Abstract
Head and neck carcinoma (HNC) are diseases arising from several tracts of the aerodigestive ways. Most HNC are squamous cell carcinoma (SCCHN). Immunotherapy is a treatment strategy aimed to reinforce the immune system. Several types of immunotherapy are available in the clinical scenario. Checkpoint inhibitors were developed later in SCCHN; nivolumab and pembrolizumab have reached the clinical approval, having both drugs demonstrated to significantly improve the overall survival, if compared with the standard of treatment (according to the results of the CheckMate 141 and KEYNOTE-040 trials). Nevertheless, immunotherapy may fail because of the genetics of SCCHN. In fact, two genetically different types of SCCHN have been discovered, one virus-related (HPV) and the other mutagens-related. They seem to show in clinical trials very different responses to immunotherapy. Given the existence of a number of factors predictive of response to immunotherapy in SCCHN, a future clinical approach may be to characterize the genetic and immunologic feature of SCCHN and to perform a well-tailored immunotherapy. This review will summarize the main immunotherapy strategies available in SCCHN, discussing their real efficacy, highlighting also the ways to improve them.
Immune System and Cancer
Immune Response Against Cancer
Immune system is not just a defense against infective pathogens but also against cancer cells. In the mid-20th century, Thomas and Burnet proposed the concept of “immunosurveillance,” in which lymphocytes acted as sentinels to protect against transformed cells [1]. This theory was supported by several lines of evidence including the detection of a high incidence of sarcomas in deeply immunocompromised mice lacking the recombination activating gene-2 (RAG-2) as demonstrated by Shankaran et al. [2].
Immunosurveillance represents just part of a dynamic process known as “cancer immunoediting,” which comprises three phases: tumor elimination, equilibrium, and escape to clinically overt disease. Immunoediting is characterized by changes in the immunogenicity of tumors because of the antitumor response of the immune system, the final step is the development of immune-resistant cancer cells.
The first phase, called “elimination,” is characterized by innate and adaptive immune responses against tumor cells. In the second phase, named “equilibrium phase,” the tumor cells escaped the elimination phase and have a nonimmunogenic phenotype. During this phase, specific T-lymphocytes and secreted cytokines (i.e., interferon gamma, IFN-γ) exert a selection pressure on tumor cells which are genetically unstable and rapidly mutating. After that, tumor cell variants which have acquired resistance to elimination enter in the third phase, called “escape phase” and during which tumor cells continue to grow and expand in an uncontrolled manner [3].
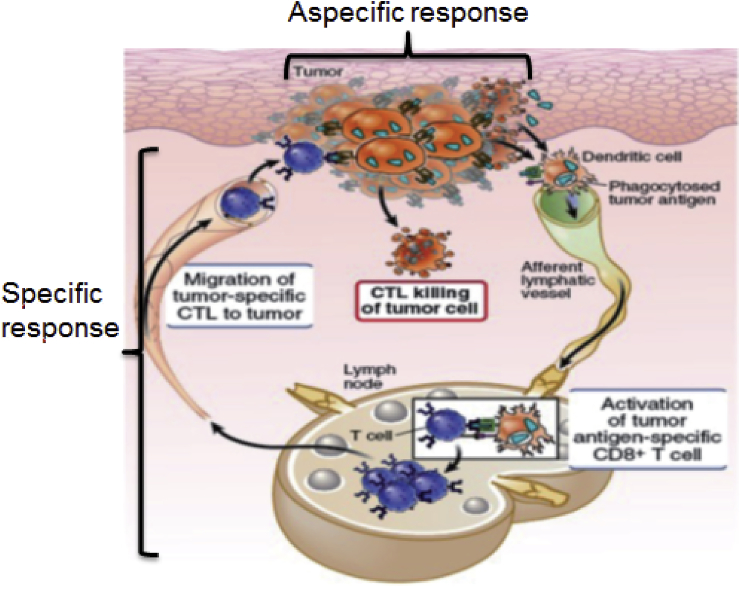
Immune response against cancer cells, which characterizes the first phase of the immunoediting process (elimination), is very complex, and it can be divided into three phases: the innate immune response, the activation of specific T-cells against cancer, and the killing of tumor cells made by the above-mentioned T-cells (Figure 1).
Figure 1.
Immune response against cancer may be divided in three phases: 1) the innate immune response that happens in the tumor site, 2) the activation of specific T-cells against cancer, in the lymph nodes and 3) the killing of tumor cells operated by the CD8+ T-cells, which migrates in the tumor site.
Tumor Escape from Immune Response
Immune response against cancer may fail, leading to the “equilibrium” and “escape” phases. There are many mechanisms underlying the failure of the immune response and most of them involve the generation of specific class of inhibitory lymphocytes and other type of immune cells, able to release cytokines suppressive for cytotoxic CD8 lymphocytes.
T-cell–dependent immune response against tumor antigens plays a crucial role in tumor immune surveillance and the critical effector cells of adaptive antitumor immunity are the activated CD8+ cytotoxic T-lymphocytes. Immune response failure develops when the effectors CD8+ T-cells are silenced from several stimuli, elicited by soluble cytokines or interaction with other immune-system cells.
The main cells able to induce anergy of the CD8+ T-cells are the regulatory T-lymphocytes (Treg). Treg cells represent a minor heterogenic subset of CD4+ T-lymphocytes. Treg cells have been well characterized by immunohistochemistry (IHC) showing the following panel of expression: CTLA-4+ (cytotoxic T-lymphocyte antigen 4), CD25-high, CD127-low, and nuclear expression of Foxp3. Normally, Tregs are committed to regulate immune response to prevent an excessive immune reactivity and their activity is mainly toward other immune cells such as effector T-cells. Tregs also play a role in cancer because the mechanisms that prevent autoimmunity are the same that limit the immune system to recognize tumor cells. Indeed, the majority of tumor-associated antigens are self-antigens or only minimal-modified self-antigens harboring genetic modifications [4]. Elevated levels of Tregs have been found in several cancer types, including lung, breast, and pancreatic cancer [5]. A number of clinical and preclinical studies show a general increase of both circulating and infiltrating Treg during cancer development [[5], [6], [7]]. Treg-mediated immunosuppression may occur by cell-to-cell contact or by cytokines secretion.
The mechanisms underlying the potential Treg-driven protumor effect remain elusive. These cells are probably recruited from peripheral circulation into the tumor by chemotactic factors released by cancer cells. Treg infiltration into the tumor microenvironment is facilitated by the binding of the chemokine receptor CCR4 (C–C chemokine receptor type 4), which is expressed on their cell surface, to its ligand CCL22 (C–C motif chemokine 22), which is secreted by many types of tumor cells [8]. Tregs are recruited into the tumor tissue where they are induced toward a highly immunosuppressive phenotype by tumor-secreted cytokines, such as TGF-β (Transforming Growth Factor), IL-35 and IL-10 [9,10]. Treg cells also dampen CD8-mediated immune response through direct mechanisms, which take into account some proteins, such as CTLA-4, T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), CD39 and CD73.
CTLA-4 (cytotoxic T-lymphocytes antigen 4) is a transmembrane protein expressed on cell surface of Treg able to link the costimulatory molecule B7 on the antigen presenting cells (APC), therefore preventing their interaction with CD28, expressed on the cell surface of CD8+ T-cells. As results, T-cells cannot be activated by APC, resulting is a significant reduction of their proliferation.
CD39 and CD73 sequentially convert ATP and ADP into the immunosuppressive factor adenosine, which inhibits DC and macrophage functions [11,12], thus inhibiting the antigen presentation phase, which is important for CD8+ T-cells maturation.
TIM-3 was firstly identified as a main driver for autoimmune diseases and it is often expressed by Treg. Tim-3 plays a key role in inhibiting Th1 response and the expression of cytokines such as TNF and IFN-γ. A number of preclinical reports have demonstrated that Treg strongly expressing TIM-3 have higher immunosuppressive function than TIM-3-negative Treg, because of their increased production of IL-10 and other suppressive molecules [13,14]. Stimulation of TIM-3 on the cell membrane of Treg may happen through its interaction with the Galectin-9, which is expressed by different tumor cells [15].
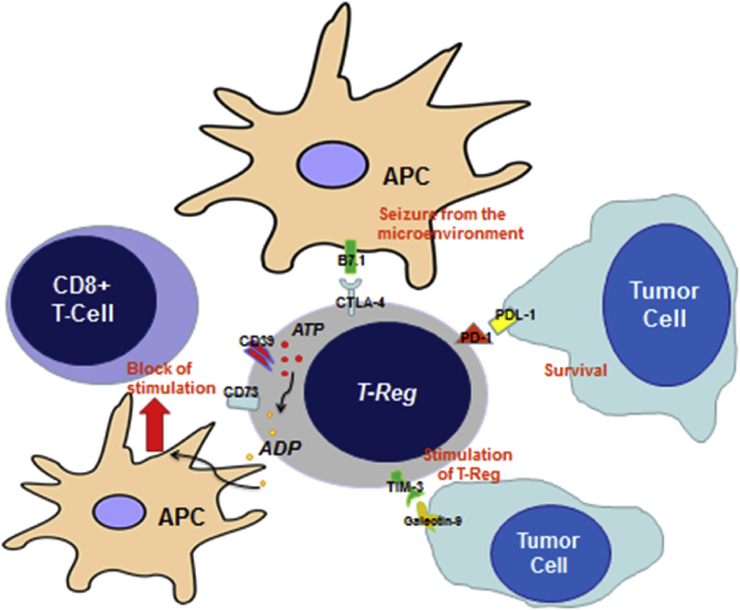
Summarizing, the cytokine mediators released by cancer cells activate and recruit circulating Treg. Treg migrate into the tumor site, where they are further activated by the cytokines produced in the tumor stroma, such as TGF-β. Ultimately, Treg suppress the activity of cytotoxic and effector cells via both cell-to-cell contact and humoral mechanisms, because of the expression of CTLA-4 and CD39 and the release of immune modulating factors such as IL-10, IL-35, and TGF-β (Figure 2).
Figure 2.
Mechanisms through which Treg can dampen the cytotoxic immune response CD8+ mediated: Tregs interact with antigen presenting cells (APC) through CTLA-4, seizing them from the microenvironment; the linkage between PD-1 and PDL-1 expressed by tumor cells allows that lasts to survive; CD39 and CD73 convert ATP in ADP, which is known to be a strong suppressor of the APC activity; finally, Treg interact directly with tumor cells through the linkage between TIM-3 and Galectin-9, resulting in their further stimulation.
Failure of immune response against cancer is made complete by the accumulation of DNA mutations in tumor cells that make them invisible to the immune system. Tumor cells may avoid immune surveillance by downregulating histocompatibility molecules, such as HLA class I gene, required to proper antigen presentation. In addition, tumor cells can release cytokines able to affect the CD8+ function [16,17].
Immune Response in Squamous Cell Carcinoma of the Head and Neck (SCCHN)
Background
Squamous cell carcinoma of the head and neck (SCCHN) are tumors originating from the first tract of aerodigestive ways and they are a not rare disease, accounting for 5–7% of all malignancies. SCCHN development is strongly related to tobacco and/or alcohol consumption. In the last 15 years, a remarkable change in SCCHN epidemiology has been observed. Indeed, the incidence of some types of SCCHN, such as oropharyngeal cancers has raised because of the increasing occurrence of human papillomavirus (HPV)–related tumors [17,18]. Several lines of evidence support the existence of at least two genetically different types of SCCHN, one virus-related and the other alcohol and/or tobacco-related, characterized by both clinical and biological opposite features [19,20].
Virus-related SCCHN are very chemo and radiosensitive, so suitable for organ preserving strategy. On the other hand, alcohol and tobacco-related SCCHN are strongly heterogeneous diseases characterized by chemo and radio-resistance [20].
Virus-related and mutagen-related SCCHNs are not only genetically different but also display diverse immunological features.
The Importance of Cancer Immune Infiltrate
Along with lung cancer and malignant melanoma, SCCHN present the highest levels of tumor immune infiltration among solid cancers [20,21]. Several evidences demonstrated that the entity of the tumor lymphocytic infiltrate correlates with prognosis [21,22]. Indeed, a high density of tumor-infiltrating lymphocytes (TILs) is associated with improved outcome in SCCHN [23]. In addition, the phenotyping of the immune infiltrate is also crucial, because TILs may be either functionally active or inactive (secondary to exhaustion or anergy). As an example, the predominant T-cell population may be represented by Treg or CD4+ Th2 lymphocytes, which are not able to elicit a response against the tumor. Karpathiou et al. demonstrated that in SCCHN, high density of CD3, CD8, and CD57 cells in the immune infiltrate was associated with better overall survival (OS) and progression-free survival (PFS) in patients treated with immunotherapy. CD3 and CD8 are markers selective for T-lymphocytes and cytotoxic T-lymphocytes, while CD57 is a marker characterizing a class of NK cells, particularly active against cancer [24]. In contrast, a tumor infiltrate rich in CD4+ cells, especially the Th2 subpopulation, inversely correlated with survival.
Taken together, this data suggest that not only the level of the immune infiltrate but also its composition is important to determine the grade of inflammation of the tumor. Notably, a more inflamed tumor, characterized by a robust immune response, is more likely to benefit from immunotherapy.
Different Etiology and Different Immune Response in SCCHN
Tobacco and alcohol (mutagens) create DNA damage, inducing mutations and potentially altering the tumor immune microenvironment. These types of genetic and immune microenvironment alterations are critical factors known to affect tumor response to immunotherapy. As a matter of fact, several studies indicate that smokers with head and neck cancer tend to have lower response rates after immunotherapy [25].
Chung and Walter demonstrated that SCCHN can be subclassified into four distinct molecular subtypes based on their expression profiles: atypical, basal, classical, and mesenchymal [26,27]. The atypical subtype contains the majority of HPV-positive tumors. The classical subtype is more frequently associated with tobacco carcinogenesis [23]. HPV-related SCCHN often belong to the atypical subtype and, interestingly, they show biomolecular features opposite respect to the mutagens-related SCCHN (alcohol and tobacco) [20].
In addition, HPV-related SCCHN are characterized also by a different immunogenicity if compared with the mutagens-related counterpart. In fact, Mandal et al. observed that the atypical (among which HPV-related) and mesenchymal subtypes had the highest degrees of immune infiltration, while the classical, which contains the almost totality of mutagens-related SCCHN, showed the lowest one [28].
Desrichard et al. correlated a specific “smoking-signature,” which characterizes the smoking-related SCCHN with the entity of the immune infiltrate in the tumor, and ultimately, with the response to immunotherapy. They found that a particular smoking-associated signature (the mutational signature framework defined by Alexandrov [29]), characterized by a high-mutational burden and specific DNA changes induced by the smoke-contained mutagens, strongly correlated with a low-immune tumor infiltrate and with poor response to immunotherapy [30]. In addition, they investigated the correlation of the CD8-mediated response against cancer, estimated by the levels of the immune effectors (i.e., granzyme and perforin expression) and interferon-gamma signaling and with the smoking status. They found that strong smokers showed the lowest activation of cytolytic activity and interferon-gamma signaling pathway. The authors concluded that smoking-related SCCHN are characterized by a low immune infiltrate and a low CD8-mediated immune response against tumor cells.
Conversely, the HPV-related SCCHN subgroup showed the opposite features, being characterized by a robust CD8-mediated response and a better response to immunotherapy.
On the basis of the abovementioned findings, we can conclude that virus-related and mutagens-related SCCHN are associated to diverse immunologic phenotypes, showing the firsts a so-called “inflamed phenotype” and the seconds a noninflamed phenotype. The immunologic phenotype strongly affects the response to immunotherapy.
Immunotherapy Strategies in SCCHN
Background
During the years several strategies aimed to reinforce immune response against cancer have been developed. The ultimate goal of these strategies is to generate a class of T-lymphocytes (CD8+) strongly and selectively able to recognize the tumor antigens and able to attack the tumor cells.
Immune-system activation is a multistep process, and the first of these steps is the direct interaction between immune cells and the tumor-associated antigens (TAA). One important principle to consider is that cancer cells express antigens that differentiate them from their nontransformed counterparts, and these antigens named TAA, are products of mutated cellular genes, aberrantly expressed normal genes, mutated self-proteins that do not contribute to cancerogenesis or genes encoding viral proteins.
The interaction between TAA and immune cells initiates the immune response. The second step is characterized by the maturation of a class of CD8+ T-lymphocytes strongly selective and restricted for TAA. These CD8+ lymphocytes, which were previously “naïve T-lymphocytes,” mature after their interaction with APC. This step may be defined as “priming” and hesitates in the generation of cytotoxic T-lymphocytes selective for TAA [20,31,32].
The third step is the migration of cytotoxic T-lymphocytes in the tumor site and the attack to tumor cells. In this last phase, the role of tumor microenvironment (TME) is crucial. TME may be defined as the set of cells and the substances released by them, surrounding the tumor. TME may exert an inhibitory effect on effector CD8+ T-cells in several ways, even on CD8 strongly activated against tumor cells during the “priming” phase [33,34].
Of note, immunotherapy may restore immune response against cancer in different ways, which we will discuss below.
Direct Administration of TAA
Unlike prophylactic vaccines that are generally administered to healthy individuals, therapeutic cancer vaccines are given to cancer patients and are designed to eradicate cancer cells through reinforcing patient's own immune responses. Several types of antitumoral vaccines have been experimented and they may be classified in different categories, including cell vaccines (tumor or immune cell), protein/peptide vaccines, and genetic (DNA, RNA and viral) vaccines [35].
Autologous tumor vaccines are the simplest type of anticancer vaccine available, and they are prepared using patient-derived tumor cells. These tumor cells are typically irradiated, combined with an immune-stimulatory adjuvant (alum or Bacillus of Calmette and Guérin, BCG), and then administered to the individual from whom the tumor cells were isolated [36,37]. Autologous tumor cells may be modified to confer higher immune-stimulatory characteristics, and some of them, for example are engineered to express IL-12, a key cytokine promoting Th1 mediated immunity.
Allogeneic whole tumor cell vaccines, which typically contain two or three established human tumor cell lines, may be used to overcome many limitations of autologous tumor cell vaccines, such as the difficulty to obtain a large number of immunogenic cancer cells from the tumor site and expensive procedures used to prepare and render them more immunogenic [38].
Most vaccination strategies for SCCHN target the HPV-positive subset where HPV antigens can be used. E6 and E7 oncoproteins are crucial for HPV-induced cell transformation, thus they have been targeted by many types of vaccines. Vaccines against these antigens are currently in clinical trials for SCCHN [39].
A phase I/II trial to assess safety and efficacy of a short peptide-based vaccine targeting HPV16 E7 [40], in combination with low dose of cyclophosphamide has recently started in patients with advanced HPV-related oropharyngeal cancer.
Vaccines should be used not only in virus-related SCCHN but also in mutagens-related SCCHN. TP53 mutations are frequently found in mutagens-related SCCHN, especially in those showing a “classical” subtype, and they usually lead to p53 intracellular accumulation [41]. Schuler et al. in a phase Ib trial enrolling 16 patients with SCCHN, used a vaccine containing autologous monocyte–derived DC loaded with selected wild type p53 peptides. DC were isolated from autologous leukapheresis, then they were matured in a cocktail containing both p53 derived antigens and different cytokine, among which IL-1β, IL-6, TNF-, and PGE2. DC was ultimately injected into patients' inguinal lymph nodes. As a result, a two-year disease-free survival (DFS) of 88% was observed, post vaccination p53-specific T-cell frequencies were increased in 11/16 patients (69%), and circulating Treg rates significantly decreased if compared with prevaccination values [42].
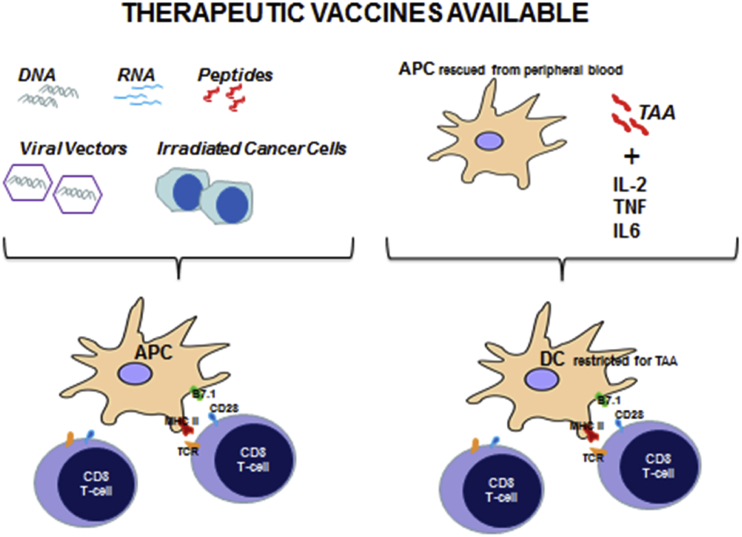
The main types of available therapeutic vaccines experimented in SCCHN are graphically represented in Figure 3.
Figure 3.
Therapeutic vaccines available. DNA, mRNA, cancer peptides, viral vector containing immunogenic constructs or irradiated cancer cells may be directly administered eliciting an immune response against cancer. In alternative, DC may be stimulated with cytokines and tumor associated antigens (TAA) and then injected in patients.
Overall, vaccines represent an attractive therapy strategy in SCCHN. However, the difficulty of stimulating a depressed immune system, which characterizes most of SCCHN, especially those virus-unrelated, represents a major obstacle. Indeed, the most relevant results in clinical trials testing vaccines have been achieved in patients with HPV-related SCCHN.
Administration of Specific T-cells, Pulsed with TAA (Adoptive Immunotherapy)
The direct activation of effector T-cells (CD8+) stimulated in vitro and reinfused intravenously is an example of “adoptive immunotherapy”. This is obtained by isolating peripheral blood mononuclear cells (PBMCs) from patient blood and stimulating them ex vivo in the presence of cytokines (IL-2) and autologous APCs expressing TAA. This procedure allows the generation of TAA-restricted cytotoxic T-lymphocytes, which after intravenous reinfusion can attack cancer cells and induce perforin-dependent apoptosis with subsequent tumor mass shrinkage. This strategy has been largely used in clinical trials enrolling patients affected by nasopharyngeal carcinoma (NPC), because of the fact that NPC cells often show EBV-associated antigens which may elicit an immune response [43]. Although the procedure may seem easy to perform, not rarely it is not possible to obtain a class of CD8+ Cells strongly selective for TAA (viral antigens in this case), thus, efforts have been made with the aim to reinforce both the selectivity and the number of effector T-cells produced. He et al., for example, obtained an effector lymphocyte population particularly enriched in CD3+/CD8+ and CD3+/CD4+ cells and with a low percentage of CD3-/CD16+ NK cells by isolating TILs directly from the tumor tissue, and by expanding them ex vivo [44].
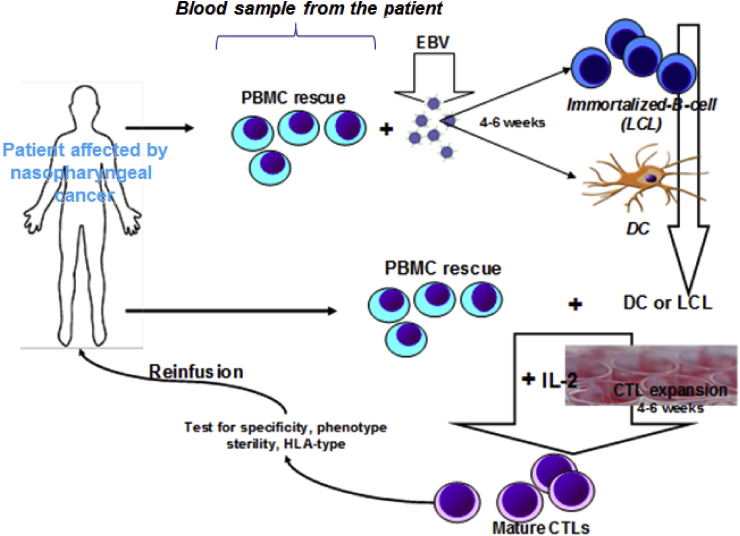
Adoptive immunotherapy is under clinical evaluation in Epstein–Barr virus–related NPC, while its use in SCCHN remains scarce. An example of adoptive immunotherapy is pictured in Figure 4.
Figure 4.
CTL-based nasopharyngeal adoptive therapy. Reinfusion of EBV-antigens restricted cytotoxic T-lymphocytes (EBV-CTLs), following ex vivo activation of autologous T-cells in presence of IL-2 and lymphoblastoid cell lines (LCLs), represented by EBV-infected immortalized B-lymphocytes. PBMC: peripheral blood mononuclear cells; EBV: Epstein–Barr virus; LCL: lymphoblastoid cell lines; DC: dendritic cells; CTL: cytotoxic T-lymphocytes; IL-2: interleukin-2.
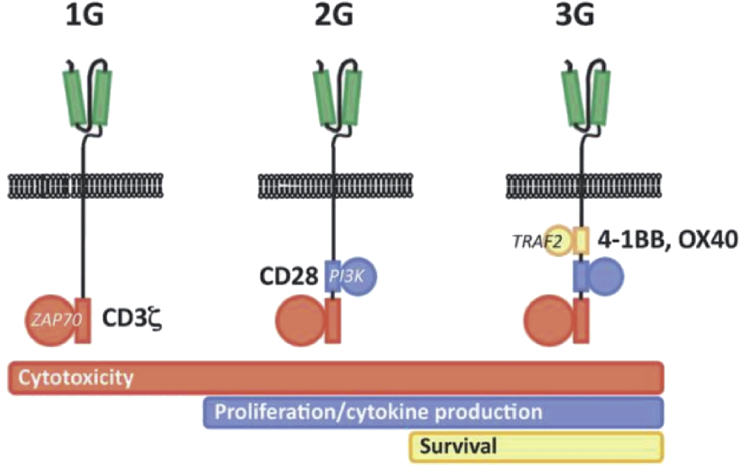
Several other strategies to increase the specificity of T-lymphocytes against TAA have been developed, though the most recent as well as the most promising is the CAR (chimeric antigen receptors) technology. CAR are chimeric transmembrane receptors constituted by an antigen specific single-chain variable fragment (against a predetermined TAA) fused with the CD3 intracellular domain (the so-called TCR, namely T-cell receptor). The aim of this strategy is to combine the specificity of a monoclonal antibody with the intracellular signal activating machinery of a T-cell, avoiding the antigen recognition from major histocompatibility complex restriction, which represents one of the hardest barriers for the immune therapy. On transfection into autologous T-cells, using viral vectors, the gene encoding for CAR leads to the synthesis and expression on the T-lymphocyte's cell membrane of a receptor highly specific for its target. CAR may be divided in three generations on the basis of their capability to exert a specific immune response. To exert its function, a T-cell requires not only the binding of a specific antigen through CD3/TCR but also the presence of costimulatory molecules as such as CD28 receptor, which binds the CD80 and CD86 expressed by the APC. Second generation CARs have been engineered to include also a costimulatory domain, usually derived by the intra-cytoplasmic portion of CD28. Third generation CARs incorporate three or more costimulatory domains as such as CD134, CD137, and DAP10 [45,46] (Figure 5). Recent clinical trials using CAR T-cell therapy have demonstrated clinical responses in several solid tumors [47,48]. An ongoing phase I trial is evaluating the intratumoral administration of CAR T-cells in locally advanced/recurrent metastatic SCCHN [49].
Figure 5.
First (1G), second (2G), and third (3G) generation CAR, depending on the presence in the molecule of none, one or more costimulatory domain. Courtesy of Perri F et al. WCRJ 2018; 5 (1): e1042.
Removing the Stimuli Able to Induce T-cells Anergy in the TME
The mechanisms underlying tumor immune evasion include the modulation of inflammatory cytokines, downregulation of antigen-processing machinery, and the expression of immune checkpoint ligands or receptors to promote immune evasion. The final result is the development of tolerance to cytotoxic T-cells [50,51]. Tumor cells in fact, develop mechanisms to thwart immune recognition and response, and one of these mechanisms is the upregulation of the so-called inhibitory checkpoint receptors (IR), which, once expressed, are able to inhibit normal T-cell activation and costimulation to maintain a homeostatic immune response.
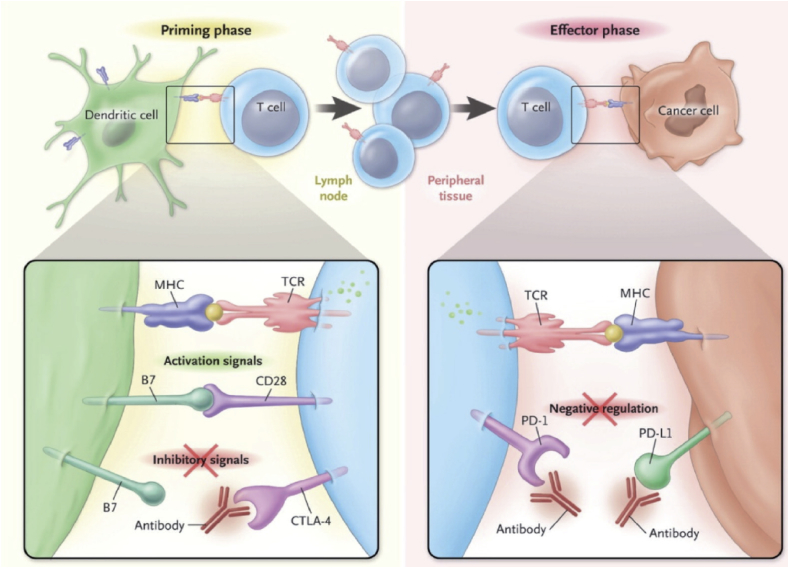
TME may block T-cells activation in different ways and in different steps, one of which is the “priming” phase. In particular, the interaction between DC and naïve T-cells takes place in the lymph nodes and consists in the delivering of two stimulatory signals. The first is the interaction between TCR (CD3) and the TAA, which have been processed and presented by the DC through the MHC-II. The second signal is a costimulatory signal and consists in the interaction between CD8, present on the cell membrane of T-cells, and the B7.1 protein. This interaction may be compromised through the expression on the T-cell surface, of the CTLA-4, which can be upregulated by several stimuli elicited by the TME, such as the production of immune-suppressive cytokines such as TGF-b. Blocking the CTLA-4/CD28 interaction using a monoclonal antibody, such as ipilimumab, may lead to an unrestricted T-cells activation by removing the inhibitory effect on T-cell priming [52].
Once activated during the priming phase, T-cells migrate in the tumor site and exert their antitumor activity on recognition of specific TAA on cancer cells. This phase can be dampened by the interaction between PD-1 on the surface of T-cells and its ligand PDL-1 on the surface of tumor cells which favors T-cells anergy. Several monoclonal antibodies inhibiting PD-1/PDL-1 interaction have been tested in clinical trials. Figure 6 describes different ways by which TME may inhibit T-cells activation.
Figure 6.
The two main phases during which TME (tumor microenvironment) can inhibit T-Cells function and the possible therapies able to circumvent this phenomenon.
While the role of CTLA-4 inhibitors is not yet established in SCCHN, anti-PD-1 has yet been registered as standard therapy because of results of two phase III clinical trials (CheckMate 141 and KEYNOTE-012). Anti-PDL-1 is yet experimental and they are currently not used in the clinical practice.
The phase III CheckMate 141 clinical trial randomized patients with recurrent SCCHN, whose disease had progressed within 6 months after platinum-based chemotherapy, to receive nivolumab or to standard single-agent systemic therapy (methotrexate, docetaxel, or cetuximab) of investigator's choice. This study showed a statistically significant improvement in overall survival (OS) from 5.1 months in standard therapy arm to 7.5 months in the nivolumab arm. The response rate (RR) was 13.3% in the nivolumab group versus 5.8% in the standard-therapy group [53].
Interestingly, a subgroup analysis revealed that among patients with p16-positive tumors, the median OS was 9.1 months in the nivolumab group versus 4.4 months in the standard-therapy group (hazard ratio for death, 0.56), and, among patients with p16-negative tumors, the median overall survival was 7.5 versus 5.8 months (hazard ratio, 0.73, P = 0.55) [53].
In conclusion, in the ITT (intent to treat) population, nivolumab appeared to be more efficacious than standard therapy, showing a longer OS than standard therapy, but, after a subgroup analysis, survival benefit seemed to be stronger in p16 positive patients. In addition, the effect of nivolumab on OS was more pronounced in PD-L1 positive patients.
The phase Ib KEYNOTE-012 enrolled 60 patients with recurrent/metastatic SCCHN, irrespective of previous therapy, but showing a PD-L1 expression of at least 1% of tumor cells. Patients received the PD-1 inhibitor pembrolizumab 10 mg/kg intravenously every 2 weeks. A subgroup analysis revealed that a significant increase in ORR was observed for PD-L1-positive versus -negative patients (22% v 4%; P = 0.021) [54].
A recently presented analysis from the KEYNOTE-012 study showed that patients with a T-cell inflamed gene expression profiling (IFN-γ gene expression profile, GEP) such as patients whose tumor showed a high mutational load (ML) achieved an increased response rate to pembrolizumab [55].
The phase 3 KEYNOTE-040 trial [56] investigated pembrolizumab in patients with recurrent/metastatic HNSCC after a platinum-based first-line chemotherapy. Four hundreds and ninety five patients were randomized to either pembrolizumab 200 mg every 3 weeks or treatment of the investigator's choice (methotrexate, docetaxel, or cetuximab). Pembrolizumab prolonged both OS and PFS, even though the statistical significance was not reached. Nevertheless, in patients with a PD-L1 combined positive score of at least 1%, median OS was 8.7 months with pembrolizumab versus 7.1 months with standard therapy (P = 0.0078), while in patients with a combined positive score (TPS) more than 50%, median OS was 11.6 versus 7.9 months with pembrolizumab and standard therapy, respectively (P = 0.0017).
Finally, the recent results of the KEYNOTE-048 trial have highlighted the significant advantage in term of response rate and survival obtained by pembrolizumab alone or in combination with the chemotherapy (cisplatin and 5-fluorouracil) if compared with the standard cisplatin-cetuximab-5fluorouracil. Notably, in the subgroup of patients whose tumor expressed high levels of PDL-1, the abovementioned advantage resulted much more strike, and in particular, the authors recommended to use pembrolizumab alone in tumor whose combined positive score (CPS) was >20, and in combination with chemotherapy in those with CPS >1 [57].
Resuming, both nivolumab (in United States and Europe) and pembrolizumab can be considered a new standard of care in the second-line treatment of patients with recurrent/metastatic SCCHN. Notably, subgroups of tumors, for example, those expressing high values of tissue PDL-1 and p16, seem to better respond to checkpoint inhibitors. Some initial data are also in favor of a better response in tumors with a so-called “inflamed gene expression profiling”.
These clinical observations pave the way to several hypotheses about the presence of predictive factors of response to immunotherapy.
Future Directions of Immunotherapy in SCCHN
Predictive Factors of Response to Immunotherapy
A number of immunomodulatory agents that target immune system checkpoints such as the CTLA-4, the PD-1 or its ligand PD-L1, have received regulatory approval for the treatment of multiple cancers including SCCHN. However, a substantial proportion of patients treated with checkpoint inhibitors have little or no benefit at a cost of high toxicity. Hence, the identification of molecular determinants of response to immunotherapy is needed.
PDL-1 Tissue Expression
CheckMate and KEYNOTE studies have investigated the impact of PDL-1 tissue expression on response to immunotherapy, finding conflicting results. The main conclusion which could be taken is that overexpression of PDL-1 is related to better response to checkpoint inhibitors [[52], [53], [54], [55], [56]]. Conceptually, overexpression of PD-L1 on tumor cells would facilitate cancer immune evasion through the inhibition of cytotoxic T-cell functions, thus, elevated expression of PD-L1 by the tumor should correlate with worse differentiation and poorer prognosis. On the other hand, PD-L1 expression on tumor cells could be secondary to IFN-production by tumor-infiltrating T-cells, which is normally associated with better outcome [29]. PD-L1 overexpression could be either a negative or positive prognostic factor depending on the cancer, and in SCCHN, it seems to be associated with good response to checkpoint inhibitors.
Characteristics of the Tumor-Immune Infiltrate
Recently, Nguyen et al. [58] demonstrated that patients with highly immune-infiltrated SCCHN had significantly superior OS compared with the patients with low tumor immune infiltration. Interestingly, highly immune infiltrated SCCHN correspond to tumors in which Th1 differentiation has occurred, and consequently, in which a wide percentage of CD8+ T-cells are detected and a high production of antitumor cytokines such as IFN-γ is observed.
A high immune infiltrate might correlate with high cell mediate immunity against cancer, thus with the development of a population of TIL composed in prevalence by CD8+ T-cells, producing high quantity of IFN-γ, perforins, and granzyme.
Lately, Hanna et al. [59] identified a so-called inflamed SCCHN phenotype, which correlates with good response to immunotherapy. The inflamed SCCHN phenotype is characterized by a high CD8+ rate and by a high expression of PD-1 and TIM-3. In the analysis conducted by Mandal et al., the presence of the NK in the immune infiltrate was also significantly correlated to immunotherapy response. NK cells do not function solely as effectors of innate immunity. Indeed, increasing evidences support the role for NK in influencing key components of the adaptive immunity through their potent secretion of IFN-γ which helps shaping the immune tumor microenvironment by activating the effectors of adaptive immunity particularly the Th1 lymphocytes [28]. The CD56+ NK subpopulation strongly influence the antitumor response and their percentage in the immune infiltrate correlates with better response to immunotherapy. The main conclusion is that SCCHN is one of the most highly immune-infiltrated cancer types, and surely, it is the most highly NK cell and Treg-infiltrated cancer type.
Interestingly, we can identify a broad diversity in the levels of immune infiltration across SCCHN, which varies based on clinical and genetic features, such as HPV status, molecular subtype (classic versus atypical) and mutational smoking signature. Generally, smoke-related SCCHN (which may be identified adopting the mutational signature framework defined by Alexandrov) are characterized by a low immune infiltrate, constituted in great part by T-Reg and CD4+ Th2 lymphocytes, while virus-related SCCHN are characterized by the reversal features. Virus-related SCCHN are more suitable to respond to checkpoint inhibitors [28].
Tumor Mutational Burden
Analyzing the genetic signatures of tumors might identify patients who have higher chances to respond to immunotherapy. Several studies have shown how the mutational burden correlates with greater efficacy of anti-PD-L1/PD-1 drugs [59,60]. Generally, a higher number of mutations corresponds to a higher number of neoplastic clones and thus to a higher number of TAA. A tumor characterized by a high mutational burden should better respond to immunotherapy, as demonstrated in different clinical trials [60,61]. This last concept is not properly true for SCCHN, because smoke and alcohol-related SCCHN, which are often characterized by a high mutational burden, have a poor response to immunotherapy is compared with HPV-related ones that instead show a lower number of DNA mutations [28]. Concluding, tumor mutational burden alone is not predictive of good response to immunotherapy in SCCHN.
Future Strategies: Combined Immunotherapy
A way to improve the efficacy of immunotherapy may be to use drugs acting on different steps of the immune response against cancer. Often, because of the development of alternative ways to circumvent immune response, the tumor escape occurs. The CheckMate 651 is an ongoing randomized trial investigating the combination of nivolumab and ipilimumab compared with standard chemotherapy (platinum, 5-fluorouracil, and cetuximab). The rationale of the study is the double unlock of the CTL at the “priming phase” and at the “effector phase” [62].
Epacadostat is an oral inhibitor of indoleamine- 2,3-dioxygenase 1 (IDO), an intracellular enzyme that initiates the first and rate-limiting step of tryptophan degradation in the kynurenine pathway. IDO-mediated depletion of tryptophan directly suppresses T-cells. In addition, IDO supports the inflammation in the TME, the development of immune tolerance in immune cells, the suppression of natural killer cells, and the generation and activation of Treg [63,64].
In the phase II ECHO-202/KEYNOTE-037 trial 38 patients with recurrent/metastatic SCCHN, progressing after at least one prior chemotherapy regimen including a platinum agent, were treated with epacadostat 100 mg daily and pembrolizumab 200 mg every 3 weeks. The combination therapy achieved a disease response of 34% and a disease control rate of 39% [65].
In addition to CTLA-4 and PD-1, TIL express a number of other coinhibitory receptors, including TIM-3, LAG-3 and KIR, which represent potential targets that could be exploited for inducing antitumor immune responses. The T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) is a type I trans-membrane protein expressed on cell surface of IFN-γ-producing Th1 cells, Treg and innate immune cells where it has been shown to suppress their responses on interaction with their ligands. A number of report have highlighted that high levels of Tim-3 expression correlate with suppression of T-cell responses and T-cell dysfunction, also referred to as T-cell exhaustion, a process of gradual loss of T-cell function during chronic infections and tumor development [66]. Studies carried out in several solid tumors have established that Tim-3 acts as a negative regulator of antitumor immunity [67]. Interestingly, Tim-3 expression on exhausted T-cells is often associated with PD-1 expression and is characteristic of the deeply exhausted T-cells, supporting the functional correlation between Tim-3 and PD-1 during the development of T-cells exhaustion [68,69]. In preclinical models the combination of Tim-3 and PD-1 pathways blockade is more effective than single agents [70]. Targeting TIM-3 represent a suggestive immunotherapy strategy in SCCHN.
Mishra et al. demonstrated that both CD8+ and CD4+ TILs coexpress inhibitory receptors, PD-1 and LAG-3 (lymphocyte activation gene-3) in preclinical models. LAG-3 is mainly expressed by CD4+ Tregs and it plays a key role in suppressing CTL functions in autoimmune responses, thus maintaining tolerance to self and tumor antigens via dampening the activity of antigen-specific CD8+ T-cells [71,72]. Overall these preclinical evidences provided the rationale for clinical trials of TIM-3 and LAG-3 inhibitors currently ongoing.
Conclusions
Despite the efficacy of chemotherapy and targeted therapy in solid tumors, including SCCHN, intrinsic or acquired resistance is common and negatively impacts patient outcome. During pharmacological treatment, DNA mutations frequently occur in tumor and may be responsible for the emergence of alternative/escape pathways.
Immunotherapy may potentially circumvent the need to target complex, redundant, and evolving molecular pathways. Immunotherapy, in fact is based on the stimulation of the immune system of the patients which may be rendered able to reject the tumor.
Several strategies of immunotherapy have been tested in head and neck tumors, with conflicting results. In fact, most head and neck tumors are resistant to immunotherapy ab initio, independently form the strategy used. Checkpoint inhibitors have shown fairly good results in clinical trials, if compared with vaccines and adoptive immunotherapy, but also in this case, tumors develop resistance.
The step forward may be to better understand the immune response which characterizes SCCHN, other than their biology, with the aim to realize a well-shaped therapy, consisting in immune drug combinations added or not to chemotherapy and or targeted therapy.
SCCHN is a very heterogeneous disease, being characterized from the biological and immunological point of view by at least two distinct entities, namely, the virus-related cancers and the mutagens-related ones. The virus-related SCCHN is often a oligoclonal disease with a low mutational burden and a low number of neoplastic clones [20], other than an high immune infiltrate, composed mainly by CD8+ T-lymphocytes and CD56+ NK cells, which exert a strong cytotoxic activity against tumor cells. On the other hand, smoke and alcohol-related SCCHN (mutagens related), is characterized by a high mutational burden, a low grade of immune cells infiltration, most of them being Treg lymphocytes, thus with a local strong immunosuppression. The knowledge of how immune response is dampened leading to tumor progression, the so called “tumor escape”, is crucial for organizing an effective immunotherapy against cancer.
Tumor escape is often the result of the T-cell exhaustion, a mechanism which occurs via T-cell–specific intrinsic mechanisms, such as the PD-1/PD-L1 pathway as well as other immunoregulatory receptors including CTLA-4, TIM-3, LAG-3, and others [[73], [74], [75]]. In addition, T-cell exhaustion can result from T-cell extrinsic pathways mediated by Treg, and myeloid-derived suppressor cells (MDSCs). These cells exert their immunoinhibitory influence primarily through secretion of immunosuppressive cytokines, such as TGF-β.
Based on these findings, a number of drugs inhibiting these pathways are currently under clinical investigation with the rationale of inhibiting the T-cells exhaustion even with combined immunotherapy strategies.
Another important issue to consider is the presence of the predictive factors of response to immunotherapy, which are still lacking. Tissue PDL-1 concentration, the mutational burden, the viral etiology and the characteristics of the immune tumor infiltrate are under investigation and in the near future, their role will be better elucidated.
Taken together clinical evidences suggest that virus-related tumors better respond to immunotherapy strategies compared with the mutagens-related ones. A step forward may be used, for the treatment of mutagens-related SCCHN, a combination of new generation checkpoint inhibitors, with the aim to reactivate a very depressed immunity, which often characterizes them.
Finally, adoptive immunotherapy (i.e., vaccines) is currently in an early stage of development and based on the available results, mainly virus-related SCCHN, such as EBV-related nasopharyngeal and HPV-related SCCHN, could benefit from them.
Funding
No funds received for this manuscript.
Conflict of Interests
All authors declared no conflicts of interests.
Biographies
Dr Francesco Perri degree in Medical Oncology and currently works at National Tumor Institute of Naples, IRCCS G. Pascale at Head and Neck Medical Oncology Unit.
Dr Franco Ionna degree in Medicine and Surgery and currently works as chief of Otolaryngology, at National Tumor Institute of Naples, IRCCS G. Pascale at Otolaryngology Unit.
Dr Francesco Longo degree in Medicine and Surgery and currently works at National Tumor Institute of Naples, IRCCS G. Pascale at Otolaryngology Unit.
Dr Giuseppina Della Vittoria Scarpati degree in Medical Oncology and currently works at Hospital of Pollena, ASL NA 3, Naples, at Medical Oncology Unit.
Dr Carmine De Angelis degree in Medical Oncology and currently works at AOU Federico II of Naples, at Medical and Experimental Oncology Unit.
Dr Alessandro Ottaiano degree in Medical Oncology and currently works at Division of innovative therapies for abdominal metastases, INT IRCCS G. Pascale, Naples.
Prof Gerardo Botti degree in Surgical Pathology and currently works as chief of Pathology, at National Tumor Institute of Naples, IRCCS G. Pascale at Surgical Pathology Unit.
Dr Francesco Caponigro degree in Medical Oncology and currently works as chief of Head and Neck Medical Oncology at National Tumor Institute of Naples, IRCCS G. Pascale at Head and Neck Medical Oncology Unit.
References
- 1.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 2.Shankaran V., Ikeda H., Bruce A.T., White J.M., Swanson P.E., Old L.J. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 3.Fridman W.H. From cancer immune surveillance to cancer immunoediting: birth of modern immuno-oncology. J Immunol. 2018;201(3):825–826. doi: 10.4049/jimmunol.1800827. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer C., Kim G.G., Albers A., Hoermann K., Myers E.N., Whiteside T.L. Characteristics of CD4CCD25C regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92(5):913–920. doi: 10.1038/sj.bjc.6602407. PMID:15714205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schipmann S., Wermker K., Schulze H.J., Kleinheinz J., Brunner G. Cutaneous and oral squamous cell carcinoma-dual immunosuppression via recruitment of FOXP3C regulatory T cells and endogenous tumour FOXP3 expression? J Craniomaxillofac Surg. 2014;42(8):1827–1833. doi: 10.1016/j.jcms.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka A., Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–118. doi: 10.1038/cr.2016.151. PMID:27995907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ichihara F., Kono K., Takahashi A., Kawaida H., Sugai H., Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9(12):4404–4408. [PubMed] [Google Scholar]
- 8.Lippitz, Bodo E. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14(6):e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 9.Maggionia D., Pignataro L., Garavelloc W. T-helper and T-regulatory cells modulation in head and neck squamous cell carcinoma. OncoImmunology. 2017;6(7) doi: 10.1080/2162402X.2017.1325066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasparoto T.H., de Souza Malaspina T.S., Benevides L., de Melo E.J., Jr., Costa M.R., Damante J.H., Ikoma M.R., Garlet G.P., Cavassani K.A., da Silva J.S. Patients with oral squamous cell carcinoma are characterized by increased frequency of suppressive regulatory T cells in the blood and tumor microenvironment. Cancer Immunol Immunother. 2010;59(6):819–828. doi: 10.1007/s00262-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Oliveira Bravo M., Carvalho J.L., Saldanha-Araujo F. Adenosine production: a common path for mesenchymal stem-cell and regulatory T-cell-mediated immunosuppression. Purinergic Signal. 2016;12(4):595–609. doi: 10.1007/s11302-016-9529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parry R.V., Chemnitz J.M., Frauwirth K.A., Lanfranco A.R., Braunstein I., Kobayashi S.V., Linsley P.S., Thompson C.B., Riley J.L. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J., Zhang H., Sun S., Sun S., Li L. The effects of Tim-3 activation on T-cells in gastric cancer progression. Oncol Lett. 2019 Feb;17(2):1461–1466. doi: 10.3892/ol.2018.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng C.C., Han F.Y. Immunoregulation effects of TIM-3 on tumors. Neoplasma. 2019 Mar 5;66(2):167–175. doi: 10.4149/neo_2018_180610N385. [DOI] [PubMed] [Google Scholar]
- 15.Gao X., Zhu Y., Li G. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuler P.J., Beorger V., Beolke E., Habermehl D., Matuschek C., Wild C.A., Greve J., Bas M., Schilling B., Bergmann C. Dendritic cell generation and CD4C CD25high FOXP3C regulatory t cells in human head and neck carcinoma during radio-chemotherapy. Eur J Med Res. 2011;16(2):57–62. doi: 10.1186/2047-783X-16-2-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bron L., Jandus C., Andrejevic-Blant S., Speiser D.E., Monnier P., Romero P., Rivals J.P. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. Int J Cancer. 2013;132(3):E85–E93. doi: 10.1002/ijc.27728. [DOI] [PubMed] [Google Scholar]
- 18.Chai R.C., Lambie D., Verma M., Punyadeera C. Current trends in the etiology and diagnosis of HPV-related head and neck cancers. Cancer Med. 2015;4(4):596–607. doi: 10.1002/cam4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hafkamp H.C., Mooren J.J., Claessen S.M., Klingenberg B., Voogd A.C., Bot F.J., Klussmann J.P., Hopman A.H., Manni J.J., Kremer B. P21 Cip1/WAF1 expression is strongly associated with HPV-positive tonsillar carcinoma and a favorable prognosis. Mod Pathol. 2009;22(5):686–698. doi: 10.1038/modpathol.2009.23. [DOI] [PubMed] [Google Scholar]
- 20.Perri F., Ionna F., Scarpati G.D.V., Longo F., Addeo R., Manzo R., Muto P., Pisconti S., Leopaldi L., Caponigro F. Translational research: a future strategy for managing squamous cell carcinoma of the head and neck? Anticancer Agents Med Chem. 2018;18(9):1220–1227. doi: 10.2174/1871520618666180411110036. [DOI] [PubMed] [Google Scholar]
- 21.Gooden M.J.M., de Bock G.H., Leffers N., Daemen T., Nijman H.W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogino S., Nosho K., Irahara N., Meyerhardt J.A., Baba Y., Shima K., Glickman J.N., Ferrone C.R., Mino-Kenudson M., Tanaka N. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15(20):6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balermpas P., Michel Y., Wagenblast J., Seitz O., Weiss C., Rödel F. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501–509. doi: 10.1038/bjc.2013.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karpathiou G., Casteillo F., Giroult J.B., Forest F., Fournel P., Monaya A., Froudarakis M., Dumollard J.M., Prades J.M., Peoc'h M. Prognostic impact of immune microenvironment in laryngeal and pharyngeal squamous cell carcinoma: immune cell subtypes, immuno-suppressive pathways and clinicopathologic characteristics. Oncotarget. 2017 Mar 21;8(12):19310–19322. doi: 10.18632/oncotarget.14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K.J., Kasper S., Vokes E.E., Even C. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung C.H., Parker J.S., Karaca G., Wu J., Funkhouser W.K., Moore D., Butterfoss D., Xiang D., Zanation A., Yin X. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004 May;5(5):489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 27.Walter V., Yin X., Wilkerson M.D., Cabanski C.R., Zhao N., Du Y., Ang M.K., Hayward M.C., Salazar A.H., Hoadley K.A. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandal R., Şenbabaoğlu Y., Desrichard A., Havel J.J., Dalin M.G., Riaz N., Lee K.W., Ganly I., Hakimi A.A., Chan T.A., Morris L.G. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016 Oct 20;1(17) doi: 10.1172/jci.insight.89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A., Behjati S., Biankin A.V., Bignell G.R., Bolli N., Borg A., Børresen-Dale A.L. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desrichard A., Kuo F., Chowell D., Lee K.W., Riaz N., Wong R.J., Chan T.A., Morris L.G.T. Tobacco smoking-associated alterations in the immune microenvironment of squamous cell carcinomas. J Natl Cancer Inst. 2018 Dec 1;110(12):1386–1392. doi: 10.1093/jnci/djy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butt S.U., Malik L. Role of immunotherapy in bladder cancer: past, present and future. Cancer Chemother Pharmacol. 2018 Apr;81(4):629–645. doi: 10.1007/s00280-018-3518-7. [DOI] [PubMed] [Google Scholar]
- 32.Della Vittoria Scarpati G., Fusciello C., Perri F., Sabbatino F., Ferrone S., Carlomagno C., Pepe S. Ipilimumab in the treatment of metastatic melanoma: management of adverse events. Onco Targets Ther. 2014 Feb 19;7:203–209. doi: 10.2147/OTT.S57335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marra A., Ferrone C.R., Fusciello C., Scognamiglio G., Ferrone S., Pepe S., Perri F., Sabbatino F. Translational research in cutaneous melanoma: new therapeutic perspectives. Anticancer Agents Med Chem. 2018;18(2):166–181. doi: 10.2174/1871520618666171219115335. [DOI] [PubMed] [Google Scholar]
- 34.Mahoney K.M., Freeman G.J., McDermott D.F. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015 Apr 1;37(4):764–782. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Circelli L., Petrizzo A., Tagliamonte M., Tornesello M.L., Buonaguro F.M., Buonaguro L. Systems biology approach for cancer vaccine development and evaluation. Vaccines (Basel) 2015;3:544–555. doi: 10.3390/vaccines3030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger M., Kreutz F.T., Horst J.L., Baldi A.C., Koff W.J. Phase I study with an autologous tumor cell vaccine for locally advanced or metastatic prostate cancer. J Pharm Pharm Sci. 2007;10:144–152. [PubMed] [Google Scholar]
- 37.Harris J.E., Ryan L., Hoover H.C., Jr., Stuart R.K., Oken M.M., Benson A.B., 3rd, Mansour E., Haller D.G., Manola J., Hanna M.G., Jr. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol. 2000;18:148–157. doi: 10.1200/JCO.2000.18.1.148. [DOI] [PubMed] [Google Scholar]
- 38.Guo C., Manjili M.H., Subjeck J.R., Sarkar D., Fisher P.B., Wang X.Y. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res. 2013;119:421–475. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C., Dickie J., Sutavani R.V., Pointer C., Thomas G.J., Savelyeva N. Targeting head and neck cancer by vaccination. Front Immunol. 2018 Apr 23;9:830. doi: 10.3389/fimmu.2018.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daemen T., Riezebos-Brilman A., Regts J., Dontje B., van der Zee A., Wilschut J. Superior therapeutic efficacy of alphavirus-mediated immunization against human papilloma virus type 16 antigens in a murine tumour model: effects of the route of immunization. Antivir Ther. 2004;9(5):733–742. [PubMed] [Google Scholar]
- 41.Perri F., Pacelli R., Della Vittoria Scarpati G., Cella L., Giuliano M., Caponigro F., Pepe S. Radioresistance in head and neck squamous cell carcinoma: biological bases and therapeutic implications. Head Neck. 2015 May;37(5):763–770. doi: 10.1002/hed.23837. [DOI] [PubMed] [Google Scholar]
- 42.Schuler P.J., Harasymczuk M., Visus C., Deleo A., Trivedi S., Lei Y., Argiris A., Gooding W., Butterfield L.H., Whiteside T.L. Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clin Cancer Res. 2014 May 1;20(9):2433–2444. doi: 10.1158/1078-0432.CCR-13-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perri F., Della Vittoria Scarpati G., Giuliano M., D'Aniello C., Gnoni A., Cavaliere C., Licchetta A., Pisconti S. Epstein-Barr virus infection and nasopharyngeal carcinoma: the other side of the coin. Anticancer Drugs. 2015 Nov;26(10):1017–1025. doi: 10.1097/CAD.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 44.He J., Tang X.F., Chen Q.Y., Mai H.Q., Huang Z.F., Li J., Zeng Y.X. Ex vivo expansion of tumor-infiltrating lymphocytes from nasopharyngeal carcinoma patients for adoptive immunotherapy. Chin J Cancer. 2012;31:287–294. doi: 10.5732/cjc.011.10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maher J.1, Brentjens R.J., Gunset G., Rivière I., Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002 Jan;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 46.Anurathapan U.1, Leen A.M.1, Brenner M.K.1, Vera J.F.2. Engineered T cells for cancer treatment. Cytotherapy. 2014 Jun;16(6):713–733. doi: 10.1016/j.jcyt.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg S.A.1, Dudley M.E. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009 Apr;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hombach A.1, Wieczarkowiecz A., Marquardt T., Heuser C., Usai L., Pohl C., Seliger B., Abken H. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001 Dec 1;167(11):6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 49.van Schalkwyk M.C.1, Papa S.E., Jeannon J.P., Guerrero Urbano T., Spicer J.F., Maher J. Design of a phase I clinical trial to evaluate intratumoral delivery of ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Hum Gene Ther Clin Dev. 2013 Sep;24(3):134–142. doi: 10.1089/humc.2013.144. [DOI] [PubMed] [Google Scholar]
- 50.Duffy S.A., Taylor J.M., Terrell J.E., Islam M., Li Y., Fowler K.E. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750–757. doi: 10.1002/cncr.23615. [DOI] [PubMed] [Google Scholar]
- 51.Höchst B., Knolle P.A. Checkpoint inhibition in head and neck cancer: immune therapeutic options, limitations, and beyond. ORL J Otorhinolaryngol Relat Spec. 2017;79(1–2):24–33. doi: 10.1159/000455810. [DOI] [PubMed] [Google Scholar]
- 52.Moy J.D., Moskovitz J.M., Ferris R.L. Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur J Cancer. 2017 May;76:152–166. doi: 10.1016/j.ejca.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016 Nov 10;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [Epub 2016 Oct 8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seiwert T.Y., Burtness B., Mehra R., Weiss J., Berger R., Eder J.P., Heath K., McClanahan T., Lunceford J., Gause C. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016 Jul;17(7):956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 55.Chow L.Q.M., Haddad R., Gupta S., Mahipal A., Mehra R., Tahara M., Berger R., Eder J.P., Burtness B., Lee S.H. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016 Nov 10;34(32):3838–3845. doi: 10.1200/JCO.2016.68.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen E.E.W., Soulières D., Le Tourneau C., Dinis J., Licitra L., Ahn M.J., Soria A., Machiels J.P., Mach N., Mehra R.1. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019 Jan 12;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 57.Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., De Castro G., Jr., Psyrri A., Baste Rotllan N., Neupane P.C., Bratland Å. First-line pembrolizumab plus platinum-based chemotherapy ‘standard of care’ for advanced HNSCC. ESMO Abstracts. 02 Nov 2018 [Google Scholar]
- 58.Nguyen N., Bellile E., Thomas D., McHugh J., Rozek L., Virani S., Peterson L., Carey T.E., Walline H., Moyer J. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38:1074–1084. doi: 10.1002/hed.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanna G.J., Liu H., Jones R.E., Bacay A.F., Lizotte P.H., Ivanova E.V., Bittinger M.A., Cavanaugh M.E., Rode A.J., Schoenfeld J.D. Defining an inflamed tumor immunophenotype in recurrent, metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2017 Apr;67:61–69. doi: 10.1016/j.oraloncology.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 60.Matsushita H., Vesely M.D., Koboldt D.C., Rickert C.G., Uppaluri R., Magrini V.J., Arthur C.D., White J.M., Chen Y.S., Shea L.K. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482:400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S. Cancer immunology: mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.An open label, randomized, two arm phase III study of nivolumab in combination with ipilimumab versus extreme study regimen (cetuximab + cisplatin/carboplatin + fluorouracil) as first line therapy in recurrent or metastatic squamous cell carcinoma of the head and neck (SCCHN). https://clinicaltrials.gov/ct2/show/NCT02741570.
- 63.Prendergast G.C., Smith C., Thomas S., Mandik-Nayak L., Laury-Kleintop L., Metz R., Muller A.J. Indoleamine 2,3-dioxygenase pathways of pathogenic indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014 Jul;63(7):721–735. doi: 10.1007/s00262-014-1549-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holmgaard R.B., Zamarin D., Munn D.H., Wolchok J.D., Allison J.P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor Tcell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamid O., Bauer T.M., Spira A.I., Olszanski A.J., Patel S.P., Wasser J.S. Epacadostat plus pembrolizumab in patients with SCCHN: preliminary phase 1/2 results from ECHO-202/KEYNOTE-037. J Clin Oncol. 2018 Sep 28 JCO2018789602. [Google Scholar]
- 66.Wherry E.J., Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fourcade J., Sun Z., Benallaoua M., Guillaume P., Luescher I.F., Sander C., Kirkwood J.M., Kuchroo V., Zarour H.M. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., Anderson A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Q., Munger M.E., Veenstra R.G., Weigel B.J., Hirashima M., Munn D.H., Murphy W.J., Azuma M., Anderson A.C., Kuchroo V.K. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ngiow S.F., von Scheidt B., Akiba H., Yagita H., Teng M.W., Smyth M.J. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 71.Mishra A.K., Kadoishi T., Wang X., Driver E., Chen Z., Wang X.J., Wang J.H. Squamous cell carcinomas escape immune surveillance via inducing chronic activation and exhaustion of CD8+ T Cells co-expressing PD-1 and LAG-3 inhibitory receptors. Oncotarget. 2016 Dec 6;7(49):81341–81356. doi: 10.18632/oncotarget.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grosso J.F., Kelleher C.C., Harris T.J., Maris C.H., Hipkiss E.L., De Marzo A., Anders R., Netto G., Getnet D., Bruno T.C. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Investig. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ott P.A., Hodi F.S., Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013;19:5300–5309. doi: 10.1158/1078-0432.CCR-13-0143. [DOI] [PubMed] [Google Scholar]
- 74.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 75.Zou W., Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]