Abstract
Background:
Alaska Native infants from the Yukon-Kuskokwim Delta (YKD) experienced respiratory syncytial virus (RSV) hospitalization rates 5 times higher and an RSV season twice as long as the general US infant population. We describe trends in hospitalization rates and seasonality during 18 years of continuous RSV surveillance in this population and explore contributions of climate and sociodemographic factors.
Methods:
We abstracted clinical and RSV test information from computerized medical records at YKD Regional Hospital and Alaska Native Medical Center from 1994 to 2012 to determine hospitalization rates and RSV season timing. Descriptive village and weather data were acquired through the US Census and Alaska Climate Research Center, University of Alaska, Fairbanks, respectively.
Results:
During 1994–2012, YKD infant RSV hospitalization rates declined nearly 3-fold, from 177 to 65 per 1000 infants/yr. RSV season onset shifted later, from mid October to late December, contributing to a significantly decreased season duration, from 30 to 11 weeks. In a multivariate analysis, children from villages with more crowded households and lacking plumbed water had higher rates of RSV hospitalizations (relative rate, 1.17; P = 0.0005 and relative rate, 1.45; P = 0.0003). No association of temperature or dew point was found with the timing or severity of RSV season.
Conclusions:
Although the RSV hospitalization rate decreased 3-fold, YKD infants still experience a hospitalization rate 3-fold higher than the general US infant population. Overcrowding and lack of plumbed water were associated with RSV hospitalization. Dramatic changes occurred in RSV seasonality, not explained by changes in climate.
Keywords: Alaska Native, respiratory syncytial virus, hospitalization, lower respiratory tract infection, Alaska, RSV, temperature
Lower respiratory tract infections (LRTI) are the number one cause of death globally in children outside of the neonatal period,1 and respiratory syncytial virus (RSV) infection is a major cause of LRTI worldwide.2,3 In the US, RSV is the most frequent cause of hospitalization for infants, with a hospitalization rate during 1993–2008 of 23 per 1000 children younger than 1 year old,4,5 although some prospective studies report half that rate.6 The burden of RSV LRTI infection in young children disproportionately affects Alaska Native (AN) children,7 and the reported RSV hospitalization rate for AN infants from the remote Yukon-Kuskokwim Delta (YKD) region of Alaska is 5 times higher than in the US general population.8–10 Compared with RSV hospitalization rates around the world,2 this rate is surpassed only by the Canadian Inuit, at 166 per 1000 infants.11
Using YKD surveillance data, we have previously shown that RSV epidemiology was characterized by a prolonged RSV season (30.5 weeks compared with 15 weeks for the US).12,13 The seasonality of RSV is an inherent characteristic of this infection.14 In temperate climates, RSV activity peaks in the winter months, whereas tropical areas have a prolonged continuous season with overall lower rates of RSV.14,15 Anticipating the timing of the RSV season is important for cost-efficient use of palivizumab prophylaxis.16 Seasonality is likely determined by weather and socio-environmental effects, which alter virus infectivity or host susceptibility, and affects social patterns, such as promoting indoor cohabitation, which can increase RSV transmission.17 Social factors are important determinants of health in YKD; prior studies documented high rates of household crowding, lack of running water and woodstove use,18,19 which have all been associated with increased risk of RSV or LRTI hospitalization.18,20,21
We analyzed 18 years of surveillance data for LRTI and RSV hospitalization in children younger than 3 years old living in YKD, an isolated population with a single health-care system, to evaluate trends in hospitalization rates and RSV seasonality over time. We looked at the relationship of climate factors to RSV season timing. We evaluated the contribution of village-level demographic characteristics to risk of RSV infection.
MATERIALS AND METHODS
The study population has been described elsewhere.8,20 Briefly, the YKD covers an area of 195,000 km2 in southwestern Alaska. It is home to ~25,000 primarily (>85%) Yupik Eskimos, who live in Bethel (population approximately 6000) or 49 small villages.22 Travel is by small aircraft, snow machine or river boats, as there are no roads between most villages. The YKD birth cohort, obtained from birth records, shows an average of 635 babies born annually.
Methods for RSV surveillance in YKD have been previously described.9 This surveillance for RSV has been conducted since late 1993 to present. The first 3 years were active surveillance; a nasopharyngeal aspirate was obtained from children younger than 3 years of age hospitalized with LRTI. Since October 1996, we obtained hospitalization data, including RSV test results, from electronic medical records of YKD Regional Hospital (YKDRH) and the referral hospital in Anchorage Alaska (Alaska Native Medical Center).9 RSV testing from 1994 to 1996 was done by rapid antigen enzyme immunoassay test pack (Abbott, Oak Park, IL) or by culture and direct immunofluorescence assay (Bartels, Issaquah, WA) on nasopharyngeal aspirate samples.12 Rapid antigen testing was done by Directogen RSV (Becton Dickson, Cockeysville, MD) during 1996–200412 and by Binax Now (Inverness Medical; Princeton, NJ) during 2005–2012.10,23 The percentage of <1 year old hospitalized LRTI patients tested for RSV was similar between all study years.
Hospitalization data (admission and discharge dates, ICD9 discharge diagnoses, village of residence, birth date, RSV test results and dates) were obtained for all YKD children younger than 3 years of age. Hospitalizations were merged if a child was read-mitted within 3 days of a previous hospitalization’s discharge date. Data from a child transferred from YKDRH to Alaska Native Medical Center were combined into 1 hospitalization. The surveillance was approved by the Alaska Area Institutional Review Board and health boards from YK Health Corporation, South central Foundation and Alaska Native Tribal Health Consortium.
For calculation of RSV rates for each season, July 1–June 30, only the first positive hospitalization in children younger than 1 year of age was used, and rates are reported per 1000 infants. We calculated season onset, offset and peak week, using the methodology of Mullins et al13 and required that >5 RSV tests were performed during the peak week. The length of the RSV season was calculated as the number of weeks from the onset week through the offset week.
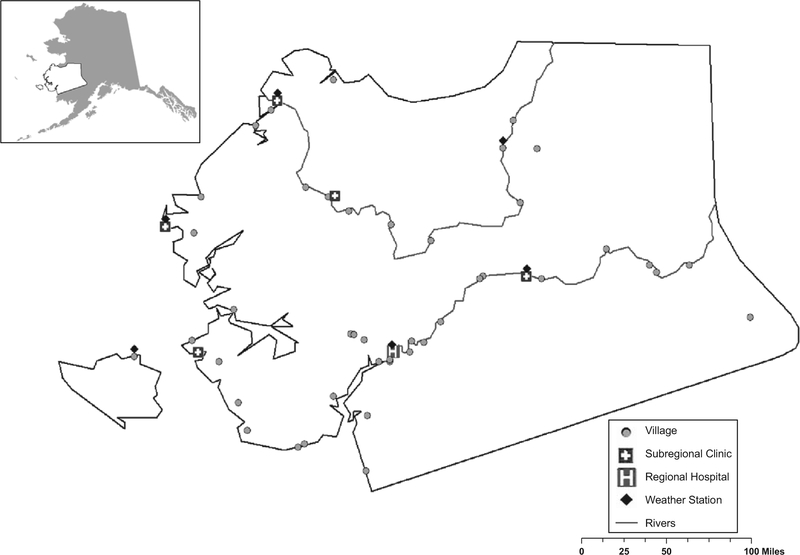
Weather data were obtained from the Alaska Climate Research Center, University of Alaska Fairbanks. We selected 6 locations geographically spread throughout YKD coast and inland that had complete daily weather records during the surveillance years of interest, shown in Figure 1. We looked at the long-term relationship between temperature and RSV hospitalizations through use of an autoregressive integrated moving average model. The dependent variable was the monthly rate of RSV hospitalization for YKD. To assess the lag between temperature fluctuations and RSV, we ran a cross-correlation analysis. Finally, to investigate year-to-year variation in the RSV rates and timing of the season, we calculated the average temperature and dew point during the winter months (October–May) and during the fall months (October–November). We tested for an association between temperature and dew point and the season’s severity (RSV rate) and onset by use of Spearman’s rank order statistic.
FIGURE 1.
Map of study region in Alaska along with locations of villages, subregional clinics and regional hospital.
In a geographic analysis, we calculated the RSV rate for each of 49 villages of residence for children younger than 1 year of age for 2 periods: July 1995–June 2005 and July 2005–June 2012. For each village, we obtained: (1) the percent of households that lack complete plumbing, (2) the percent of families below the poverty level, (3) the average number of persons per room, (4) the percent of households with >1.5 persons per room and (5) the percent of households using wood as a heat source.22 For the 1995–2005 period, we obtained all data from the Census 2000 and for the 2005–2012 period, we obtained data from the Census 2010 and the American Community Survey.22 Additionally, because a major determinant of winter weather conditions in Alaska is proximity to the ocean, we examined whether the community was geographically situated on the ocean coast. Two other variables were examined, at the village level, related to healthcare access: the presence of a subregional clinic and the distance to YKDRH. Univariate and multivariate analyses were conducted by use of Poisson regression. We calculated the percentage of the RSV decline accounted for by socio-economic factors by comparing the relative rates (RR) in the univariate and multivariate adjusted models. Variables were selected in the multivariable model through use of a purposeful backwards elimination. Variables were considered confounders and remained in the model if their exclusion changed the value of other coefficient(s) of interest by more than 15%. P values less than 0.05 were considered statistically significant.
RESULTS
RSV and LRTI Hospitalization Rates During the 18-year Surveillance Period
During 1994–2012, there were 5966 LRTI hospitalizations in YKD children younger than 3 years of age. Among the LRTI hospitalizations, 4744 (80%) were tested for RSV, and among those tested, 1903 (40%) were positive for RSV. By season, the percentage of LRTI hospitalizations positive for RSV ranged from a low of 8% (2010–2011, 16 of 209) to a high of 61% (1994–1995, 246 of 404). Among RSV hospitalizations, 78% (n = 1490) occurred in children younger than 1 year of age, 18% (n = 344) in 1 year olds and 4% (n = 69) in 2 year olds. No trends were observed over time in the age or gender distribution of RSV hospitalizations. In children younger than 1 year of age, 1335 (90%) were primary RSV hospitalizations, and 155 (10%) were repeat RSV hospitalizations for the same infant within the same RSV season.
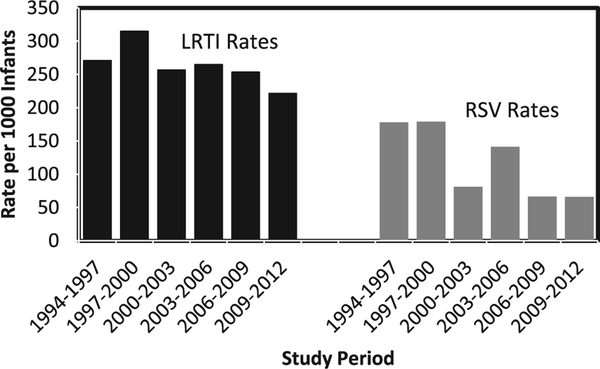
The LRTI hospitalization rate has been stable until the most recent surveillance period during which there was a slight decrease (Fig. 2, P = 0.007). The annual RSV hospitalization rate declined 60% during the surveillance period (P < 0.0001 for trend) from 177 per 1000 infants during 1994–1997 to 65 per 1000 infants during 2009–2012 (RR = 0.37). The proportion of LRTI hospitalizations positive for RSV declined from 50% during 1994–1997 to 22% during 2009–12. A discharge diagnosis of pneumonia was present for 53% (n = 1006) of RSV hospitalizations, and 70% (n = 1339) had a diagnosis of bronchiolitis. The proportion of RSV hospitalizations with a pneumonia diagnosis remained stable throughout the surveillance (52% in 1994–1997, 46% in 2009–2012), despite overall decreasing rates of RSV hospitalizations. Fifty-eight percent (275 of 476) of RSV hospitalizations had an associated diagnosis of bronchiolitis during 1994–1997, which increased to 78% (146 of 186) in 2009–2012.
FIGURE 2.
Rates of RSV and LRTI hospitalization according to 3-year surveillance periods in infants younger than 1 year of age in the YKD.
RSV Seasonal Timing
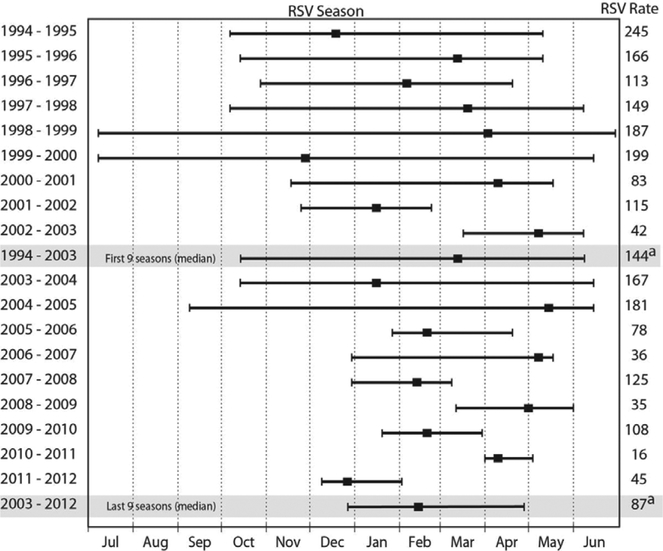
The timing of the RSV season changed over the course of surveillance to a later onset and shorter season (Fig. 3). During the first 9 seasons (1994–2003), the median season onset week was October 14–20, the peak March 10–16 and offset June 2–8. During the last 9 seasons, the season onset shifted later (P = 0.004), with the median onset moving to December 30–January 5 (Fig. 3), whereas the peak week (February 17–23, last 9 seasons) and the offset (April 28–May 4, last 9 seasons) shifted slightly earlier (not statistically significant). The length of the RSV season declined from a median of 30 weeks during the first 9 seasons to 11 weeks during the last 9 seasons (P = 0.002).
FIGURE 3.
The week of RSV season onset and offset (left and right hatch marks) along with the peak week (square marker) during 18 years of RSV hospitalization surveillance in southwest Alaska. Summary measures for the first and last 9 seasons are shown in grey. RSV hospitalization rate per 1000 among <1 year olds for each season is displayed in right hand column.
a – RSV hospitalization rate for the first and last 9 season.
From 1998 to 2009, palivizumab was administered from October through May to match the long RSV season. Since 2009, the shortened season has prompted later start dates. Palivizumab is currently administered in Alaska between November 30 and May 15,24 94% (221 of 234) of RSV hospitalizations in <1 year olds occurred during this palivizumab period during the last 5 seasons, compared with only 70% (640 of 911) during the first 9 seasons.
RSV Seasonality and Climate Parameters
Temperature was related to RSV over the 18-year time series in a second order autoregressive integrated moving average model (P = 0.003). This significant association is dominated by the peaks in RSV hospitalizations that typically occur in temperate climates during the winter months. The rate of RSV in the 3 coldest months (December–January–February) over all 18 seasons was 190.9, which is 4.3 time higher than the rate (44.0) in the 3 warmest months (June–July–August). In a cross-correlation analysis, the association between temperature and RSV hospitalizations was maximized with a lag of 36 days. Although RSV is associated with temperature, we did not find an association between the mean temperature or dew point over the winter or fall period and the severity of the RSV season over the 18 surveillance years. We did not find a statistically significant correlation between fall temperature and dew point and the onset of the RSV season.
Rates by Village
The average annual RSV hospitalizations rate for all the villages for these years was 140.2 per 1000 infants during July 1995–June 2005 and 63.3 per 1000 infants during July 2005–June 2012. At the village level, rates for these years ranged from 0 to 286 RSV hospitalizations per 1000 infants. We examined 9 factors and their relationship to the village-level RSV rates (Table 1). In univariate analyses, RSV hospitalization rate was associated with the proportion of houses that lack complete plumbing (P < 0.0001), the proportion of families below poverty (P < 0.0001), the proportion of households with >1.5 persons per room (P < 0.0001), being on the coast (P = 0.02) and closer distance to the hospital (P = 0.01; Table 1). Factors found not to be significant in univariate analyses of the RSV rates were the proportion of houses using wood as a heating source, the presence of a subregional clinic and the community size.
TABLE 1.
Village-level Factors Associated with LRTI and RSV Hospitalizations Rates in Children Younger than 1 Year in 49 Villages in the YKD, 1995–2005 and 2005–2012
| Attribute | Univariate RR | P Value | Multivariate RR | P Value |
|---|---|---|---|---|
| LRTI | ||||
| Time period (2005–2012 vs. 1995–2005) | 0.82 (0.76, 0.88) | <0.0001 | 0.88 (0.81, 0.95) | 0.001 |
| Increasing distance from hospital | 1.04 (0.98, 1.10)* | 0.25 | 0.89 (0.83, 0.96) | 0.002 |
| Subregional clinic | 0.99 (0.89, 1.10) | 0.87 | ||
| Coastal community | 1.15 (1.07, 1.24) | 0.0002 | ||
| Large community (>500 persons) | 0.99 (0.92, 1.07) | 0.78 | 1.18 (1.09, 1.28) | <0.0001 |
| % of families below poverty line | 1.29 (1.21, 1.39)† | <0.0001 | 1.15 (1.05, 1.26) | 0.003 |
| % of households >1.5 persons per room | 1.28 (1.23, 1.35)‡ | <0.0001 | 1.20 (1.13, 1.28) | <0.0001 |
| Crowding§ | 1.74 (1.57, 1.94)¶ | <0.0001 | ||
| Wood as heat source | 1.00 (0.95, 1.07)║ | 0.75 | ||
| Lack plumbing | 1.65 (1.49, 1.83)** | <0.0001 | 1.25 (1.05, 1.26) | <0.0001 |
| RSV | ||||
| Time period (2005–2012 vs. 1995–2005) | 0.48 (0.42, 0.54) | <0.0001 | 0.51 (0.45, 0.59) | <0.0001 |
| Increasing distance from hospital | 0.88 (0.79, 0.97)* | 0.01 | 0.82 (0.73, 0.92) | 0.005 |
| Subregional clinic | 0.90 (0.76, 1.07) | 0.23 | ||
| Coastal community | 1.15 (1.02, 1.29) | 0.02 | ||
| Large community (>500 persons) | 0.92 (0.82, 1.04) | 0.20 | 1.14 (1.01, 1.30) | 0.04 |
| % of families below poverty line | 1.35 (1.21, 1.51)† | <0.0001 | 1.25 (1.08, 1.45) | 0.003 |
| % of households > 1.5 persons per room | 1.30 (1.21, 1.39)‡ | <0.0001 | 1.17 (1.07, 1.28) | 0.0005 |
| Crowding§ | 1.80 (1.53, 2.11)¶ | <0.0001 | ||
| Wood as heat source | 1.02 (0.93, 1.15)║ | 0.70 | ||
| Lack plumbing | 1.85 (1.57, 2.17)** | <0.0001 | 1.45 (1.19, 1.78) | 0.0003 |
RR for 100 mile increase in the distance to the regional hospital.
RR for an increase of 20% (20% of families below poverty level to 40%, 40% to 60%, etc.).
RR for an increase of 20% (20% of households >1.5 persons per room to 40%, 40% to 60%, etc.).
Not entered into multivariate model because of collinearity with % of households >1.5 persons per room.
RR for an increase in household crowding of 1 person per room.
RR between a community where 100% use wood as a heating source and a community where 0% use wood as a home heating source.
RR between a community where 100% lack complete plumbing and a community where 0% lack complete plumbing.
On multivariate analysis, RSV hospitalization rate was higher in communities with a higher proportion of households that lack complete plumbing (P = 0.0003, Table 1). During 2005–2012, the rates were 92.3 and 57.4 for communities with >90% and <10% lacking complete plumbing, respectively. Communities with a higher proportion of households with >1.5 persons per room had higher rates of RSV on multivariate analysis (P = 0.0005, Table 1). During 2005–2012, the RSV rate was 103.4 in communities with >40% of households with >1.5 persons per room compared with 41.1 in communities where <20% of households had >1.5 persons per room. Communities with a higher proportion of families living below the poverty rate (P = 0.003), located closer to the regional hospital (P = 0.005) or with a large population size (P = 0.04) also were associated with higher RSV rates on multivariate analysis. Despite air service throughout the region, residence closer to the hospital likely reflects improved access to hospital services. The RR for time period remained significant after multivariate adjustment (RR = 0.51, 95% confidence interval: 0.45, 0.59). Although crowding, lack of plumbing, distance and poverty were significantly associated with RSV rates in these communities, there remains an almost 50% drop in the RSV hospitalization rate, over the last decades, unexplained by changes in these socio-economic factors. In contrast, after multivariate adjustment, there remains only a 12% decline in LRTI hospitalizations between the 2 time periods. Between 2000 and 2010, the percentage of homes, with >1.5 persons per room decreased from 26.8% to 22.0% and with complete plumbing increased from 50.9% to 62.6%, differences that do not account for the magnitude of the observed decline in RSV rates over time.
DISCUSSION
During 18 years of continuous surveillance in the isolated YKD region, RSV hospitalization rates declined by 60%, and there was a significant shift in the RSV season, with a later onset and shorter duration not completely explained by changes in weather or socioeconomic factors. Although rates are decreasing, RSV hospitalization rates remain 3-fold higher than the general US infant population.4,5,25 During this time period, there was a smaller decline in overall LRTI hospitalizations, suggesting the possibility of replacement with other infectious agents, including human metap-neumovirus, parainfluenza virus and influenza.10 Certain social characteristics, particularly household crowding, put children at increased risk for RSV hospitalization. Palivizumab administration since 1998 was associated with decreased RSV hospitalization rates in premature infants.26 However, from previous studies, only 7% of YKD infants were premature and they accounted for 15% of hospitalizations pre-palivizumab (1994–1998), so prophylaxis use is not likely to account for the magnitude of this rate decrease.9 Decreasing length of RSV season has led to adoption of a shorter palivizumab administration period in Alaska.24,27
RSV seasonality is a reflection of climate and environmental effects on both the survivability of the microbe and the susceptibility of the host.17 Investigators worldwide have shown a correlation of temperature to timing of RSV activity.28–31 In the United Kingdom, an increase of 1°C in mean annual temperature was associated with a shorter RSV end of season by 2–3 weeks.32 Generally, most studies in temperate regions have shown association of RSV incidence with lower temperatures and higher relative humidity.14 However, RSV surveillance data in Brazil showed shifting of the RSV season to earlier onset not associated with any changes in climate factors.33 We also documented shifts in seasonality and changes in hospitalization rates but could not attribute these changes to climate factors, even though temperature and RSV season did correlate. Although weather factors clearly influence the RSV season, likely other sociodemographic factors mitigate this effect.
Underlying determinants of health, such as poverty, poor housing, indoor air pollution and lack of running water, help explain the increased burden of LRTIs, including RSV, in indigenous populations compared with the majority population.34 Canadian studies of LRTI hospitalization in Inuit infants found RSV hospitalization rates of 166 per 1000, the highest reported in the world.11 RSV infection in Hawaii is overall lower (9.8 per 1000) compared with the US; however, Pacific Islander and Native Hawaiian infants had much higher rates of RSV hospitalization (39 per 1000 and 46 per 1000, respectively).35 In central Australia, RSV hospitalization rates in children <2 years of age was 11 per 1000 in nonaboriginal children and 30 per 1000 in aboriginal children.36 In the Navajo population, the RSV infant hospitalization rate was 91 per 1000.37 The risk factors that underlie these increased rates of disease and hospitalization are varied, based on the population, but most represent consequences of poverty and general health inequity. Canadian Inuit children had a greater risk of RSV hospitalization if their village was more remote and if their homes were overcrowded, and as well if they were not breast fed or their mothers smoked in pregnancy.38 In the US Navajo population, use of a wood stove in the home for cooking or heat was correlated with increased indoor air pollution and with risk of acute LRTI.21 Australian Aboriginal children had a greater risk of hospitalization, longer inpatient stays and a greater risk of sequelae then other Australian children.36,39 In Alaskan studies, AN children had several risk factors for RSV hospitalization, including household crowding, bottle-feeding, wood stove use, presence of fewer than 2 sinks in the home and lack of indoor running water.18–20 These risk factors are specific to the communities studied, but even general population-based studies of RSV risk factors find that more siblings and crowding at home are risk factors for severe RSV infection.40
In the village level analysis, several factors were associated with a greater risk of RSV hospitalization. Indoor crowding and large communities, which favor increased respiratory viral transmission, were both significant in the multivariate analysis. Lower family income and lack of plumbing, likely making hygiene more difficult, also contributed to higher RSV hospitalization rates. Solving these disparities will require addressing such factors as education about disease transmission, housing inequities and increasing the service of piped water. Comparison of census data during the time frame of the study shows that from 2000 to 2010, the proportion of households that were crowded decreased by 5% and the number with complete plumbing increased by 10%, both of which may contribute to the decline in RSV rates shown. Overall, the village level factors we studied can only account for 6% of the decline of RSV rates during this time. Other factors related to RSV hospitalizations that are changing over time, which we were unable to account for in this analysis, include exposure to secondhand smoke, breastfeeding, the use of pneumococcal conjugate vaccine (PCV) and the likely replacement of RSV with other infectious agents.
We report about 50% of RSV hospitalized children were also diagnosed with pneumonia, which is higher than the US average and consistent with previous studies of RSV infection in indigenous populations.34 This relationship remained constant and was not affected by the overall decrease in RSV rates we saw over time. Pneumonia in this population is a significant predictor of future sequelae, such as chronic suppurative lung disease or bronchiectasis.41 PCV has had a significant impact on pneumonia in some studies.42 YKD children experienced a sustained decrease in pneumonia and overall LRTI rates after PCV7 introduction in 2000.7 The sustained decrease in RSV hospitalizations after 2001 may also partly reflect the impact of PCV on virus-associated pneumonia as reported by Madhi and Klugman.43
Limitations to this study include the size of the birth cohort, with only 600–650 babies born each year. This study is observational, and risk factors identified in the village level analysis are associated with, but may not be causally linked to, RSV transmission, although the epidemiological literature supports most identified herein. By provider discretion, only 80% of LRTIs were tested for RSV, and consequently there could be some undetected cases. Lastly, the data were obtained retrospectively, and there may be provider variability in diagnosis and RSV testing.
RSV hospitalization is still a disproportionately large burden of disease in AN infants from the YKD region, despite recent decreases in rates. Because of the large impact of sociodemographic factors, focusing future efforts on improving plumbing and educating families about ways to limit spread will likely be important to further improve health outcomes. Continuing to monitor the season changes and administer prophylaxis based on local surveillance will be critical for appropriate use of palivizumab. Expanded use of palivizumab in newborn term infants could decrease hospitalizations further but would require careful cost analysis.
ACKNOWLEDGMENTS
We thank the children and parents of YKD and the YKHC staff. We also thank the Arctic Investigations Program—CDC staff who assisted with surveillance activities. We thank Pat Hansen for her assistance in creating figures. We gratefully acknowledge the help of Susan Gerber and Marika Iwane with this project.
Footnotes
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Liu L, Johnson HL, Cousens S, et al. ; Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 Suppl):S127–S132. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993–2008. Clin Infect Dis. 2012;54:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singleton RJ, Holman RC, Folkema AM, et al. Trends in lower respiratory tract infection hospitalizations among American Indian/Alaska Native children and the general US child population. J Pediatr. 2012;161:296–302.e2. [DOI] [PubMed] [Google Scholar]
- 8.Karron RA, Singleton RJ, Bulkow L, et al. Severe respiratory syncytial virus disease in Alaska Native children. RSV Alaska Study Group. J Infect Dis. 1999;180:41–49. [DOI] [PubMed] [Google Scholar]
- 9.Singleton RJ, Bruden D, Bulkow LR, et al. Decline in respiratory syncytial virus hospitalizations in a region with high hospitalization rates and prolonged season. Pediatr Infect Dis J. 2006;25:1116–1122. [DOI] [PubMed] [Google Scholar]
- 10.Singleton RJ, Bulkow LR, Miernyk K, et al. Viral respiratory infections in hospitalized and community control children in Alaska. J Med Virol. 2010;82:1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banerji A, Lanctôt KL, Paes BA, et al. Comparison of the cost of hospitalization for respiratory syncytial virus disease versus palivizumab prophylaxis in Canadian Inuit infants. Pediatr Infect Dis J. 2009;28:702–706. [DOI] [PubMed] [Google Scholar]
- 12.Singleton RJ, Bruden D, Bulkow LR. Respiratory syncytial virus season and hospitalizations in the Alaskan Yukon-Kuskokwim Delta. Pediatr Infect Dis J. 2007;26(11 Suppl):S46–S50. [DOI] [PubMed] [Google Scholar]
- 13.Mullins JA, Lamonte AC, Bresee JS, et al. Substantial variability in community respiratory syncytial virus season timing. Pediatr Infect Dis J. 2003;22:857–862. [DOI] [PubMed] [Google Scholar]
- 14.Tang JW, Loh TP. Correlations between climate factors and incidence–a contributor to RSV seasonality. Rev Med Virol. 2014;24:15–34. [DOI] [PubMed] [Google Scholar]
- 15.Bloom-Feshbach K, Alonso WJ, Charu V, et al. Latitudinal variations in seasonal activity of influenza and respiratory syncytial virus (RSV): a global comparative review. PLoS One. 2013;8:e54445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.From the American Academy of Pediatrics: Policy statements—modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–1701. [DOI] [PubMed] [Google Scholar]
- 17.Sloan C, Moore ML, Hartert T. Impact of pollution, climate, and sociodemographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin Transl Sci. 2011;4:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bulkow LR, Singleton RJ, DeByle C, et al. Risk factors for hospitalization with lower respiratory tract infections in children in rural Alaska. Pediatrics. 2012;129:e1220–e1227. [DOI] [PubMed] [Google Scholar]
- 19.Hennessy TW, Ritter T, Holman RC, et al. The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska Natives. Am J Public Health. 2008;98:2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulkow LR, Singleton RJ, Karron RA, et al. ; Alaska RSV Study Group. Risk factors for severe respiratory syncytial virus infection among Alaska Native children. Pediatrics. 2002;109:210–216. [DOI] [PubMed] [Google Scholar]
- 21.Robin LF, Less PS, Winget M, et al. Wood-burning stoves and lower respiratory illnesses in Navajo children. Pediatr Infect Dis J. 1996;15:859–865. [DOI] [PubMed] [Google Scholar]
- 22.American Community Survey, US Census Bureau. Available at: https://www.census.gov/acs/www/. Accessed January 9, 2014.
- 23.Miernyk K, Bulkow L, DeByle C, et al. Performance of a rapid antigen test (Binax NOW(R) RSV) for diagnosis of respiratory syncytial virus compared with real-time polymerase chain reaction in a pediatric population. J Clin Virol. 2010;50:240–243. [DOI] [PubMed] [Google Scholar]
- 24.Singleton R, Malter A. Palivizumab prophylaxis recommendations- Alaska, 2013–14 RSV season. State Alaska Epidemiol Bull. 2014;20:2014. [Google Scholar]
- 25.Holman RC, Curns AT, Cheek JE, et al. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics. 2004;114:e437–e444. [DOI] [PubMed] [Google Scholar]
- 26.Singleton R, Dooley L, Bruden D, et al. Impact of palivizumab prophylaxis on respiratory syncytial virus hospitalizations in high risk Alaska Native infants. Pediatr Infect Dis J. 2003;22:540–545. [DOI] [PubMed] [Google Scholar]
- 27.McGuiness CB, Boron ML, Saunders B, et al. Respiratory syncytial virus surveillance in the United States, 2007–2012: results from a National Surveillance System. Pediatr Infect Dis J. 2014;33:589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.du Prel JB, Puppe W, Gröndahl B, et al. Are meteorological parameters associated with acute respiratory tract infections? Clin Infect Dis. 2009;49:861–868. [DOI] [PubMed] [Google Scholar]
- 29.Noyola DE, Mandeville PB. Effect of climatological factors on respiratory syncytial virus epidemics. Epidemiol Infect. 2008;136:1328–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welliver RC Sr. Temperature, humidity, and ultraviolet B radiation predict community respiratory syncytial virus activity. Pediatr Infect Dis J. 2007;26(11 Suppl):S29–S35. [DOI] [PubMed] [Google Scholar]
- 31.Yusuf S, Piedimonte G, Auais A, et al. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol Infect. 2007;135:1077–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donaldson GC. Climate change and the end of the respiratory syncytial virus season. Clin Infect Dis. 2006;42:677–679. [DOI] [PubMed] [Google Scholar]
- 33.Paiva TM, Ishida MA, Benega MA, et al. Shift in the timing of respiratory syncytial virus circulation in a subtropical megalopolis: implications for immunoprophylaxis. J Med Virol. 2012;84:1825–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang AB, Chang CC, O’Grady K, et al. Lower respiratory tract infections. Pediatr Clin North Am. 2009;56:1303–1321. [DOI] [PubMed] [Google Scholar]
- 35.Yorita KL, Holman RC, Steiner CA, et al. Severe bronchiolitis and respiratory syncytial virus among young children in Hawaii. Pediatr Infect Dis J. 2007;26:1081–1088. [DOI] [PubMed] [Google Scholar]
- 36.Dede A, Isaacs D, Torzillo PJ, et al. Respiratory syncytial virus infections in Central Australia. J Paediatr Child Health. 2010;46:35–39. [DOI] [PubMed] [Google Scholar]
- 37.Bockova J, O’Brien KL, Oski J, et al. Respiratory syncytial virus infection in Navajo and White Mountain Apache children. Pediatrics. 2002;110 (2 Pt 1):e20. [DOI] [PubMed] [Google Scholar]
- 38.Banerji A, Greenberg D, White LF, et al. Risk factors and viruses associated with hospitalization due to lower respiratory tract infections in Canadian Inuit children: a case-control study. Pediatr Infect Dis J. 2009;28:697–701. [DOI] [PubMed] [Google Scholar]
- 39.Burgner D, Richmond P. The burden of pneumonia in children: an Australian perspective. Paediatr Respir Rev. 2005;6:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003; 143(5 Suppl):S118–S126. [DOI] [PubMed] [Google Scholar]
- 41.Singleton RJ, Valery PC, Morris P, et al. Indigenous children from three countries with non-cystic fibrosis chronic suppurative lung disease/bronchiectasis. Pediatr Pulmonol. 2014;49:189–200. [DOI] [PubMed] [Google Scholar]
- 42.Griffin MR, Zhu Y, Moore MR, et al. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madhi SA, Klugman KP; Vaccine Trialist Group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. [DOI] [PMC free article] [PubMed] [Google Scholar]