Abstract
Objectives
We present a case series of spinal cord cavernous malformations (SCCMs) to describe clinical presentation and outcomes of both surgical and conservative management.
Methods
The clinical courses of patients diagnosed with SCCMs at our institution were retrospectively reviewed. Neurologic symptoms were evaluated using the Modified McCormick Scale.
Results
A total of 18 patients were identified. Five patients (27.8%) presented with acute onset bleeding, 4 of whom underwent immediate surgical resection. Thirteen patients (72.2%) were initially managed conservatively. Eight patients (38.9%) developed a hemorrhage during follow-up, and 8 (38.9%) required surgical resection due to bleeding or neurological worsening. The mean (range) duration from primary symptoms to subsequent hemorrhage or deterioration of symptoms was 1.42 (range: 0.25–4.33) years. The mean duration from primary symptoms to surgery was 2.10 (range: 0.25–5.0) years. No patients experienced subsequent hemorrhage after surgical resection. Eleven patients (84.6%) in the surgery group showed improved neurological status, and 2 patients (15.4%) remained unchanged. The annualized subsequent hemorrhage risk was 3.7%. Patients in the immediate surgical group had a significantly larger lesion compared with those in the conservative group. There was significance between the worst Modified McCormick Scale grades at the time of debilitating symptoms and the length of hemorrhage, but not the size of the lesion.
Conclusions
Surgery for SCCMs resulted in no recurrence of hemorrhage or exacerbation of neurological symptoms, and should be considered for patients who experienced acute onset of hemorrhage or debilitating symptoms during follow-up.
Key words: Cavernous malformation, Conservative therapy, Follow-up time, Spinal cord, Surgery
Abbreviations and Acronyms: MMCS, Modified McCormick Scale; MRI, Magnetic resonance imaging; SCCM, Spinal cord cavernous malformation
Introduction
Spinal cord cavernous malformations (SCCMs) are rare and account for 5% to 12% of all spinal vascular malformations.1,2 Some SCCMs are found incidentally on magnetic resonance imaging (MRI) and others present with acute onset of severe neurological symptoms. SCCMs with severe neurological deficits or progressive symptoms are considered to require surgical management. The recurrence of hemorrhage during conservative treatment of SCCMs is also considered an indication for surgery, although symptoms with an acute onset are sometimes alleviated over time. During conservative treatment, although some SCCMs diminish, others gradually exacerbate neurological deficits with every recurrence of hemorrhage.3,4 The unclear natural history of SCCMs is a factor that complicates treatment decisions. In this study, the clinical courses of patients diagnosed with SCCMs at our institution were retrospectively reviewed. The time to hemorrhage or surgery, and functional outcomes, were examined.
Methods
Patient Population
Patients diagnosed with SCCMs from October 2007 to April 2019 in our institution were included. MRI was used to confirm the diagnosis of SCCMs and for both patients who underwent conservative and those who received surgical management. Pathologic evidence was used to confirm the diagnosis of patients who underwent surgical resection. All human studies were approved by the ethical review board of Osaka University Medical Hospital. All patients provided informed consent prior to inclusion in this study.
Data and Outcome Definitions
Clinical and radiographic data were collected at initial, perioperative, and follow-up examinations. The criteria for acute hemorrhage were based on sudden onset of neurologic symptoms. The onset time of primary subjective symptoms was determined by the history correlating it with manifestation of symptoms. The presence of recurrence was evaluated at each follow-up examination and defined as the progression of symptoms combined with radiographic evidence of disease progression. The functional status of follow-up patients was assessed with the Modified McCormick Scale (MMCS) (grade 1 = neurologically intact; 2 = mild motor or sensory deficit, but functional independence; 3 = moderate deficit and limitation of function; 4 = severe motor or sensory deficit, dependent; and 5 = paraplegia or quadriplegia).5 Follow-up evaluation was performed every 6 to 12 months with MRI. Lesion locations were categorized as either deep or superficial. A lesion was classified as superficial if it was seen to abut the pial surface either on MRI or intraoperatively. The length of hemorrhage was defined as the rostrocaudal length of low intensity on T2-weighted image, which indicated the hemosiderin deposition. The relationships among lesion size, lesion location, and debilitating symptoms were investigated.
Management Modalities
The timing of surgery was determined by a shared decision-making process involving both physicians and patients. Surgery was recommended for patients who experienced acute onset of hemorrhage or debilitating symptoms. Patients with mild symptoms who requested obliteration of the lesion to reduce subsequent hemorrhagic risk were also treated surgically. Surgical access was obtained through conventional laminectomy. All surgical procedures were performed under standard microsurgical technique with intraoperative monitoring of somatosensory-evoked and motor-evoked potentials. Different myelotomy approaches were used, depending on the location of the lesion and the intraoperative appearance of the medullary surface. For superficial intramedullary lesions in which the abnormal medullary surface was clearly differentiable, myelotomy was performed through the abnormal surface. When the lesion was deeply located, and the location was not apparent, a conventional dorsal midline approach was used. Surgical resection was performed by identification of the gliotic border of the lesion and with careful dissection.
Statistical Analysis
All statistical analyses were performed with StatMate V (version 5.01, ATMS Co. Ltd., Tokyo, Japan). The Wilcoxon signed-rank test was used for comparisons of continuous variables between the two groups. The Scheffe Test is a post hoc test and it was used in an analysis of variance. Statistical significance was defined as P less than or equal to 0.05. Values are presented as the mean ± standard deviation.
Results
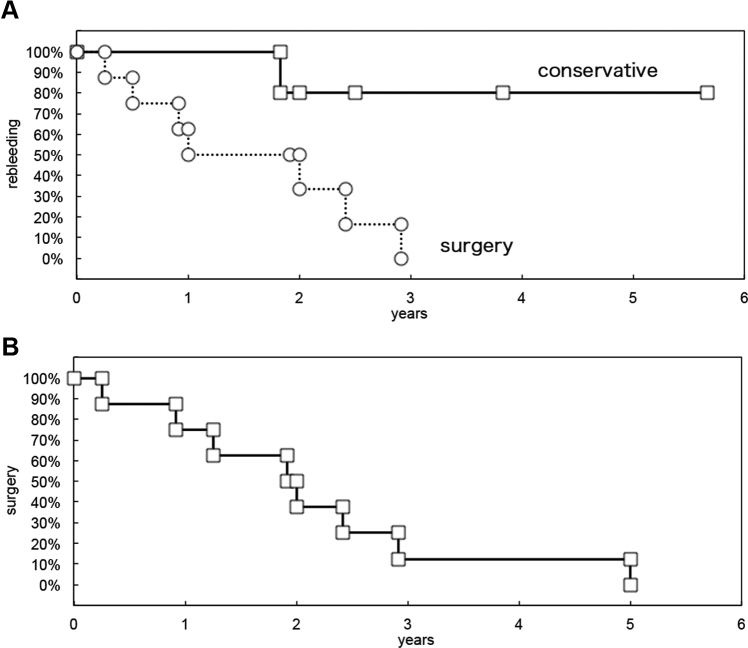
A total of 18 patients (11 men, 61.1%) were identified. The mean age of all patients was 36.9 years. Of this group, 5 (27.7%) underwent conservative management. All patients had at least 1 hemorrhage prior to their initial clinical visit at our hospital. The primary symptoms included sensory disturbances in a lower extremity (n = 15, 83.3%), motor disturbances in a lower extremity (n = 6, 33.3%), pain (n = 5, 27.7%), bladder-related symptoms (n = 3, 16.6%), and bowel-related symptoms (n = 3, 16.6%). Pain and motor weakness were significant in the conservative group (P = 0.0005, P = 0.0194, respectively). Bladder and rectal dysfunction was a significantly high in the immediate group (P = 0.022). All patients treated with immediate surgical resection had experienced pain (neck pain, back pain, chest pain, abdominal pain) 1–2 months before the acute onset of bleeding. The mean duration from primary symptoms to subsequent hemorrhage or deterioration of symptoms was 1.95 years (range: 0.25–4.33 years) (Figure 1). The mean duration from primary symptoms to surgery was 2.10 years (range: 0.25–5.0 years) (Figure 1). For the 5 patients who underwent conservative management, during a follow-up period of 5.3 years (26.6 patient-years), 1 patient experienced new hemorrhages, corresponding to an annualized subsequent hemorrhage risk of 3.7%.
Figure 1.
Kaplan-Meier plots of (A) time to subsequent hemorrhage or deterioration of symptoms and (B) surgery.
Lesion location was cervical in 6 patients and thoracic in 12 patients. Mean lesion size was 15.6 ± 6.3 mm. Patients in the immediate surgical group had a significantly larger lesion (19.8 ± 5.9 mm) than those in the conservative group (10.8 ± 4.1 mm, P = 0.0390). Detailed descriptions of the patients' baseline characteristics are shown in Table 1. Complete resection was achieved in all patients. The mean follow-up period was 4.6 years: 4.3 years (range: 0.5–11.8 years) in the surgical group and 5.3 years (range: 2–12.6 years) in the conservative group. None of these patients experienced recurrent hemorrhage and none exhibited evidence of lesion recurrence in the follow-up period. No surgical revisions were performed.
Table 1.
Baseline Patient Characteristics
| Parameter | All Patients (n = 18) | Non-operation (n = 5) | Immediate Surgery (n = 5) | Surgery (n = 8) | P Value |
|---|---|---|---|---|---|
| Age, years | 36.9 (9–68) | 43.4 (25–68) | 25.6 (9–49) | 39.9 (15–68) | 0.2986 |
| Male | 11 (61.1) | 4 (80) | 3 (60) | 4 (50) | 0.6043 |
| Location | 0.2231 | ||||
| Cervical | 6 (33.3) | 3 (60) | 2 (40) | 1 (12.5) | |
| Thoracic | 12 (66.7) | 2 (40) | 3 (60) | 7 (87.5) | |
| Size, mm | 15.6 ± 6.3 | 10.8 ± 4.1 | 19.8 ± 5.9 | 14.5 ± 4.9 | 0.0390∗ |
| Location | 0.7881 | ||||
| Superficial | 6 (33.3) | 2 (40) | 1 (20) | 3 (37.5) | |
| Deep | 12 (66.7) | 3 (60) | 4 (80) | 5 (62.5) | |
| Pain | 5 (27.7) | 5 (100) | 0 (0) | 0 (0) | 0.0005∗ |
| Weakness | 12 (66.6) | 1 (13.3) | 5 (100) | 7 (87.5) | 0.0194∗ |
| Sensory deficits | 15 (83.3) | 3 (60) | 5 (100) | 8 (100) | 0.1825 |
| Bladder and rectal dysfunction | 4 (22.2) | 0 (0) | 4 (80) | 0 (0) | 0.022∗ |
| Presentation interval, years | 1.95 (0.25–4.33) | 1.83 | - | 1.97 (0.25–4.33) | |
| Time to surgery, years | 2.10 (0.25–5.00) | - | - | 2.10 (0.25–5.00) |
Values are presented as: mean (range); n (%); or mean ± SD.
Significant difference (P < 0.05) between groups.
Functional outcomes of all patients evaluated at last follow-up were as follows: MMCS grade 1, n = 2 (11.1%); grade 2, n = 10 (55.6%); grade 3, n = 4 (22.2%); grade 4, n = 0 (0%); and grade 5, n = 2 (11.1%) (Table 2). Compared with their preoperative status, as assessed by the MMCS, 11 patients experienced improvement in neurologic function and 2 patients remained unchanged. One patient with conservative treatment recovered to baseline at the last follow-up evaluation. Figure 2 shows a typical presentation and post-treatment outcome of surgically treated SCCMs. In comparison, Figure 3 shows the natural course of a conservatively managed case.
Table 2.
Preoperative and Postoperative Modified McCormick Scale (MMCS) Grades
| Parameter | All Patients (n = 18) | Non-operation (n = 5) | Immediate Surgery (n = 5) | Surgery (n = 8) |
|---|---|---|---|---|
| Pretreatment MMCS | ||||
| 1 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 2 | 6 (33.3) | 5 (100) | 0 (0) | 1 (0) |
| 3 | 4 (22.2) | 0 (0) | 1 (20) | 3 (0) |
| 4 | 5 (27.8) | 0 (0) | 2 (30) | 3 (0) |
| 5 | 3 (16.7) | 0 (0) | 2 (30) | 1 (0) |
| Final follow-up MMCS | ||||
| 1 | 2 (11.1) | 1 (20) | 0 (0) | 1 (0) |
| 2 | 10 (55.6) | 3 (60) | 3 (60) | 4 (0) |
| 3 | 4 (22.2) | 1 (20) | 1 (20) | 2 (0) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 5 | 2 (11.1) | 0 (0) | 1 (20) | 1 (0) |
| P Value | 0.539 | 0.057 | 0.061 |
Values are presented as n (%).
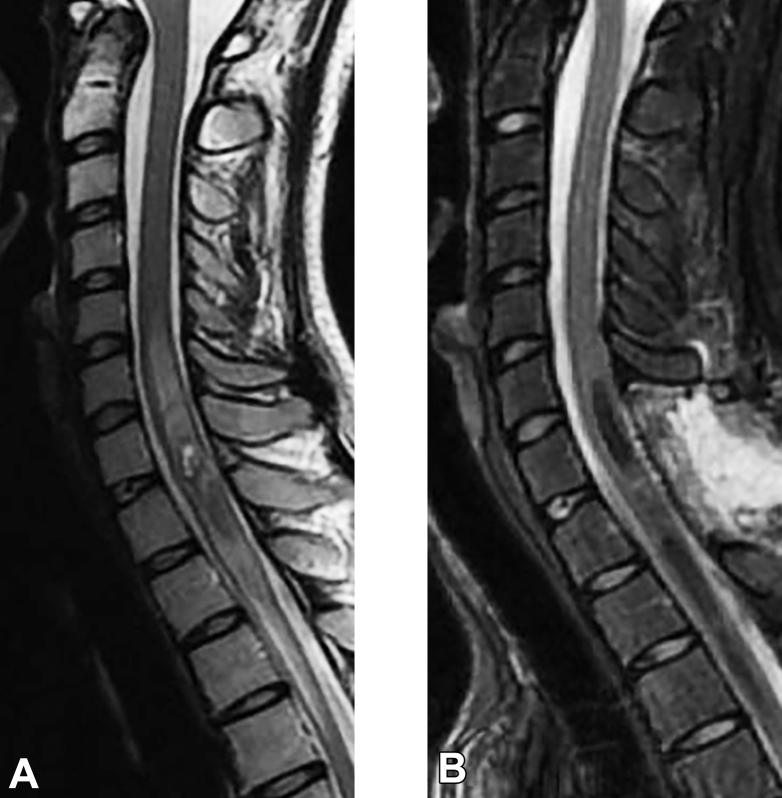
Figure 2.
Example magnetic resonance images of conservative cases. (A) Onset magnetic resonance image. (B) Final follow-up magnetic resonance image.
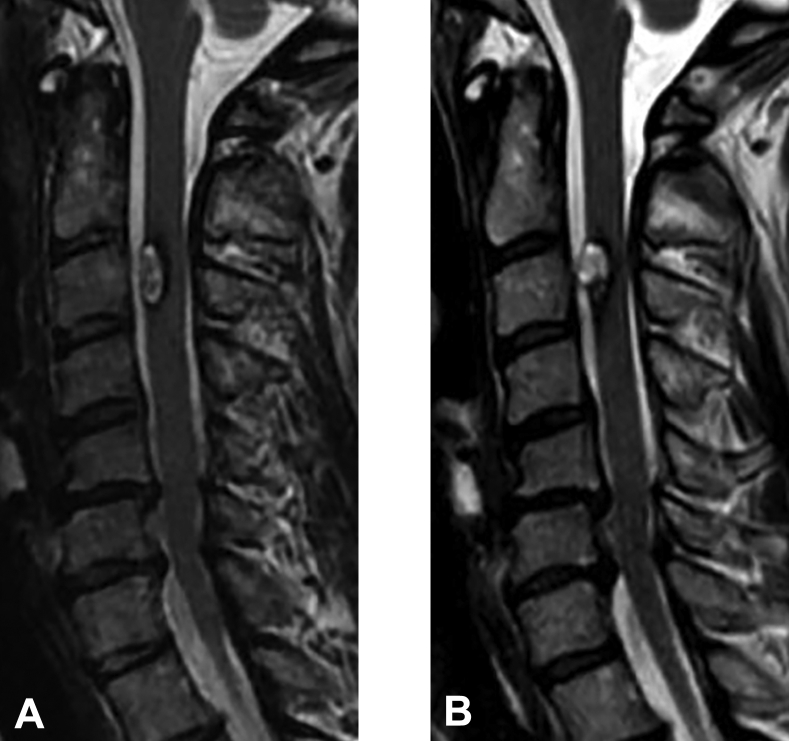
Figure 3.
Example magnetic resonance images of surgical cases. (A) Onset magnetic resonance image. (B) Final follow-up magnetic resonance image.
Superficial lesions were seen in 2 patients (40%) in the conservative treatment group, 3 patients (37.5%) in the surgery group, and 1 patient (20%) in the immediate surgery group. Superficially located lesions did not have significantly better outcomes at follow-up as measured by the MMCS (P = 0.43). The worst MMCS grades at the time of debilitating symptoms were significantly related to length of hemorrhage (P < 0.05), but not lesion size (P = 0.3193) (Table 3). The locations of superficial and deeper lesions were also not significantly related to the MMCS grades at the time of debilitating symptoms (P = 0.4259), but deeper lesions were associated with worse MMCS grades (3.5 ± 1.1) than superficial lesions (2.6 ± 0.9).
Table 3.
Differences Between MMCS Grade in the Length of Lesion, Length of Hemorrhage, and Location
| Parameter | MMCS Grade 2 (n = 5) | MMCS Grade 3 (n = 5) | MMCS Grade 4 (n = 4) | MMCS Grade 5 (n = 3) | P Value |
|---|---|---|---|---|---|
| Length of lesions, mm | 11.5 ± 4.0 | 18.0 ± 4.0 | 15.2 ± 7.6 | 17.3 ± 6.8 | 0.3193 |
| Length of hemorrhage, mm | 25.5 ± 18.0 | 31.1 ± 9.6 | 58.6 ± 31.5 | 132.4 ± 19.8∗ | 0.0004 |
| Location | 0.4259 | ||||
| Superficial | 3 | 2 | 1 | 0 | |
| Deep | 2 | 3 | 3 | 3 |
Values are presented as mean ± SD or n.
Significant difference (P < 0.05) between groups.
Discussion
Compared with cranial cavernous malformations, aggressive symptoms are observed in SCCMs because of the narrow spinal cavity, which contributes to a low tolerance for space-occupying lesions. Resection is the definitive treatment and can provide good outcomes (Table 4).1,3,5, 6, 7,9,11, 12, 13, 14, 15, 16, 17, 18,20, 21, 22, 23, 24, 25, 26 In a retrospective review of conservatively treated SCCMs, 14.8% of the patients worsened during a mean follow-up duration of 33.9 months.10 In a systematic review and meta-analysis reported in the literature, 11.3% of conservatively treated patients showed worse functions after follow-up than at initial presentation.3 Kharkar et al.27 reported 14 conservatively managed patients who had symptomatic SCCMs, of whom 71% were clinically stable throughout a mean follow-up of 80 months. The present data show that 1 patient in the conservative group improved, 3 patients remained unchanged, and 1 patient's functional status deteriorated. These findings highlight the importance of conservative management of patients with asymptomatic or mildly symptomatic SCCMs.
Table 4.
Clinical Data From the 17 Eligible Articles That Had Over 10 Patients
| Study | No. of Patients | Male (%) | Mean Age (Years) | Mean Size (mm) | Location (%) |
Outcome (%) |
Annualized Bleeding Risks (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C | T | Improvement | Same | Worse | ||||||
| Cristante and Hermann, 19986 | 12 | 33.3 | 33.5 | 58.3 | 33.3 | 58.3 | 25.0 | 16.7 | ||
| Sandalcioglu et al., 20037 | 10 | 30 | 37.5 | 50.0 | 50.0 | 40.0 | 60.0 | 0 | 4.5 | |
| Santoro et al., 20048 | 10 | 50 | 40.9 | 9.6 | 40.0 | 60.0 | 90.0 | 10.0 | 0 | |
| Jallo et al., 20069 | 26 | 65.4 | 38.0 | 16.1 | 34.6 | 65.4 | 50.0 | 42.3 | 7.7 | |
| Bian et al., 20093 | 16 | 77.8 | 38.2 | 5.5 | 50.0 | 50.0 | 75.0 | 25.0 | 0 | 3.1 |
| Matsuyama et al., 200910 | 17 | 41.2 | 41.6 | 41.2 | 52.9 | 35.3 | 47.1 | 17.6 | ||
| Park et al., 200911 | 14 | 64.3 | 34.3 | 28.6 | 50.0 | 78.6 | 0 | 21.4 | 1.7 | |
| Lu and Lawton, 201012 | 22 | 59.1 | 42.0 | 27.3 | 72.7 | 40.9 | 50.0 | 9.1 | ||
| Steiger et al., 201013 | 20 | 45.0 | 39.2 | 35.0 | 60.0 | 47.1 | 41.2 | 11.7 | ||
| Aoyama et al. 201014 | 12 | 58.3 | 34.1 | 41.7 | 50 | 58.3 | 25.0 | 16.7 | ||
| Choi et al., 20105 | 21 | 38.1 | 39.3 | 11.7 | 47.6 | 47.6 | 47.6 | 42.9 | 9.5 | 2.1 |
| Liang et al., 201115 | 64 | 62.5 | 35.2 | 32.8 | 67.2 | 36.0 | 55.0 | 9.0 | ||
| Maslehaty et al., 201116 | 11 | 37.5 | 41.6 | 45.4 | 36.4 | 63.6 | 36.4 | 0 | ||
| Reitz et al., 201517 | 48 | 52.1 | 41.3 | 39.5 | 56.3 | 22.9 | 70.8 | 6.3 | ||
| Zhang et al., 201618 | 58 | 60.3 | 38.7 | 12.9 | 46.6 | 50.0 | 67.2 | 29.3 | 3.5 | 3.9 |
| Azad et al., 20181 | 32 | 41.0 | 44.2 | 7.1 | 50.0 | 50.0 | 23.0 | 73.0 | 4.0 | |
| Goyal et al., 201919 | 21 | 71.4 | 48.0 | 10.5 | 28.6 | 71.4 | 78.2 | 21.8 | 5.5 | |
| Present cases | 13 | 53.8 | 32.7 | 15.6 | 23.0 | 77.0 | 84.6 | 15.4 | 0 | 3.7 |
C, cervical; T, thoracic.
In the present study, although the annualized hemorrhagic risk may have seemed acceptable at 3.7% in the conservative group, this is still a lifelong risk that may expose younger patients to a high cumulative hemorrhagic risk. Furthermore, SCCMs have the potential to result in more aggressive than their intracranial counterpart because the spinal canal is much less tolerant of space-occupying lesions.19 Goyal et al.20 indicated that large and symptomatic SCCMs appear to pose an increased risk for subsequent hemorrhage. In the present study, patients in the surgical group had significantly larger lesions than those in the conservative group. In particular, lesion size in the immediate surgical resection group was significantly larger than in the conservative group, whereas no significant relationships were seen between MMCS grades and lesion location or size.
There were no clinical differences among the no operation, immediate surgery, and surgery group. Although pain was statistically significant in no operation group, all patients in the immediate surgery group had presented with back pain corresponding to the lesion prior to the acute onset. The ratio of weakness and bladder dysfunction was significantly high in the immediate surgery and surgery group due to the surgical indication in our case series. In our case series, although the majority of lesions were located in the thoracic spinal cord (77%), the review of literature revealed the relatively larger distribution in the thoracic spinal cord (Table 4). Consistent with prior reports, mean age corresponded to the third and fourth decades of life. The slightly higher male to female sex ratio in our series was similar to that described in the literatures. The mean size of lesions was distributed between 5.5 mm and 16.1 mm, whereas our case series noted a relatively larger size (15.6 mm).
The annual rates of hemorrhage of SCCMs are approximately 1.7% to 5.5%, as reported in previous studies. Badhiwala et al.2 calculated the annual bleeding rate of 2.1% from the meta-analysis of 632 patients. The annual rates of hemorrhage of symptomatic patients is 9.5% to 17.6%.19,28 Annual risk for hemorrhage of asymptomatic patients is 0.8%.20 Other reported bleeding rates of asymptomatic lesions are 1.4%–1.6% per year.20,23
According to the literature, 22.9% to 90.0% of all patients showed improvement in functional status after resection of the lesion, whereas 10.0% to 73.0% remained unchanged. A total of 21.4% were reported to have deteriorated postoperatively compared with the baseline condition.3,8,17,27 In the present study, 84.6% of surgically treated patients improved, 15.4% remained unchanged, and none showed deteriorated functional status.
In this study, surgery was performed for the acute onset of hemorrhage or debilitating symptoms. Patients in the surgical group had a significantly larger lesion (Table 1). Therefore, it was suggested that the length of lesion was associated to the onset risk of symptoms. MMCS was applied to assess the neurological function and severity. Although, in this study, no significant relationships were seen between MMCS grades and lesion size, the worst MMCS grades at the time of debilitating symptoms was related to the length of hemorrhage (Table 3). These findings suggested that the length of hemorrhage was associated with the neurologic severity.
Conclusions
There were limitations to this study. It was retrospective and included observational data of small sample size in a single institution. The quality of data and clinical course was limited by patient recall bias. Nonetheless, surgery for SCCMs resulted in no recurrence of hemorrhage or exacerbation of neurological symptoms, and should be considered for patients who experienced acute onset of hemorrhage or debilitating symptoms during follow-up.
Declaration of Competing Interest
The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank J. Shinya for administrative assistance.
Supplementary Data
References
- 1.Azad T.D., Veeravagu A., Li A. Long-term effectiveness of gross-total resection for symptomatic spinal cord cavernous malformations. Neurosurgery. 2018;83:1201–1208. doi: 10.1093/neuros/nyx610. [DOI] [PubMed] [Google Scholar]
- 2.Badhiwala J.H., Farrokhyar F., Alhazzani W. Surgical outcomes and natural history of intramedullary spinal cord cavernous malformations: a single-center series and meta-analysis of individual patient data: clinical article. J Neurosurg Spine. 2014;21:662–676. doi: 10.3171/2014.6.SPINE13949. [DOI] [PubMed] [Google Scholar]
- 3.Bian L.G., Bertalanffy H., Sun Q.F. Intramedullary cavernous malformations: clinical features and surgical technique via hemilaminectomy. Clin Neurol Neurosurg. 2009;111:511–517. doi: 10.1016/j.clineuro.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Canavero S., Pagni C.A., Duca S. Spinal intramedullary cavernous angiomas: a literature meta-analysis. Surg Neurol. 1994;41:381–388. doi: 10.1016/0090-3019(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 5.Choi G.H., Kim K.N., Lee S. The clinical features and surgical outcomes of patients with intramedullary spinal cord cavernous malformations. Acta Neurochir (Wien) 2011;153:1677–1685. doi: 10.1007/s00701-011-1016-3. [DOI] [PubMed] [Google Scholar]
- 6.Cristante L., Hermann H.D. Radical excision of intramedullary cavernous angiomas. Neurosurgery. 1998;43:424–430. doi: 10.1097/00006123-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Sandalcioglu I.E., Wiedemayer H., Gasser T. Intramedullary spinal cord cavernous malformations: clinical features and risk of hemorrhage. Neurosurg Rev. 2003;26:253–256. doi: 10.1007/s10143-003-0260-2. [DOI] [PubMed] [Google Scholar]
- 8.Santoro A., Piccirilli M., Frati A. Intramedullary spinal cord cavernous malformations: report of ten new cases. Neurosurg Rev. 2004;27:93–98. doi: 10.1007/s10143-003-0302-9. [DOI] [PubMed] [Google Scholar]
- 9.Jallo G.I., Freed D., Zareck M. Clinical presentation and optimal management for intramedullary cavernous malformations. Neurosurg Focus. 2006;21:e10. doi: 10.3171/foc.2006.21.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Matsuyama Y., Sakai Y., Katayama Y. Surgical results of intramedullary spinal cord tumor with spinal cord monitoring to guide extent of resection. J Neurosurg Spine. 2009;10:404–413. doi: 10.3171/2009.2.SPINE08698. [DOI] [PubMed] [Google Scholar]
- 11.Park S.B., Jahng T.A., Chung C.K. The clinical outcomes after complete surgical resection of intramedullary cavernous angiomas: changes in motor and sensory symptoms. Spinal Cord. 2009;47:128–133. doi: 10.1038/sc.2008.89. [DOI] [PubMed] [Google Scholar]
- 12.Lu D.C., Lawton M.T. Clinical presentation and surgical management of intramedullary spinal cord cavernous malformations. Neurosurg Focus. 2010;29:E12. doi: 10.3171/2010.6.FOCUS10139. [DOI] [PubMed] [Google Scholar]
- 13.Steiger H.J., Turowski B., Hänggi D. Prognostic factors for the outcome of surgical and conservative treatment of symptomatic spinal cord cavernous malformations: a review of a series of 20 patients. Neurosurg Focus. 2010;29:E13. doi: 10.3171/2010.6.FOCUS10123. [DOI] [PubMed] [Google Scholar]
- 14.Aoyama T., Hida K., Houkin K. Intramedullary cavernous angiomas of the spinal cord: clinical characteristics of 13 lesions. Neurol Med Chir (Tokyo) 2011;51:561–566. doi: 10.2176/nmc.51.561. [DOI] [PubMed] [Google Scholar]
- 15.Liang J.T., Bao Y.H., Zhang H.Q. Management and prognosis of symptomatic patients with intramedullary spinal cord cavernoma: clinical article. J Neurosurg Spine. 2011;15:447–456. doi: 10.3171/2011.5.SPINE10735. [DOI] [PubMed] [Google Scholar]
- 16.Maslehaty H., Barth H., Petridis A.K. Symptomatic spinal cavernous malformations: indication for microsurgical treatment and outcome. Eur Spine J. 2011;20:1765–1770. doi: 10.1007/s00586-011-1898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reitz M., Burkhardt T., Vettorazzi E. Intramedullary spinal cavernoma: clinical presentation, microsurgical approach, and long-term outcome in a cohort of 48 patients. Neurosurg Focus. 2015;39:E19. doi: 10.3171/2015.5.FOCUS15153. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L., Yang W., Jia W. Comparison of outcome between surgical and conservative management of symptomatic spinal cord cavernous malformations. Neurosurgery. 2016;78:552–561. doi: 10.1227/NEU.0000000000001075. [DOI] [PubMed] [Google Scholar]
- 19.Goyal A., Rinaldo L., Alkhataybeh R. Clinical presentation, natural history and outcomes of intramedullary spinal cord cavernous malformations. J Neurol Neurosurg Psychiatry. 2019;90:695–703. doi: 10.1136/jnnp-2018-319553. [DOI] [PubMed] [Google Scholar]
- 20.Ghogawala Z., Ogilvy C.S. Intramedullary cavernous malformations of the spinal cord. Neurosurg Clin N Am. 1999;10:101–111. [PubMed] [Google Scholar]
- 21.Gross B.A., Du R., Popp A.J. Intramedullary spinal cord cavernous malformations. Neurosurg Focus. 2010;29:E14. doi: 10.3171/2010.6.FOCUS10144. [DOI] [PubMed] [Google Scholar]
- 22.Kharkar S., Shuck J., Conway J. The natural history of conservatively managed symptomatic intramedullary spinal cord cavernomas. Neurosurgery. 2007;60:865–872. doi: 10.1227/01.NEU.0000255437.36742.15. [DOI] [PubMed] [Google Scholar]
- 23.Kondziella D., Brodersen P., Laursen H., Hansen K. Cavernous hemangioma of the spinal cord conservative or operative management? Acta Neurol Scand. 2006;114:287–290. doi: 10.1111/j.1600-0404.2006.00675.x. [DOI] [PubMed] [Google Scholar]
- 24.McCormick P.C., Torres R., Post K.D. Intramedullary ependymoma of the spinal cord. J Neurosurg. 1990;72:523–532. doi: 10.3171/jns.1990.72.4.0523. [DOI] [PubMed] [Google Scholar]
- 25.Mitha A.P., Turner J.D., Spetzler R.F. Surgical approaches to intramedullary cavernous malformations of the spinal cord. Neurosurgery. 2011;68:317–324. doi: 10.1227/NEU.0b013e3182138d6c. [DOI] [PubMed] [Google Scholar]
- 26.Tong X., Deng X., Li H. Clinical presentation and surgical outcome of intramedullary spinal cord cavernous malformations. J Neurosurg Spine. 2012;16:308–314. doi: 10.3171/2011.11.SPINE11536. [DOI] [PubMed] [Google Scholar]
- 27.Gordon C.R., Crockard H.A., Symon L. Surgical management of spinal cord cavernoma. Br J Neurosurg. 1995;9:459–464. doi: 10.1080/02688699550041098. [DOI] [PubMed] [Google Scholar]
- 28.Vishteh A.G., Zabramski J.M., Spetzler R.F. Patients with spinal cord cavernous malformations are at an increased risk for multiple neuraxis cavernous malformations. Neurosurgery. 1999;45:30–33. doi: 10.1097/00006123-199907000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.