Abstract
Once thought to have revolutionized therapeutic intervention in surgery, Recombinant Human Bone Morphogenic Protein 2 (rhBMP2) is now in its second decade of sustained controversy over the side effects associated with its use. Side effects associated with clinical use of rhBMP2 (Infuse, Medtronic Inc) include a marked inflammatory response, pain, therapeutic failures, ectopic bone, tissue degradation, and death. What is missing, despite the depth of literature on the subject, is a direct interrogation of rhBMP2, specifically for inflammation. Here we set out to determine if rhBMP2 alters traditional macrophage markers associated with pro-inflammatory responses, and pro-reparative responses to injury. Based on our previous work, we hypothesized there would be no direct effect of peptide on macrophage polarization. Here we utilized commercially available murine macrophages, RAW 264.7, and treated these cells with rhBMP2 in standard growth media or macrophage polarizing media (M1 and M2) at several doses of the peptide. Our readouts were cell viability, apoptosis, gene expression of M1 and M2 markers, and ELISA for M1 marker iNOS, and M2 marker Arg1. Our data give very little evidence to support an alteration in macrophage phenotype by rhBMP2 alone, or alteration of the phenotype when cultured in enriched M1 or M2 media. These results further suggest that other factors associated with the clinical use of Infuse, likely supraphysiological rhBMP2 doses and off label usage, are more likely the culprit for poor outcomes. This further reinforces the utility of rhBMP2 and other peptides in tissue engineering therapies when conditions are tightly controlled.
Keywords: rhBMP2, Macrophage, Polarization, Inflammation
1. Introduction1
Controversy exists for the use of biologicals and the products of bioengineering technology in medical therapy (Badylak et al., 2011; Krzyszczyk et al., 2018; Lowenthal and Sugarman, 2015; Lu et al., 2015; Nerem, 2006; Sabetkish et al., 2015; Taylor et al., 2014; Vaquette et al., 2018; Yano et al., 2018). Concerns primarily center on spatial and temporal control of combinatory therapies that often include some combination of cell, growth factor, and scaffold delivery for the treatment of disease or augmentation of surgical intervention and tissue repair. Possibly one of the biggest controversies has centered around the use of recombinant human bone morphogenic proteins (rhBMP) more specifically rhBMP2 which has been patented and marketed as Infuse by Medtronic (Cooper and Kou, 2018; Hopper and Kapadia, 2018; Schnurman et al., 2016; Vorrasi and Kolokythas, 2017). Infuse is FDA approved for several indications including closed tibial fracture, spinal fusion, alveolar ridge augmentation, and maxillary sinus lift. However, greater than 90% of reported use of Infuse is off label. This off label use may potentially be fueling the controversy (Bauer and Muschler, 2000; Giannoudis et al., 2005; Kaiser et al., 2014; Miyazaki et al., 2009; Sarkar and Lee, 2015). In 2008 the FDA issued a black box warning concerning the side effects of Infuse and removed cervical spinal fusion as an indication after several deaths were attributed to the use of Infuse (Molinari et al., 2016; Molinari and Molinari, 2016). Nevertheless, Infuse continues to be used regularly in the fields of orthopedics and dentistry. Reported side effects of Infuse treatment, particularly in dental procedures, include rampant inflammation, pain, seroma formation ,and even surgical failures (James et al., 2016; Suarez-Lopez Del Amo et al., 2015; Vaquette et al., 2018; Vorrasi and Kolokythas, 2017).
Marshall Urist identified rhBMP2 in 1965 after experimentation definitively showed its ability to produce ectopic bone within a muscle pocket in a surgical lagomorph model (Canalis, 1985; De Biase and Capanna, 2005; Einhorn, 2003; Ripamonti, 2017; Ronga et al., 2013; Urist, 1965; Urist et al., 1983). Decades of improvement followed with research primarily focused on how to make delivery more efficient in order to augment bone healing and create bone in spaces that previously could only be treated with autologous bone graft, allograft, xenograft, or alloplastic graft. Although there remains disagreement over whether the rhBMP2 delivery schema is appropriate due to purported poor binding of the growth factor to scaffold (Howie et al., 2018a; Hsu et al., 2013; Suarez-Gonzalez et al., 2012) and the supraphysiological doses delivered (James et al., 2016; Krishnan et al., 2017; Zara et al., 2011) it remains a fact that use of Infuse precipitates bone fill when used for appropriate clinical indications.
Our group and others have been focused on the pathway of effect for delivery of rhBMP2 using preclinical surgical models (Durham et al., 2018a; Durham et al., 2018b; Grey et al., 2019; Herberg et al., 2017; Herberg et al., 2014; Howie et al., 2018a; Howie et al., 2018b). Within our models, we have been relatively unable to replicate the side effect of rampant inflammation that is described in the clinical literature case studies. In fact, much of our preclinical data has even suggested that not only does rhBMP2 not precipitate an abnormal inflammatory response to injury, in some conditions it may even act in an anti-inflammatory manner (Durham et al., 2018a; Grey et al., 2019). These data precipitated our focus on rhBMP2 in the inflammatory axis. In this work we hypothesize that rhBMP2 does not alter macrophage activity, specifically polarization, when modeled in vitro. We utilize murine derived macrophages and culture conditions that mimic an M1, pro-inflammatory environment and M2 pro-reparative environment and utilize transcriptional and translational readouts to address this controversy.
2. Materials and Methods
2.1. Cells and Growth Conditions
Raw 264.7 cells (ATCC, TIB-71) were obtained as a gift from Dr. Beth Lee, The Ohio State University. These cells were maintained in a 75cm2 flask using standard culture media (Dulbecco’s Modified Eagle’s Medium (DMEM, Lonza, Walkersvile Inc., MD, 12604F) with 10% Fetal Bovine Serum (FBS, Atlanta Biologicals, Atlanta, GA, S11150H) and 1% Penicillin/Streptomycin (Lonza, 10k/10k 17–602E)) until 85% confluent, when they were moved to a 175cm2 flask after cell scraper dissociation. Cells were sub-cultured as necessary throughout the experimentation.
To induce M1 pro-inflammatory macrophage polarization standard culture media (as defined above) was supplemented with 100 ng/ml Lipopolysaccharides (LPS, Sigma-Aldrich, St. Louis, MO, L2880) and 100 ng/ml Interferon-gamma (IFN-Gamma, R&D Systems, Minneapolis, MN, 485-MI/CV). For M2 pro-reparative macrophage polarization, standard culture media was supplemented with 100 ng/ml Interleukin-4 (IL4, R&D Systems, 404-Ml/CV)(Wei et al., 2018).
For rhBMP2 treatment, recombinant human bone morphogenic protein 2 (rhBMP2, Medtronic, Warsaw, IN) was reconstituted in sterile water per manufacturer protocol. For cell treatment, rhBMP2 was diluted in cell culture media to the following concentrations: 0 ng/ml, 10 ng/ml, 50 ng/ml, and 100 ng/ml.
2.2. Immunocytochemistry
To assess presence of iNOS (inducible nitic oxide synthase) and Arginase, RAW 264.7 cells were seeded at a density of 25,000 per well in 8 well chamber slides (Thermo Fisher, Waltham, MA,154534). Cells were treated for 24 hours with standard culture media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2, M1 polarization media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2, or M2 polarization media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2. For immunocytochemistry, cells were washed with phosphate buffered saline (PBS, HyClone, Fisher Scientific, SH30256.01), fixed with 10% Neutral Buffered Formalin, permeabilized with 0.1 % Triton-x, and blocked with 1% Bovine Serum Albumin. Cells were incubated overnight at 4°C with Anti-iNOS antibody (iNOS, AbCam, Cambridge, MA, ab15323, 1:100) to confirmM1 polarization and Anti-Arginase antibody (Arginase, AbCam, ab91279, 1:200) to confrimM2 polarization. After primary antibody incubation, cells were washed and incubated with fluorescent tagged (FITC) secondary antibody (AbCam, ab96899, 1:250) for one hour at room temperature in the dark. Cells were then washed, chambers were removed, and slides were mounted with Vectashield with DAPI nuclear counterstain (Vector Laboratories, Burlingame, CA, H-1500).
All stained slides were photographed for analysis using FITC and DAPI epifluorescence detection (Olympus, Miami, FL, USA, TH4–100). A region of interest including the center of each chamber on three replicate slides for each treatment was imaged at 10x magnification for analysis. Positive cell counts were assessed using Image J Software to convert color images to greyscale, set a standard threshold, create a binary mask, and analyze particles, to count single color images. Counts for positive iNOS and Arginase were standardized to total cell counts (DAPI).
2.3. Cell Activity Assays
To determine the effects of rhBMP2 on RAW 264.7 cell polarization, cells were seeded into 96 well plates at a density of 4,000 cells per well. Cells were treated with standard culture media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2, M1 polarization media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2, or M2 polarization media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2 for 24 hours. Cell viability (proliferation) was assessed with the colorimetric MTS assay (Promega, Madison, WI, G3581) and apoptosis was assessed using the Apo-ONE Caspase-3/7 assay (Promega, G7791) per manufacturer’s protocol using a gen5 plate reader (BioTek, Winooski, VT).
2.4. Quantitative Real-Time PCR
RAW 264.7 cells were seeded at a density of 150,000 cells per well in 6 well culture plates, and treated for 24 hours with standard culture media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2, M1 polarization media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2, or M2 polarization media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2. RNA was isolated using the OMEGA bio-tek E.Z.N.A. Total RNA Kit 1 (Omega Bio-tek, Norcross, GA, R6834–02) according to manufacturer’s protocol. Quality and quantity of RNA was assessed using a Synergy Hi Microplate reader and a Take3 Microvolume Plate (BioTek). Complimentary DNA synthesis was performed using Quanta qScript cDNA Synthesis reagents following manufacturer’s protocol (Quanta Biosciences, Beverly, MA, 95047–025).
To quantify expression of M1 and M2 macrophage polarization targets, cDNA was subjected to quantitative PCR using Applied Biosystems TaqMan Gene Expression Master Mix and targeted TaqMan gene expression assays for the following M1 macrophage targets; Nos2 (Mm00440502_m1), Il6 (Mm00446190_m1), Tnf-Alpha (Mm00443258_m1), Sosc3 (Mm00545913_s1), Il1β (Mm00434228_m1), and Il12b (Mm001288989_m1) and M2 macrophage targets; Arg1 (Mm00475988_m1), Il10 (Mm01288386_m1), Mrc1 (Mm01329362_m1), Ppar (Mm00440940_m1). Data were normalized to 18S (Mm03928990_g1) ribosomal RNA expression by ΔCT. Quantitative data were compared for gene expression changes due to rhBMP2 treatment by ΔΔCT methodology. Previously published statistical analysis methodology was used to determine differences for gene expression after rhBMP treatment (Yuan et al., 2006). Differences were considered significant if p≤0.05 and are represented by fold change compared to control 0 ng/ml rhBMP2 in Figures 1–3. To confirm the function of M1 and M2 polarization media, gene expression was compared to standard culture media alone (Sup Fig 1) and differences were considered significant if p≤0.05
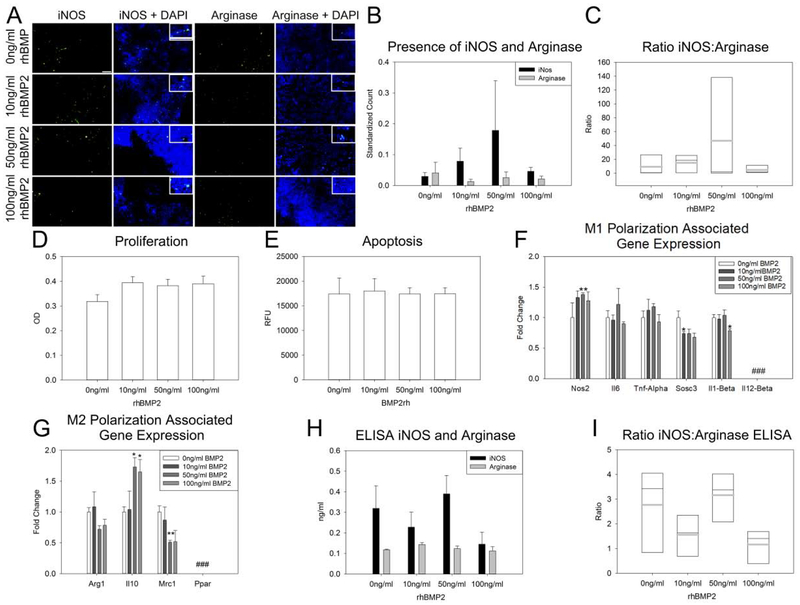
Figure 1: BMP2 does not affect macrophage polarization.
A. Representative images of iNOS (first column) and Arginase (third column) stained RAW 264.7 cells treated with 0 ng/ml, 10 ng/ml, 50 ng/ml and 100 ng/ml BMP2 (descending order). Higher magnification inset for cellular localization of staining. B. Count of positive INOS and Arginase cells normalized to total cells (DAPI). C. Ratio of iNOS:Arginase positive cells natively in the macrophage population. BMP2 treatment did not affect cell proliferation (D) or apoptosis (E). F. BMP2 treatment did increase expression of M1 pro-inflammatory gene Nos2 with the 50 ng/ml dose, reduced expression of Sosc3 with the 10 ng/ml dose, and reduced expression of Il1-Beta at the 100 ng/ml dose. No native expression of Il12-Beta was detected in these cells. G. M2 pro-reparative gene expression with BMP2 treatment indicates an increase in Il10 expression with 50 ng/ml and 100 ng/ml BMP2 and a decrease in expression of Mrc1 with 50 ng/ml BMP2. No native expression of Ppar was identified in these cells. H–I. Quantity of iNOS and Arginase were not changed with BMP2 treatment (H), nor did BMP2 treatment affect macrophage polarization (I). n=3 replicates per assay Scale= 100 μm Grey horizontal bar = mean Black horizontal bar = media (C,I) ### Expression below detectable threshold *p≤0.05 **p≤0.01
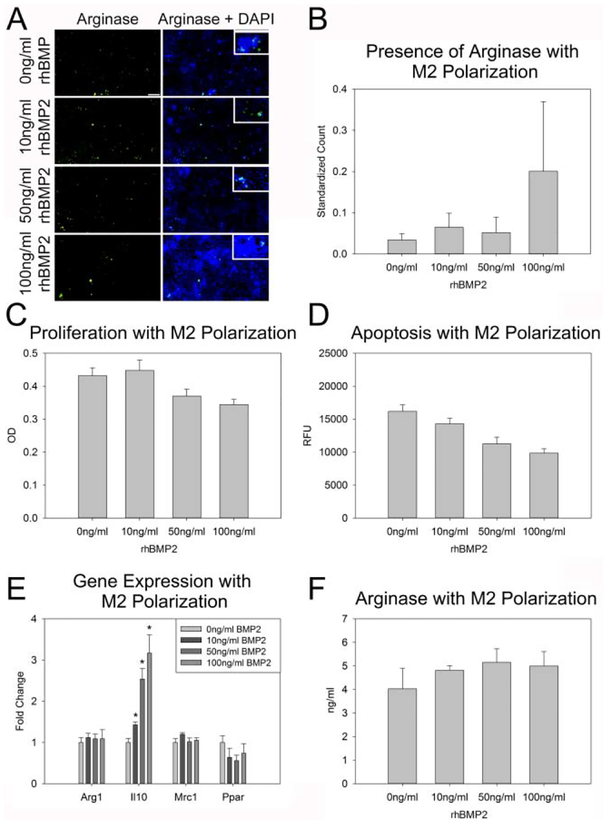
Figure 3: BMP2 does not augment pro-reparative M2 macrophage activity.
A. Representative images of Arginase stained RAW 264.7 cells treated with M2 polarization media and 0 ng/ml, 10 ng/ml, 50 ng/ml and 100 ng/ml BMP2 (descending order). Higher magnification inset for cellular localization of staining. B. Count of Arginase cells positive cells normalized to total cells (DAPI) indicates an increasing trend with increasing BMP2 treatment. C–D. Treatment of M2 polarized RAW 264.7 cells with BMP2 did not affect proliferation (C), or apoptosis (D). E. Assessment of M2 pro-reparative associated gene expression indicates a stepwise increase in IL10 expression in M2 polarized cells with BMP2 treatment. F. Quantity of Arginase protein did not vary with BMP2 treatment. n=3 replicates per assay Scale= 100 μm *p≤0.05
2.5. Protein Analysis
Protein was isolated form RAW 264.7 cells seeded at a density of 300,000 cells per well and treated with standard culture media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2, M1 polarization media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2, or M2 polarization media supplemented with 0 ng/ml, 10 ng/ml, 50 ng/ml 100 ng/ml rhBMP2 for 24 hours. For protein isolation, cells were washed with PBS and protein was extracted using radioimmunoprecipitation buffer (RIPA, G Biosciences, St. Louis, MO, 786–490) for 30 mins at 4°C with agitation. Cell lysate was used for iNOS (LSBio, Seattle, WA, LS-F4110) and Arginase (LSBio, LS-F6864) enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s protocol.
2.6. Statistics
Standard two-way Analysis of Variance (ANOVA) with post-hoc Bonferoni analyses were conducted for all comparisons based on means where appropriate. Violations of homogeneity of variance and normality were corrected through transformations of the data where needed. Differences were considered significant with p≤0.05. All discrete data are presented as means ± standard error of the mean (SEM).
3. Results
3.1. rhBMP2 Does Not Affect Macrophage Polarization
To determine the effect of rhBMP2 treatment on macrophage cell polarization and inflammation, RAW 264.7 cells were treated in vitro with 0 ng/ml (control), 10 ng/ml, 50 ng/ml, and 100 ng/ml rhBMP2 for 24 hours. Assessment of native iNOS and Arginase activity under these conditions indicated no significant modulation of these markers of macrophage polarization with rhBMP2 treatment (Figure 1A–C). Further, 24-hour treatment with rhBMP2 did not affect cell proliferation or apoptosis (Figure 1D–E). Assessment of native expression of M1 pro-inflammatory associated genes indicated an increase in Nos2 expression with 50 ng/ml rhBMP2 treatment as compared to control (0 ng/ml) (p=0.005). Sosc3 expression was decreased with 10 ng/ml rhBMP2 treatment as compared to control (0 ng/ml) (p=0.020) Il1-Beta expression was also decreased (p=0.030) as compared to control (0 ng/ml) but only for the 100 ng/ml rhBMP2 treatment. Expression of Il12-Beta was below the threshold of detection in unpolarized RAW 264.7 cells (Figure 1F). Native expression of M2 pro-reparative macrophage associated gene Il10 was increased with both 50 ng/ml and 100 ng/ml rhBMP2 treatment as compared to control (0 ng/ml) (p=0.023, p=0.050 respectively). Expression of Ppar was below the threshold of detection in unpolarized RAW 264.7 cells (Figure 1G). Quantification of iNOS and Arginase protein indicated no significant change in the amounts of these proteins with rhBMP2 treatment, and no polarization of macrophages due to rhBMP2 treatment alone (Figure 1H–I).
3.2. rhBMP2 does not augment pro-inflammatory M1 macrophage activity
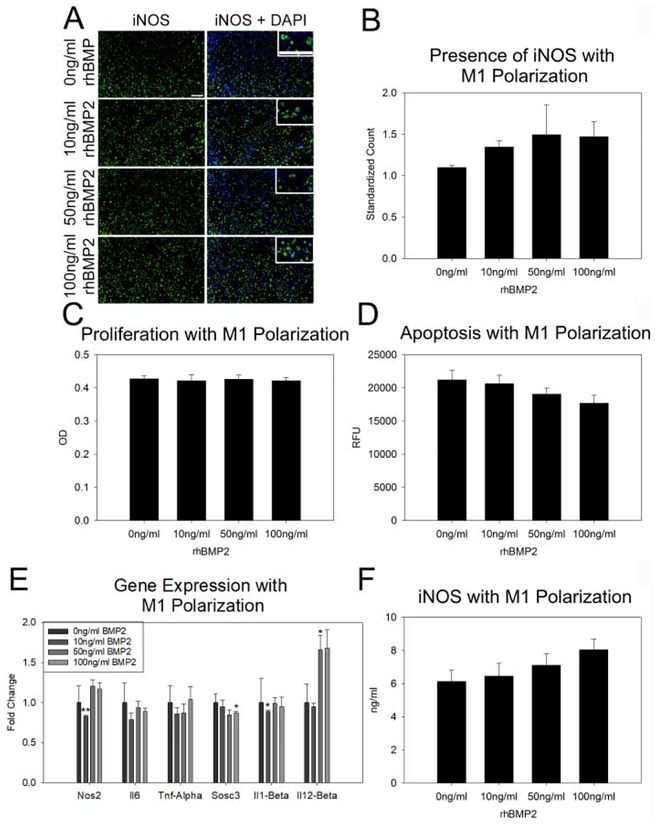
As rhBMP2 alone did not affect macrophage polarization, we next sought to determine if rhBMP2 treatment increased M1 pro-inflammatory macrophage activity. iNOS positive cells were increased with M1 polarization media as compared to control media (p≤0.001) (Sup Fig. 1) however, combined treatment with rhBMP2 and M1 polarization media did not change iNOS presence in RAW 264.7 cells (Figure 2A–B). Neither M1 polarization media nor rhBMP2 treatment affected cell proliferation or apoptosis (Figure 2C–D). Assessment of M1 pro-inflammation associated gene expression when M1 polarization was induced indicated a decrease in Nos2 expression with 10 ng/ml rhBMP2 treatment as compared to control M1 polarization media alone (p=0.009). Sosc3 expression was also reduced as compared to control M1 polarization media alone with 10 ng/ml rhBMP2 treatment (p=0.029). Il1-Beta expression was reduced with 10 ng/ml rhBMP2 as compared to control M1 media (p=0.021) and Il12-Beta expression was increased with 50 ng/ml rhBMP2 treatment as compared to M1 control media (p=0.037)(Figure 2E). Quantification of iNOS protein with M1 polarization indicated no change in protein levels with rhBMP2 treatment (Figure 2F).
Figure 2: BMP2 does not augment pro-inflammatory M1 macrophage activity.
A. Representative images of iNOS stained RAW 264.7 cells treated with M1 polarization media and 0 ng/ml, 10 ng/ml, 50 ng/ml and 100 ng/ml BMP2 (descending order). Higher magnification inset for cellular localization of staining. B. Count of positive INOS cells normalized to total cells (DAPI) indicates an increasing trend with increasing BMP2 treatment. C–D. Treatment of M1 polarized RAW 264.7 cells with BMP2 did not affect proliferation (C), or apoptosis (D). E. Assessment of M1 pro-inflammatory associated gene expression indicates a reduction in Nos2 with 10 ng/ml and Sosc3 with 100ng/ml BMP2 treatment and an increase in Il12-Beta with 50 ng/ml treatment. F. Quantity of iNOS protein did not vary with BMP2 treatment. n=3 replicates per assay Scale= 100 μm *p≤0.05 **p≤0.01
3.3. rhBMP2 does not augment pro-reparative M2 macrophage activity
As rhBMP2 did not augment M1 pro-inflammatory macrophage activity, we assessed if rhBMP2 treatment increased M2 pro-reparative macrophage activity. Arginase positive cells were increased with M2 polarization media as compared to control media (p=0.04) however, combined treatment with rhBMP2 and M2 polarization media did not significantly change Arginase presence in RAW 264.7 cells (Figure 3A–B). M2 polarization media did not change proliferation or apoptosis of these cells, nor did concurrent treatment with rhBMP2 (Figure 3C–D). Expression of the M2 pro-reparative gene IL10 increased over control M2 polarization alone in a stepwise fashion with increasing BMP2 treatment (p=0.017, p=0.012, p=0.013 respectively) (Figure 3E). Quantification of Arginase protein via ELISA revealed no change in Arginase with rhBMP2 treatment in M2 polarized cells (Figure 3F).
4. Discussion
Here we set out to test the hypotheses that rhBMP2 does not drive alterations in macrophage polarization and that under M1 or M2 mock conditions rhBMP2 does not drive an accentuation or decrease in the expected macrophage responses. When macrophages were simply treated with rhBMP2 low levels of iNOS and even lower levels of Arg1 protein were detected and no statistically significant alterations were observed due to rhBMP2 treatment. Gene expression results were mixed with an increase in Nos2 at 50 ng/ml of rhBMP2 but a decrease in Sosc3 at the lower 10 ng/ml dose and Il1-Beta at the higher 100 ng/ml dose. The M2 gene expression data were similarly mixed with increases observed for Il10 at higher rhBMP2 doses (50 and 100 ng/ml) but decreases in those same doses of Mrc1. Note also we observed no amplification of Il12-Beta, an M1 target, or Pparγ, an M2 target, when standard growth media was used. Overall, rhBMP2 appears to have no great effect on macrophage activity in these baseline conditions.
Our M1 conditioned media (Wei et al., 2018) had the intended effect of increased markers associated with pro-inflammation compared with standard growth media (Sup Fig 1). Thus, these growth conditions are deemed appropriate to mimic a pro-inflammatory environment associated with injury, injury repair, or iatrogenic intervention. In these conditions, rhBMP2 did appear to drive small but not statistically significant increases in iNos at the protein level associated with increased dose. However, gene expression data was not confirmatory in the case of Nos2, which appeared to have some decreased expression at 10ng/ml, or Sosc3 at 100ng/ml. Only l12-Beta expression was significantly increased as compared to M1 media alone with 50 ng/ml rhBMP2. Thus, data here do not support rhBMP2 as a factor that greatly alters M1 polarization and overall is inconsistent with clinical reports of rampant inflammatory symptomology being driven solely by rhBMP2.
Our M2 conditioned media (Wei et al., 2018) had the intended effect of increased markers associated with pro-reparative macrophage activity compared with standard growth media, especially Pparγ gene expression (Sup Fig 1). Thus, these growth conditions are deemed appropriate to mimic a pro-reparative environment associated with injury repair and healing. Like the results for the M1 studies, there were no major changes at the protein level with the exception of an increase in Arg1 at 100ng/ml rhBMP2 that was not statistically different, likely due to the variability in response. Gene expression did show IL10 increases with rhBMP2 in a dose dependent manner. Also noted, though not significant, was a dose dependent decrease in apoptosis of the macrophages with increasing rhBMP2 concentration. Thus, there is no evidence to suggest rhBMP2 decreases M2 macrophage polarization or pro-reparative macrophage activity and may support an alternative hypothesis that rhBMP2 can be partially harnessed to increase pro-reparative response.
5. Conclusions
Overall our data do not support the supposition that rhBMP2 alone or in response to caustic M1 or repetitive M2 environments drives changes that may impact healing. It remains unexplained why rhBMP2 might drive the observed patient responses, but these data suggest the peptide alone is not likely solely responsible. What is still very much up for debate is the supraphysiological doses used in human therapies that are difficult to mimic in culture and in preclinical models (Durham et al., 2018a; Grey et al., 2019; Herberg et al., 2014; Krishnan et al., 2017; Molinari et al., 2016; Molinari and Molinari, 2016; Zara et al., 2011). As rhBMP2 is thought to act in a feedback loop where the delivered peptide is cleared within 24–48 hours and then the endogenous system begins to make more bone growth factors in response at that site (Kim et al., 2014; Lissenberg-Thunnissen et al., 2011; Tsuji et al., 2006), future research should interrogate the delivered doses of rhBMPs in clinical scenarios. Further this is even more important as emerging peptides are being patented for use including rhBMPs 4, 7 and 9 aimed at bone therapies (Jiang et al., 2006; Kang et al., 2004; Nakashima, 2005; Nakashima and Akamine, 2005; Xiang et al., 2012). As there is no evidence to suggest that the delivered peptide will not precipitate the desired fill over a longer recovery period, reduced doses may reduce side effects and restore confidence in the use of these important drugs.
Supplementary Material
Supplemental Figure 1: Macrophages can be polarized. A. Representative images of iNOS (Top) and Arginase (Bottom) stained RAW 264.7 cells treated with control media (left) and polarization media (right). Higher magnification inset for cellular localization of staining. B. Standardized count of positive iNOS and Arginase cells indicates significantly more iNOS staining with M1 media (p≤0.001) and Arginase staining was not significantly increased with M2 media (p=0.908) due to high variability in control images and low abundance of Arginase in general. C. Assessment of mRNA expression for M1 related genes indicates that M1 media drives an increase in Nos2 (p=0.002), IL6 (p<0.001), Tnf-Alpha (p=0.016) Sosc3 (p<0.001), II1-Beta (p=0.007) gene expression, thus confirming polarization of RAW 264.7 macrophages for a pro-inflammatory phenotype. D. Assessment of M2 related gene expression also confirmed that M2 media drives polarization towards a reparative phenotype as expression of Arg1 (p<0.001) and Mrc1 (p=0.002) were significantly increased with M2 media as compared to control media. E. ELISA data for iNOS (p=0.001) and Arginase (p=0.011) also confirmed successful M1 and M2 polarization of RAW 264.7 cells. n=3 replicates per assay. Scale= 100 μm. ### Expression below detectable threshold *p≤0.05 **p≤0.01 ***p≤0.001.
Highlights.
rhBMP2 does not affect macrophage polarization
rhBMP2 does not augment pro-inflammatory M1 macrophage activity
rhBMP2 does not augment pro-reparative M2 macrophage activity
Supraphysiological dosing may be responsible for poor clinical outcomes
6. Acknowledgements
This work was made possible by grants from the AO Foundation [S-16-108 to Cray] and the NIH/NIDCR [F31DE026684 to Durham]. Products provided by Medtronic Sofamore Danek USA were used in this research. The authors would like to thank Dr. Beth S. Lee, Ph.D. at The Ohio State University for RAW 264.7 cells.
Abbreviations
- BMP2
Recombinant human bone morphogenic protein 2
- DAPI
4’,6-diamidino-2-phenylindole
- DMEM
Dulbecco’s Modified Eagle’s Medium
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal Bovine Serum
- FITC
Fluorescein isothiocyanate
- IFN - Gamma
Interferon Gamma
- IL4
Interleukin 4
- iNOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharide
- RIPA
Radioimmunoprecipitation assay buffer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflicts of interest.
7. References
- Badylak SF, Taylor D, Uygun K, 2011. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 13, 27–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer TW, Muschler GF, 2000. Bone graft materials. An overview of the basic science. Clin Orthop Relat Res, 10–27. [PubMed] [Google Scholar]
- Canalis E, 1985. Effect of growth factors on bone cell replication and differentiation. Clin Orthop Relat Res, 246–263. [PubMed] [Google Scholar]
- Cooper GS, Kou TD, 2018. Risk of Cancer Following Lumbar Fusion Surgery With Recombinant Human Bone Morphogenic Protein-2 (rhBMP-2): An Analysis Using a Commercially Insured Patient Population. Int J Spine Surg 12, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase P, Capanna R, 2005. Clinical applications of BMPs. Injury 36 Suppl 3, S43–46. [DOI] [PubMed] [Google Scholar]
- Durham EL, Howie RN, Hall S, Larson N, Oakes B, Houck R, Grey Z, Steed M, LaRue AC, Muise-Helmericks R, Cray J, 2018a. Optimizing bone wound healing using BMP2 with absorbable collagen sponge and Talymed nanofiber scaffold. J Transl Med 16, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham EL, Howie RN, Houck R, Oakes B, Grey Z, Hall S, Steed M, LaRue A, Muise-Helmericks R, Cray J, 2018b. Involvement of calvarial stem cells in healing: A regional analysis of large cranial defects. Wound Repair Regen 26, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn TA, 2003. Clinical applications of recombinant human BMPs: early experience and future development. J Bone Joint Surg Am 85-A Suppl 3, 82–88. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Dinopoulos H, Tsiridis E, 2005. Bone substitutes: an update. Injury 36 Suppl 3, S20–27. [DOI] [PubMed] [Google Scholar]
- Grey ZJ, Howie RN, Durham EL, Hall SR, Helke KL, Steed MB, LaRue AC, Muise-Helmericks RC, Cray JJ, 2019. Sub-clinical dose of bone morphogenetic protein-2 does not precipitate rampant, sustained inflammatory response in bone wound healing. Wound Repair Regen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S, Aguilar-Perez A, Howie RN, Kondrikova G, Periyasamy-Thandavan S, Elsalanty ME, Shi X, Hill WD, Cray JJ, 2017. Mesenchymal stem cell expression of SDF-1beta synergizes with BMP-2 to augment cell-mediated healing of critical-sized mouse calvarial defects. J Tissue Eng Regen Med 11, 1806–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg S, Susin C, Pelaez M, Howie RN, Moreno de Freitas R, Lee J, Cray JJ Jr., Johnson MH, Elsalanty ME, Hamrick MW, Isales CM, Wikesjo UM, Hill WD, 2014. Low-dose bone morphogenetic protein-2/stromal cell-derived factor-1beta cotherapy induces bone regeneration in critical-size rat calvarial defects. Tissue Eng Part A 20, 1444–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper RA, Kapadia H, 2018. Discussion: Cost-Effectiveness Analysis of Demineralized Bone Matrix and rhBMP-2 versus Autologous Iliac Crest Bone Grafting in Alveolar Cleft Patients. Plast Reconstr Surg 142, 744–745. [DOI] [PubMed] [Google Scholar]
- Howie RN, Durham E, Oakes B, Grey Z, Smith J, Campbell P, LaRue A, Steed M, Muise-Helmericks R, Cray J, 2018a. Testing a novel nanofibre scaffold for utility in bone tissue regeneration. J Tissue Eng Regen Med 12, 2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie RN, Herberg S, Durham E, Grey Z, Bennfors G, Elsalanty M, LaRue AC, Hill WD, Cray JJ, 2018b. Selective serotonin re-uptake inhibitor sertraline inhibits bone healing in a calvarial defect model. Int J Oral Sci 10, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu EL, Ghodasra JH, Ashtekar A, Nickoli MS, Lee SS, Stupp SI, Hsu WK, 2013. A comparative evaluation of factors influencing osteoinductivity among scaffolds designed for bone regeneration. Tissue Eng Part A 19, 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, Soo C, 2016. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev 22, 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Gittens SA, Chang Q, Zhang X, Chen C, Zhang Z, 2006. The use of tissue-engineered bone with human bone morphogenetic protein-4-modified bone-marrow stromal cells in repairing mandibular defects in rabbits. Int J Oral Maxillofac Surg 35, 1133–1139. [DOI] [PubMed] [Google Scholar]
- Kaiser MG, Groff MW, Watters WC 3rd, Ghogawala Z, Mummaneni PV, Dailey AT, Choudhri TF, Eck JC, Sharan A, Wang JC, Dhall SS, Resnick DK, 2014. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: bone graft extenders and substitutes as an adjunct for lumbar fusion. J Neurosurg Spine 21, 106–132. [DOI] [PubMed] [Google Scholar]
- Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He TC, 2004. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther 11, 1312–1320. [DOI] [PubMed] [Google Scholar]
- Kim RY, Oh JH, Lee BS, Seo YK, Hwang SJ, Kim IS, 2014. The effect of dose on rhBMP-2 signaling, delivered via collagen sponge, on osteoclast activation and in vivo bone resorption. Biomaterials 35, 1869–1881. [DOI] [PubMed] [Google Scholar]
- Krishnan L, Priddy LB, Esancy C, Klosterhoff BS, Stevens HY, Tran L, Guldberg RE, 2017. Delivery vehicle effects on bone regeneration and heterotopic ossification induced by high dose BMP-2. Acta Biomater 49, 101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyszczyk P, Acevedo A, Davidoff EJ, Timmins LM, Marrero-Berrios I, Patel M, White C, Lowe C, Sherba JJ, Hartmanshenn C, O’Neill KM, Balter ML, Fritz ZR, Androulakis IP, Schloss RS, Yarmush ML, 2018. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci) 6, 79–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissenberg-Thunnissen SN, de Gorter DJ, Sier CF, Schipper IB, 2011. Use and efficacy of bone morphogenetic proteins in fracture healing. Int Orthop 35, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J, Sugarman J, 2015. Ethics and policy issues for stem cell research and pulmonary medicine. Chest 147, 824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Arbit HM, Herrick JL, Segovis SG, Maran A, Yaszemski MJ, 2015. Tissue engineered constructs: perspectives on clinical translation. Ann Biomed Eng 43, 796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Tsumura H, Wang JC, Alanay A, 2009. An update on bone substitutes for spinal fusion. Eur Spine J 18, 783–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari RW, Kerr C, Kerr D, 2016. Bone morphogenetic protein in pediatric spine fusion surgery. J Spine Surg 2, 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari RW, Molinari C, 2016. The Use of Bone Morphogenetic Protein in Pediatric Cervical Spine Fusion Surgery: Case Reports and Review of the Literature. Global Spine J 6, e41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, 2005. Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev 16, 369–376. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Akamine A, 2005. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod 31, 711–718. [DOI] [PubMed] [Google Scholar]
- Nerem RM, 2006. Tissue engineering: the hope, the hype, and the future. Tissue Eng 12, 1143–1150. [DOI] [PubMed] [Google Scholar]
- Ripamonti U, 2017. Functionalized Surface Geometries Induce: “Bone: Formation by Autoinduction”. Front Physiol 8, 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronga M, Fagetti A, Canton G, Paiusco E, Surace MF, Cherubino P, 2013. Clinical applications of growth factors in bone injuries: experience with BMPs. Injury 44 Suppl 1, S34–39. [DOI] [PubMed] [Google Scholar]
- Sabetkish S, Kajbafzadeh AM, Sabetkish N, Khorramirouz R, Akbarzadeh A, Seyedian SL, Pasalar P, Orangian S, Beigi RS, Aryan Z, Akbari H, Tavangar SM, 2015. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix liver scaffolds. J Biomed Mater Res A 103, 1498–1508. [DOI] [PubMed] [Google Scholar]
- Sarkar SK, Lee BT, 2015. Hard tissue regeneration using bone substitutes: an update on innovations in materials. Korean J Intern Med 30, 279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurman Z, Smith ML, Kondziolka D, 2016. Off-label innovation: characterization through a case study of rhBMP-2 for spinal fusion. J Neurosurg Spine 25, 406–414. [DOI] [PubMed] [Google Scholar]
- Suarez-Gonzalez D, Barnhart K, Migneco F, Flanagan C, Hollister SJ, Murphy WL, 2012. Controllable mineral coatings on PCL scaffolds as carriers for growth factor release. Biomaterials 33, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez Del Amo F, Monje A, Padial-Molina M, Tang Z, Wang HL, 2015. Biologic Agents for Periodontal Regeneration and Implant Site Development. Biomed Res Int 2015, 957518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DA, Caplan AL, Macchiarini P, 2014. Ethics of bioengineering organs and tissues. Expert Opin Biol Ther 14, 879–882. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V, 2006. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38, 1424–1429. [DOI] [PubMed] [Google Scholar]
- Urist MR, 1965. Bone: formation by autoinduction. Science 150, 893–899. [DOI] [PubMed] [Google Scholar]
- Urist MR, DeLange RJ, Finerman GA, 1983. Bone cell differentiation and growth factors. Science 220, 680–686. [DOI] [PubMed] [Google Scholar]
- Vaquette C, Pilipchuk SP, Bartold PM, Hutmacher DW, Giannobile WV, Ivanovski S, 2018. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv Healthc Mater 7, e1800457. [DOI] [PubMed] [Google Scholar]
- Vorrasi JS, Kolokythas A, 2017. Controversies in Traditional Oral and Maxillofacial Reconstruction. Oral Maxillofac Surg Clin North Am 29, 401–413. [DOI] [PubMed] [Google Scholar]
- Wei F, Zhou Y, Wang J, Liu C, Xiao Y, 2018. The Immunomodulatory Role of BMP-2 on Macrophages to Accelerate Osteogenesis. Tissue Eng Part A 24, 584–594. [DOI] [PubMed] [Google Scholar]
- Xiang L, Liang C, Zhen-Yong K, Liang-Jun Y, Zhong-Liang D, 2012. BMP9-induced osteogenetic differentiation and bone formation of muscle-derived stem cells. J Biomed Biotechnol 2012, 610952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Speidel AT, Yamato M, 2018. Four Food and Drug Administration draft guidance documents and the REGROW Act: A litmus test for future changes in human cell-and tissue-based products regulatory policy in the United States? J Tissue Eng Regen Med 12, 1579–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN Jr., 2006. Statistical analysis of real-time PCR data. BMC bioinformatics 7, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, Ting K, Soo C, 2011. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A 17, 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Macrophages can be polarized. A. Representative images of iNOS (Top) and Arginase (Bottom) stained RAW 264.7 cells treated with control media (left) and polarization media (right). Higher magnification inset for cellular localization of staining. B. Standardized count of positive iNOS and Arginase cells indicates significantly more iNOS staining with M1 media (p≤0.001) and Arginase staining was not significantly increased with M2 media (p=0.908) due to high variability in control images and low abundance of Arginase in general. C. Assessment of mRNA expression for M1 related genes indicates that M1 media drives an increase in Nos2 (p=0.002), IL6 (p<0.001), Tnf-Alpha (p=0.016) Sosc3 (p<0.001), II1-Beta (p=0.007) gene expression, thus confirming polarization of RAW 264.7 macrophages for a pro-inflammatory phenotype. D. Assessment of M2 related gene expression also confirmed that M2 media drives polarization towards a reparative phenotype as expression of Arg1 (p<0.001) and Mrc1 (p=0.002) were significantly increased with M2 media as compared to control media. E. ELISA data for iNOS (p=0.001) and Arginase (p=0.011) also confirmed successful M1 and M2 polarization of RAW 264.7 cells. n=3 replicates per assay. Scale= 100 μm. ### Expression below detectable threshold *p≤0.05 **p≤0.01 ***p≤0.001.