Abstract
Ovarian cancer (OC) is an important cause of gynecologic cancer-related deaths. In Mexico, around 4700 new cases of OC are diagnosed per year and it represents the second cause of gynecological cancer mortality with more than 2700 deaths. Germline mutations in BRCA1/2 genes are present in 13–18% of OC cases. Few studies have evaluated the presence of mutations in BRCA genes in a population of OC Mexican patients and their relationship with clinical response and survival rates.
A total of 179 OC patients were studied by molecular testing for BRCA1/2 through next-generation sequencing and multiplex ligation-dependent probe amplification. Recurrence-free survival (RFS) was estimated by the Kaplan–Meier method. BRCA mutation was detected in 33% of patients. A percentage of 66.1% were BRCA1 mutated and 33.9% were BRCA2 mutated. BRCA1 mutation carriers had a worst RFS compared with BRCA2 mutation carriers (37.6 [29–46.2] vs 72.7 [38.4–107.2]; P = 0.030). The most common mutation for BRCA1 was ex9-12del (28.2%) (Mexican founder mutation). The Mexican founder mutation had a better RFS than other BRCA1 mutations (86.1 [37.2–135.1] vs 34.5 [20.7–48.2]; P = 0.033). The presence of BRCA2 mutations in the ovarian cancer cluster region (OCCR) had a significantly better RFS than mutations in breast cancer cluster regions (BCCR) and not-related risk region (NRR) (NR vs 72.8 [39–106.6] vs 25.8 [8.3–43.2]; P = 0.013). These results demonstrate that the prevalence of BRCA1/2 positive patients in OC Mexican patients are the highest reported. Patients with mutations in BRCA2 have a better prognosis than those mutated in BRCA1. The Mexican founder mutation has an important role in clinical outcomes. These results highlight the importance to test all the HGSP (high-grade serous papillary) OC patients with or without cancer family history (CFH) in Mexican population.
Abbreviations: OC, ovarian cancer; CFH, cancer family history; CS, clinical stage; HGSP, high-grade serous papillary; CBP/TXL, carboplatin/paclitaxel; NGS, next-generation sequencing; OCCR, ovarian cancer cluster region; BCCR, breast cancer cluster region; NRR, not-related risk region; RFS, recurrence-free survival; OS, overall survival; NR, non reached
Introduction
In the world, there are 295,414 new cases and 184,799 ovarian cancer (OC) deaths per year. In Mexico, around 4759 OC new cases are diagnosed and it represents the second cause of gynecological cancer mortality with 2765 deaths (GLOBOCAN 2018).
A family history of breast and ovarian cancer has been associated with an increased probability of a genetic predisposition to these cancers [1]. This risk is increased twice when the history of an affected second-degree relative is present and increased up to four times greater when dealing with a relative in the first degree [2]. A Mexican population study found that less than 10% of OC patients had cancer family history (CFH) [3]. The exploration of familial associations of OC with other cancers suggests that OC shares susceptibility with colorectal, breast, endometrium, liver cancer, and cancer of unknown primary [4]. This evidence suggests that these associations should have implications in genetic counseling. According to previous studies, 13–18% of OC is associated with germline mutations in BRCA1/2 genes [5], [6], [7].
BRCA1/2, also known as breast cancer susceptibility genes 1 and 2, are tumor suppressor genes. More than one thousand mutations have been described and may be inherited in an autosomal dominant manner [8]. BRCA1 is a DNA damage response protein and works in both checkpoint activation and DNA repair. BRCA2 is a mediator of homologous recombination [9], [10]. These are essential activities to prevent tumor development.
A study in Mexican OC patients found a prevalence of 28% of mutations in BRCA1/2 genes [3].
In some specific populations such as Ashkenazi Jews, there is a higher prevalence of mutations in the BRCA1/2 genes, which in turn increases the risk by 65% for breast cancer and 40% for ovarian cancer. This risk is because of the presence of three founder mutations (BRCA1 185delAG, BRCA1 538insC, and BRCA2 6174delT). Around 2.5% of that population carries at least one of those mutations [11], [12].
There are five OC histological subtypes: high-grade serous papillary (HGSP), endometrioid, mucinous, clear cells, and low-grade serous papillary (LGSP); BRCA1/2 mutation is more commonly associated with the HGSP subtype. Patients carrying mutations in BRCA have a better response to treatment and are less likely to have a progression of the disease within six months after the end of primary therapy compared with those who do not have the mutation (14.9% vs 31.7%; P < 0.0001) [5].
There are currently few studies that assessed the presence of BRCA mutations in the Mexican population. The purpose of this study was to corroborate, in a population of Mexican OC patients, the presence of germline mutations in the BRCA1/2 genes and their correlation with clinicopathological characteristics, clinical response, and survival rates.
Material and Methods
Study Design
A total of 179 patients with OC in clinical stages (CS) IA to IVB were enrolled from October 2015 to August 2017 at the National Institute of Cancer in Mexico. All patients provided written informed consent before entering the study. Inclusion criteria were adult patients (>18 years), diagnosed with OC with a histopathological confirmation. Clinicopathological characteristics (age, sex, born city, CFH), medical treatment, and clinical outcomes of patients were recorded. Patients received pre- and posttest genetic counseling, according to international recommendations, as well as follow-up by geneticists. Cascade screening for relatives was also provided.
Genetic Testing for BRCA Mutations
Samples of 16 mL of blood from each patient were obtained and drawn into two 8 mL EDTA tubes (BD Biosciences). The next-generation sequencing (NGS) that interrogates all coding regions and up to 50 bases in each intronic region to detect small mutations in the BRCA1/2 genes was carried out at the clinical laboratory (Quest Diagnostics, US). To identify exon deletions and duplications, named large rearrangements of BRCA1/2, a multiplex ligation-dependent probe amplification (MLPA) was used. The mutation status was correlated with the standard clinicopathological characteristics of the patients. The analytical sensitivity was >99% of relevant mutations occurring in the described regions.
The clinical significance of the variants was determined according to the guidelines established by the international consortium ENIGMA (evidence-based network for the interpretation of germline mutant alleles) and ClinVar database which was provided by the Quest Diagnostics laboratory.
Statistical Analyses
Continuous variables were tabulated as medians with ranges, or as means with standard deviations, depending on data's distribution. The distribution was assessed using the Shapiro–Wilk test with a P-value higher than 0.05 considered as normally distributed. Two groups' comparisons were tested using Student's T-test or Mann–Whitney U. Nominal data were analyzed using the chi-square (χ2) test. RFS was calculated as the difference between the date of recurrence or the last follow-up and the beginning of surveillance of first-line treatment. RFS curves were estimated by the Kaplan–Meier method, whereas comparisons among groups were analyzed with log-rank or Breslow tests. Statistically significant and borderline variables (P ≤ 0.05) were included in the multivariate analyses. All data were analyzed using the SPSS software package version 23 (SPSS, Inc., Chicago, IL, US).
Results
Total Population Characteristics
A total of 179 OC patients were studied. Median patients' age was 48 years, with the majority between 35 and 54 years old. We tested patients from 19 out of 32 states in our country. Positive CFH was reported in 63.7% of patients, and the most common familial cancer type reported was breast cancer (51.8%). The most common histological subtype and CS were HGSP and IIIC, respectively (69.3% and 45.8%). Twenty-four patients (13.4%) had a double primary malignancy, the most frequent being breast/ovarian (79.2%). The most common chemotherapy schedule administrated as a primary treatment was carboplatin and paclitaxel every three weeks (87.7%). Rates of complete response, partial response, stable disease, and progression of the disease with primary treatment were 57%, 27.4%; 5.6%, and 10.1%, respectively (Table 1).
Table 1.
Baseline and Clinical Characteristics of Ovarian Cancer Patients
| Characteristics | Total |
|---|---|
| Age (years) at diagnosis | |
| Median (range) | 48 (18–76) |
| Mean ± SD | 49.4 ± 10.6 |
| Group of age at diagnosis | |
| ≤34 | 4.5 (8/179) |
| 35–44 | 32.4 (58/179) |
| 45–54 | 32.4 (58/179) |
| 55–64 | 21.2 (38/179) |
| ≥65 | 9.5 (17/179) |
| Born city | |
| Aguascalientes | 0.6 (1/179) |
| CDMX | 38 (68/179) |
| Chihuahua | 0.6 (1/179) |
| Coahuila | 0.6 (1/179) |
| Guanajuato | 1.1 (2/179) |
| Guerrero | 1.7 (3/179) |
| Hidalgo | 8.9 (16/179) |
| Jalisco | 1.1 (2/179) |
| México | 24.6 (44/179) |
| Michoacán | 4 (7/179) |
| Morelos | 2.8 (5/179) |
| Oaxaca | 1.7 (3/179) |
| Puebla | 4.5 (8/179) |
| Querétaro | 2.2 (4/179) |
| San Luis Potosí | 1.1 (2/179) |
| Tabasco | 0.6 (1/179) |
| Tlaxcala | 3.4 (6/179) |
| Veracruz | 2.2 (4/179) |
| Zacatecas | 0.6 (1/179) |
| CFH | |
| Negative | 36.3 (65/179) |
| Positive | 63.7 (114/179) |
| Type of CFH | |
| Breast | 51.8 (59/114) |
| Prostate | 19.3 (22/114) |
| Ovarian | 13.2 (15/114) |
| Melanoma | 9.6 (11/114) |
| Pancreas | 6.1 (7/114) |
| Endometrium | 3.5 (4/114) |
| Number of related cancer associated to BRCA | |
| Not associated | 26.3 (30/114) |
| Associated | 73.7 (84/114) |
| 1 | 64.3 (54/84) |
| 2 | 29.8 (25/84) |
| 3 | 6 (5/84) |
| Clinical stage at diagnosis | |
| IA | 3.9 (7/179) |
| IC | 7.8 (14/179) |
| IIA/B | 7.7 (3/179) |
| IIIA | 3.4 (6/179) |
| IIIB | 8.4 (15/179) |
| IIIC | 45.8 (82/179) |
| IVA | 11.7 (21/179) |
| IVB | 17.3 (31/179) |
| Histological subtypes | |
| Clear cells | 4.5 (8/179) |
| HGSP | 69.3 (124/179) |
| LGSP | 5.6 (10/179) |
| Mucinous | 0.6 (1/179) |
| Endometroid | 12.8 (23/179) |
| G1 | 17.4 (4/23) |
| G2 | 69.6 (16/23) |
| G3 | 13 (3/23) |
| Adenocarcinoma | 2.8 (5/179) |
| Mixed | 4.5 (8/179) |
| HGSP/endometroid | 62.5 (5/8) |
| HGSP/clear cells | 12.5 (1/8) |
| Endometroid/clear cells | 12.5 (1/8) |
| Endometroid/HGSP | 12.5 (1/8) |
| Double primary malignancy | |
| Negative | 86.6 (155/179) |
| Positive | 13.4 (24/179) |
| Breast/ovarian | 79.2 (19/24) |
| Endometrium/ovarian | 20.8 (5/24) |
| 1° Line treatment | |
| CBP/TXL per week | 7.8 (14/179) |
| CBP/TXL 3 weeks | 87.7 (157/179) |
| Other | 7.8 (14/179) |
| Line of treatment | |
| 1° Line | 38 (68/179) |
| 2° Line | 27.9 (50/179) |
| ≥3 | 34.1 (61/179) |
| Treatment response at 1° line of treatment | |
| CR | 57 (102/179) |
| PR | 27.4 (49/179) |
| SD | 5.6 (10/179) |
| PD | 10.1 (18/179) |
| Platinum-based therapy | |
| Platinum sensitive | 91.6 (164/179) |
| Platinum resistant | 8.4 (15/179) |
CFH = cancer family history.
Presence of Germinal BRCA Mutations
BRCA mutations were detected in 33% of the patients (59/179); 66.1% (39/59) were BRCA1, and 33.9% (20/59) were BRCA2 mutations. The most common mutation for BRCA1 carrier patients was BRCA1 ex9-12del (Mexican founder mutation), which was found in 28% (11/39) of cases. Among BRCA2 mutations, c.8168 A > G was the most prevalent in 25% (5/20) of cases (Table 2).
Table 2.
BRCA Status of Ovarian Cancer Patients
| BRCA status | % (N) | |
|---|---|---|
| Wild-type | 67 (120/179) | |
| Mutated | 33 (59/179) | |
| BRCA1 | 66.1 (39/59) | |
| BRCA2 |
33.9 (20/59) |
|
| Mutation |
% (N) |
Location |
| BRCA1 | ||
| 1) ex9-12del (Mexican Founder Mutation) | 28.2 (11/39) | OCCR |
| 2) c.2806–2809 del GATA | 5.1 (2/39) | |
| 3) c.1860 del T | 5.1 (2/39) | |
| 4) c.1723 dup G | 2.6 (1/39) | |
| 5) c.1961 del A | 2.6 (1/39) | |
| 6) c.2101 A > T | 2.6 (1/39) | |
| 7) c.2551 G > T | 2.6 (1/39) | |
| 8) c.3598 C > T | 2.6 (1/39) | |
| 9) c.3648 dup A | 2.6 (1/39) | |
| 10) c.3858–3861 del TGAG | 2.6 (1/39) | |
| 11) c.4868 C > G | 5.1 (2/39) | BCCR |
| 12) c.211 A > G | 2.6 (1/39) | |
| 13) c.5353 C > T | 2.6 (1/39) | |
| 14) exon 18–19 del | 5.1 (2/39) | |
| 15) c.4327 C > T | 7.7 (3/39) | NRR |
| 16) c.798–799 del TT | 5.1 (2/39) | |
| 17) c.4976 del C | 2.6 (1/39) | |
| 18) c.68–69 del AG | 2.6 (1/39) | |
| BRCA2 | ||
| 19) c.4325 C > A | 10 (2/20) | OCCR |
| 20) c.5116–5119 del AATA | 10 (2/20) | |
| 21) c.4749–4750 del AG | 5 (1/20) | |
| 22) c.5542 del A | 5 (1/20) | |
| 23) c.5616–5620 del AGTAA | 5 (1/20) | |
| 24) c.5631 del C | 5 (1/20) | |
| 25) c.8168 A > G | 25 (5/20) | BCCR |
| 26) c.1796–1800 del CTTAT | 10 (2/20) | |
OCCR = ovarian cancer cluster region; BCCR = breast cancer cluster region; NRR = not-related risk region.
Location of BRCA1 and BRCA2 Mutations
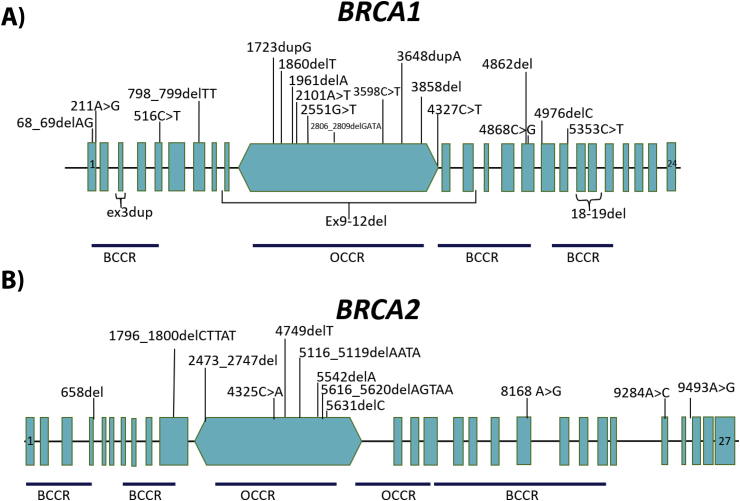
A total of 34 variants or mutations were detected in the BRCA genes, 22 mutations in BRCA1, and 12 in BRCA2. The majority of the mutations (74%) were located in the areas known as the ovarian cancer cluster regions (OCCR) and breast cancer cluster regions (BCCR) in both genes (BRCA1/2). Among BRCA1 gene mutations, 22 (56.5%) were in the OCCR region, 9 (23%) in the BCCR region, and 8 (20.5%) in the not-related risk region (NRR) (Table 2, Figure 1A). For BRCA2 gene mutations, locations in the OCCR, BCCR, and NRR regions were 8 (40%), 7 (35%), and 5 (25%) (Table 2, Figure 1B).
Figure 1.
Location of 34 mutations detected inBRCA1/2genes (BRCA exchange database and verified in the ClinVar database). (A) Location of 22 reported mutations in BRCA1 gene. (B) Location of the 12 mutations in BRCA2 gene. OCCR, ovarian cancer cluster region; BCCR, breast cancer cluster region; NRR, not-related risk region.
Clinical Significance of Mutations
Genetic variants of BRCA1/2 are classified according to the possibility of increasing the risk of developing cancer. Among 22 BRCA1 mutation types detected, 81.8% (18/22) were pathogenic and affected 32 of 39 patients (Table 2), and 18.1% (4/22) were not yet reviewed variants and affected 7 out of 39 patients (Supplementary Table 1).
With respect to BRCA2, 12 different mutations types were detected, 66.7% (8/12) were pathogenic mutations and affected 15 of 20 patients (Table 2), and 33.3% (4/12) were not yet reviewed variants and affected 5 of 20 patients (Supplementary Table 1).
Correlation Analysis Between Clinicopathological Characteristics and BRCA Mutations
For BRCA mutations carriers, median age was 50 years (range = 27–73). The most common state of birth of BRCA mutations carriers detected was Mexico City (30.5%), followed by the Estado de Mexico (27.1%), Oaxaca and Puebla (5.1%); P = 0.034) (Table 3).
Table 3.
Bivariate Analysis of Clinical Characteristics of Ovarian Cancer Patients with BRCA Status
| Characteristics | BRCA − |
BRCA + |
P |
|---|---|---|---|
| % (N) | % (N) | ||
| Age (years) at DX | |||
| Median (range) | 47 (18–76) | 50 (27–73) | 0.467 |
| Mean ± SD | 49.1 ± 11.3 | 49.9 ± 9.1 | 0.583 |
| Group of age at DX | 0.041 | ||
| ≤34 | 5.8 (7/120) | 1.7 (1/59) | |
| 35–44 | 35.8 (43/120) | 25.4 (15/59) | |
| 45–54 | 25 (30/120) | 47.5 (28/59) | |
| 55–64 | 22.5 (27/120) | 18.6 (11/59) | |
| ≥65 | 10.8 (13/120) | 6.8 (4/59) | |
| Born city | 0.034 | ||
| Aguascalientes | 0 (0/120) | 1.7 (1/59) | |
| CDMX | 41.7 (50/120) | 30.5 (18/59) | |
| Chihuahua | 0.8 (1/120) | 0 (0/59) | |
| Coahuila | 0 (0/120) | 1.7 (1/59) | |
| Guanajuato | 0 (0/120) | 3.4 (2/59) | |
| Guerrero | 0.8 (1/120) | 3.4 (2/59) | |
| Hidalgo | 11.7 (14/120) | 3.4 (2/59) | |
| Jalisco | 1.7 (2/120) | 0 (0/59) | |
| México | 23.3 (28/120) | 27.1 (16/59) | |
| Michoacán | 5 (6/120) | 1.7 (1/59) | |
| Morelos | 2.5 (3/120) | 3.4 (2/59) | |
| Oaxaca | 0 (0/120) | 5.1 (3/59) | |
| Puebla | 4.2 (5/120) | 5.1 (3/59) | |
| Querétaro | 1.7 (2/120) | 3.4 (2/59) | |
| San Luis Potosí | 0 (0/120) | 3.4 (2/59) | |
| Tabasco | 0 (0/120) | 1.7 (1/59) | |
| Tlaxcala | 3.3 (4/120) | 3.4 (2/59) | |
| Veracruz | 2.5 (3/120) | 1.7 (1/59) | |
| Zacatecas | 0.8 (1/120) | 0 (0/59) | |
| Patients with CFH | 0.034 | ||
| Negative | 41.7 (50/120) | 25.4 (15/59) | |
| Positive | 58.3 (70/120) | 74.6 (44/59) | |
| Patients with CFH associated with BRCA | 0.015 | ||
| No associated | 34.3 (24/70) | 13.6 (6/44) | |
| Associated | 65.7 (46/70) | 86.4 (38/44) | |
| Type of cancer reported in CFH patients | |||
| Breast | 37.1 (26/70) | 75 (33/44) | <0.0001 |
| Prostate | 22.9 (16/70) | 13.6 (6/44) | 0.225 |
| Ovarian | 8.6 (6/70) | 20.5 (9/44) | 0.068 |
| Melanoma | 10 (7/70) | 9.1 (4/44) | 0.873 |
| Pancreas | 8.6 (6/70) | 2.3 (1/44) | 0.173 |
| Endometrium | 4.3 (3/70) | 2.3 (1/44) | 0.570 |
| 1 | 42.9 (30/70) | 54.5 (24/44) | |
| 2 | 18.6 (13/70) | 27.3 (12/44) | |
| 3 | 4.3 (3/70) | 4.5 (2/44) | |
| Clinical stage at Dx | |||
| IA | 5 (6/120) | 1.7 (1/59) | 0.123 |
| IC | 10 (12/120) | 3.4 (2/59) | |
| IIA | 0.8 (1/120) | 0 (0/59) | |
| IIB | 0 (0/120) | 3.4 (2/59) | |
| IIIA | 5 (6/120) | 0 (0/59) | |
| IIIB | 9.2 (11/120) | 6.8 (4/59) | |
| IIIC | 44.2 (53/120) | 49.2 (29/59) | |
| IVA | 10 (12/120) | 15.3 (9/59) | |
| IVB | 15.8 (19/120) | 20.3 (12/59) | |
| Histological subtype | |||
| Clear cells | 5 (6/120) | 3.4 (2/59) | 0.111 |
| HGSP | 64.2 (77/120) | 79.7 (47/59) | |
| LGSP | 7.5 (9/120) | 1.7 (1/59) | |
| Mucinous | 0.8 (1/120) | 0 (0/59) | |
| Endometroid | 16.7 (20/120) | 5.1 (3/59) | |
| G1 | 15 (3/20) | 33.3 (1/3) | |
| G2 | 70 (14/20) | 66.7 (2/3) | |
| G3 | 15 (3/20) | 0 (0/3) | |
| Adenocarcinoma | 1.7 (2/120) | 5.1 (3/59) | |
| Mixed | 4.2 (5/8) | 5.1 (3/8) | |
| HGSP/endometroid | 40 (2/5) | 100 (3/3) | |
| HGSP/clear cells | 20 (1/5) | 0 (0/3) | |
| Endometroid/clear cells | 20 (1/5) | 0 (0/3) | |
| Endometroid/HGSP | 20 (1/5) | 0 (0/3) | |
| Double primary malignancy | |||
| Negative | 91.7 (110/120) | 76.3 (45/59) | 0.004 |
| Positive | 8.3 (10/120) | 23.7 (14/59) | |
| Breast/ovarian | 50 (5/10) | 100 (14/14) | 0.003 |
| Endometrium/ovarian | 50 (5/10) | 0 (0/14) | |
| 1° line treatment | |||
| CBP/TXL per week | 8.3 (10/120) | 6.8 (4/59) | 0.410 |
| CBP/TXL 3 weeks | 85.8 (103/120) | 91.5 (54/59) | |
| Other | 5.8 (7/120) | 1.7 (1/59) | |
| Lines of treatment | |||
| 1° Line | 40 (48/120) | 33.9 (20/59) | 0.256 |
| 2° Line | 30 (36/120) | 23.7 (14/59) | |
| ≥3° Line | 30 (36/120) | 42.4 (25/59) | |
| Treatment response at 1° line of Tx | |||
| CR | 58.3 (70/120) | 61 (36/59) | 0.375 |
| PR | 25.8 (31/120) | 30.5 (18/59) | |
| SD | 15.8 (19/120) | 8.5 (5/59) | |
| Platinum-based therapy | |||
| Platinum sensitive | 93.3 (112/120) | 88.1 (52/59) | 0.238 |
| Platinum resistant | 6.7 (8/120) | 11.9 (7/59) | |
CFH = cancer family history.
Almost 75% of the patients with BRCA mutations reported CFH in at least one relative (P = 0.034). The most frequently reported CFH type associated with BRCA mutation was breast (P < 0.0001) and we only found a trend for the presence of OC CFH (P = 0.068). Fourteen BRCA positive mutated patient (23.7%) had double primary malignancy (P = 0.004), and all of them were breast/ovarian (P = 0.003) (Table 3).
Clinicopathological Characteristics Associated with BRCA1/2 Mutations
According to BRCA mutations types, BRCA1 mutations were more commonly detected in younger patients than in those with BRCA2 mutations (median age, 46 vs 54 years; P = 0.001) (Supplementary Table 1). The clinicopathological characteristics according to the BRCA1 mutation subtypes (Mexican founder mutation vs other BRCA1 mutations) analysis showed that all patients with the Mexican founder mutation (11/39) had CFH (P = 0.047) (Supplementary Table 2).
Association of Clinical Characteristics of the Whole Study Population and Recurrence-free Survival
At the time of data cutoff, 123 patients (68.7%) had recurrence disease and 56 patients (31.3%) had no recurrence (both groups were ultimately included in the analysis). The mean follow-up was 41 months (SD 29.7 months) and the median of RFS was 47.7 months [95% CI 40.4–55] for all the patients. Patients with clinical stage I–II had better RFS compared with those with stages IIIA–B, IIIC, IV (96.9 vs 46.3 vs 43.4 vs 41.8 months; P = 0.001). Patients with endometroid histological subtype also had better RFS compared with those with HGSP, clear cell and LGSP (91.8 vs 37.2 vs 40.3 vs 33.8 months; P = 0.004) (Table 4).
Table 4.
Clinical Characteristics Associated Factors with Recurrence-free Survival
| Variable | Total (N = 179) |
BRCA (−) (N = 120) |
BRCA (+) (N = 59) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 95% Confidence interval |
P | Median | 95% Confidence interval |
P | Median | 95% Confidence interval |
P | ||||
| Lower bound | Upper bound | Lower bound | Upper bound | Lower bound | Upper bound | |||||||
| RFS (months) | 47.7 | 40.4 | 55.0 | 49.2 | 40.6 | 57.8 | 43.7 | 34.8 | 52.6 | |||
| Age (years) | 0.970 | 0.818 | 0.873 | |||||||||
| ≤48 | 46.3 | 34.2 | 58.4 | 46.3 | 31.2 | 61.4 | 46.5 | 20.1 | 72.9 | |||
| >48 | 48.1 | 39.9 | 56.2 | 51.4 | 30.9 | 72.0 | 41.8 | 33.1 | 50.5 | |||
| Group of age at DX | <0.0001 | <0.0001 | <0.0001 | |||||||||
| ≤34 | 14.7 | 7.4 | 22.1 | 16.3 | 12.3 | 20.2 | – | – | – | |||
| 35–44 | 51.7 | 42.5 | 61.0 | 51.8 | 31.7 | 72.0 | 46.5 | 20.5 | 72.5 | |||
| 45–54 | 49.0 | 29.1 | 68.9 | 57.7 | 34.6 | 80.8 | 47.7 | 34.7 | 60.7 | |||
| 55–64 | 61.4 | 32.3 | 90.4 | 51.4 | 25.0 | 77.9 | 37.2 | 0.0 | 85.1 | |||
| ≥65 | 43.7 | 20.7 | 66.7 | 30.4 | 22.8 | 69.2 | 56.6 | 36.0 | 77.2 | |||
| Clinical stage at Dx | 0.001 | 0.001 | 0.199 | |||||||||
| I & II | 96.9 | 86.6 | 107.3 | 96.9 | 86.5 | 107.3 | NR | NR | NR | |||
| III (A & B) | 46.3 | 21.2 | 71.4 | 38.4 | 16.8 | 60.0 | 59.6 | 5.4 | 113.8 | |||
| IIIC | 43.4 | 28.5 | 58.2 | 50.8 | 37.7 | 63.9 | 37.2 | 23.2 | 51.1 | |||
| IV | 41.8 | 23.9 | 59.6 | 28.5 | 8.2 | 48.9 | 43.7 | 34.4 | 49.2 | |||
| Histology | 0.004 | 0.004 | <0.0001 | |||||||||
| Clear cells | 40.3 | 26.7 | 53.8 | 40.3 | 26.5 | 54.1 | – | – | – | |||
| HGSP | 37.2 | 27.0 | 47.3 | 33.4 | 19.4 | 47.5 | 40.1 | 30.7 | 49.5 | |||
| LGSP | 33.8 | 28.5 | 84.6 | 62.5 | 16.3 | 132.3 | – | – | – | |||
| Endometroid | 91.8 | 50.7 | 132.8 | 96.9 | 86.2 | 107.6 | – | – | – | |||
| Adenocarcinoma | 88.1 | 69.7 | 106.5 | NR | NR | NR | 79.5 | 29.2 | 129.8 | |||
| Mixed | 54.5 | 33.6 | 75.4 | 102.9 | 43.6 | 110.9 | 54.5 | 37.2 | 71.9 | |||
| Double primary malignancy | 0.887 | 0.720 | 0.897 | |||||||||
| Negative | 47.7 | 40.4 | 54.9 | 49.2 | 41.4 | 57.0 | 43.7 | 35.0 | 52.3 | |||
| Positive | 80.6 | 11.6 | 149.5 | NR | NR | NR | 34.5 | 29.2 | 96.0 | |||
| Breast/ovarian | 34.5 | 4.7 | 93.5 | 0.868 | NR | NR | NR | – | 34.5 | 29.2 | 96.0 | – |
| Endometrium/ovarian | NR | NR | NR | NR | NR | NR | – | – | – | |||
| 1° line treatment | 0.793 | 0.875 | 0.937 | |||||||||
| CBP/TXL per week | 42.8 | 32.5 | 53.1 | 42.8 | 3.4 | 49.4 | 40.1 | 9.2 | 71.0 | |||
| CBP/TXL 3 weeks | 46.5 | 37.5 | 55.4 | 49.2 | 6.3 | 61.6 | 43.7 | 32.3 | 55.1 | |||
| Other | 61.4 | 9.2 | 113.5 | 61.4 | 26.6 | 113.5 | – | – | – | |||
| Line of treatment | 0.043 | 0.023 | 0.659 | |||||||||
| 1° Line | 62.3 | 49.8 | 74.9 | 62.5 | 48.6 | 76.4 | 34.5 | 21.0 | 48.0 | |||
| 2° Line | 47.7 | 37.9 | 57.4 | 46.3 | 23.0 | 69.6 | 43.4 | 20.9 | 65.9 | |||
| ≥3° Line | 37.6 | 30.02 | 45.21 | 33.8 | 20.8 | 46.8 | 47.7 | 30.0 | 65.4 | |||
CHF = cancer family history.
RFS Analysis According to BRCA1/2 Status
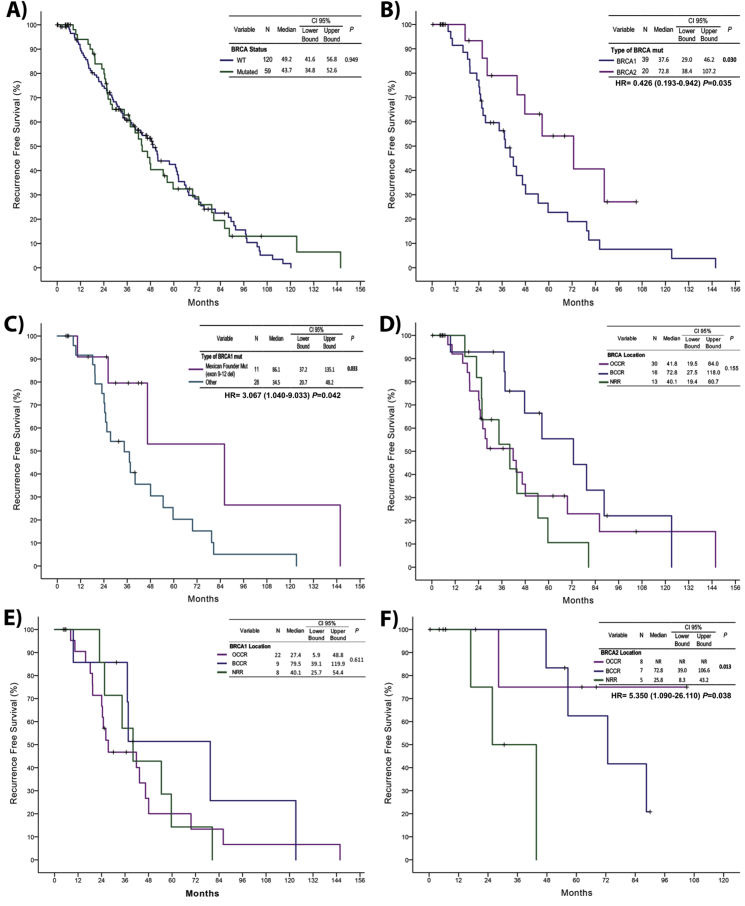
In BRCA1/2 mutated patients (59/179), there was a trend toward a better RFS in those patients without CFH compared with those with CFH (56.6 [44.1–69.1] vs 37.6 [28.4–46.9]; P = 0.096). There was no significant difference in RFS between BRCA mutated carriers and no mutation carriers (wild-type patients) (P = 0.949) (Table 5, Figure 2A), but BRCA1 mutated patients group did show a worse RFS than its counterpart of patients with BRCA2 mutation (37.6 [29–46.2] vs 72.8 [38.4–107.2]; P = 0.030) (Table 5, Figure 2B). Particularly, in the BRCA1 patient subpopulation, the specific mutation ex9-12del (Mexican founder mutation) showed a better RFS than those with other types of BRCA1 mutations (86.1 [37.2–135.1] vs 34.5 [20.7–48.2]; P = 0.033) (Figure 2C).
Table 5.
BRCA Mutations Associated Factors with Recurrence-free Survival
| Variable | N | Median | 95% Confidence interval |
P | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| RFS (months) | 179 | 47.7 | 40.4 | 55.0 | |
| BRCA status | 0.949 | ||||
| WT | 120 | 49.2 | 41.59 | 56.8 | |
| Mutated | 59 | 43.7 | 34.8 | 52.6 | |
| Type of BRCA mut | 0.030 | ||||
| BRCA1 | 39 | 37.6 | 29.0 | 46.2 | |
| BRCA2 | 20 | 72.77 | 38.37 | 107.17 | |
| BRCA location | 0.080 | ||||
| OCCR | 30 | 41.8 | 19.5 | 64.0 | |
| BCCR | 16 | 72.8 | 27.5 | 118.1 | |
| NRR | 13 | 40.1 | 19.4 | 60.7 | |
| Type of BRCA1 mut | 0.033 | ||||
| Endemic (exon 9–12 del) | 11 | 86.1 | 37.2 | 135.1 | |
| Other | 28 | 34.5 | 20.7 | 48.2 | |
| Type of BRCA2 mut | 0.442 | ||||
| c.8168 A > G | 5 | 72.8 | 51.9 | 93.6 | |
| Other | 15 | 62.3 | 19.2 | 105.3 | |
| BRCA1 location | 0.584 | ||||
| OCCR | 22 | 27.4 | 4.4 | 50.5 | |
| BCCR | 9 | 79.5 | 39.1 | 119.9 | |
| NRR | 8 | 40.1 | 25.7 | 54.4 | |
| BRCA2 location | 0.013 | ||||
| OCCR | 8 | NR | NR | NR | |
| BCCR | 7 | 72.8 | 39.0 | 106.6 | |
| NRR | 5 | 25.8 | 8.3 | 43.2 | |
OCCR = ovarian cancer cluster region; BCCR = breast cancer cluster region; NRR = not-related risk.
Figure 2.
Impact of BRCA mutations on recurrence-free survival of Mexican ovarian cancer patients. (A) RFS of patients with BRCA wild-type (blue line) vs mutated BRCA (green line) (49.2 [41.6–56.8] vs 43.7 [34.8–52.6] P = 0.949). (B) RFS compared between BRCA1 mutations carriers (blue line) vs BRCA2 mutations carriers (green line) (37.6 [29–46.2] vs 72.8 [38.4–107.2]; P = 0.030). (C) BRCA1 mutation carriers, comparing survival between carriers of the Mexican founder mutation (ex9-12del) (purple line) vs other mutations in BRCA1 (blue line) (86.1 [37.2–135.1] vs 12.0, 95% C.I. [11.7–12.3]; P = 0.033). (D) RFS comparison in BRCA OCCR (violet line), BCCR (purple line), and NRR (green line) (41.6 [19.5–64.0] vs 72.8 [27.5–118.0] vs 40.1 [19.4–60.7]; P = 0.155). (E) RFS comparison of BRCA1 locations: OCCR (violet line), BCCR (purple line) and NRR (green line) (27.4 [5.9–48.8] vs 79.5 [39.1–119.9] vs 40.1 [25.7–54.4]; P = 0.611). (F) RFS BRCA2 locations: OCCR (violet line), BCCR (purple line) and NRR (green line); (NR vs 72.8 [39–106.6] vs 25.8 [8.3–43.2]; P = 0.013). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
RFS Depending on the Location of BRCA1/2 Mutation
The analysis considering the location of the mutations globally in the OCCR, BCCR and NRR regions, showed a superior benefit on RFS for those mutations located in BCCR (41.6 [19.5–64.0] vs 72.8 [27.5–118.0] vs 40.1 [19.4–60.7]; P = 0.155; Figure 1D). RFS analysis of BRCA1 mutated subpopulation when classified by mutation location (OCCR, BCCR, NRR) do not shown differences (27.4 [5.9–48.8] vs 79.5 [39.1–119.9] vs 40.1 [25.7–54.4]; P = 0.611) (Table 5, Figure 2E). In contrast, the RFS analysis of mutation locations for BRCA2 has shown a better RFS to OCCR than those with mutations in BCCR and NRR (NR vs 72.8 [39–106.6] vs 25.8 [8.3–43.2]; P = 0.013) (Table 5, Figure 2F).
Multivariate analysis showed that BRCA2-mutated OC patients are likely to have better RFS than those with BRCA1 mutations (HR = 0.426; P = 0.035) and patients with a BRCA1 mutation other than ex9-12del (Mexican founder mutation) have a higher recurrence risk (HR = 3.07; P = 0.042).
Discussion
BRCA genes are important elements in the suppression of tumors [13]. In neoplasms such as breast and ovarian cancer, mutation of BRCA1/2 genes is linked to the hereditary development of diseases [14]. Analysis of BRCA status has quickly become a standard clinical test in OC in developed countries. Benefit is being reflected in risk determination and appropriate treatment decisions [15]. The NCCN has concentrated a series of guidelines to be used in the assessment and evaluation of genetic risk. The Society of Gynecologic Oncology (SGO) and the American College of Obstetricians and Gynecologists have joined in a consensus statement regarding genetic counseling as well [16]. According to these guidelines, all women diagnosed with OC are advised to receive genetic counseling and testing for germline BRCA mutations.
Integrated models of close collaborations between geneticists and oncologists has allowed the exploration of BRCA and other OC-associated genes [17], but despite the clinical importance of germline BRCA mutations, widespread use of genetic testing is still low in some regions of the world.
This work is the largest analysis of BRCA genes status in a Mexican OC population.
Frequency of BRCA1/2 mutations was considerably higher than previously reported by studies in other predominantly Caucasian population studies [7]. In the same way, our study reported a higher frequency of BRCA mutations carriers than a cohort of Hispanic cancer patients with breast and ovarian cancer [3].
In our study, most of the mutations were located in the central regions of both genes. The OCCR and BCCR regions were characterized as risk regions for developing ovarian and breast cancer, respectively. For the BRCA1 gene, it was determined that the mutations that occurred between exons 1–11, and particularly in the central region of exon 11, conferred an elevated risk of OC compared with the mutations present in exons 12–24. It was also observed that mutations in the OCCR region decreased the risk of breast cancer and increased the risk of OC [18], [19]. Similarly, for the BRCA2 gene, it was observed that the mutations in exon 11 confer an increased risk of OC compared with a lower risk of breast cancer [19], [20]. The advance in the study of mutations in the BRCA genes has made it possible to elucidate new regions OCCR and BCCR, which amplifies the inclusion of mutations in new regions now proven to contribute to the risk of OC and breast cancer [21].
Among BRCA1 mutations, those located in OCCR regions represented a higher percentage than mutations in BCCR and mutations in NRR regions. For BRCA2, half of the mutations were located in OCCR regions; however, mutations in BCCR occurred less frequently than mutations in NRR regions. It should be mentioned that in the present work were found OCCR and BCCR regions mutations that were not previously reported, which indicates that these mutations can be part of the characteristic mutations of carrier patients with OC.
The possible effect that a certain mutation may have on the risk of cancer is of clinical significance. Among the mutations detected in our study, we observed the predominance of pathogenic variants in both BRCA1/2. This finding represents more than double than the observed in a study of 333 nonselected cases of OC in a Polish population [22]. Another study that included 158 nonselected Brazilian OC patients identified a proportion of BRCA1 pathogenic variants similar to our study [23].
Almost 75% of the patients with mutations in a BRCA gene had a CFH and most of these cases were related to breast cancer. This data could serve to support the recommendation of providing genetic counseling to OC patients. The risk of OC in BRCA1/2 mutation carriers considering the position of the mutation has been previously analyzed. In some studies, mutations in OCCR and BCCR were found to be associated with a higher incidence of OC compared with the incidence of breast cancer [19]; in addition, it has been found a slightly lower association between CFH and the risk of OC compared with the correlation analysis performed independently of the cluster regions for OCCR [24].
Several studies have reported that the presence of mutations in BRCA genes correlates with better survival in patients with OC [5], [25]. This finding may correspond to a better response to chemotherapy treatment [26], [27], which becomes more successful because of deficiency in the mechanisms of damage repair to DNA in which BRCA1/2 are involved [28], [29], [30].
Our results do not show a significant difference in survival analyses between BRCA mutated and wild-type carriers. In this context, a study of 1421 OC patients showed an initial survival advantage among BRCA mutation carriers, but this response did not predict long-term (10 years) survival) [31]. Also, another study that analyzed the survival of OC patients carrying germline mutations in the BRCA1/2 genes compared with survival of patients with strong family history for breast or ovarian cancer and with a negative genetic testing for BRCA mutation showed no survival advantage for BRCA mutation carrier patients [32].
In our study population, the RFS analysis between BRCA1 mutation and BRCA2 mutation carriers detected a better prognosis for those with the BRCA2 mutation. This finding is consistent with previous reports [33].
Finally, we observed a BRCA1 ex9-12del prevalence of 28.2% (11/39) as well as an association with a better RFS compared with RFS of other mutations in BRCA1. The Mexican founder mutation BRCA1 ex9-12del was first detected in 2013, in a study that sought to analyze the prevalence and types of mutations in BRCA. In that study, 46 Hispanic patients with a personal or familial history of ovarian and breast cancer were included. A subpopulation of 492 breast cancer patients was analyzed to characterize the large rearrangements of BRCA1 ex9-12del. This analysis found that this mutation accounted for 10–12% of all mutations in BRCA1 [33]. On the other hand, a study by Garza-Villarreal included 92 patients with ovarian cancer found that the prevalence of Mexican founder mutation was 35% (considering all BRCA mutated OC patients) [3]. The difference in the prevalence of the BRCA1 ex9-12del mutation compared with the reported in our study (39.1% vs 28.2%) may be because of the methodological characteristics of both studies. The association of BRCA1 ex9-12del mutation with a better RFS may be because of the biological effect of the deletion, which causes the loss of a vast extension of thousands of base pairs of nucleotides in the BRCA1 gene [34], generating a truncated and less functional form of the protein compared with those generated by other mutations that give rise to not so radical changes [34]. The association of Mexican founder mutation and better RFS rate could result in a very useful tool in genetic counseling to predict the prognosis of OC in patients with this specific mutation.
In conclusion, this study reports the highest prevalence of BRCA1/2 mutations in an OC patient population. Patients with mutations in BRCA2 have a better prognosis than those mutated in BRCA1. The Mexican founder mutation, BRCA1 ex9-12del, has an important role in the clinical outcomes. These results highlight the importance to test all the OC patients with CFH and HGSP histology in to integrate into the national health system as a diagnostic test.
Conflict of Interest
DGR, RMAG, and GAG have participated in Speakers' Bureau to AstraZeneca. DGR also has participated as speaker with Roche and Lilly. The other authors have no conflicts of interest to declare.
Acknowledgments/Funding Source
The authors sincerely thank Paulina Maria Núñez-Martinez for the revision and comments made to this manuscript, and they also thank Ernesto González-Ibarra for all his technical support. Astra Zeneca supported this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2019.11.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jones M.R., Kamara D., Karlan B.Y., Pharoah P.D.P., Gayther S.A. Genetic epidemiology of ovarian cancer and prospects for polygenic risk prediction. Gynecol Oncol. 2017;147(3):705–713. doi: 10.1016/j.ygyno.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Kerber R.A., Slattery M.L. The impact of family history on ovarian cancer risk. The Utah Population Database. Arch Intern Med. 1995;155(9):905–912. [PubMed] [Google Scholar]
- 3.Villarreal-Garza C., Alvarez-Gomez R.M., Perez-Plasencia C., Herrera L.A., Herzog J., Castillo D. Significant clinical impact of recurrent BRCA1 and BRCA2 mutations in Mexico. Cancer. 2015;121(3):372–378. doi: 10.1002/cncr.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng G., Yu H., Kanerva A., Forsti A., Sundquist K., Hemminki K. Familial Ovarian Cancer Clusters with Other Cancers. Sci Rep. 2018;8(1):11561. doi: 10.1038/s41598-018-29888-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alsop K., Fereday S., Meldrum C., deFazio A., Emmanuel C., George J. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S., Royer R., Li S., McLaughlin J.R., Rosen B., Risch H.A. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol Oncol. 2011;121(2):353–357. doi: 10.1016/j.ygyno.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 7.Walsh T., Casadei S., Lee M.K., Pennil C.C., Nord A.S., Thornton A.M. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108(44):18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godet I., Gilkes D.M. BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integr Cancer Sci Ther. 2017;4(1) doi: 10.15761/ICST.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy R., Chun J., Powell S.N. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12(1):68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Couch F.J., Wang X., McGuffog L., Lee A., Olswold C., Kuchenbaecker K.B. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet. 2013;9(3) doi: 10.1371/journal.pgen.1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank T.S., Deffenbaugh A.M., Reid J.E., Hulick M., Ward B.E., Lingenfelter B. Clinical characteristics of individuals with germline mutations in BRCA1 and BRCA2: analysis of 10,000 individuals. J Clin Oncol. 2002;20(6):1480–1490. doi: 10.1200/JCO.2002.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 12.Gabai-Kapara E., Lahad A., Kaufman B., Friedman E., Segev S., Renbaum P. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111(39):14205–14210. doi: 10.1073/pnas.1415979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, E.Y. and W.J. Muller, Oncogenes and tumor suppressor genes. Cold Spring Harb Perspect Biol. 2(10): p. a003236. [DOI] [PMC free article] [PubMed]
- 14.Paul, A. and S. Paul, The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers. Front Biosci (Landmark Ed). 19: p. 605-618. [DOI] [PMC free article] [PubMed]
- 15.Wallace A.J. New challenges for BRCA testing: a view from the diagnostic laboratory. Eur J Hum Genet. 2016;24(Suppl 1):S10–S18. doi: 10.1038/ejhg.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lancaster J.M., Powell C.B., Chen L.M., Richardson D.L., S.G.O.C.P. Committee Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2015;136(1):3–7. doi: 10.1016/j.ygyno.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 17.George A. UK BRCA mutation testing in patients with ovarian cancer. Br J Cancer. 2015;113(Suppl 1):S17–S21. doi: 10.1038/bjc.2015.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gayther S.A., Warren W., Mazoyer S., Russell P.A., Harrington P.A., Chiano M. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995;11(4):428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- 19.Thompson D., Easton D., Breast Cancer Linkage C. Variation in cancer risks, by mutation position, in BRCA2 mutation carriers. Am J Hum Genet. 2001;68(2):410–419. doi: 10.1086/318181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gayther S.A., Mangion J., Russell P., Seal S., Barfoot R., Ponder B.A. Variation of risks of breast and ovarian cancer associated with different germline mutations of the BRCA2 gene. Nat Genet. 1997;15(1):103–105. doi: 10.1038/ng0197-103. [DOI] [PubMed] [Google Scholar]
- 21.Rebbeck T.R., Mitra N., Wan F., Sinilnikova O.M., Healey S., McGuffog L. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA. 2015;313(13):1347–1361. doi: 10.1001/jama.2014.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koczkowska M., Krawczynska N., Stukan M., Kuzniacka A., Brozek I., Sniadecki M. Spectrum and Prevalence of Pathogenic Variants in Ovarian Cancer Susceptibility Genes in a Group of 333 Patients. Cancers (Basel) 2018;10(11) doi: 10.3390/cancers10110442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cotrim D.P., Ribeiro A.R.G., Paixao D., de Queiroz Soares D.C., Jbili R., Pandolfi N.C. Prevalence of BRCA1 and BRCA2 pathogenic and likely pathogenic variants in non-selected ovarian carcinoma patients in Brazil. BMC Cancer. 2019;19(1):4. doi: 10.1186/s12885-018-5235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teixeira N, van der Hout A, Oosterwijk JC, Vos JR, Devilee P, van Engelen K, et al. The association between cancer family history and ovarian cancer risk in BRCA1/2 mutation carriers: can it be explained by the mutation position? Eur J Hum Genet26(6), 848–857. [DOI] [PMC free article] [PubMed]
- 25.Rudaitis V., Zvirblis T., Kanopiene D., Janulynaite D., Griskevicius L., Janavicius R. BRCA1/2 mutation status is an independent factor of improved survival for advanced (stage III-IV) ovarian cancer. Int J Gynecol Cancer. 2014;24(8):1395–1400. doi: 10.1097/IGC.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 26.Vencken P.M., Kriege M., Hoogwerf D., Beugelink S., van der Burg M.E., Hooning M.J. Chemosensitivity and outcome of BRCA1- and BRCA2-associated ovarian cancer patients after first-line chemotherapy compared with sporadic ovarian cancer patients. Ann Oncol. 2011;22(6):1346–1352. doi: 10.1093/annonc/mdq628. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher D.J., Konner J.A., Bell-McGuinn K.M., Bhatia J., Sabbatini P., Aghajanian C.A. Survival in epithelial ovarian cancer: a multivariate analysis incorporating BRCA mutation status and platinum sensitivity. Ann Oncol. 2011;22(5):1127–1132. doi: 10.1093/annonc/mdq577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharyya A., Ear U.S., Koller B.H., Weichselbaum R.R., Bishop D.K. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275(31):23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 29.Foulkes W.D. BRCA1 and BRCA2: chemosensitivity, treatment outcomes and prognosis. Fam Cancer. 2006;5(2):135–142. doi: 10.1007/s10689-005-2832-5. [DOI] [PubMed] [Google Scholar]
- 30.Venkitaraman A.R. Linking the cellular functions of BRCA genes to cancer pathogenesis and treatment. Annu Rev Pathol. 2009;4:461–487. doi: 10.1146/annurev.pathol.3.121806.151422. [DOI] [PubMed] [Google Scholar]
- 31.Kotsopoulos J., Rosen B., Fan I., Moody J., McLaughlin J.R., Risch H. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol Oncol. 2016;140(1):42–47. doi: 10.1016/j.ygyno.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Artioli G., Borgato L., Cappetta A., Wabersich J., Mocellin S., Dalla Palma M. Overall survival in BRCA-associated ovarian cancer: case-control study of an Italian series. Eur J Gynaecol Oncol. 2010;31(6):658–661. [PubMed] [Google Scholar]
- 33.Liu J., Cristea M.C., Frankel P., Neuhausen S.L., Steele L., Engelstaedter V. Clinical characteristics and outcomes of BRCA-associated ovarian cancer: genotype and survival. Cancer Genet. 2012;205(1-2):34–41. doi: 10.1016/j.cancergen.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weitzel J.N., Lagos V.I., Herzog J.S., Judkins T., Hendrickson B., Ho J.S. Evidence for common ancestral origin of a recurring BRCA1 genomic rearrangement identified in high-risk Hispanic families. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1615–1620. doi: 10.1158/1055-9965.EPI-07-0198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.