Highlights
-
•
Self- and other-reflection in schizophrenia were studied with fMRI.
-
•
Patients failed to activate the right temporo-parietal junction in other-reflection.
-
•
They also hyperactivated lateral prefrontal cortex for self and other-reflection.
-
•
These findings might be linked to altered self/other processing in schizophrenia.
Keywords: Psychosis, Schizophrenia, Self-reflection, Other-reflection, fMRI, Temporo-parietal junction
Abstract
Background
An alteration in self/other differentiation has been proposed as a basis for several symptoms in schizophrenia, including delusions of reference and social functioning deficits. Dysfunction of the right temporo-parietal junction (TPJ), a region linked with social cognition, has been proposed as the basis of this alteration. However, imaging studies of self- and other-processing in schizophrenia have shown, so far, inconsistent results.
Methods
Patients with schizophrenia and healthy controls underwent fMRI scanning while performing a task with three conditions: self-reflection, other-reflection and semantic processing.
Results
Both groups activated similar brain regions for self- and other-reflection compared to semantic processing, including the medial prefrontal cortex, the precuneus and the TPJ. Compared to healthy subjects, patients hyperactivated the left lateral frontal cortex during self- and other-reflection. In other-reflection, compared to self-reflection, patients failed to increase right TPJ activity.
Conclusions
Altered activity in the right TPJ supports a disturbance in self/other differentiation in schizophrenia, which could be linked with psychotic symptoms and affect social functioning in patients. Hyperactivity of the lateral frontal cortex for self- and other-reflection suggests the presence of greater cognitive demand to perform the task in the patient group.
1. Introduction
A disturbance in the process of distinguishing the self from others is proposed as a relevant factor contributing to the symptom profile in schizophrenia: poor self/other distinction may lead to incorrect attribution of one's own thoughts or intentions to others, resulting in paranoia and ideas of reference, or to a disruption of the sense of agency of behavior leading to delusions of control (Eddy, 2018). Self/other differentiation has been proposed to involve both motor processes underpinning imitation and cognitive processes linked to mentalizing and reasoning about the other's beliefs and emotions (Eddy, 2018), and its failure (self/other blending) might impair perspective taking, among other processes (Eddy, 2016). As a result, it can also affect social interaction in terms of increasing social anhedonia and social withdrawal (Eddy, 2016; van der Meer et al., 2010; van der Weiden et al., 2015). Social functioning deficits are common in schizophrenia and can result in the misinterpretation of others’ intentions and difficulties in daily social interactions (Green et al., 2015). These deficits are closely linked to worse functional outcomes (Fett et al., 2011). Therefore, a better understanding of the alterations in processing self- and other-related information will be an important contribution to the improvement of therapeutic interventions and patients’ quality of life.
Brain activity related to self-reflection is typically assessed with tasks that require the participant to decide whether a particular trait adjective or descriptive statement applies to themselves. For other-reflection, the same kind of decision is applied to another personally or publicly known person. Both conditions are associated with activation in a network of regions which include the medial prefrontal cortex (mPFC), the temporo-parietal junction (TPJ), and the posterior cingulate cortex (PCC) (Denny et al., 2012), very similar to the regions associated with social cognition, theory of mind, autobiographical memory or future planning, which are often referred to as the default-mode network (DMN, Buckner et al., 2008). The involvement of DMN regions in such a broad range of cognitive functions has led to the hypothesis that one of the core functions of the network is to process self-relevant or social information (Andrews-Hanna et al., 2014; Molnar-Szakacs and Uddin, 2013). Patients with schizophrenia have shown altered brain activity in DMN regions during self-reflection, including the PCC (Holt et al., 2011; van der Meer et al., 2013), the mPFC (Tan et al., 2015), and the temporal cortex (Pauly et al., 2014). Other-reflection has been associated with altered activation of the PCC (van der Meer et al., 2013), the temporal cortex (Murphy et al., 2010), and the mPFC and cuneus (Pauly et al., 2014), but also in regions not traditionally linked to other-reflection like the insula (Pauly et al., 2014) and the fusiform and lingual gyri (Tan et al., 2015). These findings suggest that there is altered DMN function during self- and other-reflection in schizophrenia, although the lack of consistency among the results makes it difficult to characterize the nature of the alteration precisely.
Although self- and other-reflection tend to activate a similar set of brain regions, studies have also shown differences between the two conditions in the mPFC (with ventral mPFC more linked to self-reflection and dorsal mPFC to other-reflection) and the TPJ, among other areas (Denny et al., 2012). The TPJ (especially in the right hemisphere) has been proposed as a key brain structure for self/other differentiation, with its disruption generating deficits in self and other representations in several psychiatric disorders (Eddy, 2016). Literature on the function of the right TPJ has highlighted its involvement in tasks that require processing information about others’ intentions and mental states, like theory of mind or moral judgments tasks (Saxe, 2010), which is consistent with such a role. Alteration of TPJ function by means of non-invasive brain stimulation affects third-person perspective taking (Elk et al., 2016), which has signaled this region also as a potential target for intervention (Donaldson et al., 2015). It is important to note, though, that the TPJ is a functionally defined region that can be subdivided in different portions (Mars et al., 2012): the most posterior and dorsal parts of the TPJ (i.e. angular gyrus) seem to be more linked with social information processing and tend to co-activate with other regions involved in self-reflection and theory of mind, while anterior parts are associated with externally cued attention (Scholz et al., 2009). There also seems to be a hemispheric asymmetry in the TPJ, since social cognition has been especially linked to the right TPJ while the left has been attributed a more general role in meta-representation (for a review, see Saxe, 2010). However, this remains an open issue since the left TPJ is sometimes also activated in social processing tasks (Krall et al., 2015).
Alterations in self/other differentiation in schizophrenia may be observed in the comparison between self- and other-reflection. For example, Jardri et al. (2011), using a task where participants heard their own pre-recorded voice alternated with someone else's voice, found that the ‘self’ and ‘non-self’ cortical maps were more overlapped in patients with schizophrenia than controls, affecting the medial frontal cortex and PCC/precuneus, the right middle temporal cortex and the right inferior parietal cortex (adjacent to the TPJ). This was interpreted as impairment for self/other differentiation in the patient group. Bedford et al. (2012) found an alteration in the dorsomedial prefrontal cortex in patients, who showed similar levels of activity in this region for self and other-processing, while the controls significantly increased its activity for self-reflection compared to other-reflection. However, other studies have reported no differences between patients and controls (Holt et al., 2011; Tan et al., 2015; van der Meer et al., 2013; Zhang et al., 2015), or found differences outside the typical self/other-reflection areas (e.g., the precentral gyrus in Pauly et al., 2014).
The studies reviewed above reveal that, although self/other processing and differentiation are proposed as a basis for several symptoms in schizophrenia, consistent deficits in the neural correlates of these cognitive processes are yet to be characterized. This heterogeneity in previous studies might be a result of the combination of different factors: first, most of the studies on self/other reflection in schizophrenia have used tasks where trait adjectives or sentences are presented to the participants, who have to decide whether these traits apply to themselves or to another person. However, the control conditions against which self- and other-reflection are contrasted vary from affect labeling (i.e. judging whether the adjective is positive or negative) (Holt et al., 2011; Murphy et al., 2010; Tan et al., 2015), semantic knowledge (i.e. answering Yes or No to sentences about general facts) (van der Meer et al., 2013; Zhang et al., 2015) or perceptual tasks (Bedford et al., 2012; Pauly et al., 2014). Contrasting self- or other-reflection against conditions that might impose different cognitive demands (e.g. semantic knowledge is likely to be more demanding than perceptual tasks) may result in differences in the self and other activation maps. Secondly, the degree of familiarity with the individual chosen for other-reflection also ranges from close relatives and friends (Holt et al., 2011; Murphy et al., 2010; Pauly et al., 2014; van der Meer et al., 2013) to public figures (Bedford et al., 2012; Tan et al., 2015), which could also affect activation patterns (e.g. reflecting upon close relatives may rely more in autobiographical memory, while reflecting upon public figures may involve more semantic memory, and involve different brain regions, see Murray et al., 2012). Finally, significant differences in patient status and small sample sizes might also explain the lack of consistency among results.
The present study aims to further examine self- and other-processing in schizophrenia, with an emphasis on self/other differentiation (i.e. the direct comparison between self- and other-reflection). We will use a self/other-reflection task based on previous studies (Modinos et al., 2009; van der Meer et al., 2013) that we have already used successfully in healthy subjects (Fuentes-Claramonte et al., 2019), where we observed significant differences between self- and other-reflection in DMN regions with the specific involvement of the right posterior TPJ (angular gyrus) in other, but not self-reflection. Considering the variability in the tasks used in the previous literature, we have selected this task to best isolate the components of interest for our study. We consider that the present work will allow us to broaden the knowledge on the neural basis of self/other differentiation in schizophrenia. Although there is no consistent pattern of findings in the existing schizophrenia literature, we expect that alterations will arise in relevant DMN areas involved in self/other differentiation, namely the mPFC, the PCC and the TPJ.
2. Methods
2.1. Subjects
Thirty-two patients with a DSM-IV-TR diagnosis of schizophrenia recruited from three psychiatric hospitals (Benito Menni CASM, Sagrat Cor de Martorell and Hospital Sant Rafael) in Barcelona participated in the study. They all underwent diagnostic evaluation by trained raters using the Spanish version of the Structured Clinical Interview for DSM Disorders (SCID). Psychotic symptoms were also scored using the Positive and Negative Syndrome Scale (PANSS, Kay et al., 1987) and the The Clinical Global Impressions Scale (CGI, Guy, 1976). Patients were excluded if they (a) were younger than 18 or older than 65 years, (b) had a history of brain trauma or neurological disease, and (c) had shown alcohol/substance abuse within 12 months prior to participation. With respect to the last criterion, all participants were questioned about alcohol and drug use during the previous year by a psychiatrist. As well as excluding patients who showed evidence of substance abuse/dependence, we also excluded those who reported habitual use of cannabis. All patients were right-handed and all were taking antipsychotic medication.
The control sample consisted of 33 right-handed healthy individuals recruited from non-medical staff working in the hospital, their relatives and acquaintances, plus independent sources in the community. They were selected to be similar to the patients in age, sex and IQ (premorbid IQ in the patients). This last was estimated using the Word Accentuation Test (Test de acentuación de palabras (TAP) Del Ser et al., 1997; Gomar et al., 2011), which requires pronunciation of Spanish words whose accents have been removed. This test is conceptually similar to the UK National Adult Reading Test (NART) (Nelson, 1982) and the US Wide Range of Achievement Test used in the USA (Jastak and Wilkinson, 1984). Scores can be converted into full scale IQ estimates (Gomar et al., 2011). The control sample met the same exclusion criteria as the patients. They were also questioned and excluded if they reported a history of mental illness and/or treatment with psychotropic medication and the SCID was also used to exclude current psychiatric disorders.
All participants gave written informed consent prior to participation. All the study procedures had been previously approved by the Research Ethics Committee FIDMAG Sisters Hospitallers (Comité de Ética de la Investigación de FIDMAG Hermanas Hospitalarias) and complied with its ethical standards on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Healthy controls received a gift-card as a compensation for their participation in the study.
2.2. Self-other task
The task used in the present study was adapted from the one described in Modinos et al. (2009) and has already been used in healthy subjects by our group (Fuentes-Claramonte et al., 2019) to investigate the processing of information related to the self and another known person, compared to general semantic knowledge. To best isolate the components of interest for our study, this task uses (1) a semantic control condition (matched in perceptual and motor requirements and in statement complexity with the conditions of interest) to separate self- and other-reflection from processing of externally-oriented information, and (2) an ‘other’ condition aimed to compare reflection upon oneself to reflection on other individuals within the participant's environment, for which we selected an ‘other’ personally known by the participant (so responses might be based on previous interactions and not so much on semantic knowledge, and so exposure to this ‘other’ might be more uniform across participants than with public figures), but not a very close ‘other’ to avoid strong emotional investment.
Before scanning, participants were given detailed task instructions and were asked to choose an acquaintance to think about inside the scanner for the other-reflection condition. The chosen individual had to be familiar to the participant, but not too close in order to avoid eliciting strong feelings towards them (valid examples were classmates or co-workers), similar to the original implementation of the task (Modinos et al., 2009). The final choice of acquaintance was made in consultation with one of the researchers to ensure the degree of closeness was similar for all participants.
During the task, participants viewed a series of statements which were either about themselves (Self), about an acquaintance (Other) or about general knowledge (Facts), which were similar in length and complexity in the three conditions. Participants had to respond whether they considered the sentence to be true or false with a button press. In the Self condition, sentences referring to personal qualities, attributes or attitudes were presented (e.g. “In general, I like order”, “I am a tense or very nervous person”). Similarly, in the Other condition sentences referred to the personality traits and behavior of the chosen acquaintance. Examples of sentences included in the Other condition are “OTHER often makes decisions without thinking” or “OTHER usually has very good ideas”, where OTHER was substituted by the chosen person's name. In the Facts condition, sentences referred to general knowledge, such as “A decade is a period of ten years” or “Insects only have four legs”. As in the original version, half of the sentences in the Self and Other conditions had a positive valence and the rest had a negative quality, while in the Facts condition half of the sentences were true and the other half were false. Different from the original, the Self and Other conditions only included statements referring to personality, behavior and attitudes, but not physical appearance (these were a minority in Modinos et al., 2009). All statements were written in Spanish, and all participants were fluent Spanish speakers.
The task consisted of 54 trials (18 per condition) arranged in a block design. Each block started with an instruction screen indicating the condition that corresponded to that block (“Sentences about Me”, “Sentences about Other”, “Sentences about Facts”), which lasted 3s. After a 1s delay, three trials were presented, each lasting 9s, where the sentence appeared in the center of the screen and the options “Yes” and “No” appeared at the bottom-right and bottom-left corners, respectively, to act as a reminder of the required response (“Yes” with the right index finger, and “No” with the left index finger). Trials were separated by a 1s blank screen. After the three trials, the next block started, for a total of 6 blocks per condition. Every 3 blocks there was a resting period of 16s in which only a crosshair was presented as fixation point. Block order was pseudorandomized, with each of the three conditions occurring once between resting periods. Total task duration was 12 min and 12 s. This design deviates from the task design used by Modinos et al. (2009) in that the trials were longer, resulting in fewer trials overall, but with a similar amount of time spent in each condition. This change was made to ensure participants had time to read and reflect on each statement before responding. As demonstrated in Fuentes-Claramonte et al. (2019), this version of the task is able to elicit activation of relevant DMN brain regions in self and other-reflection.
2.3. Image acquisition
Images were acquired with a 3T Philips Achieva scanner (Philips Medical Systems, Best, the Netherlands). Functional data were acquired using a T2*-weighted echo-planar imaging (EPI) sequence with 364 volumes and the following acquisition parameters: TR = 2000 ms, TE = 30 ms, Flip angle = 78O, in-plane resolution= 3 × 3 mm, FOV = 240 mm, slice thickness = 3 mm, inter-slice gap = 1mm. Slices (32 per volume) were acquired with an interleaved order parallel to the AC-PC plane. Before the functional sequence, a high-resolution anatomical 3D volume was acquired using a TFE (Turbo Field Echo) sequence for anatomical reference and inspection (TR = 8.15ms; TE = 3.73ms; Flip angle = 8O; voxel size = 0.9375 × 0.9375 mm; slice thickness = 1mm; slice number = 160; FOV = 240 mm).
2.4. Image preprocessing and analysis
Preprocessing and analysis was carried out with the FEAT module included in the FSL (FMRIB Software Library) software, version 5.0 (Smith et al., 2004). The first 20 s (10 volumes) of the sequence, corresponding to signal stabilization, were discarded. Preprocessing included motion correction (using the MCFLIRT algorithm) and co-registration and normalization to a common stereotactic space (Montreal Neurological Institute template). Before group analyses, normalized images were spatially filtered with a Gaussian filter (FWHM = 5 mm). To minimize unwanted movement-related effects, individuals with an estimated maximum absolute movement >3.0 mm or an average absolute movement >0.3 mm were excluded from the study.
Statistical analysis was performed by means of a General Linear Model (GLM) approach. At the first level, three regressors of interest were defined in the GLM corresponding to the three task conditions (Self, Other, Facts) in a block-design fashion. Instruction screens were modeled by an additional nuisance regressor. Fixation periods were not modeled and thus acted as an implicit baseline. GLMs were fitted to generate activation maps for each of the three conditions of interest compared to baseline and for the comparisons between conditions (Self vs. Facts, Other vs. Facts, Other vs. Self). Second level analyses and group comparisons between patients and controls were performed within the FEAT module, with mixed-effects GLMs (Beckmann et al., 2003). All statistical tests were carried out at the cluster level with a corrected p value of 0.05 using Gaussian random field methods. The default threshold of z = 2.3 was used to define the initial set of clusters.
3. Results
Demographic and clinical characteristics of the final samples included in the analyses are detailed in Table 1. As can be seen, the patients and the controls did not differ significantly in terms of age, sex and estimated premorbid IQ. Nine patients and 6 controls were excluded from the analyses due to excessive head movement. All patients were on antipsychotic treatment (21 on atypical neuroleptics and 2 on both typical and atypical) and were admitted to inpatient (14 patients) or outpatient (9 patients) units.
Table 1.
Demographic and clinical sample characteristics.
| Patients (N = 23) | Controls (N = 27) | Significance | |
|---|---|---|---|
| Sex (M/F) | 16/7 | 17/10 | p = 0.623 |
| Age | 37.00 (8.06) | 38.74 (10.20) | p = 0.512 |
| range 22-55 | range 23-61 | ||
| Estimated pre-morbid IQ (TAP) | 100.64 (10.23) | 104.07 (6.34) | p = 0.178 |
| range 81-114 | range 91-114 | ||
| PANSS Total score | 69 (18.19) | ||
| range 39-115 | |||
| PANSS Positive | 16.04 (5.49) | ||
| range 8-28 | |||
| PANSS Negative | 19.74 (5.79) | ||
| range 11-33 | |||
| PANSS General | 33.22 (9.08) | ||
| range 18-59 | |||
| CGI | 4.17 (0.89) | ||
| range 3-6 | |||
| Treatment (chlorpromazine equivalent, daily dose in mg) | 498.77 (490.27) | ||
| range 62.5-2400 |
Measures are means (SD). Group differences were tested with a Chi-square test for sex and unpaired two sample t-tests for age and pre-morbid IQ.
3.1. Self vs. facts mean group results
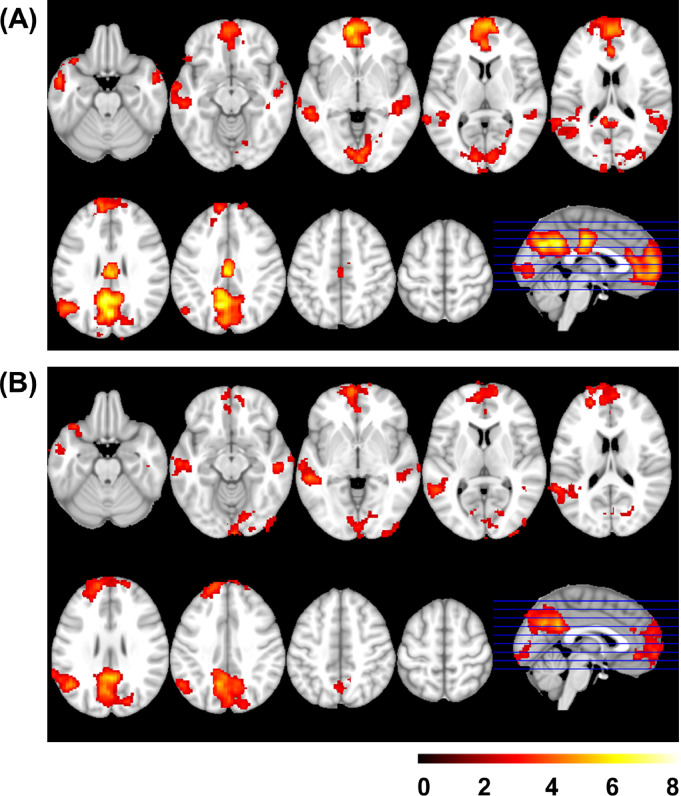
Brain activity associated to self-reflection was identified by the Self > Facts contrast, where both patients and controls showed similar regions of activation in the medial prefrontal cortex, the PCC and precuneus, the left angular gyrus and TPJ area, the middle temporal cortex (bilateral), and parts of the visual cortex (calcarine cortex and lingual gyrus, see Fig. 1 and Supplementary Table S1 for details).
Fig. 1.
Mean activation in Self > Facts control group (A) and in the patient group (B). Images are displayed in neurological convention (right is right). Color bar depicts z values.
3.2. Self vs. facts group comparison
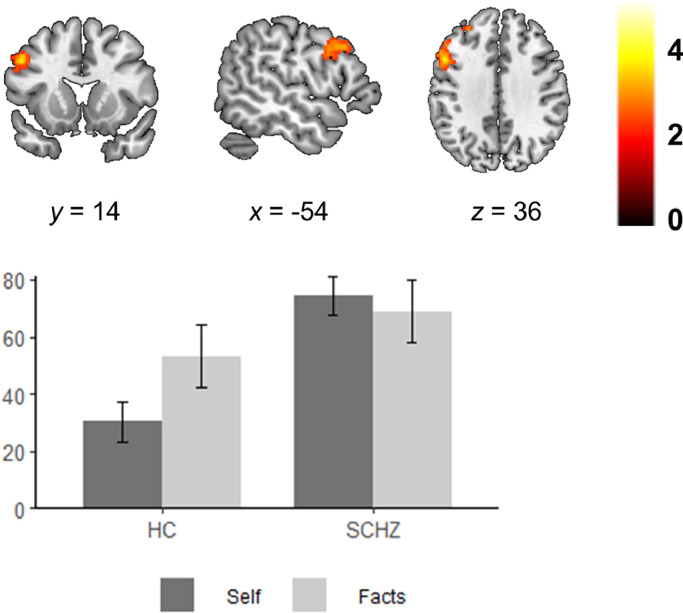
Group comparison in the Self vs. Facts contrast showed a cluster of differences in the left DLFPC/middle frontal gyrus (MNI coordinates x = -52, y = 14, z = 36; Z = 3.94; cluster size = 527 voxels; p = 0.008). As seen in Fig. 2, the patients hyperactivated this region in self-reflection. Moreover, controls increased activity in the DLPFC in Facts with respect to Self, but this increase was not observed in the patients, who displayed similar (heightened) levels of activation in both conditions.
Fig. 2.
Group differences between healthy subjects and schizophrenic patients in the Self vs. Facts contrast. Plot shows the mean parameter estimates (average of all beta weights in the cluster) for the left DLFPC in the Self and Facts conditions (respective to baseline) for each group in the cluster of group differences. Error bars represent standard error of the mean. Images are displayed in neurological convention (right is right). Color bar depicts z values.
3.3. Other vs. facts mean group results
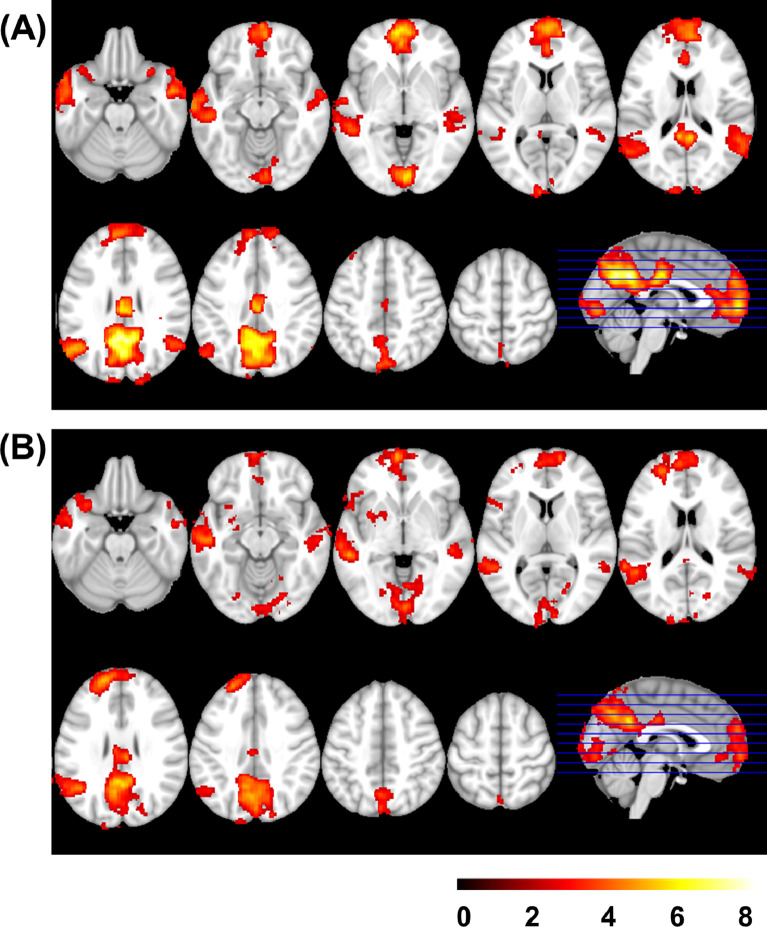
Other-reflection (Other > Facts contrast) yielded a very similar pattern of activation to that of Self-reflection, involving mostly the same regions plus the right angular gyrus in the control group. In patients, other-reflection also activated the left insula and putamen (see Fig. 3 and Supplementary Table S2 for details).
Fig. 3.
Mean activation in Other > Facts in the control group (A) and in the patient group (B). Images are displayed in neurological convention (right is right). Color bar depicts z values.
3.4. Other vs. facts group comparison
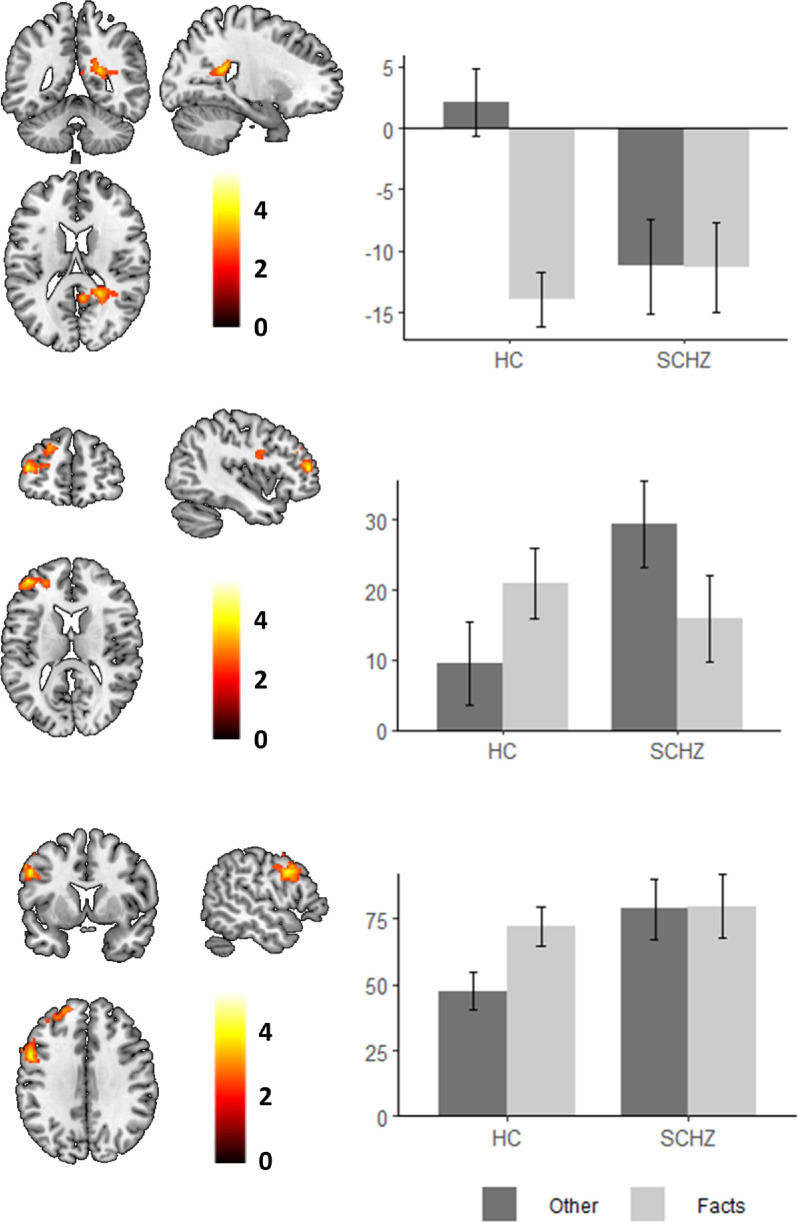
This group comparison showed three clusters of differences between patients and controls: one in the precuneus (MNI coordinates x = 22, y = -46, z = 18; Z = 3.94; cluster size = 502 voxels; p = 0.02), one in the left DLPFC (MNI coordinates x = -42, y = 50, z = 14; Z = 4.06; cluster size = 594 voxels; p = 0.008), and one in the left precentral gyrus (MNI coordinates x = -54, y = 8, z = 34; Z = 4.21; cluster size = 552 voxels; p = 0.01). We observed that patients deactivated the precuneus during the Other condition while controls did not (although both groups deactivated this region in the Facts condition, as shown in Fig. 4). In the DLPFC and precentral gyrus, we observed increased activation in the patient group in Other-processing (while activation in the Facts condition was similar in both groups). Interestingly, these last two clusters were located very close to the area of differences found in the Self vs. Facts contrast and displayed a similar pattern of alteration in the patient group.
Fig. 4.
Group differences between healthy subjects and schizophrenic patients in the Other vs. Facts contrast. Plots show the mean parameter estimates (beta weights) for the precuneus (upper row), the DLPFC (middle row) and the precentral gyrus (lower row) in the Other and Facts conditions (respect to baseline) for each group. Error bars represent standard error of the mean. Images are displayed in neurological convention (right is right). Color bars depict z values.
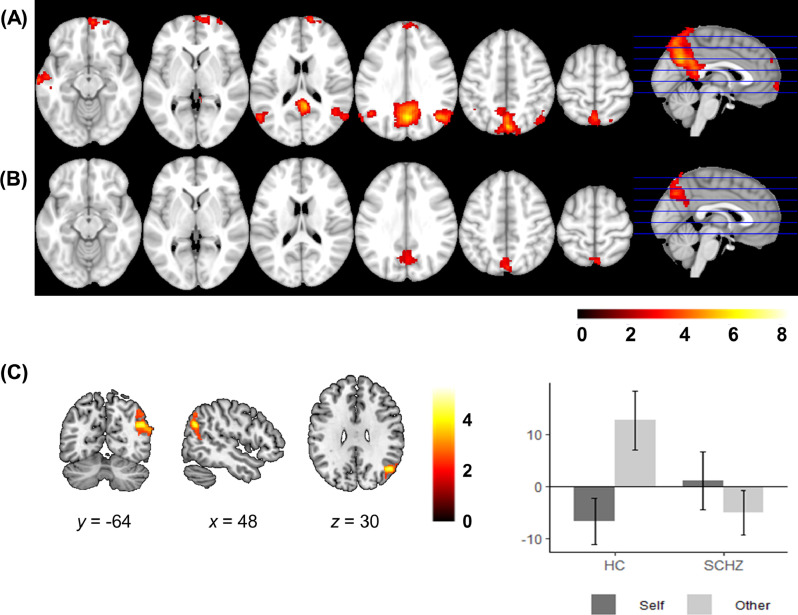
3.5. Self vs. other mean group results
No region showed increased activity in Self trials when compared to the Other condition (Self> Other contrast), neither in the patients nor in the controls. On the other hand, several brain areas were more active in the Other than in the Self condition: healthy subjects showed activation in the precuneus and PCC, mPFC extending into the right hemisphere, angular gyrus, temporal pole and amygdalae, and right superior frontal cortex (Supplementary Table S3, Fig. 5). Patients with schizophrenia, however, only showed activation in the PCC and the left temporal pole (Supplementary Table S3, Fig. 5).
Fig. 5.
Mean activation in Other > Self in the control group (A) and in the patient group (B). (C) Group differences between healthy subjects and schizophrenic patients in the Other vs. Self contrast. Plot shows the mean parameter estimates (beta weights) for the cluster in the right angular gyrus in the Self and Other conditions (respect to baseline) for each group. Patients fail to activate the right angular gyrus in the Other condition. Error bars represent standard error of the mean. Images are displayed in neurological convention (right is right). Color bars depict z values.
3.6. Self vs. other group comparison
This group comparison showed a cluster of differences in the right angular gyrus (MNI coordinates x = 48, y = -64, z = 30; Z = 4.54; cluster size = 547 voxels; p = 0.007), which was activated for other-reflection in the control group, but not in the patients (Fig. 5).
4. Discussion
This study aimed to examine the brain correlates of self/other differentiation in schizophrenia by comparing brain activation during self- and other-reflection. We report, for the first time, a significant alteration in right TPJ activity in patients with schizophrenia in other-reflection. In healthy controls, self- and other-reflection activated a similar set of brain regions when compared with a semantic control condition, although direct comparison of self and other revealed that some of them were relatively more active in other-reflection. However, the right TPJ was not activated for self, but only for other-reflection, which is consistent with the role attributed to this area in self/other differentiation and social cognition (Eddy, 2016; Sowden and Shah, 2014). In contrast, patients with schizophrenia did not activate right TPJ for either self- or other-reflection. TPJ hypoactivation in schizophrenia has been previously described with social cognition tasks (Benedetti et al., 2009; de Achával et al., 2012; Makowski et al., 2016; Thakkar et al., 2014; but see also Kronbichler et al. (2017), which shows meta-analytic evidence of both aberrant hypo and hyperactivation of the TPJ in mentalizing tasks). The present finding supports the hypothesis that TPJ dysfunction may underlie a disturbance in self/other differentiation processes in schizophrenia (Eddy, 2016).
Self/other differentiation is believed to initially involve motor mechanisms underpinning imitation and recognition of action goals, as well as interpretation of facial emotions and non-verbal communication; followed by mentalizing processes that include inferring the mental state of the other or reasoning about their beliefs and emotions (Eddy, 2018). Successfully decoupling the self and other may aid higher level perspective taking. In contrast, when these processes are impaired, self/other blending can occur and the person may have reduced ability in perspective taking, or may not be able to suppress imitation and this could lead to confusion, personal distress and depersonalization (Eddy, 2016). The right TPJ is associated with decoupling mechanisms, referring to the ability to dissociate an agent's mental state from one's own beliefs and to differentiate between belief and reality (van Veluw and Chance, 2014). This could conceivably provide a basis for patients with schizophrenia being impaired in identifying the origin of beliefs, intentions, or actions, and so having difficulties testing them against reality, resulting not only in difficulties in social interaction but also in delusional explanations of others’ behavior (e.g. ideas/delusions of reference or persecution, grandiosity).
It should be noted that the TPJ is not a unitary region, and at least two subdivisions have been found using resting-state functional connectivity: a more anterior part that shows connectivity with attentional regions (ventral PFC and anterior insula) and a more posterior subdivision connected with DMN regions (mPFC, PCC/precuneus) (Mars et al., 2012). In the present study, group differences were found in the angular gyrus, which belongs to the posterior subdivision of the TPJ, and is usually described as belonging to the DMN (Buckner et al., 2008). Interestingly, though, there was a laterality effect: the left angular gyrus was activated both by self- and other-reflection (although BOLD signal was higher in the second), but the right was only active during other-reflection. This adds specificity to our results, since the right TPJ has been more strongly linked to self/other differentiation and social cognition in the previous literature than the left (Eddy, 2016). Consistently, there were no group differences in the left TPJ.
The findings concerning the TPJ are also relevant because this region has been proposed as a target for therapeutic intervention. Several drugs may affect its function, for instance, drugs that alter norepinephrine (Strange and Dolan, 2007), selective serotonin reuptake inhibitors (Cremers et al., 2016) and intranasal oxytocin (Hu et al., 2016). In addition, the TPJ is a promising target for neuromodulation techniques such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) (Donaldson et al., 2015).
In the present study, we also observed hyperactivation of the left lateral frontal cortex (including the DLPFC and the precentral gyrus) during self- and other-reflection. Detailed examination of the activation pattern in this area revealed that hyperactivation was specific to self/other processing, while activation levels were similar between groups in the semantic condition. Given the attentional role that the lateral frontal cortex has been assigned (Badre, 2008), and its involvement in semantic memory (Raposo et al., 2012), activation of this region would arguably be expected during semantic processing (i.e. Facts condition), but not in self- and other-reflection. Moreover, we observed that the patients deactivated a cluster in the precuneus for both semantic and other processing, while controls only did so for semantic processing. Deactivation of the precuneus is characteristic of tasks that place cognitive demands on the subject, such as classical attention or working memory tasks (Cavanna and Trimble, 2006), even in schizophrenic patients (Pomarol-Clotet et al., 2008). In the patients in our study, self- and other-reflection seem to have imposed greater cognitive demands than in controls, leading to hyperactivity of the lateral prefrontal cortex and deactivation of the precuneus in conditions that should not require these changes (more akin to cognitive, attention-demanding tasks). An alternative, or perhaps complementary interpretation of this finding, would be that healthy participants treat self- and other-related information differently from semantic knowledge, as evidenced by the obvious differences in patterns of brain activity between these conditions. In schizophrenia, however, the ability to distinguish between these two kinds of information may be disturbed, leading to difficulties in the management of social information.
This activation pattern is also interesting because DMN regions involved in self/other-reflection tend to decrease their activation when attention is externally focused, which in turn involves an increase in activity of attentional or executive networks, where the lateral prefrontal cortex is a relevant node (Fox et al., 2005). Thus, adequate balance between these intrinsic networks seems necessary for adaptive cognitive functioning, and a failure in its regulation might be linked to psychiatric symptoms. Alterations in the DMN are well-established in schizophrenia (Dreher et al., 2012; Haatveit et al., 2016; Pomarol-Clotet et al., 2008; Salgado-Pineda et al., 2011; Schneider et al., 2011). Some authors have proposed that there is a stable difference in the DMN structure and its connections with the salience network and the central executive network (Menon, 2011; Woodward et al., 2011). Given that healthy subjects showed activation differences between task conditions in the lateral prefrontal cortex, the precuneus and the right angular gyrus, and these changes were not found in patients, our results could also be linked to a failure in network balance between the DMN and the executive network in schizophrenia. These results may suggest that the process of functional specialization of the DMN may be altered in schizophrenia.
The present results add evidence to the range of alterations in self/other-reflection reported in schizophrenia. However, our findings converge only partially with previous results. In the Other vs. Facts contrast, we found altered activation of a region close to the PCC as also found by Tan et al. (2015) and van der Meer et al. (2013), but other findings have not been replicated. Our task was based on the design used by van der Meer et al. (2013); however, we did not include sentences about physical attributes in the Self or the Other conditions. A second reason for differences might be the degree of familiarity with the individual chosen as ‘other’ in the Other condition (Murray et al., 2012). In our study, participants were instructed to choose a known person but not someone with a close relationship with them, so that the ‘other’ was personally known and had a history of past interactions with the participant, but not as close as in other studies where the ‘other’ was required to be a close friend or family member. Other studies have used a public ‘other’; however, this may impose significant variation in the amount of exposure to such individuals. Although we tried to control the level of closeness between the participant and the ‘other’, it is important to keep in mind that there is some degree of variability that could not be controlled. It might also be important to bear in mind that relationships and attachments might be different in schizophrenia patients than in controls. It is also of course possible that discrepancies between studies could be explained in terms of prevalence of positive and negative symptoms in the schizophrenia samples, stage of the disorder, or medication use.
4.1. Conclusions
The present study is the first to reveal diminished activation of the right TPJ, a brain region with a relevant proposed role for self/other differentiation and social cognition, in patients with schizophrenia during other-reflection. Additionally, it found evidence that schizophrenic patients rely more on cognitive control areas (i.e. left lateral prefrontal cortex) for self- and other-reflection, suggesting that this type of cognitive process might place greater cognitive demands in patients with this disorder. Taken together, the results support a failure in self- and other-related information processing in schizophrenia and provide evidence for disturbances in self/other differentiation, which might lead to altered judgments of others’ behavior and personality. An alteration in the right TPJ activity is a potential neural mechanism for this disturbance. This latter finding may be relevant for future studies, particularly those aiming to examine psychosocial treatments and neuromodulation techniques in schizophrenia.
Financial support
This work was supported by Generalitat de Catalunya (2017 SGR 01271 to EP-C and 2017 SGR 1265 to PF-C from AGAUR). Also by grants from Ministerio de Ciencia, Innovación y Universidades: Juan de la Cierva-formación contract (FJCI-2015-25278 to PF-C) and from Ministerio de Economía y Competitividad (FFI2016-77647-C2-2-P to PS-P). And by the Instituto de Salud Carlos III, co-funded by European Regional Development Fund/European Social Fund “Investing in your future”: Miguel Servet Research contract (CPII16/00018 to EP-C), Rio Hortega contract (CM15/00024 to MM-S), and Research Project Grants (PI14/01151 to RS, PI14/01148 to EP-C, PI18/00810 to EP-C, PI18/00877 to RS and PI18/00880 to PM).
CRediT authorship contribution statement
Paola Fuentes-Claramonte: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Marta Martin-Subero: Investigation, Data curation, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Pilar Salgado-Pineda: Conceptualization, Methodology, Investigation, Data curation. Aniol Santo-Angles: Investigation. Isabel Argila-Plaza: Investigation. Josep Salavert: Investigation. Antoni Arévalo: Investigation. Clara Bosque: Investigation. Carmen Sarri: Investigation. Amalia Guerrero-Pedraza: Investigation. Antoni Capdevila: Investigation, Resources. Salvador Sarró: Resources, Project administration, Funding acquisition, Supervision. Peter J. McKenna: Conceptualization, Funding acquisition, Writing - review & editing. Edith Pomarol-Clotet: Conceptualization, Funding acquisition, Writing - review & editing, Supervision. Raymond Salvador: Conceptualization, Methodology, Software, Writing - original draft, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
None
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2019.102134.
Appendix. Supplementary materials
References
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn. Sci. 2008;12:193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Bedford N.J., Surguladze S., Giampietro V., Brammer M.J., David A.S. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC Psychiatry. 2012;12 doi: 10.1186/1471-244X-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F., Bernasconi A., Bosia M., Cavallaro R., Dallaspezia S., Falini A., Poletti S., Radaelli D., Riccaboni R., Scotti G., Smeraldi E. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr. Res. 2009;114:154–160. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cremers H., Lee R., Keedy S., Phan K.L., Coccaro E. Effects of escitalopram administration on face processing in intermittent explosive disorder: an fMRI Study. Neuropsychopharmacology. 2016;41:590–597. doi: 10.1038/npp.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Achával D., Villarreal M.F., Costanzo E.Y., Douer J., Castro M.N., Mora M.C., Nemeroff C.B., Chu E., Bär K.J., Guinjoan S.M. Decreased activity in right-hemisphere structures involved in social cognition in siblings discordant for schizophrenia. Schizophr. Res. 2012;134:171–179. doi: 10.1016/j.schres.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Del Ser T., Gonzalez-Montalvo J., Martinez-Espinosa S., Delgado-Villapalos C., Bermejo F. Estimation of premorbid intelligence in spanish people with the word accentuation test and its application to the diagnosis of dementia. Brain Cogn. 1997;33:343–356. doi: 10.1006/brcg.1997.0877. [DOI] [PubMed] [Google Scholar]
- Denny B.T., Kober H., Wager T.D., Ochsner K.N. A meta-analysis of functional neuroimaging studies of self and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J. Cogn. Neurosci. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson P.H., Rinehart N.J., Enticott P.G. Noninvasive stimulation of the temporoparietal junction: a systematic review. Neurosci. Biobehav. Rev. 2015;55:547–572. doi: 10.1016/j.neubiorev.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Dreher J.C., Koch P., Kohn P., Apud J., Weinberger D.R., Berman K.F. Common and differential pathophysiological features accompany comparable cognitive impairments in medication-free patients with schizophrenia and in healthy aging subjects. Biol. Psychiatry. 2012;71:890–897. doi: 10.1016/j.biopsych.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy C.M. Social cognition and self-other distinctions in neuropsychiatry: insights from schizophrenia and Tourette syndrome. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;82:69–85. doi: 10.1016/j.pnpbp.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Eddy C.M. The junction between self and other? Temporo-parietal dysfunction in neuropsychiatry. Neuropsychologia. 2016;89:465–477. doi: 10.1016/j.neuropsychologia.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Elk M.Van, Duizer M., Sligte I., Schie H.Van. Transcranial direct current stimulation of the right temporoparietal junction impairs third-person perspective taking. Cogn. Affect. Behav. Neurosci. 2016:9–23. doi: 10.3758/s13415-016-0462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fett A.K.J., Viechtbauer W., Dominguez M., de G., Penn D.L., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Fuentes-Claramonte P., Martín-Subero M., Salgado-Pineda P., Alonso-Lana S., Moreno-Alcázar A., Argila-Plaza I., Santo-Angles A., Albajes-Eizagirre A., Anguera-Camós M., Capdevila A., Sarró S., McKenna P.J., Pomarol-Clotet E., Salvador R. Shared and differential default-mode related patterns of activity in an autobiographical, a self-referential and an attentional task. PLoS One. 2019;14:1–21. doi: 10.1371/journal.pone.0209376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomar J.J., Ortiz-Gil J., McKenna P.J., Salvador R., Sans-Sansa B., Sarró S., Guerrero A., Pomarol-Clotet E. Validation of the Word Accentuation Test (TAP) as a means of estimating premorbid IQ in Spanish speakers. Schizophr. Res. 2011;128:175–176. doi: 10.1016/j.schres.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Green M.F., Horan W.P., Lee J. Social cognition in schizophrenia. Nat. Rev. Neurosci. 2015;16:620–631. doi: 10.1038/nrn4005. [DOI] [PubMed] [Google Scholar]
- Guy W. National Institute of Mental Health; Rockville, MD: 1976. Clinical Global Impression. ECDEU Assessment Manual for Psychopharmacology. [Google Scholar]
- Haatveit B., Jensen J., Alnes D., Kaufmann T., Brandt C.L., Thoresen C., Andreassen O.A., Melle I., Ueland T., Westlye L.T. Reduced load-dependent default mode network deactivation across executive tasks in schizophrenia spectrum disorders. NeuroImage Clin. 2016;12:389–396. doi: 10.1016/j.nicl.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt D.J., Cassidy B.S., Andrews-Hanna J.R., Lee S.M., Coombs G., Goff D.C., Gabrieli J.D., Moran J.M. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol. Psychiatry. 2011;69:415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Scheele D., Becker B., Voos G., David B., Hurlemann R., Weber B. The effect of oxytocin on third-party altruistic decisions in unfair situations: an fMRI study. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R., Pins D., Lafargue G., Very E., Ameller A., Delmaire C., Thomas P. Increased overlap between the brain areas involved in self-other distinction in schizophrenia. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastak S., Wilkinson G.S. Jastak Associates; Wilmington, Del: 1984. WRAT-R : wide range achievement test-revised administration manual. [Google Scholar]
- Kay S.R., Flszbein A., Opfer L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Krall S.C., Rottschy C., Oberwelland E., Bzdok D., Fox P.T., Eickhoff S.B., Fink G.R., Konrad K. The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct. Funct. 2015;220:587–604. doi: 10.1007/s00429-014-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler L., Tschernegg M., Martin A.I., Schurz M., Kronbichler M. Abnormal brain activation during theory of mind tasks in schizophrenia: a meta-analysis. Schizophr. Bull. 2017;43:1240–1250. doi: 10.1093/schbul/sbx073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski C.S., Lepage M., Harvey P.-O. Functional neural correlates of social approval in schizophrenia. Soc. Cognit. Affect. Neurosci. 2016;11:445–457. doi: 10.1093/scan/nsv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars R.B., Sallet J., Schüffelgen U., Jbabdi S., Toni I., Rushworth M.F.S. Connectivity-based subdivisions of the human right “temporoparietal junction area”: Evidence for different areas participating in different cortical networks. Cereb. Cortex. 2012;22:1894–1903. doi: 10.1093/cercor/bhr268. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cognit. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Modinos G., Ormel J., Aleman A. Activation of anterior insula during self-reflection. PLoS One. 2009;4:e4618. doi: 10.1371/journal.pone.0004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Szakacs I., Uddin L.Q. Self-processing and the default mode network: interactions with the mirror neuron system. Front. Hum. Neurosci. 2013;7:571. doi: 10.3389/fnhum.2013.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E.R., Brent B.K., Benton M., Pruitt P., Diwadkar V., Rajarethinam R.P., Keshavan M.S. Differential processing of metacognitive evaluation and the neural circuitry of the self and others in schizophrenia: a pilot study. Schizophr. Res. 2010;116:252–258. doi: 10.1016/j.schres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Murray R.J., Schaer M., Debbané M. Degrees of separation: a quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neurosci. Biobehav. Rev. 2012;36:1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Nelson H.E. NFER-Nelson; UK: 1982. The National Adult Reading Test (NART): Test Manual. Wind. https://doi.org/Thesis_references-Converted#319. [Google Scholar]
- Pauly K.D., Kircher T.T.J., Schneider F., Habel U. Me, myself and I: Temporal dysfunctions during self-evaluation in patients with schizophrenia. Soc. Cogn. Affect. Neurosci. 2014;9:1779–1788. doi: 10.1093/scan/nst174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomarol-Clotet E., Salvador R., Sarró S., Gomar J., Vila F., Martínez Á., Guerrero A., Ortiz-Gil J., Sans-Sansa B., Capdevila A., Cebamanos J.M.M., McKenna P.J.P.J., Sarro S., Gomar J., Vila F., Martinez A., Guerrero A., Ortiz-Gil J., Sans-Sansa B., Capdevila A., Cebamanos J.M.M., McKenna P.J.P.J. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol. Med. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- Raposo A., Mendes M., Marques J.F. The hierarchical organization of semantic memory: executive function in the processing of superordinate concepts. Neuroimage. 2012;59:1870–1878. doi: 10.1016/j.neuroimage.2011.08.072. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P., Fakra E., Delaveau P., McKenna P.J., Pomarol-Clotet E., Blin O. Correlated structural and functional brain abnormalities in the default mode network in schizophrenia patients. Schizophr. Res. 2011;125:101–109. doi: 10.1016/j.schres.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Saxe R. The right temporo-parietal junction: a specific brain region for thinking about thoughts. Handb. Theory Mind. 2010:1–35. [Google Scholar]
- Schneider F.C., Royer A., Grosselin A., Pellet J., Barral F.G., Laurent B., Brouillet D., Lang F. Modulation of the default mode network is task-dependant in chronic schizophrenia patients. Schizophr. Res. 2011;125:110–117. doi: 10.1016/j.schres.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Scholz J., Triantafyllou C., Whitfield-Gabrieli S., Brown E.N., Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One. 2009;4 doi: 10.1371/journal.pone.0004869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sowden S., Shah P. Self-other control: a candidate mechanism for social cognitive function. Front. Hum. Neurosci. 2014;8:1–5. doi: 10.3389/fnhum.2014.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange B.A., Dolan R.J. Β-adrenergic modulation of oddball responses in humans. Behav. Brain Funct. 2007;3:1–5. doi: 10.1186/1744-9081-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S., Zhao Y., Fan F., Zou Y., Jin Z., Zen Y., Zhu X., Yang F., Tan Y., Zhou D. Brain correlates of self-evaluation deficits in schizophrenia: a combined functional and structural MRI study. PLoS One. 2015;10:1–12. doi: 10.1371/journal.pone.0138737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar K.N., Peterman J.S., Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am. J. Psychiatry. 2014;171:539–548. doi: 10.1176/appi.ajp.2013.13040498. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L., Costafreda S., Aleman A., David A.S. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci. Biobehav. Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- van der Meer L., De Vos A.E., Stiekema A.P.M., Pijnenborg G.H.M., Van Tol M.J., Nolen W.A., David A.S., Aleman A. Insight in schizophrenia: involvement of self-reflection networks? Schizophr. Bull. 2013;39:1352–1362. doi: 10.1093/schbul/sbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weiden A., Prikken M., van Haren N.E.M. Self-other integration and distinction in schizophrenia: a theoretical analysis and a review of the evidence. Neurosci. Biobehav. Rev. 2015;57:220–237. doi: 10.1016/j.neubiorev.2015.09.004. [DOI] [PubMed] [Google Scholar]
- van Veluw S.J., Chance S.A. Differentiating between self and others: an ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging Behav. 2014;8:24–38. doi: 10.1007/s11682-013-9266-8. [DOI] [PubMed] [Google Scholar]
- Woodward N.D., Rogers B., Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr. Res. 2011;130:86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Opmeer E.M., Ruhé H.G., Aleman A., Van Der Meer L. Brain activation during self- and other-reflection in bipolar disorder with a history of psychosis: comparison to schizophrenia. NeuroImage Clin. 2015;8:202–209. doi: 10.1016/j.nicl.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.