Abstract
The middle longitudinal fascicle (MdLF) is a recently delineated association cortico-cortical fiber pathway in humans, connecting superior temporal gyrus and temporal pole principally with the angular gyrus, and is likely to be involved in language processing. However, the MdLF has not been studied in language disorders as primary progressive aphasia (PPA). We hypothesized that the MdLF will exhibit evidence of neurodegeneration in PPA patients. In this study, 20 PPA patients and 25 healthy controls were recruited in the Primary Progressive Aphasia program in the Massachusetts General Hospital Frontotemporal Disorders Unit. We used diffusion tensor imaging (DTI) tractography to reconstruct the MdLF and extract tract-specific DTI metrics (fractional anisotropy (FA), radial diffusivity (RD), mean diffusivity (MD) and axial diffusivity (AD)) to assess white matter changes in PPA and their relationship with language impairments. We found severe WM damage in the MdLF in PPA patients, which was principally pronounced in the left hemisphere. Moreover, the WM alterations in the MdLF in the dominant hemisphere were significantly correlated with impairments in word comprehension and naming, but not with articulation and fluency. In addition, asymmetry analysis revealed that the DTI metrics of controls were similar for each hemisphere, whereas PPA patients had clear laterality differences in MD, AD and RD. These findings add new insight into the localization and severity of white matter fiber bundle neurodegeneration in PPA, and provide evidence that degeneration of the MdLF contribute to impairment in semantic processing and lexical retrieval in PPA.
Keywords: Diffusion tensor imaging, Middle longitudinal fascicle, Middle longitudinal fasciculus, Primary progressive aphasia, Semantic processing
Abbreviations: MDLF, middle longitudinal fascicle; PPA, primary progressive aphasia; PPVT, peabody picture vocabulary test; DTI, diffusion tensor imaging; FA, fractional anisotropy, RD, radial diffusivity, MD, mean diffusivity and AD, axial diffusivity
Highlights
-
•
Integrity loss of middle longitudinal fascicle (MdLF) in PPA.
-
•
MdLF degeneration correlated with impairments in word comprehension and retrieval.
-
•
MdLF not significantly correlated with articulation or fluency.
-
•
Connectivity model: gray/white matter areas involved in human semantic processing.
1. Introduction
The middle longitudinal fascicle (MdLF) is a recently delineated association cortico-cortical fiber pathway in humans (Makris, 1999; Makris et al., 2009). As defined originally by Makris and colleagues (Makris, 1999; Makris et al., 2009), the MdLF courses through the superior temporal gyrus (STG) and inferior parietal lobule (IPL) connecting STG (BA 22, 42) and temporal pole (TP, BA 38) principally with the angular (AG, BA 39) gyrus. The MdLF is distinct from other long association fiber tracts connecting the frontal, parietal or temporal lobes such as the superior longitudinal fascicle II (SLF II) and SLF III or the arcuate fascicle (AF), respectively, and the extreme capsule (Makris et al., 2009). Although recent reports have advanced our understanding of human STG structural connectivity and enriched further the dialog on the MdLF (Makris, 1999; Makris et al., 2009; De Champfleur et al., 2013, 2013a; Makris et al., 2013b; Maldonado et al., 2013; Martino et al., 2013; Wang et al., 2013; Kamali et al., 2014), a critical issue to be demonstrated is the MdLF's involvement in language processing (De Witt Hamer et al., 2011; Makris et al., 2013a, 2013b; Wang et al., 2013). Since the discovery of this fiber tract in humans, our group has suggested the MdLF plays an important role in the perisylvian language system in the dominant hemisphere (Makris, 1999; Makris et al., 2009) and has proposed an alternative model of language processing, complementary to the original Wernicke–Geschwing model, including the MdLF (Makris and Pandya, 2009). We also hypothesized its involvement in such language disorders as primary progressive aphasia (PPA) (Makris et al., 2013a, 2013b).
Primary progressive aphasia (PPA) is a clinical neurodegenerative syndrome characterized by progressive degradation of language function, with initial relative sparing of other cognitive domains (Gorno-Tempini et al., 2011). Degeneration of language regions in the dominant hemisphere has been associated with progressive deficits in speech and/or language function, and gray matter (GM) atrophy has been demonstrated in fronto-temporal-parietal regions in PPA, primarily in the left hemisphere (Gorno-Tempini et al., 2004; Sapolsky et al., 2010). However, language functions rely not only on cortical regions, but also on the white matter (WM) fiber bundles connecting them (Friederici, 2009). Previous studies investigating WM bundles in PPA patients mainly focused on the classical fronto-temporo-parietal dorsal and ventral language tracts (Whitwell et al., 2010; Acosta-Cabronero et al., 2011; Galantucci et al., 2011; Agosta et al., 2012, 2013; Grossman et al., 2013; Mahoney et al., 2013; Sajjadi et al., 2013; Schwindt et al., 2013). WM damage in those tracts has been revealed in PPA with differential involvement of tracts in the subtypes of PPA. The MdLF has not yet been studied in PPA; given its location and connectivity, it is very likely that WM integrity of the MdLF in PPA patients would be damaged and associated to language deficits.
Based on our previous investigations of MdLF structural connectivity (Makris, 1999; Makris et al., 2009, 2013a, 2013b) and PPA (Sapolsky et al., 2010; Mesulam et al., 2014, 2014) and the rationale that the connectivity of the superior temporal gyrus and inferior parietal lobule is critical for semantic aspects of language processing (Mesulam et al., 2014), we hypothesized that the MdLF should be structurally damaged in PPA and that these alterations should be correlated with such aspects of semantics as word comprehension and object naming. To this end, we used diffusion tensor imaging (DTI) tractography to reconstruct the MdLF and compared measures of microstructural integrity including fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) between patients with PPA and controls. Given the lateralization of language, we also hypothesized that the MdLF alterations in PPA and behavioral relationships would be asymmetrical, mainly localized in the dominant hemisphere. Furthermore, we hypothesized that damage to the MdLF, would be associated with impairments in specific language domains within the group of PPA patients, with semantic memory and lexical retrieval being the predominant associated impairments with relative sparing of the fluency and grammaticality of speech.
2. Materials and methods
2.1. Subjects
Patients with PPA (n = 20) were recruited from an ongoing longitudinal study being conducted in the Primary Progressive Aphasia Program in the Massachusetts General Hospital Frontotemporal Disorders Unit, and were evaluated using a structured clinical assessment performed by a behavioral neurologist and speech and language pathologist. The diagnosis of PPA was made if (1) a gradually progressive language disturbance was the most salient symptom prompting the patient/family to seek clinical evaluation; (2) the progressive nature of the deficits and the fact that the language disorder was the chief problem during the initial few years of the disease were documented by the history obtained from the patient and an informant who knows the patient well; and (3) the presence of aphasia was documented by a structured clinical evaluation which also demonstrated the absence of other salient deficits. PPA subtypes (agrammatic [PPA-G], semantic [PPA-S], logopenic [PPA-L], and mixed/other [PPA-O]) were diagnosed using an approach similar to that previously described (Mesulam et al., 2009). The severity of language impairment in patients was evaluated by the Progressive Aphasia Severity Scale (PASS). In addition, twenty-five age-matched healthy controls (HCs) were included for MRI analyses. All participants gave written informed consent in accordance with guidelines established by the Partners Human Research Committee.
2.2. MRI acquisition
In this sample, MRI data were acquired using a Siemens Trio 3.0 Tesla scanner (Siemens Medical Systems, Erlangen, Germany). Procedures for data collection included head movement restriction using expandable foam cushions and automated scout and shimming procedures. Whole-brain DTI was performed using a single-shot echo-planar imaging sequence in 64 axial planes with 60 non-collinear diffusion sensitization gradients (b = 700 s/mm2), and 10 reference image with no diffusion weighting (b0 image). Imaging parameters were as follows: TR/TE: 8020/83 msec; field of view: 256 × 256 mm2; acquisition matrix: 128 × 128, and 2-mm section thickness without intersection gaps. Axial sections were acquired parallel to the anterior–posterior commissural line to cover the entire brain. A fluid-attenuated inversion recovery sequence was visually inspected to rule out nondegenerative pathologies.
2.3. DTI analysis
The functional MRI of the brain (FMRIB) software library was employed to process and analyze diffusion tensor imaging data (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). The diffusion-weighted data were registered using an affine registration to the b0 volume to correct for eddy currents and motion artifacts. Prior to fitting the tensor, brain masks of each b0 image were generated using the brain-extraction tool (BET). BET is based on regional properties of the image, where the forces pushing the template outward are locally computed at each vertex. The Diffusion Toolkit (http://trackvis.org/dtk) was then used to fit the tensor and compute the diagonal elements (λ1, λ2 and λ3) at each brain voxel, from which fractional anisotropy (FA), radial diffusivity (RD), axial diffusivity (AD) and mean diffusivity (MD) were subsequently calculated and exported. Whole brain tractography was performed using a b-spline interpolated streamline algorithm (stepsize 0.5 mm; fractional anisotropy threshold 0.15; angle threshold 35) implemented in TrackVis.
2.4. Virtual dissections and tract-specific measurements
Virtual in vivo dissections of the MdLF were performed using TrackVis in 20 PPA patients and 25 controls. Based on priori knowledge of the anatomy of the MdLF, regions of interest (ROI) were defined manually in the native diffusion space on the coronal fractional anisotropy images of each participant, and were used as seed regions for tracking. To dissect the MdLF, we first identified the coronal slice that unambiguously depicted the frontotemporal transition on FA map. Then, a ROI was drawn on a coronal slice that was 18 mm (9 slices) posterior to the frontotemporal transition, and located in the white matter of STG including all STG WM voxels of that slice. Tractography was then performed using a single-seed approach. In order to exclude fibers from neighboring tracts, we used exclusion masks for spurious virtual fibers that were crossing to the opposite hemisphere. Fig. 1 illustrates the topographic relationships between the MdLF and other neighboring long cortico-cortical association fiber tracts such as the inferior longitudinal fascicle and the arcuate fascicle. For each subject's tracts in native space, average FA, MD, RD and AD values were obtained and entered in the statistical analysis (Makris et al., 2013b).
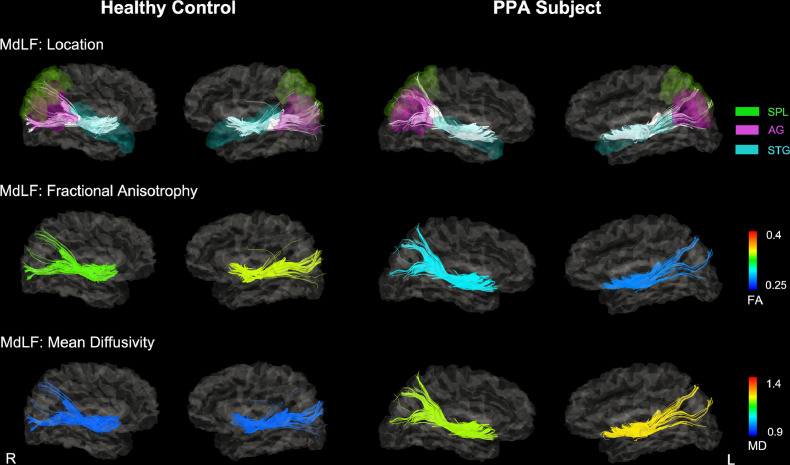
Fig. 1.
Reconstructions of the MdLF in a single healthy control and a PPA subject. Tracts are superimposed onto the subject's T1-weighted image normalized into the DTI space. A. The color of these tracts was scaled by the mean FA value of each tract. B. The color of these tracts was scaled by the mean MD value of each tract. The mean FA value of the MdLF was lower (more blue) in this PPA patient than in healthy control, especially in left hemisphere. The mean MD value of the MdLF was higher (more yellow) in this PPA patient than in healthy control, especially in left hemisphere. Abbreviations: MdLF = middle longitudinal fascicle; PPA = primary progressive aphasia; FA = fractional anisotropy; MD = mean diffusivity; R = right; L= left; STG = superior temporal gyrus; SPL = superior parietal lobe; AG = angular gyrus.
The symmetry of the measured FA, MD, RD and AD by a symmetry Index (Galaburda et al., 1990): where the Leftdiffusivity and Rightdiffusivity are the diffusivity measurements (FA, MD, RD and AD) in the left and right MdLF. Positive values indicate the diffusivity measurements is larger in the left hemisphere.
2.5. Statistical analysis
The statistical analyses of demographic, cognitive data and DTI metric were performed using SPSS software (Version 23, SPSS, Inc., Chicago, IL, USA). A student's t-test (two-tailed) for independent samples or Chi-squared test was used to investigate differences between controls and patients with PPA. We specifically focused on single word comprehension and word retrieval function, and examined their relationship with the averaged DTI-derived metrics of the MdLF. Articulation and fluency were chosen as “control processes” not expected to demonstrate relationships with the MdLF, and their relationship with DTI-derived metrics of the MdLF were also explored. To control the confounding effect of age, partial correlation analysis was used to examine the relationship between DTI-derived metrics of the MdLF (FA, MD, AD, RD) and PASS subscores (articulation, fluency, single word retrieval and word retrieval) in the PPA group.
3. Results
Demographic information and clinical data for these subjects are shown in Table 1. There were no significant differences between groups in age and sex.
Table 1.
. Demographic and clinical characteristics.
| PPA | Controls | |
|---|---|---|
| N | 20 | 25 |
| Age | 63.95 ± 8.00 | 64.88 ± 7.23 |
| Gender (M:F) | 8:12 | 10:15 |
| PPA Subtype (G:S:L:O) | 8:6:5:1 | – |
| Disease Duration (Months) | 48.76 ± 27.96 | – |
| PASS sum of boxes score (N = 18) | 6.42 ± 5.42 | – |
| Articulation (0:0.5:1:2:3) | 12:1:1:2:2 | – |
| Fluency (0:0.5:1:2:3) | 4:8:2:2:2 | – |
| Word retrieval (0:0.5:1:2:3)l | 3:7:5:3:0 | – |
| Single word comprehension (0:0.5:1:2:3) | 11:4:2:1:0 | – |
Abbreviations: PPA = primary progressive aphasia; M = male; F = female; G = agrammatic PPA; S = semantic PPA; L = logopenic PPA; O = Mixed or Uncertain PPA type; PASS = Progressive Aphasia Severity Scale.
3.1. Abnormalities in MdLF integrity in PPA
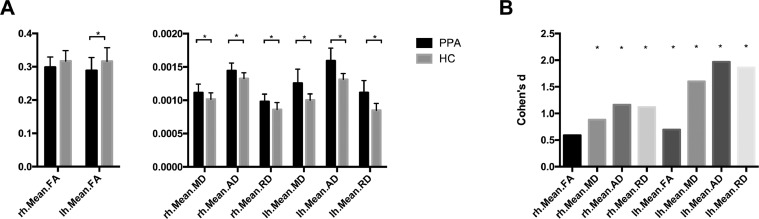
Fig. 1 shows an example of the reconstructed trajectories of the MdLF of a single healthy control and a PPA subject. Visual assessments of these maps showed that the MdLF had lower mean FA values and higher mean MD values in the PPA group than the control groups, especially in the left hemisphere. Group comparison between two groups revealed that PPA subjects had higher mean FA value in the left MdLF (two-tailed t(43) = 2.32, p = 0.025), and lower mean MD (left: two-tailed t(43) = 5.33, p < 0.001; right: two-tailed t(43) = 2.94, p = 0.005), AD (left: two-tailed t(43) = 6.56, p < 0.001; right: two-tailed t(43) = 3.86, p < 0.001) and RD (left: two-tailed t(43) = 6.21, p < 0.001; right: two-tailed t(43) = 3.73, p < 0.001) of the bilateral MdLF compared with HCs. Fig. 2A shows the bar graphs of averaged DTI-derived metrics of the MdLF (FA, MD, AD, RD) for PPA patients and HCs. We also calculated the Cohen's d score (McGrath and Meyer, 2006) for each measure to compare the effect sizes between the measures. Fig. 2B shows the Bar graphs of Cohen's d score. For all effects, the group differences were larger in the left hemisphere than in the right hemisphere.
Fig. 2.
A: Bar graphs of averaged DTI-derived metrics of the MdLF (FA, MD, AD, RD) for PPA patients and HCs. B: Bar graphs of Cohen's d effect size indicating the standardized difference between PPA patients and HCs. * indicate significant group difference between PPA patients and HCs (p < 0.05). Abbreviations: MdLF = middle longitudinal fascicle; PPA = primary progressive aphasia; HC = healthy control; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; AD = axial diffusivity; rh = right hemisphere; lh = left hemisphere.
3.2. Relationship between MdLF integrity and language function
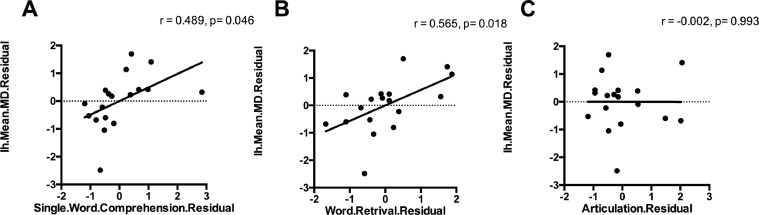
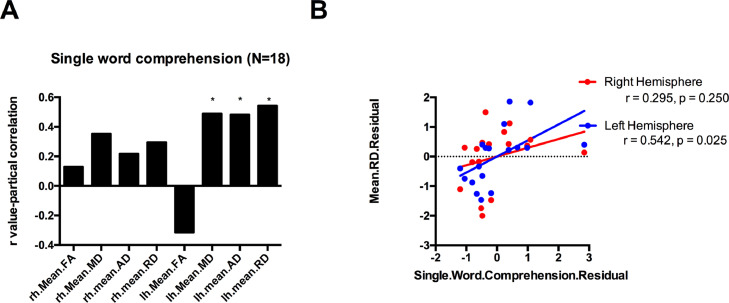
The PASS single word comprehension subscore was correlated with mean MD, AD and RD of the MdLF in left hemisphere (r = 0.565, p = 0.018; r = 0.627, p = 0.007; r = 0.617, p = 0.008), but not in the right hemisphere (p > 0.05). The word retrieval subscore was also correlated with mean MD, AD and RD of the MdLF in left hemisphere (r = 0.489, p = 0.046; r = 0.483, p = 0.049; r = 0.542, p = 0.025), but not in the right side (p > 0.05). There was no significant correlation between articulation or fluency subscores and DTI-derived metrics of the MdLF (p > 0.05). Fig. 3 illustrates this dissociation: semantic and lexical retrieval functions are related to MdLF integrity (panels A and B) but articulation is not (panel C). Fig. 4 provides more detail on the asymmetry of these relationships, with the largest effects between left hemisphere MdLF diffusivity measures and single word comprehension and smaller, non-significant effects in the same direction in the right hemisphere.
Fig. 3.
Scatterplots show the relationship between articulation/single word comprehension/word retrieval subscore and mean MD value of the left MdLF. Mean MD value of the left MdLF have significant correlation with single word comprehension and word retrieval subscore, but not with articulation subscore. Abbreviations: MdLF = middle longitudinal fascicle; MD = mean diffusivity; rh = right hemisphere; lh= left hemisphere.
Fig. 4.
A: Bar graphs of r-values, which illustrates the size of the relationship between mean FA/MD/AD/RD of the MdLF and single word comprehension subscore in PPA patients. Single word comprehension subscore was significantly correlated with mean MD, AD and RD of the MdLF in left hemisphere, but not in the right side. B: Scatterplot shows the relationship between single word comprehension and mean RD of the MdLF. * (p<0.05). Abbreviations: MdLF = middle longitudinal fascicle; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; AD = axial diffusivity; rh = right hemisphere; lh= left hemisphere.
3.3. Hemispheric asymmetry of MdLF integrity in PPA
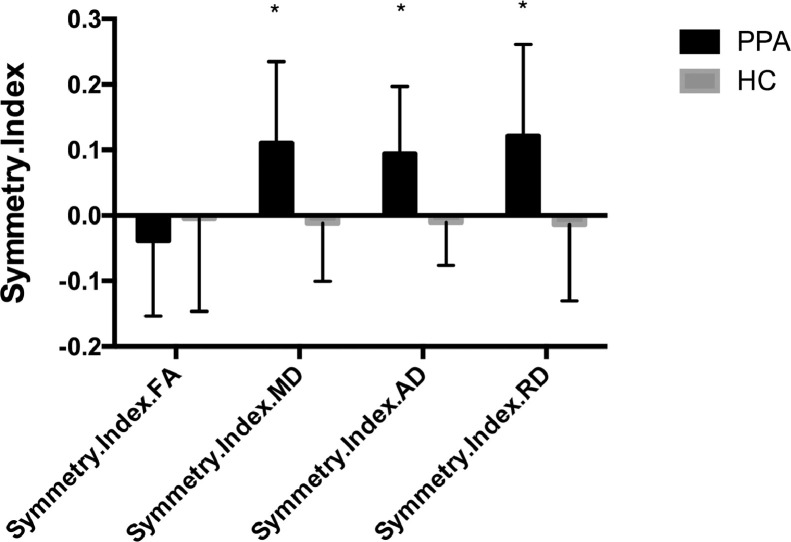
Asymmetry is here defined as a distribution of symmetry indices that is significantly non-zero. MdLF FA in the PPA group showed a marginal, non-significant rightward asymmetry (MD: −0.039 ± 0.115 [two-tailed t(19) = 1.46; p < 0.05]), whereas the FA in the control group was symmetric. MD, AD and RD in the PPA group demonstrated a leftward asymmetry (MD: 0.111 ± 0.124 [two-tailed t(19) = 3.98; p < 0.001]; AD: 0.094 ± 0.103 [two-tailed t(19) = 4.11; p < 0.001]; RD: 0.111 ± 0.140 [two-tailed t(19) = 3.86; p = 0.001]), whereas those of the control group were symmetric (MD: −0.092 ± 0.407 [two-tailed t(24) = 1.13]; AD: −0.011 ± 0.065 [two-tailed t(24) = 0.83]; RD: −0.014 ± 0.116 [two-tailed t(24) = 0.60]; all p values > 0.05). The symmetry indices of MD, AD and RD were different between groups (MD: two-tailed t(43) = 2.15, p = 0.038; AD: two-tailed t(43) = 4.18, p < 0.001; RD: two-tailed t(43) = 3.53, p = 0.001). Fig. 5 shows the averaged symmetry indices in the PPA and control groups.
Fig. 5.
Bar graphs illustrating Symmetry Index in the PPA and control groups. * indicates that the average symmetry index is different from zero (P < 0.05). Abbreviations: PPA = primary progressive aphasia; HC = healthy control; FA = fractional anisotropy; MD = mean diffusivity; RD = radial diffusivity; AD = axial diffusivity.
4. Discussion
In this study, we delineated the MdLF tract connecting STG and TP principally with AG and SMG in PPA patients and their matched controls by using DTI tractography. We found severe WM abnormalities in the MdLF in PPA patients, which were most prominent in the left hemisphere. Moreover, the WM alterations of the MdLF in the dominant hemisphere were associated with impairments in word comprehension and word retrieval in PPA patients, indicating a more specific role of the MdLF in semantic and lexical aspects of language processing. These relationships were specific: there was no relationship between MdLF integrity and articulation or fluency language measures. Furthermore, the symmetry of the DTI metrics of this tract present in healthy controls was lost in PPA, with a clear left-lateralized pattern of abnormality in patients with impaired language function.
Current theories on brain organization suggest that language functions are organized as distributed, segregated, and overlapping networks (Mesulam, 1990; Petrides, 2013; Mesulam et al., 2014). This network-based approach suggests that not only cortical lesions, but also damage to the WM connections between cortical areas can lead to a neural dysfunction and, hence, language impairments. Using DTI, studies have demonstrated the presence of WM damage in the PPA patients (Agosta et al., 2010; Acosta-Cabronero et al., 2011; Galantucci et al., 2011; Agosta et al., 2012, 2013; Schwindt et al., 2013). In voxel-based exploratory studies, PPA patients showed consistent WM damage in several frontal, temporal and parietal regions, where cortical atrophy is also evident (Acosta-Cabronero et al., 2011; Agosta et al., 2012; Schwindt et al., 2013). However, due to the nature of the voxel-based analysis, these studies could only infer in general terms which specific tracts were involved. Tractography analyses are instead helpful to quantify the WM damage in specific white matter pathways, and have successfully examined the microstructure of white matter in PPA patients (Agosta et al., 2010; Acosta-Cabronero et al., 2011; Galantucci et al., 2011; Agosta et al., 2013; Mandelli et al., 2014). Most investigations focused on the standard fronto-temporo-parietal dorsal and ventral language pathways: the dorsal pathway mainly consists of superior longitudinal fascicle (SLF), while the ventral pathway mainly includes inferior longitudinal fascicle (ILF) and uncinate fascicle (UF). These studies showed WM damage in ventral tracts was most evident in the semantic variant PPA patients, whereas WM damage in dorsal tracts was more prominent in non-fluent variant PPA patients (Agosta et al., 2010; Galantucci et al., 2011, 2013). Although the role of standard dorsal and ventral tracts in language has been well documented in PPA patients, there are other tracts connecting to language areas that remain to be explored. The MdLF was originally demonstrated in the rhesus monkey by Seltzer and Pandya (1984), and its existence in the human brain has been demonstrated only recently (Makris and Pandya, 2009). This fiber bundle courses through the STG and IPL connecting STG and TP principally with the AG (Makris, 1999; Makris et al., 2009; De Champfleur et al., 2013; Kamali et al., 2014). It also connects STG and TP with other parietal and occipital regions, specifically the supramarginal gyrus (SMG) (Makris et al., 2013a, 2016), the superior parietal lobule (SPL) (Makris et al., 2013a; Wang et al., 2013), precuneus (Makris et al., 2013a; Wang et al., 2013), cuneus (Makris et al., 2013a; Wang et al., 2013), and lateral occipital areas (Makris et al., 2013a; Maldonado et al., 2013). The present study provides novel evidence for the involvement of the MdLF in PPA patients.
While diffusivity measures showed a clear increase in the left-greater-than-right bilateral MdLF of PPA patients, the changes in FA were less evident. Similarly, Agosta et al. (2010) reported in a previous study on semantic variant patients that FA did not change in another white matter tract of the temporal lobe—the inferior longitudinal fasciculus—while changes of other diffusivity metrics were evident. FA is a function of the ratio between parallel to transverse diffusivities. If changes along the direction of the major axis of the diffusion ellipsoid (AD) were proportional to those of the minor axes (RD), then FA would remain relatively unchanged. This likely explains why FA changes are not always found. This pattern of DTI changes could reflect a combination of decreased axonal packing in white matter structures and demyelination, allowing for increased diffusivity in all orientations within a voxel (Agosta et al., 2010). Atrophic changes, gliosis and demyelination have been observed in post-mortem studies of patients with PPA (Mesulam et al., 2014). These pathological changes are likely the basis of the DTI abnormalities in the present study, although this deserves systematic study. Despite evident cortical atrophy, white matter changes are often not detectable by conventional MRI in early PPA. Thus, the underlying mechanism for white matter abnormalities in PPA is most likely to be axonal degeneration of with matter fibers secondary to neuronal degeneration, although when PPA patients have tau pathology there is often substantial axonal pathology.
In our previous work, our group has indicated the MdLF as an important component of the perisylvian language system in the dominant hemisphere and may be related to language processing (Makris et al., 2009, 2013a, 2013b; Makris et al., 2016). However, the MdLF's involvement in language processing has been questioned by other investigators (De Witt Hamer et al., 2011; Wang et al., 2013). Specifically, De Witt Hamer et al. (2011) suggested the MdLF may participate but is not essential for language processing based on their intraoperative brain electrostimulation observations of no interference with picture naming. Besides, Wang and colleagues (Wang et al., 2013) suggested against a role for the MdLF in language as their results showed minimal connections between STG and AG. In the present study, we found that severity of diffusion abnormalities of the MdLF in the dominant hemisphere were correlated with performance in word comprehension and naming in PPA patients, confirming the functional role of the MdLF in language processing. There is growing body of evidence suggesting that the left anterior temporal lobe needs to be concerned as part of the canonical language network with a critical role in single word comprehension and object naming (Mesulam et al., 2015). Damage to the MdLF could disconnect this region with parietal language-related regions, thus contributing to the impairments in word comprehension and naming in PPA patients.
Here the MdLF was found to be symmetrical for DTI metrics in controls. Given that the MdLF may be involved in language in the left hemisphere, but may also play an important role in visuospatial attention in the right hemisphere, it is possible the left and right MdLFs are equally robust and, therefore, show no significant laterality difference in their biophysical characteristics. Instead, PPA patients showed clear laterality differences in MD, AD and RD, while FA was not statistically significant. This finding is in line with more pronounced WM damage of the MdLF in the left hemisphere, indicating the underlying asymmetrical neurodegeneration of the left hemisphere in PPA.
5. Limitations and future studies
There are certain limitations that should be considered when interpreting the results of this study. One issue relates to the use of the diffusion tensor-based technique. Diffusion tensor models deal poorly with crossing fibers, which result in a more spherical shape of the diffusion tensor ellipsoid, even in the absence of white matter damage. Another issue is that assessments were exclusively cross-sectional. A more complete understanding of how the MdLF correlates with the PPA syndromes will require larger disease cohorts followed longitudinally. Moreover, other cortico-cortical association fiber pathways such as the inferior longitudinal fasciculus (ILF) and the arcuate fasciculus (AF) may show abnormalities in PPA. The MdLF, nevertheless has not received attention in this disorder. As shown in Fig. 6, whereas the MdLF is clearly a distinctly different fiber tract from other language related long association fiber tracts, there is also co-sharing of origins and terminations of their fibers. The latter would explain the overlap of some functions as well. It should also be noted that the anatomy of MdLF in humans is currently a topic of debate and that its definition has been expanded since its discovery in humans (Makris, 1999; Makris et al., 2009), in which MdLF was demonstrated to be a long corticocortical association connection between the superior temporal gyrus (including the temporal pole) and the inferior parietal lobule (i.e., AG and SMG). We view the present study as the first step in the investigation of the major language related pathways including the MdLF in PPA. Future studies need to clarify further the individual contributions of the different long association fiber tracts associated with the temporo-parietal cortical regions. Lastly, our results lack histopathologic confirmation. Interpretation of WM damage as an indication of pathological substrates therefore remains speculative. A further crucial issue that was not directly addressed here is the relation between tract degeneration and GM atrophy. Further studies are needed to elucidate this question.
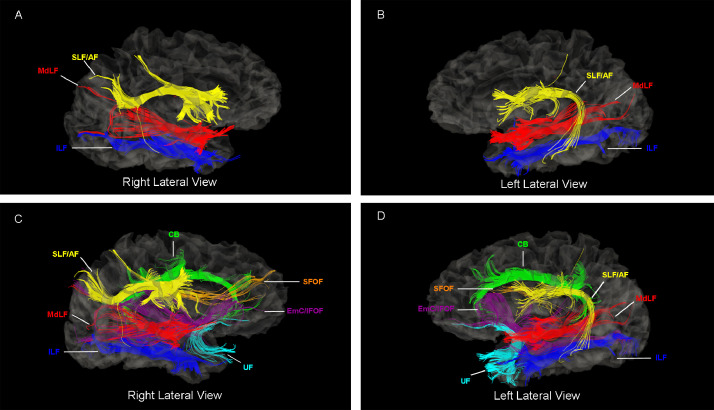
Fig. 6.
Illustration of the middle longitudinal fascicle (MdLF) and its neighboring cortico-cortical long association fiber tracts. Specifically, the inferior longitudinal fasciculus (ILF), superior longitudinal fascicle/arcuate fascicule (SLF/AF), cingulum bundle (CB), uncinate fasciculus (UF), superior longitudinal fasciculus (SFOF) and the extreme capsule/inferior longitudinal fasciculus (EmC/IFOF) complex are shown in the left hemisphere of a representative subject that participated in the present study as a healthy control. As shown in this figure, whereas the MdLF is clearly a distinctly different fiber tract from ILF and SLF/AF, there may be also co-sharing of origins and terminations of their fibers. The latter notion has been demonstrated in previous studies of our group (see e.g., Figure 8 in Makris et al., 2016).
6. Conclusion
In conclusion, this is the first study to document WM damage of the MdLF in PPA patients, adding new insight into the localization and relative severity of white matter fiber connections involved in PPA. These changes have significant correlations with deficits in word comprehension and naming, although the exact correspondence to the underlying neuropathology remains to be elucidated.
Funding
Supported by R01DC014296 from National Institute on Aging & National Institute of Deafness and Other Communication Disorders, R21NS077059 & R21NS079905 from National Institute of Neurological Disorders and Stroke, R01AG042512 from National Institute on Aging & National Institute of Mental Health, R21AT008865 from National Center for Complementary and Integrative Health, R01MH111917 from National Institute of Mental Health, R21DA042271 from National Institute on Drug Abuse and the MGH Morphometric Analysis Center core. Imaging was performed at the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital.
Informed consent
Informed consent was obtained from all patients that participated in this study.
CRediT authorship contribution statement
Chunyan Luo: Formal analysis, Validation, Investigation, Data curation, Methodology, Conceptualization, Writing - original draft, Visualization. Sara Makaretz: Data curation, Formal analysis, Software, Resources. Michael Stepanovic: Data curation, Formal analysis, Software, Resources. George Papadimitriou: Data curation, Formal analysis, Software, Resources. Megan Quimby: Validation, Investigation, Data curation. Senthil Palanivelu: Visualization, Formal analysis, Writing - review & editing. Bradford C. Dickerson: Validation, Investigation, Methodology, Conceptualization, Writing - original draft, Visualization, Investigation, Project administration, Supervision, Writing - review & editing, Funding acquisition, Resources. Nikos Makris: Validation, Investigation, Methodology, Conceptualization, Writing - original draft, Visualization, Investigation, Project administration, Supervision, Writing - review & editing, Funding acquisition, Resources.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- Acosta-Cabronero J., Patterson K., Fryer T.D., Hodges J.R., Pengas G., Williams G.B. Atrophy, hypometabolism and white matter abnormalities in semantic dementia tell a coherent story. Brain. 2011;134(Pt 7):2025–2035. doi: 10.1093/brain/awr119. [DOI] [PubMed] [Google Scholar]
- Agosta F., Galantucci S., Canu E., Cappa S.F., Magnani G., Franceschi M. Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia: a DT MRI study and a literature review. Brain Lang. 2013;127(2):157–166. doi: 10.1016/j.bandl.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Agosta F., Henry R.G., Migliaccio R., Neuhaus J., Miller B.L., Dronkers N.F. Language networks in semantic dementia. Brain. 2010;133(1):286–299. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F., Scola E., Canu E., Marcone A., Magnani G., Sarro L. White matter damage in frontotemporal lobar degeneration spectrum. Cereb. Cortex. 2012;22(12):2705–2714. doi: 10.1093/cercor/bhr288. [DOI] [PubMed] [Google Scholar]
- De Champfleur N., Lima Maldonado I., Moritz-Gasser S., Machi P., Le Bars E., Bonafe A. Middle longitudinal fasciculus delineation within language pathways: a diffusion tensor imaging study in human. Eur. J. Radiol. 2013;82(1):151–157. doi: 10.1016/j.ejrad.2012.05.034. [DOI] [PubMed] [Google Scholar]
- De Witt Hamer P.C., Moritz-Gasser S., Gatignol P., Duffau H. Is the human left middle longitudinal fascicle essential for language? A brain electrostimulation study. Hum. Brain Mapp. 2011;32(6):962–973. doi: 10.1002/hbm.21082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. Pathways to language: fiber tracts in the human brain. Trends Cogn. Sci. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Galaburda A.M., Rosen G.D., Sherman G.F. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28(6):529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- Galantucci S., Tartaglia M.C., Wilson S.M., Henry M.L., Filippi M., Agosta F. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134(Pt 10):3011–3029. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Dronkers N.F., Rankin K.P., Ogar J.M., Phengrasamy L., Rosen H.J. Cognition and anatomy in three variants of primary progressive aphasia. Ann. Neurol. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M., Powers J., Ash S., McMillan C., Burkholder L., Irwin D. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang. 2013;127(2):106–120. doi: 10.1016/j.bandl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali A., Flanders A.E., Brody J., Hunter J.V., Hasan K.M. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct. Funct. 2014;219(1):269–281. doi: 10.1007/s00429-012-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney C.J., Malone I.B., Ridgway G.R., Buckley A.H., Downey L.E., Golden H.L. White matter tract signatures of the progressive aphasias. Neurobiol. Aging. 2013;34(6):1687–1699. doi: 10.1016/j.neurobiolaging.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N. Behavioral Neuroscience. Boston University; Boston: 1999. Delineation of human association fiber pathways using histologic and magnetic resonance methodologies. [Google Scholar]
- Makris N., Pandya D.N. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct. Funct. 2009;213(3):343–358. doi: 10.1007/s00429-008-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Papadimitriou G.M., Kaiser J.R., Sorg S., Kennedy D.N., Pandya D.N. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb. Cortex. 2009;19(4):777–785. doi: 10.1093/cercor/bhn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Preti M.G., Asami T., Pelavin P., Campbell B., Papadimitriou G.M. Human middle longitudinal fascicle: variations in patterns of anatomical connections. Brain Struct. Funct. 2013;218(4):951–968. doi: 10.1007/s00429-012-0441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Preti M.G., Wassermann D., Rathi Y., Papadimitriou G.M., Yergatian C. Human middle longitudinal fascicle: segregation and behavioral-clinical implications of two distinct fiber connections linking temporal pole and superior temporal gyrus with the angular gyrus or superior parietal lobule using multi-tensor tractography. Brain Imaging Behav. 2013;7(3):335–352. doi: 10.1007/s11682-013-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N., Zhu A., Papadimitriou G.M., Mouradian P., Ng I., Scaccianoce E. Mapping temporo-parietal and temporo-occipital cortico-cortical connections of the human middle longitudinal fascicle in subject-specific, probabilistic, and stereotaxic Talairach spaces. Brain Imaging Behav. 2016;11(5):1258–1277. doi: 10.1007/s11682-016-9589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado I.L., de Champfleur N.M., Velut S., Destrieux C., Zemmoura I., Duffau H. Evidence of a middle longitudinal fasciculus in the human brain from fiber dissection. J. Anat. 2013;223(1):38–45. doi: 10.1111/joa.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli M.L., Caverzasi E., Binney R.J., Henry M.L., Lobach I., Block N. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J. Neurosci. 2014;34(29):9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J., da Silva-Freitas R., Caballero H., Marco de Lucas E., Garcia-Porrero J.A., Vazquez-Barquero A. Fiber dissection and diffusion tensor imaging tractography study of the temporoparietal fiber intersection area. Neurosurgery. 2013;72(1 Suppl Operative):87–97. doi: 10.1227/NEU.0b013e318274294b. discussion -8. [DOI] [PubMed] [Google Scholar]
- McGrath R.E., Meyer G.J. When effect sizes disagree: the case of r and d. Psychol. Methods. 2006;11(4):386–401. doi: 10.1037/1082-989X.11.4.386. [DOI] [PubMed] [Google Scholar]
- Mesulam M., Wieneke C., Rogalski E., Cobia D., Thompson C., Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch. Neurol. 2009;66(12):1545–1551. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M.M. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann. Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mesulam M.M., Rogalski E.J., Wieneke C., Hurley R.S., Geula C., Bigio E.H. Primary progressive aphasia and the evolving neurology of the language network. Nat. Rev. Neurol. 2014;10(10):554–569. doi: 10.1038/nrneurol.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M.M., Thompson C.K., Weintraub S., Rogalski E.J. The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain. 2015;138(Pt 8):2423–2437. doi: 10.1093/brain/awv154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Academic Press; 2013. Neuroanatomy of Language Regions of the Human Brain. [Google Scholar]
- Sajjadi S.A., Acosta-Cabronero J., Patterson K., Diaz-de-Grenu L.Z., Williams G.B., Nestor P.J. Diffusion tensor magnetic resonance imaging for single subject diagnosis in neurodegenerative diseases. Brain. 2013;136(Pt 7):2253–2261. doi: 10.1093/brain/awt118. [DOI] [PubMed] [Google Scholar]
- Sapolsky D., Bakkour A., Negreira A., Nalipinski P., Weintraub S., Mesulam M.M. Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology. 2010;75(4):358–366. doi: 10.1212/WNL.0b013e3181ea15e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky D., Domoto-Reilly K., Dickerson B.C. Use of the progressive aphasia severity scale (PASS) in monitoring speech and language status in PPA. Aphasiology. 2014;28(8–9):993–1003. doi: 10.1080/02687038.2014.931563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt G.C., Graham N.L., Rochon E., Tang-Wai D.F., Lobaugh N.J., Chow T.W. Whole-brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Hum. Brain Mapp. 2013;34(4):973–984. doi: 10.1002/hbm.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B., Pandya D.N. Further observations on parieto-temporal connections in the rhesus monkey. 1984;55(2):301–312. doi: 10.1007/BF00237280. [DOI] [PubMed] [Google Scholar]
- Wang Y., Fernandez-Miranda J.C., Verstynen T., Pathak S., Schneider W., Yeh F.C. Rethinking the role of the middle longitudinal fascicle in language and auditory pathways. Cereb. Cortex. 2013;23(10):2347–2356. doi: 10.1093/cercor/bhs225. [DOI] [PubMed] [Google Scholar]
- Whitwell J.L., Avula R., Senjem M.L., Kantarci K., Weigand S.D., Samikoglu A. Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology. 2010;74(16):1279–1287. doi: 10.1212/WNL.0b013e3181d9edde. [DOI] [PMC free article] [PubMed] [Google Scholar]