Abstract
BACKGROUND: Breast cancer (BC) is a heterogeneous disease, and patients with apparently similar clinicopathological characteristics in clinical practice show different outcome. This study evaluated in primary BCs and in the subgroup of the triple-negative breast cancers (TNBCs) the level of tumor infiltrating lymphocytes (TILs), Na+/H+ exchanger regulatory factor 1 (NHERF1) expression, and their association respect to the clinical outcome of patients. MATERIAL AND METHODS: NHERF1 expression was assessed by immunohistochemistry in 338 BC samples; the analysis of TILs was examined using hematoxylin and eosin stained slides, according to International TILs Working Group 2014. RESULTS: Multivariate analysis identified TILs as an independent prognostic factor for DFS in the entire cohort and in the TNBC subgroup (HR, 0.32; 95% CI, 0.12–0.87; P = 0.026; and HR, 0.22; 95% CI, 0.06–0.80; P = 0.022, respectively). Univariate and survival analysis by Kaplan–Meier method revealed that patients with cytoplasmic (c) NHERF1-/TILs+ expression had better DFS than other patients (P = 0.049), and this result was also found in the TNBC subgroup (P = 0.031). Moreover, TNBC patients with cNHERF1−/TILs− expression had a worse DFS and OS than other patients (P = 0.057 and P = 0.002, respectively). CONCLUSIONS: In the complex scenario of BC and in the era of tumor immunogenicity and immunotherapy, we found an association of TIL levels and cNHERF1 expression that could be useful to identify BCs and particularly TNBC patients with different prognosis and clinical outcome.
Introduction
Breast cancer (BC) is a heterogeneous disease, and patients with apparently similar tumors for the clinicopathological characteristics in clinical practice show different clinical outcome. In this scenario, the need to find new biological markers for a more precise characterization of the disease for prognosis and therapeutic purposes has become mandatory.
Recently, the study of tumor microenvironment has acquired more and more importance in different human cancers [1], also in consequence of the contemporary development and spread of the new immunotherapeutic treatments. Even if historically BC was not considered an immunologically active cancer, recently it was observed that tumor infiltrating lymphocytes (TILs) can predict response to chemotherapy and improved prognosis [2]. TILs are considered a selected population of immune cells, consisting of cytotoxic and helper T cells, B cells, macrophages, dendritic cells, and natural killer cells with a higher specific immunological reactivity against tumor cells. Loi S et al. have previously shown that a higher quantity of immune infiltrate present in both early stage triple-negative breast cancers (TNBCs) and human epidermal growth factor receptor 2 (HER2)–positive BC samples was associated with significantly improved clinical outcome [3]. The quantification of TILs, as the percentage infiltration in the tumor and surrounding stroma, has thus been demonstrated to be a reproducible biomarker that could be implemented in the standard clinical pathology [4].
During the last ten years, our group has focused own studies on Na+/H+ exchanger regulatory factor 1 (NHERF1), a scaffold protein able to link different molecules and involved, for this peculiarity, in many human cancers as BC [5]. For the first time, on a clinical cohort of BC patients with a long-term follow-up, we showed the prognostic significance of nuclear NHERF1 (nNHERF1) expression and that the loss of nNHERF1 was associated with poor DFS [6].
Little is known about the involvement of NHERF1 in the new scenario of immunotherapeutic cells. Our pioneering study in a group of 55 BC patients revealed that NHERF1 was also expressed in the cytoplasm of the tumor cells and of the majority of the lymphocytes that were present in tumoral and lymphonodal stroma. Furthermore, we analyzed the expression of NHERF1 in circulating peripheral blood lymphocytes between BC patients and healthy control group and found a significantly higher expression in the first group [7]. These evidences underlined a hypothetical involvement of NHERF1 in immunological events associated with neoplastic disease.
This study aimed to evaluate in a series of primary BCs and in the subgroup of the TNBCs, with a long follow-up, the level of TILs, NHERF1 expression, and their association with respect to the prognosis and clinical outcome of patients.
Materials and Methods
Patient's Characteristics
The cohort included a retrospective and nonconsecutive series of 338 patients with primary BC, surgically treated at the Istituto Tumori “Giovanni Paolo II” of Bari between 1998 and 2012. The patients were enrolled retrospectively on the basis of the availability of the biological material and the clinical follow-up. Patients were excluded if they had a previous history of invasive BC or other previous or concomitant malignancies or concomitant diseases. The median follow-up time was 72 months (range 1–207). Estrogen receptor (ER), progesterone receptor (PgR), HER2 status, and proliferative activity (Ki67) were determined by the pathology department of our institute. ER and PgR positivity was defined with a cutoff >10%. A Ki67 labeling index >20% was used to classify a tumor sample as positive [8]. HER2 protein expression was scored in accordance with the Hercep Τest scoring system (Food and Drug Administration). HER2 negativity was defined as immunohistochemistry (IHC) 0/1+; HER2 was considered positive if immunostaining was 3+ or if a score 2+ showed gene amplification by fluorescence in situ hybridization (FISH) in accordance with the criteria of the ASCO/CAP guidelines 2007 [9]. The HER2 IHC and HER2 FISH results were interpreted by molecular pathologists. On the basis of the hormonal receptors and HER2 status, 112 of these tumors were classified as TNBCs.
All patients provided an informed consent form to utilize their removed biological tissue for research purposes, according to ethical standards. This study was approved by the Ethic Committee of our institute (number 657/CE 13-02-2018).
Immunohistochemistry and Immunohistochemical Assessment of NHERF1
To perform the immunohistochemical assessment of NHERF1 in 338 tumor samples from primary BC patients, we followed the previous method [8]. The slides were processed and stained by the indirect immunoperoxidase method using the BenchMark XT automated staining instrument (Ventana Medical Systems, Tucson, AZ, USA). The slides of 4–5 μm were incubated at 37° with the specific primary antibody diluted in antibody diluent (Ventana Medical Systems, Tucson, AZ, USA): rabbit polyclonal NHERF1 antibody (anti-EBP50; ThermoFisher Scientific, Rockford, IL, USA) at 1:350 for 16 min. Finally, tissues were counterstained with hematoxylin and bluing reagent (Ventana Medical Systems, Tucson, AZ, USA) for 8 min and 4 min, respectively, and then were dehydrated and mounted. Positive and negative controls were included in each staining run as indicated in the data sheet.
The data from IHC assay were examined independently by two investigators who had no prior knowledge of the clinicopathological data. Any discrepancies between the two observers were resolved by reexamination and consensus.
NHERF1 immunostaining was predominantly cytoplasmic (cNHERF1); however, in some cases, an intense nuclear (nNHERF1) staining was also observed. This was scored separately, and its significance was evaluated. The median value of immunoreactive cells was used as cutoff. The cases were classified positive when cNHERF1 immunoreactivity was present in ≥70% of tumor cells, and when nNHERF1 immunoreactivity was present in (>0%) of tumor cells observed.
Quantification of TILs
The analysis of TILs within the borders of the invasive tumor was assessed in full-face hematoxylin and eosin–stained sections (4–5 μm, magnification ×200) from the surgical specimen, according to TILs Working Group recommendations [4]. TILs were scored in the stromal compartment as the percentage of all mononuclear cells in the area of stromal tissue. Tumors with stromal TILs score of ≥50% were considered lymphocyte predominant BC.
Statistical Analysis
NHERF1 and TILs analysis was carried out in relation to disease-free survival (DFS) and overall survival (OS). DFS (in months) was calculated as the time frame between the date of surgery and the date of locoregional/distant relapse (second invasive BC, second primary cancer, and/or death without evidence of BC) to the date of last contact. OS (in months) was calculated as the time frame between the date of surgery and date of last contact or the date of death from any cause. DFS and OS survival curves were computed by Kaplan–Meier method and compared by the log rank test. Cox proportional hazard regression model was performed to assess prognostic factors, including the variables that were statistically significant in univariate analysis. The association between categorical variables was investigated using chi-square test and between continuous variable using Spearman correlation test. Statistical significance level was p-values <0.05. Statistical analyses were made using the SAS statistical software, version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Patient Characteristics
In this study, we included 338 patients with primary BC which had the clinicopathological characteristic summarized in Table S1. The median age of the patients was 51 years. The more representative tumor histotype was the ductal infiltrating carcinoma (90.2%), with a tumor size larger than 2.0 cm (59.2%), nuclear grade 3 (69.1%), and the positivity of the lymphonodal status (53.6%). The majority of cases were negative for the ER (52%), PgR (58.6%), and for HER2 (75.6%), while they were positive for the expression of Ki67 (75%).
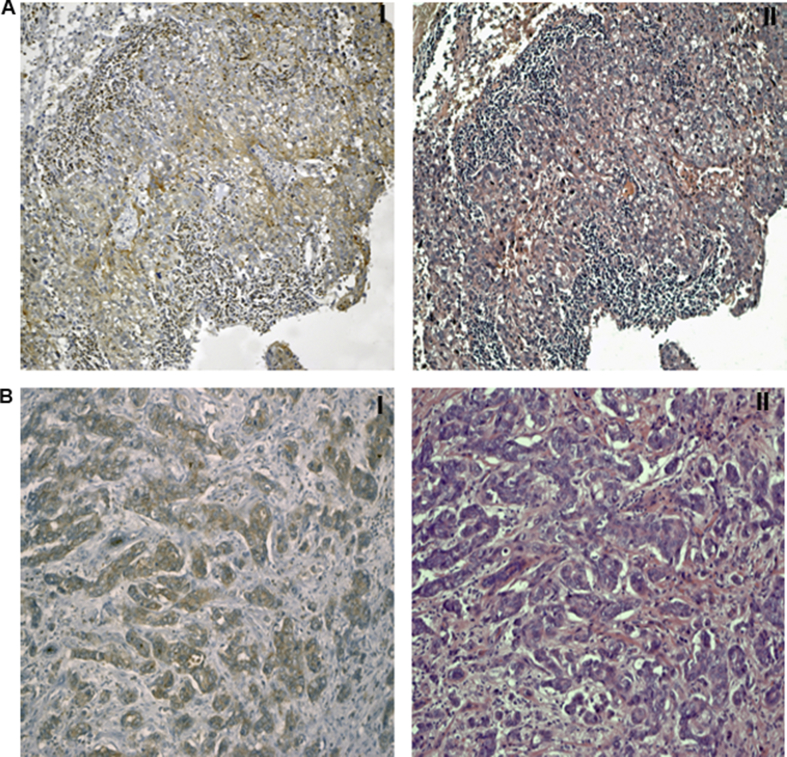
In the entire cohort of patients, we evaluated NHERF1 expression in the cytoplasmic and nuclear compartment and the presence or absence of TILs. For 12 cases, of which 4 were TNBCs, cNHERF1 and nNHERF1 staining was not evaluable. Cytoplasmic NHERF1 overexpression was observed in 50.6% of the tumors, and nNHERF1 expression was absent in 72.7% of the tumors. The 27.3% of tumors with nNHERF1 expression also showed a cNHERF1 immunoreactivity. The majority of cases, at last, had the lack of TILs (73.7%). Representative images of NHERF1 expression and TIL levels were reported in Figure 1.
Figure 1.
Representative images of NHERF1 expression and TIL levels. (A-I) Low NHERF1 expression and (A-II) high TIL levels; (B-I) low NHERF1 expression and (B-II) low TIL levels (magnification, ×100).
About clinical treatment, 173 (51.2%) patients received only adjuvant chemotherapy, while 165 (48.8%) were treated by both chemotherapy and hormonotherapy.
Association of Cytoplasmic NHERF1 and TILs with Clinicopathological Characteristics
We considered, in particular, the association of cNHERF1 expression and the level of TILs with the clinicopathological patient characteristics, as reported in Tables 1 and 2.
Table 1.
Association of cNHERF1 and TILs with Tumor Clinicopathological Characteristics in Overall Cohort of Patients.
| cNHERF1 <70% |
cNHERF1 ≥70% |
p | TILs <50% |
TILs ≥50% |
p | |
|---|---|---|---|---|---|---|
| N. (%) | N. (%) | N. (%) | N. (%) | |||
| Age (years) | ||||||
| ≤51 | 86 (53.4) | 80 (48.5) | 120 (48.2) | 50 (56.2) | ||
| >51 | 75 (46.6) | 85 (51.5) | 0.374 | 129 (51.8) | 39 (43.8) | 0.196 |
| Histotype | ||||||
| Ductal | 143 (88.8) | 151 (91.6) | 230 (92.4) | 76 (85.4) | ||
| Lobular | 10 (6.2) | 7 (4.2) | 14 (5.6) | 3 (3.4) | ||
| Other | 8 (5.0) | 7 (4.2) | 0.506 | 5 (2.0) | 10 (11.2) | 0.004 |
| Tumor Size (cm) | ||||||
| ≤2.0 | 70 (47.3) | 56 (34.8) | 106 (45.1) | 28 (32.6) | ||
| >2.0 | 78 (52.7) | 105 (65.2) | 0.026 | 129 (54.9) | 58 (67.4) | 0.044 |
| Node | ||||||
| Negative | 73 (46.8) | 75 (46.0) | 108 (44.6) | 45 (51.1) | ||
| Positive | 83 (53.2) | 88 (54.0) | 0.889 | 134 (55.4) | 43 (48.9) | 0.295 |
| Grade | ||||||
| 1–2 | 53 (33.3) | 46 (28.6) | 89 (36.5) | 17 (19.3) | ||
| 3 | 106 (66.7) | 115 (71.4) | 0.358 | 155 (63.5) | 71 (80.7) | 0.003 |
| ER (%) | ||||||
| ≤10 | 75 (46.6) | 94 (57.3) | 122 (49.2) | 54 (60.7) | ||
| >10 | 86 (53.4) | 70 (42.7) | 0.053 | 126 (50.8) | 35 (39.3) | 0.063 |
| PgR (%) | ||||||
| ≤10 | 81 (50.6) | 109 (66.5) | 139 (56.3) | 58 (65.2) | ||
| >10 | 79 (49.4) | 55 (33.5) | 0.004 | 108 (43.7) | 31 (34.8) | 0.145 |
| Ki67 (%) | ||||||
| ≤20 | 40 (25.3) | 40 (24.7) | 74 (30.1) | 12 (13.9) | ||
| >20 | 118 (74.7) | 122 (75.3) | 0.897 | 172 (69.9) | 74 (86.1) | 0.003 |
| HER2 | ||||||
| Negative | 116 (78.4) | 104 (72.7) | 174 (78.7) | 55 (68.7) | ||
| Positive | 32 (21.6) | 39 (27.3) | 0.263 | 47 (21.3) | 25 (31.3) | 0.073 |
Bold indicates P < 0.05.
Table 2.
Association of cNHERF1 and TILs with Tumor Clinicopathological Characteristics in TNBC Subgroup.
| cNHERF1 <70% |
cNHERF1 ≥70% |
p | TILs <50% |
TILs ≥50% |
p | |
|---|---|---|---|---|---|---|
| N. (%) | N. (%) | N. (%) | N. (%) | |||
| Age (years) | ||||||
| ≤51 | 28 (52.8) | 29 (52.7) | 38 (46.3) | 20 (66.7) | ||
| >51 | 25 (47.2) | 26 (47.3) | 0.991 | 44 (53.7) | 10 (33.3) | 0.058 |
| Histotype | ||||||
| Ductal | 48 (90.6) | 47 (85.5) | 76 (92.6) | 23 (76.7) | ||
| Lobular | 1 (1.9) | 2 (3.6) | 3 (3.7) | 0 | ||
| Other | 4 (7.5) | 6 (10.9) | 0.461 | 3 (3.7) | 7 (23.3) | 0.004 |
| Tumor Size (cm) | ||||||
| ≤2.0 | 22 (51.2) | 22 (40.7) | 39 (53.4) | 9 (32.1) | ||
| >2.0 | 21 (48.8) | 32 (59.3) | 0.308 | 34 (46.6) | 19 (67.9) | 0.056 |
| Node | ||||||
| Negative | 30 (61.2) | 31 (57.4) | 41 (54.0) | 23 (76.7) | ||
| Positive | 19 (38.8) | 23 (42.6) | 0.695 | 35 (46.0) | 7 (23.3) | 0.032 |
| Grade | ||||||
| 1–2 | 8 (15.1) | 9 (16.7) | 16 (19.7) | 3 (10.0) | ||
| 3 | 45 (84.9) | 45 (83.3) | 0.825 | 65 (80.3) | 27 (90.0) | 0.228 |
| Ki67 (%) | ||||||
| ≤20 | 4 (7.7) | 4 (7.3) | 10 (12.2) | 0 | ||
| >20 | 48 (92.3) | 51 (92.7) | 0.935 | 72 (87.8) | 29 (100) | 0.061 |
Bold indicates P < 0.05.
We found that the majority of cases with cNHERF1-positive expression (median value ≥ 70%) had tumor size larger than 2.0 cm (65.2%, p = 0.026) and were ER and PgR negative (57.3%, p = 0.053 and 66.5%, p = 0.004, respectively). The majority of cases with TILs negative level (cutoff < 50%) had ductal histotype (92.4%, p = 0.004), tumor size larger than 2.0 cm (54.9%, p = 0.044), nuclear grade 3 (63.5%, p = 0.003), and high Ki67 expression (69.9%, p = 0.003).
Considering also the TNBC subgroup, NHERF1 expression was not associated with any clinicopathological characteristic, while the low level of TILs was associated with ductal histotype (92.6%, p = 0.004) and the high level of TILs with negative lymphonodal status (76.7%, p = 0.032).
When we analyzed the continuous variables of NHERF1 expression and TIL level in the overall population, we observed that there was an inverse statistically significant correlation between cNHERF1 expression and TILs (p = 0.0003) and a direct statistically significant correlation between nNHERF1 expression and TILs (rs = 0.12, p = 0.027).
Survival Analyses
The possible impact of NHERF1 expression and TILs status on patient outcome was investigated with respect to DFS and OS.
Multivariate analysis of the entire cohort, and then of the only TNBC population (Table 3), identified TILs as independent prognostic variables for DFS (HR, 0.32; 95% CI, 0.12–0.87; p = 0.026 and HR, 0.22; 95% CI, 0.06–0.80; p = 0.022, respectively).
Table 3.
Multivariate Analysis of DFS in the Overall Series and TNBC Patients.
| HR (95% CI) | p | |
|---|---|---|
| Overall Patients | ||
| Age (>51 vs ≤51) | 0.77 (0.38–1.54) | 0.460 |
| Tumor size >2.0 vs ≤2.0) | 1.74 (0.84–3.61) | 0.137 |
| Grade (3 vs 1–2) | 2.41 (0.93–6.23) | 0.069 |
| cNHERF1 (≥70% vs <70%) | 0.93 (0.46–1.86) | 0.831 |
| nNHERF1 (>0% vs 0%) | 1.09 (0.40–2.98) | 0.865 |
| TILs (≥50% vs <50%) | 0.32 (0.12–0.87) | 0.026 |
| TNBC Patients | ||
| Age (>51 vs ≤51) | 0.39 (0.13–1.14) | 0.086 |
| Tumor size >2.0 vs ≤2.0) | 2.04 (0.78–5.35) | 0.145 |
| Grade (3 vs 1–2) | 3.37 (0.41–27.51) | 0.257 |
| cNHERF1 (≥70% vs <70%) | 0.57 (0.22–1.43) | 0.229 |
| nNHERF1 (>0% vs 0%) | 1.76 (0.44–6.98) | 0.423 |
| TILs (≥50% vs <50%) | 0.22 (0.06–0.80) | 0.022 |
Bold indicates P < 0.05.
We then investigated by univariate analysis the relationship between the combination of cNHERF1 expression and TIL level with respect to DFS and OS (Table 4).
Table 4.
Univariate Analysis of DFS and OS Considering the Combination of cNHERF1 Expression and TILs Levels in the Overall Group and TNBC Patients.
| Characteristics | N. Pts | DFS |
OS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N. Events | 5-yr % DFS | p | HR (95% CI) | p | N. Events | 5-yr % OS | p | HR (95% CI) | P | ||
| Overall Patients | |||||||||||
| Other | 277 | 58 | 84 | 1.00 | 23 | 93 | 1.00 | ||||
| cNHERF1−/TILs+ | 49 | 5 | 93 | 0.049 | 0.41 (0.16–1.03) | 0.057 | 3 | 93 | 0.483 | 0.65 (0.20–2.17) | 0.487 |
| TNBC Patients | |||||||||||
| Other | 95 | 26 | 76 | 1.00 | 10 | 89 | 1.00 | ||||
| cNHERF1−/TILs+ |
13 |
0 |
100 |
0.031 |
Not estimable |
– |
0 |
100 |
0.209 |
Not estimable |
|
| Overall Patients | |||||||||||
| Other | 214 | 42 | 85 | 1.00 | 15 | 94 | 1.00 | ||||
| cNHERF1−/TILs− | 112 | 21 | 86 | 0.861 | 1.05 (0.62–1.77) | 0.860 | 11 | 90 | 0.276 | 1.54 (0.70–3.35) | 0.280 |
| TNBC Patients | |||||||||||
| Other | 68 | 13 | 84 | 1.00 | 2 | 97 | 1.00 | ||||
| cNHERF1−/TILs− | 40 | 13 | 72 | 0.057 | 2.08 (0.96–4.49) | 0.063 | 8 | 78 | 0.002 | 8.15 (1.73–38.47) | 0.008 |
Bold indicates P < 0.05.
In the overall cohort of patients, the tumor phenotype cNHERF1−/TILs+ was significantly associated with better DFS (p = 0.049), and this finding was also verified when we considered only the TNBC subgroup (p = 0.031). No significant association was found, instead, with respect to OS.
Analyzing cNHERF1−/TILs− tumors, in the overall cohort of patients, no significant association resulted with respect to DFS and OS. But, in the TNBC subgroup, we found that patients with cNHERF1−/TILs− expression had worse DFS (p = 0.057) and OS (p = 0.002).
Differently, the cNHERF1-positive expression in association with TIL level did not show any significant result with respect to patient outcome.
Patients with cNHERF1−/TILs+ expression had a higher DFS than the DFS of other patients (5 years %, 93% versus 84%, p = 0.049) (Figure 2A), and this result was also found in the TNBC subgroup (5 years %, 100% versus 76%, p = 0.031) (Figure 2B). Moreover, Kaplan–Meier curves, relative to TNBC patients, showed that the ones with cNHERF1−/TILs− expression had a worse survival, both DFS and OS, compared with other TNBC patients (p = 0.057 and p = 0.002, respectively) (Figure 2C and D).
Figure 2.
Kaplan–Maier curve analysis and log-rank test.A Kaplan–Maier curve for disease-free survival according to cNHERF1 <70% TIL ≥50% (cNHERF1−/TILs+) versus others in all patients; B Kaplan–Maier curve for disease-free survival according to cNHERF1 <70% TIL ≥50% (cNHERF1−/TILs+) versus others in TNBC subgroup; C Kaplan–Maier curve for disease-free survival according to cNHERF1 <70% TIL <50% (cNHERF1-/TILs−) versus others in TNBC subgroup; D Kaplan–Maier curve for overall survival according to cNHERF1 <70% TIL <50% (cNHERF1−/TILs−) versus others in TNBC subgroup.
When we considered separately TILs and NHERF1 expression in the overall cohort of patients, no statistically significant difference has been observed respect to DFS an OS.
Discussion
There are recent evidences that support the role of immune-related factors in BC prognosis and treatment, and in particular, TILs represent a crucial factor in this scenario. Most of TNBCs and HER2-positive BCs have dense immune infiltrates, and some studies showed that the presence of TILs in tumor tissue may predict response to neoadjuvant therapy [10] and also may have a significant prognostic value after adjuvant chemotherapy [[11], [12], [13], [14]]. Although the concept of evaluating TILs in BC and making correlations with clinical outcome is not new, yet there are few consistent data to consider TILs as reliable prognostic factors because of same complex and controversial aspects [15]. The present study would be another further contribution in this contest, to add new knowledge in the scenario of prognosis and future possible new treatments of BC patients. Our results suggest that TILs are an independent prognostic factor for DFS in TNBCs, supporting the studies in which TILs were able to identify a subset of patients with good prognosis [16,17]. Moreover, Yu X et al. reported that high value of TILs was associated with better prognosis in BC patients [18]. Our finding was true also for the entire cohort of BC patients suggesting that all tumors with high immunological setting have a chance of better clinical outcome [19]. The negative expression of TILs, instead, was associated with some worse clinicopathological characteristics as larger tumor size, high nuclear grade, and high proliferative activity, as previous found [20].
Considering only TIL level, however, in our study, Kaplan–Meir curves did not show any significant results respect to OS. Interestingly, it is the association of TILs with NHERF1 expression capable to stratifying BC patients for prognosis. We previously found that the loss of nNHERF1 was associated with reduced survival [6] and that the TNBC patients with cNHERF1 and nuclear PARP1 coexpression had a shorter OS. This last result suggests that NHERF1 in association with other biological markers could be useful to identify patients with different prognosis [8].
It is the first time that the interaction of NHERF1 and TILs has been studied in BC patients. Even if cNHERF1-positive expression was associated with some worse tumor clinicopathological characteristics, confirming previous results [6,[21], [22], [23]], this marker alone was not related to patient survival. However, in the whole series and in the TNBC subgroup, the low cNHERF1 expression combined with TILs level was able to select patients for their outcome. In specific, in the subset of patients with negative cNHERF1 expression, the contextual TILs predominance was associated with a better DFS, both in the entire cohort and in the TNBCs. Moreover, the TNBC patients with negative cNHERF1 expression and the contextual absence of TILs had worse clinical outcome with respect to the other subgroups, for both DFS and OS. These results underlined the important role of TILs as prognostic biomarker, both in all BCs and particularly in TNBC patients. In BCs and in TNBC patients who have earlier relapse and worse survival compared with nonTNBCs, the positive expression of TILs remarks that immunogenic tumors had a good chance of survival.
These findings are true even in a context in which an aggressive characteristic, as the positive expression of cNHERF1, was lost. Differently, considering the expression of positive cNHERF1 in relation to TILs, we did not observe any significant result with respect to the clinical patient outcome. Probably, the cNHERF1-positive expression counterbalances the protective effect of TILs presence, interfering with their activity and in general with the tumor microenvironment, as already demonstrated [23].
TILs were also analyzed in different studies in relation to the adjuvant setting, and they were associated to better prognosis and decreased distant recurrence, also in TNBC subgroup [3,17]. However, the association of high TILs with improved outcome in the setting of chemotherapy may also be related to the ability of chemotherapy to enhance immune response [24]. A limit of our study was that we had no clinical data, according to response evaluation criteria in solid tumors (RECIST) criteria, about the patient response to clinical treatment, based on adjuvant chemotherapy or chemotherapy plus hormonotherapy. So we could not correlate our results and particularly TIL's role in the setting of response to therapy and also as possible predictive biomarker, which could influence at last general patient prognosis and outcome.
Conclusion
In the complex scenario of the BCs heterogeneity, and the new actual field of tumor immunogenicity and immunotherapy, we find a new interesting association between TIL level and cNHERF1 expression able to identify TNBC and also BC patients with different prognosis and clinical outcome. Next future studies need to clarify these results.
Funding Statement
This work was supported by funding from the Italian Ministry of Health “Ricerca Corrente 2018–2020.”
Conflict of Interest
The authors declare no potential conflict of interest.
Acknowledgments
The authors would like to thank Francesco Fanelli for the technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2019.10.020.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Badalamenti G., Fanale D., Incorvaia L., Barraco N., Listì A., Maragliano R., Vincenzi B., Calò V., Iovanna J.L., Bazan V. Role of tumor-infiltrating lymphocytes in patients with solid tumors: can a drop dig a stone? Cell Immunol. 2018 doi: 10.1016/j.cellimm.2018.01.013. pii: S0008-8749 (18): 30014-30015. [DOI] [PubMed] [Google Scholar]
- 2.Stanton S.E., Disis M.L. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loi S., Drubay D., Adams S., Pruneri G., Francis P.A., Lacroix-Triki M., Joensuu H., Dieci M.V., Badve S., Demaria S. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–569. doi: 10.1200/JCO.18.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salgado R., Denkert C., Demaria S., Sirtaine N., Klauschen F., Pruneri G., Wienert S., Van den Eynden G., Baehner F.L., Penault-Llorca F., International TILs Working Group 2014 The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centonze M., Saponaro C., Mangia A. NHERF1 between promises and hopes: overview on cancer and prospective openings. Transl Oncol. 2018;11(2):374–390. doi: 10.1016/j.tranon.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paradiso A., Scarpi E., Malfettone A., Addati T., Giotta F., Simone G., Amadori D., Mangia A. Nuclear NHERF1 expression as a prognostic marker in breast cancer. Cell Death Dis. 2013;4:e904. doi: 10.1038/cddis.2013.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellizzi A., Mangia A., Malfettone A., Cardone R.A., Simone G., Reshkin S.J., Paradiso A. Na+/H+ exchanger regulatory factor 1 expression levels in blood and tissue predict breast tumour clinical behaviour. Histopathology. 2011;58(7):1086–1095. doi: 10.1111/j.1365-2559.2011.03844.x. [DOI] [PubMed] [Google Scholar]
- 8.Mangia A., Scarpi E., Partipilo G., Schirosi L., Opinto G., Giotta F., Simone G. NHERF1 together with PARP1 and BRCA1 expression as a new potential biomarker to stratify breast cancer patients. Oncotarget. 2017;8(39):65730–65742. doi: 10.18632/oncotarget.19444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff A.C., Hammond M.E., Schwartz J.N., Hagerty K.L., Allred D.C., Cote R.J., Dowsett M., Fitzgibbons P.L., Hanna W.M., Langer A. American Society of Clinical Oncology, College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 10.Denkert C., Loibl S., Noske A., Roller M., Müller B.M., Komor M., Budczies J., Darb-Esfahani S., Kronenwett R., Hanusch C. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 11.Denkert C., Wienert S., Poterie A., Loibl S., Budczies J., Badve S., Bago-Horvath Z., Bane A., Bedri S., Brock J. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29(10):1155–1164. doi: 10.1038/modpathol.2016.109. [DOI] [PubMed] [Google Scholar]
- 12.Denkert C., von Minckwitz G., Darb-Esfahani S., Lederer B., Heppner B.I., Weber K.E., Budczies J., Huober J., Klauschen F., Furlanetto J. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. doi: 10.1016/S1470-2045(17)30904-X. [DOI] [PubMed] [Google Scholar]
- 13.Pfitzner B.M., Lederer B., Lindner J., Solbach C., Engels K., Rezai M., Dohnal K., Tesch H., Hansmann M.L., Salat C. Clinical relevance and concordance of HER2 status in local and central testing-an analysis of 1581 HER2-positive breast carcinomas over 12 years. Mod Pathol. 2018;31(4):607–615. doi: 10.1038/modpathol.2017.171. [DOI] [PubMed] [Google Scholar]
- 14.Baxevanis C.N., Sofopoulos M., Fortis S.P., Perez S.A. The role of immune infiltrates as prognostic biomarkers in patients with breast cancer. Cancer Immunol Immunother. 2019;68(10):1671–1680. doi: 10.1007/s00262-019-02327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wein L., Savas P., Luen S.J., Virassamy B., Salgado R., Loi S. Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol. 2017;7:156. doi: 10.3389/fonc.2017.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loi S., Michiels S., Salgado R., Sirtaine N., Jose V., Fumagalli D., Kellokumpu-Lehtinen P.L., Bono P., Kataja V., Desmedt C. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 17.Adams S., Gray R.J., Demaria S., Goldstein L., Perez E.A., Shulman L.N., Martino S., Wang M., Jones V.E., Saphner T.J. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu X., Zhang Z., Wang Z., Wu P., Qiu F., Huang J. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: a systematic review and meta-analysis. Clin Transl Oncol. 2016;18(5):497–506. doi: 10.1007/s12094-015-1391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Criscitiello C., Esposito A., Trapani D., Curigliano G. Prognostic and predictive value of tumor infiltrating lymphocytes in early breast cancer. Cancer Treat Rev. 2016;50:205–207. doi: 10.1016/j.ctrv.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Jang N., Kwon H.J., Park M.H., Kang S.H., Bae Y.K. Prognostic value of tumor-infiltrating lymphocyte density assessed using a standardized method based on molecular subtypes and adjuvant chemotherapy in invasive breast cancer. Ann Surg Oncol. 2018;25(4):937–946. doi: 10.1245/s10434-017-6332-2. [DOI] [PubMed] [Google Scholar]
- 21.Cardone R.A., Bellizzi A., Busco G., Weinman E.J., Dell'Aquila M.E., Casavola V., Azzariti A., Mangia A., Paradiso A., Reshkin S.J. The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor cells. Mol Biol Cell. 2007;18(5):1768–1780. doi: 10.1091/mbc.E06-07-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karn T., Pusztai L., Holtrich U., Iwamoto T., Shiang C.Y., Schmidt M., Müller V., Solbach C., Gaetje R., Hanker L. Homogeneous datasets of triple negative breast cancers enable the identification of novel prognostic and predictive signatures. PLoS One. 2011;6(12):e28403. doi: 10.1371/journal.pone.0028403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saponaro C., Vagheggini A., Scarpi E., Centonze M., Catacchio I., Popescu O., Pastena M.I., Giotta F., Silvestris N., Mangia A. NHERF1 and tumor microenvironment: a new scene in invasive breast carcinoma. J Exp Clin Cancer Res. 2018;37(1):96. doi: 10.1186/s13046-018-0766-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leon-Ferre R.A., Polley M.Y., Liu H., Gilbert J.A., Cafourek V., Hillman D.W., Elkhanany A., Akinhanmi M., Lilyquist J., Thomas A. Impact of histopathology, tumor-infiltrating lymphocytes, and adjuvant chemotherapy on prognosis of triple-negative breast cancer. Breast Cancer Res Treat. 2018;167(1):89–99. doi: 10.1007/s10549-017-4499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.