationship between the age of severe asthmatic patients and the efficacy of anti-IL-5/IL-5R mABs with respect to the risk of exacerbation and change in FEV1.
A pairwise meta-analysis was performed in order to calculate the effect estimates induced by anti-IL-5/IL-5R mABs on the outcome variables, namely the risk of asthma exacerbations and change in FEV1, Asthma Control Questionnaire (ACQ) score, St. George's Respiratory Questionnaire (SGRQ) score, Asthma Quality of Life Questionnaire (AQLQ) score, Asthma Symptom Utility Index (ASUI) score, Total Asthma Symptom Score, use of oral glucocorticoids, and use of as needed short-acting β2-agonist (SABA).
Since data were selected from a series of studies performed by researchers operating independently, and a common effect size cannot be assumed, the random-effect model was used to perform the pair-wise meta-analysis in order to balance the study weights and adequately estimate the 95%CI of the mean distribution of drugs effect on the investigated variables. Although the mathematics behind the fixed-effects model are much simpler that those of the random-effect model, results of this quantitative synthesis cannot be generalized via fixed-effect model as the included studies were dissimilar.14 Indeed, the greater the degree of difference across the studies included in the analysis, the more important becomes the use the random-effect model.15
A sensitivity analysis was performed in the population with high blood Eos cell count (≥300 cells per μl). Data were reported as relative risk (RR) or mean difference (MD) and 95% confidence interval (95%CI). Meta-regression analysis via random-effect method was carried out by plotting the effect estimates (outcome variables) resulting from the pairwise meta-analysis with the age of asthmatic patients (explanatory variable). Meta-regression analysis was also used to assess whether atopy, comorbidities, oral corticosteroid, and smoking habit represented potential source of bias.
OpenMetaAnalyst was used to perform pair-wise meta-analysis and meta-regression analysis,16 and ImageJ to extract data from figures when necessary17; the statistical significance was set at P < 0.05.
Results
A total of 34 studies were initially selected: 15 for mepolizumab, 15 for benralizumab and 4 for reslizumab. We therefore included only phase 3 studies and those which had the approved FDA dosage drug and administration. Data obtained from 5,763 severe asthmatic patients (63.9% treated with anti-IL-5/IL-5R mABs, 36.1% treated with placebo) were selected from 10 studies published between 2014 and 2017: 4 on benralizumab,18, 19, 20, 21 3 on mepolizumab,22, 23, 24 and 3 on reslizumab.25, 26, 27 Patients over 65 years of age were 9.62% (554 subjects), in agreement with previous data concerning the age distribution by asthma severity.28,29 The summary of baseline characteristics of the studies included in this quantitative synthesis are reported in Table 1.
Table 1.
Summary of baseline characteristics in included studies. Q4W: every 4 weeks; Q8W: every 8 weeks
| N° PATIENTS RANDOMIZED | AGE RANGE | BLOOD EOS COUNT | EXACERBATION/YEAR | FEV1% PRED | |
|---|---|---|---|---|---|
| Mepolizumab | |||||
| Ortega et al. (2014) | 576 | 12–81 | 290 ± 1050 | 3.8 ± 2.7 | 59.3 ± 7.5 |
| Bel et al. (2014) | 135 | 16–74 | 250 ± 1245 cell/μl | 3.3 ± 3.4 | 59.6 ± 17.0 |
| Chupp GL et al. (2017) | 556 | ≥12 | 234 (≥150 cell/μl); 186 (≥300 cell/μl) | 2.9 ± 1,9 | 55.5 ± 14.4 |
| Benralizumab | |||||
| Bleecker et al. (2016) | 1205 | 12–75 | 390 Q4W 360 Q8W | 2.9 ± 1.8 Q4W 2.8 ± 1.5 Q8W | 57.4% ± 14.1 Q4W; 56.1% ± 14.6 Q8W |
| Fitzgerald et al. (2016) | 1306 | 12–75 | 370 Q4W 400 Q8W | 2.7 (1.9) Q4W 2.7 (1.4) Q8W | 58.9% ± 14.8 Q4W; 57.9% ± 14.9 Q8W |
| Nair et al. (2017) | 220 | 18–75 | 462 Q4W 437 Q8W | 2.8 ± 2.0 Q4W 3.1 ± 2.8 Q8W | 57.4 ± 18.0 Q4W 59.0 ± 17.9 Q8W |
| Ferguson et al. (2017) | 351 | 18–75 | 205 | 0.3 ± 0.8 | 69.7% ± 10.9 |
| Reslizumab | |||||
| Castro et al. (2015) | 489 | 12–75 | 696 | 1.9 ± 1.6 | 63.6 ± 18.6 |
| Bjermer et al. (2016) | 315 | 12–75 | 648 | 57 (%) | 70.4 |
| Corren et al. (2016) | 496 | 18–65 | 281 | 66 (%) | 66.8 |
As expected, the pairwise meta-analysis indicated that all anti-IL-5/IL-5R mABs were effective in reducing the risk of asthma exacerbation (overall RR 0.55, 95%CI 0.49–0.62; P < 0.001 vs. placebo), increasing FEV1 (overall MD +102 ml 95%CI 78–126; P < 0.001 vs. placebo), reducing ACQ score (overall MD -0.26 points 95%CI -0.34 to −0.18; P < 0.001 vs. placebo), reducing SGRQ score (overall MD -7.15 points 95%CI -9.83 to −4.47; P < 0.001 vs. placebo), increasing AQLQ score (overall MD +0.24 points 95%CI 0.14–0.33; P < 0.001 vs. placebo), increasing ASUI score (overall MD +0.05 points 95%CI 0.03–0.07; P < 0.001 vs. placebo), reducing Total Asthma Symptom Score (overall MD -0.15 points 95%CI -0.26 to −0.03; P < 0.05 vs. placebo), and reducing the use of oral glucocorticoids (overall RR 0.67 95%CI 0.59–0.80; P < 0.001 vs. placebo). No reduction in the use of as needed SABA was detected (overall MD -0.20 puffs/day 95%CI -0.45 – 0.04; P > 0.05 vs. placebo). The efficacy profile of benralizumab, mepolizumab, and reslizumab was not significantly different between each other (P > 0.05).
The meta-regression analysis showed that age did not modulate the efficacy of anti-IL-5/IL-5R mABs against the risk of exacerbation neither in the overall population (coefficient −0.007, P = 0.89), nor in patients with high blood Eos cell count (coefficient 0.075, P = 0.30). Conversely, the level of blood Eos cell count drove the efficacy of anti-IL-5/IL-5R mABs against the risk of exacerbation regardless of age (coefficient −0.27, P < 0.001): in high Eos patients, anti-IL-5/IL-5R mABs were more effective than in asthmatic patients with blood Eos count <300 cells per μl.
Age did not modulate the efficacy of anti-IL-5/IL-5R mABs with respect to the change in FEV1 (coefficient −7.15, P = 0.190), ACQ score (coefficient −0.01, P = 0.722), SGRQ score (coefficient −1.22, P = 0.697), AQLQ score (coefficient −0.01, P = 0.834), Total Asthma Symptom Score (coefficient 0.05, P = 0.354), the use of oral glucocorticoids (coefficient −0.03, P = 0.830), and the use of as needed SABA (coefficient 0.07, P = 0.389). No meta-regression analysis was performed on ASUI as this outcome was investigated in only two RCTs.25,26
A signal of relationship between the age and the impact on FEV1 was detected in high eosinophilic patients (coefficient −15.18, P = 0.087): in subjects with blood Eos count ≥300 cells per μl the efficacy of anti-IL-5/IL-5R mABs was greater in younger patients than in older patients (Fig. 2). As for exacerbations, the level of blood Eos count modulated the efficacy of anti-IL-5/IL-5R mABs with respect to the improvement in FEV1 (coefficient 87.39, P < 0.01): the higher the level of blood Eos the greater the efficacy of anti-IL-5/IL-5R mABs regardless of age. Atopy, comorbidities, oral corticosteroid, and smoking habit did not represent significant (P > 0.05) effect modifiers and, thus, they were not a source of bias.
Fig. 2.
Graphical representation of the meta-regression analysis for the relationship between the age and the impact of anti-IL-5/IL-5R mABs on FEV1 in high eosinophilic asthmatic patients. The Y-axis indicates the change in FEV1 (ml) from baseline compared to placebo
Discussion
The introduction in clinical practice of new biological drugs for severe uncontrolled asthma has led to exploration of factors that could influence the response to treatment: in this respect, older age-associated changes could play a relevant role. The main finding of the current investigation is that age does not affect the efficacy of anti-IL-5/IL-5R mABs in RCTs conducted in eosinophilic severe asthmatics. This is true when assessing the reduction in exacerbations and improvement in lung function, as well as changes in clinical variables and in quality of life. Interestingly, anti-IL-5/IL-5R mABs seemed to be less efficient in older asthmatics with high eosinophilic levels with regards to the lung function improvement. In addition, the analysis confirmed that the level of blood Eos predicts the response to all anti-IL-5/IL-5R mABs.
Epidemiological studies confirm that asthma in older populations is as frequent as in younger ages and is characterized by features of more severe and difficult to treat disease.30 The use of biological drugs in this population could strongly improve the outcomes of asthma treatment. It is therefore logical to analyze whether ageing influences the response to biological treatment. Of note, current asthma medications have never been tested in older asthmatics, since the most advanced ages have been excluded by enrollment in clinical trials.11 At present, the impact of age-related changes on the response to biological treatment for severe asthma is largely unknown. On the one hand, data from omalizumab are reassuring, since this drug has been found to be as effective in older asthmatics as in younger patients, and it is able to reduce exacerbations and symptoms in patients aged 50 years and over.31 On the other hand, however, very little is known with regard to the efficacy of novel anti-IL-5/IL-5R mABs in older subjects.
With the aim to review the evidence on the efficacy and safety of new biological anti IL-5/IL-5R treatments, with specific focus on the influence of different ages, the current meta-analysis was performed, and selected studies were analyzed in order to explore whether a relationship between age and efficacy of mABs exists. The investigation included mepolizumab, which was approved for severe asthmatics aging over 12 years, with an Eos concentration of 150/μL at screening or 300/μL within the past 12 months. Early studies proved that mepolizumab reduced the number of blood and sputum Eos and allowed prednisone-sparing in asthmatics with sputum eosinophilia.20 Later, the Dose Ranging Efficacy and Safety with Mepolizumab in Severe Asthma (DREAM) study32 further confirmed its effectiveness and tolerability in subjects with severe eosinophilic asthma. The Efficacy and Safety Study of Mepolizumab Adjunctive Therapy in Subjects with Severe Uncontrolled Refractory Asthma (MENSA) study demonstrated the lack of difference between intravenous or subcutaneous administrations every 4 weeks, with respect to the reduction of asthma exacerbations and improvement in markers of asthma control.23 Reslizumab was evaluated in patients older than 18 years with severe persistent asthma. Castro and colleagues25 demonstrated that the drug at an intravenous dose of 3.0 mg/kg every 4 weeks induces reduction in rates of asthma exacerbations and improvement in FEV1 and asthma control. Benralizumab is able to induce Eos depletion in blood, sputum and airway mucosa. In a phase 2b study, Castro et al.33 showed that the administration of benralizumab at 20 mg and 100 mg reduced asthma exacerbations in adults with a baseline blood Eos count of at least 300 cells/μL. Phase 3 studies confirmed the efficacy and safety of benralizumab for individuals with severe uncontrolled asthma with elevated blood Eos.
To our knowledge, this is the first study that evaluates age as a potential factor influencing the efficacy of anti-IL-5/IL-5R mAbs. Recently, Busse and colleagues34 indirectly compared the efficacy of mepolizumab, benralizumab and reslizumab according to blood Eos counts, showing that the efficacy is proportional to the level of blood Eos. However, no comparison between age ranges was made. Furthermore, a recent systematic review35 compared the three regimens in terms of reductions in rate of exacerbations, as well as improvements in health-related quality of life and pulmonary function, and confirmed that patients with severe asthma and high Eos blood counts benefit from taking biological treatment to improve asthma control; adults and children over 12 years were included, but no formal analysis taking age into account was performed.
The results of the current study confirmed that the efficacy of all explored drugs was dependent on the blood levels of Eos. By weighting the specific contribution of age ranges, the rigorous analytic process ruled out the role of age on the beneficial effects of the biologic drugs, both in terms of reduction in the risk of exacerbations and improvement in lung function, as assessed by changes in FEV1, thus reinforcing the use of anti-IL-5/IL-5R mABs in older individuals. In addition, changes in clinical parameters and quality of life were not affected by age. It should be emphasized, however, that only few studies included subjects in the most advanced ages: the paucity of older asthmatics limits the generalizability of the current findings to the extreme ages and advocates for complementary pragmatic studies specifically designed for older populations. In fact, asthmatics in the most advanced ages often present with comorbidities and consequent polypharmacy. Both factors can interact with current pharmacological treatments and negatively impact on their efficacy and safety. We showed that, in real-life settings, more than 40% of asthmatic subjects are currently treated by protocols based on results of RCTs for which they would have not been eligible, and this proportion increases in older patients with comorbidities.11 In the current investigation, comorbid condition, as well as atopy and cigarette smoke exposure, did not significantly affect the explored relationships.
The observation that the efficacy of the anti-IL-5/IL-5R mABs tended to be lower in older populations when looking at asthmatics with high Eos levels with respect to lung function is novel and deserves some speculation. Younger asthmatics with high Eos could be representative of a specific phenotype of asthma that is actively responsive to anti-IL-5/IL-5R drugs, and this feature decreases with ageing. One potential explanation lies in the nature of airway hyperresponsiveness, which is strongly related to inflammatory cells and mediators in younger asthmatics,36 whereas in older subjects it is mainly influenced by structural changes of the airway wall,37,38 thus being less responsive to lung function improvements. Perhaps, the age-related immunological changes, namely immunosenescence, counteract the beneficial effect associated with the depletion of Eos. This has to be tested in specifically designed studies.
Since the meta-analytical approach was based on published literature, and not on individual data, caution should be made in the interpretation and generalizability of the findings. The heterogeneity of the studied populations may indeed limit the applicability of the current results. In this respect, we specifically extended the search to subjects over 65 years of age, with the aim to include older subjects whenever indicated. With this limit in mind, the current meta-analysis offers the opportunity to corroborate the findings obtained by single investigations, which are more open to selection and interpretation biases.
Meta-regression analysis allows relating the size of effect (i.e. the risk of exacerbations and changes in FEV1) to one or more characteristics of the studies included (i.e age). The main advantage of such a methodological approach is that it is possible to identify the trial-level covariates that may influence the effect estimate of a certain treatment.39 However, it should be acknowledged that the relationship described by a meta-regression is an observational association across trials that do not benefit from randomization to underpin a causal interpretation. Furthermore, in the current study meta-regression analysis was used to relate the results of the trials to published age averages of patient characteristics within trials, and not with individual patient data. This is a potential limitation; however, since the patient averages were based on large samples, the relationship was not attenuated by measurement error.39 Another limitation is that, although the natural history of asthma in men and women is different across the life-course, it was not possible to perform a subset analysis based on gender because separate data for men and women were not reported in the selected studies.
In conclusion, age was not demonstrated to affect the efficacy of anti-IL-5/IL-5R mABs. In high Eos asthmatics, anti-IL-5/IL-5R mABs appeared to be more effective to reduce the risk of exacerbations than in those with blood Eos count <300 cells/μl regardless of age. The current investigation provides robust findings to support the use of anti-IL-5/IL-5R mABs in asthmatics of different age ranges. The implementation of RCTs and pragmatic studies including the most advanced ages will further allow extending applicability of the current results to real-life settings.
Funding
The authors state there has been no significant financial support for this work that could have influenced its outcome.
Consent for publication
The authors give their consent for publication.
Competing Interests
The authors confirm that there are no known conflicts of interest associated with this publication.
Ethics approval
Not applicable.
Author contributions
Stefania Principe participated in the design of the study, performed the literature search, collected the data, contributed to the interpretation of the results and the writing of the manuscript, and gave the final approval of the version to be published.
Luigino Calzetta participated in the design of the study, performed the study analysis, contributed to the writing of the manuscript, and gave final approval of the version to be published.
Alida Benfante participated in the design of the study, contributed to the writing of the manuscript, and gave final approval of the version to be published.
Paola Rogliani participated in the design of the study, contributed to the interpretation of the findings and the writing of the manuscript, and gave final approval of the version to be published.
Nicola Scichilone conceived and design the study, participated in the interpretation of the results and wrote the first version of the manuscript. He is the guarantor of the paper, and he gave final approval of the version to be published.
Acknowledgements
Not applicable.
References
- 1.To T., Stanojevic S., Moores G. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC Public Health. 2012 Mar 19;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung K.F., Wenzel S.E., Brozek J.L. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014 Feb;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 3.Oraka E., Kim H.J., King M.E., Callahan D.B. Asthma prevalence among US elderly by age groups: age still matters. J Asthma. 2012;49:593–599. doi: 10.3109/02770903.2012.684252. [DOI] [PubMed] [Google Scholar]
- 4.Bellia V., Pedone C., Catalano F. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest. 2007;132:1175–1182. doi: 10.1378/chest.06-2824. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma (GINA) 2017. Global strategy for asthma management and prevention.http://ginasthma.org/2016-gina-report-global-strategy-for-asthma-management.and.prevention [Google Scholar]
- 6.Fernandes A.G., Souza-Machado C., Coelho R.C. Risk factors for death in patients with severe asthma. Journal brasileiro de pneumologia. 2014;40(4):364–372. doi: 10.1590/S1806-37132014000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadatsafavi M., Lynd L., Marra C. Direct health care costs associated with asthma in British Columbia. Can Respir J. 2010 Mar-Apr;17(2):74–80. doi: 10.1155/2010/361071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price D., Wilson A.M., Chisholm A. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 2016 Jan 7;9:1–12. doi: 10.2147/JAA.S97973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talini D., Novelli F., Bacci E. Sputum eosinophilia is a determinant of FEV1 decline in occupational asthma: results of an observational study. BMJ Open. 2015 Jan 5;5(1) doi: 10.1136/bmjopen-2014-005748. e005748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nejjari C., Tessier J.F., Barberger-Gateau P., Jacqmin H., Dartigues J.F., Salamon R. Functional status of elderly people treated for asthma-related symptoms: a population based case-control study. Eur Respir J. 1994;7:1077–1083. [PubMed] [Google Scholar]
- 11.Battaglia S., Basile M., Spatafora M., Scichilone N. Are asthmatics enrolled in randomized trials representative of real-life outpatients? Respiration. 2015;89(5):383–389. doi: 10.1159/000375314. [DOI] [PubMed] [Google Scholar]
- 12.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inf Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedder H., Sarri G., Keeney E., Nunes V., Dias S. Data extraction for complex meta-analysis (DECiMAL) guide. Syst Rev. 2016;5:212. doi: 10.1186/s13643-016-0368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeCoster J. 2004. Meta-analysis notes.http://www.stat-help.com/meta.pdf [Google Scholar]
- 15.Turner J.R., Durham T.A. Meta-methodology: conducting and reporting meta-analyses. J Clin Hypertens. 2014;16:91–93. doi: 10.1111/jch.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace B.C., Dahabreh I.J., Trikalinos T.A., Lau J., Trow P., Schmid C.H. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49:1–15. [Google Scholar]
- 17.Abramoff M.D., Magalhaes P.J., Ram S.J. Image processing with ImageJ. Biophot Int. 2004;11:1081–8693. [Google Scholar]
- 18.Bleecker E.R., FitzGerald J.M., Chanez P. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 19.FitzGerald J.M., Bleecker E.R., Nair P. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 20.Nair P., Wenzel S., Rabe K.F. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson G.T., FitzGerald J.M., Bleecker E.R. Benralizumab for patients with mild to moderate, persistent asthma (BISE): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Respiratory Medicine. 2017;5:568–576. doi: 10.1016/S2213-2600(17)30190-X. [DOI] [PubMed] [Google Scholar]
- 22.Chupp G.L., Bradford E.S., Albers F.C. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. The Lancet Respiratory Medicine. 2017;5:390–400. doi: 10.1016/S2213-2600(17)30125-X. [DOI] [PubMed] [Google Scholar]
- 23.Ortega H.G., Liu M.C., Pavord I.D. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 24.Bel E.H., Wenzel S.E., Thompson P.J. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 25.Castro M., Zangrilli J., Wechsler M.E. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. The Lancet Respiratory Medicine. 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 26.Bjermer L., Lemiere C., Maspero J., Weiss S., Zangrilli J., Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789–798. doi: 10.1016/j.chest.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 27.Corren J., Weinstein S., Janka L., Zangrilli J., Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150:799–810. doi: 10.1016/j.chest.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Zein J.G., Dweik R.A., Comhair S.A. Asthma is more severe in older adults. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133490. e0133490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zein J.G., Udeh B.L., Teague W.G. Impact of age and sex on outcomes and hospital cost of acute asthma in the United States, 2011-2012. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157301. e0157301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braman S.S. Asthma in the elderly. Clin Geriatr Med. 2017 Nov;33(4):523–537. doi: 10.1016/j.cger.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Humbert M., Busse W., Hanania N.A. Omalizumab in asthma: an update on recent developments. J Allergy Clin Immunol Pract. 2014 Sep-Oct;2(5):525–536.e1. doi: 10.1016/j.jaip.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Pavord I.D., Korn S., Howarth P. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012 Aug 18;380(9842):651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 33.Castro M., Wenzel S.E., Bleecker E.R. Benralizumab, an anti-interleukin 5 receptor α monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014 Nov;2(11):879–890. doi: 10.1016/S2213-2600(14)70201-2. [DOI] [PubMed] [Google Scholar]
- 34.Busse W., Chupp G., Nagase H. Anti-IL-5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019 Jan;143(1):190–200.e20. doi: 10.1016/j.jaci.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 35.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev 2017, Issue 9. Art. No.: CD010834. [DOI] [PMC free article] [PubMed]
- 36.Ward C., Pais M., Bish R. Airway inflammation, basement membrane thickening and bronchial hyperresponsiveness in asthma. Thorax. 2002 Apr;57(4):309–316. doi: 10.1136/thorax.57.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macklem P.T. Mechanical factors determining maximum bronchoconstriction. Eur Respir J Suppl. 1989 Jun;6:516s–519s. [PubMed] [Google Scholar]
- 38.Verbeken E.K., Cauberghs M., Mertens I., Clement J., Lauweryns J.M., Van de Woestijne K.P. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest. 1992 Mar;101(3):793–799. doi: 10.1378/chest.101.3.793. [DOI] [PubMed] [Google Scholar]
- 39.Thompson S.G., Higgins J.P.T. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]