Abstract
Purpose
The purpose of this electronic health record (EHR)–based retrospective cohort study was to characterize a population of patients participating in a 12-month, lifestyle change program in a community-based health system and to examine longitudinal weight outcomes.
Methods
Program participants were identified in the EHRs of a health care delivery system across 18 sites between 2010 and 2017. Outcomes were mean weight change and proportion of patients with ≥5% weight loss through 24 months from program initiation.
Results
Among 4463 program participants, 3156 met study eligibility criteria, with a mean ± SD age of 53.5 ± 13.1 years; 77.7% were women. Mean baseline weight ± SD was 101.3 ± 23.8 kg. Three main cardiometabolic risk groups were identified: prediabetes/high risk for diabetes (47.3%), overweight/obese in the absence of elevated diabetes risk (27.2%), and existing diabetes (23.9%). Maximal mean weight loss was 3.9% at 6 months from baseline. At 12 and 24 months from baseline, mean weight loss was 3.2% and 2.3%, respectively, with 31% and 29% of participants attaining ≥5% weight loss. Long-term weight outcomes were similar across risk groups.
Conclusions
A lifestyle change program in a clinical practice setting is associated with modest weight loss, sustained through 24 months, among participants with a range of cardiometabolic risk factors. More than one-quarter of participants achieve ≥5% weight loss, regardless of cardiometabolic risk.
More than 70% of adults in the United States are overweight or obese,1 a major modifiable risk factor for type 2 diabetes (T2D).2 The Diabetes Prevention Program (DPP), a landmark multicenter randomized controlled trial (RCT), demonstrated that an intensive, predominantly one-on-one behavioral lifestyle intervention that promotes healthy eating habits, calorie reduction, physical activity, and weight loss lowers the risk of developing T2D by 58% over 3 years.3 Weight loss was the dominant predictor of decreased incidence of T2D in the DPP trial, with each kilogram of weight loss corresponding to a 16% risk reduction.4 Weight management is an important strategy for cardiometabolic risk reduction and the prevention of T2D and cardiovascular disease (CVD).
Numerous translational, group-based lifestyle change programs, modeled from the original DPP curriculum, have been developed for community and clinical settings as a more cost-efficient approach to diabetes prevention.5–10 RCTs have demonstrated the efficacy of these programs in promoting weight loss and reducing cardiometabolic risk factors among individuals at high risk for T2D.5–10 Such programs, however, have been less commonly evaluated once integrated into clinical practice, outside the context of a research study.11–13
The implementation of a lifestyle change program within a health care delivery system and its integration with routine care provides a unique opportunity to examine the effects of such programs on weight outcomes among real-world patients. To date, there are few, if any, large-scale studies that have examined long-term weight outcomes among program participants in a clinical practice setting. The ability to leverage electronic health record (EHR) data in the evaluation of this program is a pragmatic and efficient approach to contribute to the evidence base, allowing this work to more rapidly inform diabetes prevention strategies in clinical practice and future health policy. The purpose of this EHR-based retrospective cohort study was to characterize a population of patients participating in a 12-month, lifestyle change program in a community-based health system and to examine longitudinal weight outcomes.
Methods
Study Design and Setting
This EHR-based retrospective cohort study was conducted at Sutter Health, a mixed-payer health care delivery system in Northern California. This study design allows for access to a large volume of data on program participants who are cared for in routine clinical practice and was selected over prospective analysis as the program under evaluation was implemented prior to the initiation of the study.
Sutter Health provides comprehensive medical services across 130 ambulatory clinics and 24 acute-care hospitals, with approximately 11 million outpatient visits and 200 000 hospital discharges, annually. Sutter Health clinics and hospitals are linked by a single EHR system (Epic, Verona, Wisconsin). This study used an EHR research database that included health care information on patients between January 1, 2001, and December 31, 2017. Data from this study were derived from the EHR of Sutter Health. Given the nature of the study and the use of existing data, this study was approved by Sutter Health’s Institutional Review Board with a Health Insurance Portability and Accountability Act (HIPAA) waiver of authorization and informed consent.
Lifestyle Change Program
Sutter Health uses a group-based, 12-month lifestyle change program that is aligned with Centers for Disease Control and Prevention (CDC) recommendations for T2D prevention, based on the original DPP curriculum.14 The curriculum is conducted in person and is composed of 3 phases: (1) the core phase includes 12 weekly sessions, (2) the transition phase includes weekly/biweekly sessions over an additional 12 weeks, and (3) the support phase includes monthly/bimonthly sessions for the remainder of the year. The core and transition phases promote modest weight loss through healthy eating, calorie reduction, and increased physical activity. The support phase reinforces lifestyle behavior changes, facilitates problem-solving skills, and increases social support and motivation for long-term weight management.
Sutter Health began implementing the lifestyle change program at 7 outpatient clinics in 2010. As of 2017, the program was offered at 18 clinics. The intended population for CDC-aligned lifestyle change programs is individuals at high risk for T2D, based on clinical evidence of prediabetes or a validated screening tool developed by the American Diabetes Association.15,16 At Sutter Health, the program is open to a range of patients with elevated cardiometabolic risk, including those with evidence of diabetes.
Cohort Identification
The authors identified lifestyle change program participants in the EHR between January 1, 2010 (first implementation of the program at Sutter Health), and December 31, 2017 (end of study database). The date of the first program visit was defined as baseline. For inclusion in this analysis, participants were required to be ≥18 years of age as of baseline and to have EHR activity ≥12 to 36 months prior to baseline to capture medical history. Participants with International Classification of Diseases, Ninth Revision (ICD-9) or Tenth Revision (ICD-10) diagnoses in their medical record for conditions or procedures associated with substantial changes in weight, including metastatic cancer, pregnancy, gastric bypass surgery, and end-stage kidney disease, in the 12 months prior or up to 24 months after baseline were excluded.
Data Collection and Management
Demographic information was extracted from the EHR, including participants’ date of birth, sex, race/ethnicity, and preferred spoken language, which all are self-reported, and primary insurance. Census tract median household income was determined from participants’ home addresses and was used as a proxy for socioeconomic status.
Clinical characteristics recorded in the EHR within the 12-month period prior to baseline, including weight, body mass index (BMI), smoking status, and blood pressure, were also extracted. Individuals were categorized into the following BMI groups: healthy weight (18.5 to <25 kg/m2), overweight (25 to <30 kg/m2), obese (30 to <35 kg/m2), or severely obese (≥35 kg/m2). For participants who identified as Asian, BMI categories were shifted downward by 2 kg/m2, given higher cardiometabolic risk at a lower BMI among this group.17
Comorbidities were identified in the EHR in the 12 months prior to baseline, including prediabetes, diabetes, hypertension, dyslipidemia, metabolic syndrome, atherosclerotic cardiovascular disease, and depression. Conditions were identified by ≥1 of the following: (1) ICD-9 or ICD-10 diagnoses from the problem list, encounters, or medical claims; (2) laboratory values; and (3) medication orders. See the online supplement for algorithms used to identify conditions (Supplementary Table). A Charlson comorbidity index (CCI) score was calculated for each participant as a measure of overall disease burden based on established methods.18 The CCI was originally developed for predicting mortality in the inpatient setting; however, it has been used extensively as a measure of multimorbidity in the outpatient setting.19 Individuals were also classified as having a high risk for T2D in the absence of documented prediabetes based on the American Diabetes Association (ADA) screening tool.16
The authors obtained information from the EHR on patients’ medication orders active as of the index date, including prescription-based weight loss products, appetite suppressants, and diabetes medications. Data were also collected on participants’ health care utilization as potential measures of engagement with their health care.20 Participants were classified as having an established primary care provider within the health care system and quantified the number of outpatient encounters and telephonic/electronic encounters, as well as whether the individual had a preventive visit or influenza immunization in the 12 months prior to baseline based on Current Procedural Terminology codes. The number of program sessions completed by participants was also quantified.
Outcome Measures
The primary outcome was mean percent change in weight from baseline at the 12- and 24-month follow-up. Weight measurements are recorded in the EHR at each program visit and at routine health care encounters; however, values may be unmeasured due to lack of a program visit or routine encounter during follow-up or due to insufficient follow-up (eg, patients who initiated the program as of July 1, 2017, have ≤6 months of follow-up). Short-term follow-up weight measurements were categorized into discrete intervals: 1 to 4 months and 5 to 7 months. For each interval, the value recorded closest to 3 and 6 months, respectively, was used. Long-term follow-up weight measurements were captured closest to 12, 18, and 24 months from baseline (± 3 months at each time point). The secondary outcome was the proportion of participants with ≥5% weight loss at 12 months from baseline, which is considered clinically meaningful.21 The researchers also examined the proportion of patients with ≥7% weight loss, which is a primary goal for the original DPP lifestyle intervention and translational lifestyle change programs.3,22
Statistical Analyses
All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, North Carolina). Between-group differences in baseline characteristics were examined by independent t tests for continuous variables and χ2 tests of independence for categorical variables. Mean weight changes at each time point from baseline were calculated with 95% confidence intervals (CIs). Missing weights were not imputed; however, the relationship between unmeasured weight values and baseline characteristics among participants with available follow-up was examined. Logistic regression was used to identify patient characteristics associated with ≥5% weight loss at 12 months, corresponding to the completion of the curriculum. Unadjusted odds ratios (ORs) and 95% CIs were generated for the relationship between each baseline variable (Table 1) and the outcome. Subsequently, adjusted ORs and 95% CIs were generated from multivariable regression models, which included all patient characteristics listed in Table 1. P < .05 was considered statistically significant.
Table 1.
Baseline Characteristics
| Characteristic | All Program Participants (N = 3156) | High Risk for T2D (n = 1493) | Overweight/Obese Low T2D Risk (n = 858) | Existing T2D (n = 755) | P Valuea |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean ± SD, y | 53.47 ± 13.10 | 57.28 ± 11.15 | 43.39 ± 11.82 | 57.56 ± 11.80 | <.0001 |
| Female, No. (%) | 2452 (77.69) | 1125 (75.35) | 769 (89.63) | 515 (68.20) | <.0001 |
| Race/ethnicity, No. (%) | <.0001 | ||||
| Non-Hispanic black | 140 (4.44) | 70 (4.69) | 24 (2.80) | 43 (5.70) | |
| Non-Hispanic Asian | 180 (5.70) | 68 (4.55) | 62 (7.23) | 49 (6.49) | |
| Non-Hispanic white | 2149 (68.09) | 1077 (72.14) | 541 (63.05) | 494 (65.43) | |
| Hispanic | 391 (12.39) | 150 (10.05) | 142 (16.55) | 92 (12.19) | |
| Other | 72 (2.28) | 28 (1.88) | 20 (2.33) | 24 (3.18) | |
| Unknown | 224 (7.10) | 100 (6.70) | 69 (8.04) | 53 (7.02) | |
| Prefers English, No. (%) | 3080 (97.59) | 1464 (98.06) | 828 (96.50) | 739 (97.88) | .1113 |
| Insurance payer, No. (%) | <.0001 | ||||
| Commercial FFS/PPO | 1422 (45.06) | 639 (42.80) | 488 (56.88) | 275 (36.42) | |
| Commercial HMO | 560 (17.74) | 279 (18.69) | 159 (18.53) | 110 (14.57) | |
| Medicare (FFS/HMO) | 613 (19.42) | 364 (24.38) | 39 (4.55) | 205 (27.15) | |
| Medicaid | 57 (1.81) | 17 (1.14) | 26 (3.03) | 13 (1.72) | |
| Other/self | 88 (2.78) | 38 (2.55) | 35 (4.08) | 12 (1.59) | |
| Unknown | 416 (13.18) | 156 (10.45) | 111 (12.94) | 140 (18.54) | |
| Median household income, No. (%) | <.0001 | ||||
| <$50 000 | 510 (16.16) | 224 (15.00) | 127 (14.80) | 143 (18.94) | |
| ≥$50 000 to <$75 000 | 939 (29.75) | 419 (28.06 | 257 (29.95) | 250 (33.11) | |
| ≥$75 000 to <$100 000 | 770 (24.40) | 364 (24.38) | 218 (25.41) | 177 (23.44) | |
| ≥$100 000 | 684 (21.67) | 369 (24.72) | 193 (22.49) | 114 (15.10) | |
| Missing | 253 (8.02) | 117 (7.84) | 63 (7.34) | 71 (9.40) | |
| Clinical characteristics | |||||
| Weight, mean ± SD, kg | 101.33 ± 23.84 | 103.22 ± 24.33 | 94.40 ± 20.55 | 106.10 ± 24.23 | <.0001 |
| BMI categories, No. (%) | <.0001 | ||||
| Healthy | 49 (1.55) | 28 (1.88) | 0 (0) | 3 (0.40) | |
| Overweight | 456 (14.45) | 157 (10.52) | 219 (25.52) | 77 (10.20) | |
| Obese | 1810 (57.35) | 870 (58.27) | 502 (58.51) | 418 (55.36) | |
| Severely obese | 841 (26.65) | 438 (29.34) | 137 (15.97) | 257 (34.04) | |
| Systolic BP, mean ± SD, mm Hg | 126.81 ± 15.36 | 129.43 ± 14.98 | 119.92 ± 12.58 | 129.47 ± 16.59 | <.0001 |
| Diastolic BP, mean ± SD, mm Hg | 77.33 ± 9.33 | 78.39 ± 9.19 | 75.99 ± 8.84 | 76.97 ± 9.92 | <.0001 |
| Smoking status, No. (%) | <.0001 | ||||
| Current | 189 (5.99) | 61 (4.09) | 65 (7.58) | 59 (7.81) | |
| Ever | 831 (26.33) | 432 (28.94) | 143 (16.67) | 242 (32.05) | |
| Never | 2080 (65.91) | 975 (65.30) | 630 (73.43) | 446 (59.07) | |
| Unknown | 56 (1.77) | 25 (1.67) | 20 (2.33) | 8 (1.06) | |
| Comorbidities | |||||
| Metabolic syndrome, No. (%) | 772 (24.46) | 250 (16.74) | 5 (0.58) | 514 (68.08) | <.0001 |
| Hypertension, No. (%) | 1391 (44.07) | 733 (49.10) | 132 (15.38) | 508 (67.28) | <.0001 |
| Dyslipidemia, No. (%) | 1380 (43.73) | 653 (43.74) | 151 (17.60) | 552 (73.11) | <.0001 |
| ASCVD, No. (%) | 214 (6.78) | 102 (6.83) | 18 (2.10) | 86 (11.39) | <.0001 |
| Depression, No. (%) | 661 (20.94) | 304 (20.36) | 187 (21.79) | 157 (20.79) | .6908 |
| CCI score | <.0001 | ||||
| 0 | 1729 (54.78) | 1009 (67.58) | 677 (78.90) | 28 (3.71) | |
| 1–2 | 1240 (39.29) | 447 (29.94) | 176 (20.51) | 589 (78.01) | |
| 3–4 | 157 (4.97) | 32 (2.14) | 5 (0.58) | 114 (15.10) | |
| 5–6 | 25 (0.79) | 1 (0.07) | 0 (0) | 23 (3.05) | |
| >6 | 5 (0.16) | 4 (0.27) | 0 (0) | 1 (0.13) | |
| Prescriptions | |||||
| Active Rx, mean ± SD, count | 3.99 ± 3.63 | 3.61 ± 3.20 | 2.49 ± 2.66 | 6.38 ± 4.16 | <.0001 |
| Weight loss drug, No. (%) | 200 (6.34) | 89 (5.96) | 68 (7.93) | 38 (5.03) | .0630 |
| Any diabetes drug, No. (%) | 559 (17.71) | 21 (1.41) | 9 (1.05) | 502 (66.49) | <.0001 |
| Weight loss diabetes drug, No. (%) | 453 (14.35) | 21 (1.41) | 9 (1.05) | 413 (54.70) | <.0001 |
| Other diabetes drugs, No. (%) | 280 (8.87) | 0 (0) | 0 (0) | 259 (34.30) | <.0001 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; BP, blood pressure; CCI, Charlson comorbidity index; FFS, fee for service; HMO, health maintenance organization; PPO, preferred provider organization; Rx, prescription; SD, standard deviation; T2D, type 2 diabetes.
P values are derived from analysis of variance for comparison of means or χ2 tests of independence for a comparison of proportions for participants across cardiometabolic risk groups.
Results
Participant Identification
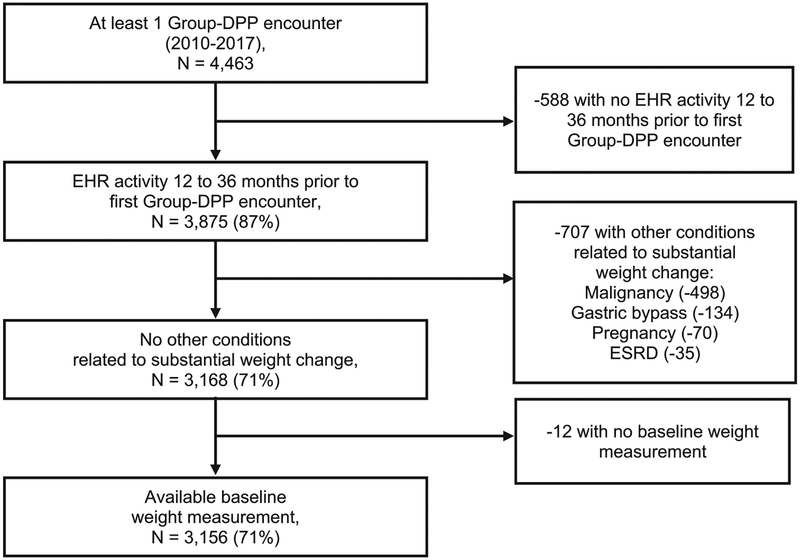
Among 4463 program participants with ≥1 lifestyle change program encounter across 18 clinical sites between 2010 and 2017, 3156 (71%) met full cohort eligibility criteria (Figure 1). Participants attended a mean ± SD of 8.5 ± 8.8 sessions over 12 months. At 3, 6, 12, 18, and 24 months from baseline, respectively, 3104, 3011, 2839, 2656, and 2379 participants had sufficient follow-up. Among these individuals, weight measurements were available for 2851 (92%), 2106 (70%), 2277 (80%), 1986 (75%), and 1678 (71%) at 3, 6, 12, 18, and 24 months, respectively. Participants with unmeasured weight values did not differ from those with measured values in terms of baseline weight or BMI; however, those with follow-up weight measurements tended to be older with more comorbidities (data not shown).
Figure 1.
Eligibility criteria flow diagram. EHR, electronic health record; ESRD, end-stage renal disease; Group-DPP, group-based diabetes prevention program lifestyle intervention.
Baseline Characteristics
Participants had a mean ± SD age of 53.5 ± 13.1 years, 77.7% were female, and 68.1% were non-Hispanic white (NHW) (Table 1), with a mean ± SD baseline weight of 101.3 ± 23.8 kg. A majority (84%) of participants were obese or severely obese. Among program participants, there were 3 major cardiometabolic risk groups: (1) high risk for T2D based on clinical evidence of prediabetes or the ADA screening tool (47.3%), (2) overweight/obese in the absence of elevated T2D risk (27.2%), and (3) existing T2D (23.9%). An additional 1.6% of program participants (n = 50) who did not fall into the above risk groups either had evidence of type 1 diabetes (T1D; 33 of 50) or had a healthy BMI and no evidence of diabetes or elevated diabetes risk but had other cardiometabolic risk factors, such as metabolic syndrome, hypertension, or dyslipidemia (17 of 50). The most common comorbid conditions among program participants were hypertension (44.1%) and dyslipidemia (43.7%). Additional characteristics are shown in Table 2.
Table 2.
Additional Characteristics of Participants
| Health care utilization | All Group-DPP Participants (N = 3156) | High Risk for T2D (n = 1493) | Overweight/Obese Low T2D Risk (n = 858) | Evidence of T2D (n = 755) | Other Risk (n = 50) | P Value |
|---|---|---|---|---|---|---|
| Established PCP, No. (%) | 2890 (91.57) | 1384 (92.70) | 775 (90.33) | 690 (91.39) | 41 (82.00) | .0578 |
| Year of index visit, No. (%) | .0722 | |||||
| 2010 | 86 (2.72) | 34 (2.28) | 24 (2.80) | 26 (3.44) | 2 (4.00) | |
| 2011 | 253 (8.02) | 116 (7.77) | 67 (7.81) | 65 (8.61) | 5 (10.00) | |
| 2012 | 451 (14.29) | 190 (12.73) | 120 (13.99) | 134 (17.75) | 7 (14.00) | |
| 2013 | 515 (16.32) | 234 (15.67) | 139 (16.20) | 132 (17.48) | 10 (20.00) | |
| 2014 | 456 (14.45) | 221 (14.80) | 116 (13.52) | 114 (15.10) | 5 (10.00) | |
| 2015 | 572 (18.12) | 283 (18.96) | 155 (18.07) | 122 (16.16) | 12 (24.00) | |
| 2016 | 4798 (15.78) | 259 (17.35) | 136 (15.85) | 96 (12.72) | 7 (14.00) | |
| 2017 | 325 (10.30) | 156 (10.45) | 101 (11.77) | 66 (8.74) | 2 (4.00) | |
| Season of index visit, No. (%) | .1857 | |||||
| Spring (January-February) | 837 (26.52) | 417 (27.93) | 231 (26.92) | 177 (23.44) | 12 (24.00) | |
| Summer (March-May) | 787 (24.94) | 356 (23.84) | 220 (25.64) | 196 (25.96) | 15 (30.00) | |
| Fall (June-August) | 702 (22.24) | 550 (36.84) | 300 (34.97) | 169 (22.38) | 18 (36.00) | |
| Winter (September-December) | 830 (26.30) | 170 (11.39) | 107 (12.47) | 213 (28.21) | 5 (10.00) | |
| Outpatient ambulatory visits, mean ± SD, count | 8.47 ± 8.82 | 8.59 ± 8.23 | 7.06 ± 9.33 | 9.67 ± 8.62 | 11.04 ± 14.53 | <.0001a |
| Telephone/electronic visits, mean ± SD, count | 13.12 ± 12.93 | 13.69 ± 13.63 | 10.01 ± 9.98 | 15.47 ± 13.95 | 14.06 ± 10.55 | <.0001a |
| Preventive visit, No. (%) | 1087 (34.44) | 596 (39.92) | 301 (35.08) | 177 (23.44) | 13 (26.00) | <.0001a |
| Influenza immunization, No. (%) | 746 (23.64) | 390 (26.12) | 153 (17.83) | 195 (25.83) | 8 (16.00) | <.0001a |
| Group-DPP classes, mean ± SD, count | 9.87 ± 5.31 | 10.34 ± 5.46 | 9.20 ± 5.09 | 9.75 ± 5.14 | 9.64 ± 5.30 | <.0001a |
Abbreviations: Group-DPP, group-based diabetes prevention program lifestyle intervention; PCP, primary care provider; SD, standard deviation; T2D, type 2 diabetes.
Participants across the 3 major cardiometabolic risk groups differed markedly on many baseline demographics and clinical characteristics (Table 1). Participants at high risk for T2D or those with existing T2D were, on average, older (57.3 and 57.6 years, respectively) than those who were overweight/obese in the absence of elevated T2D risk (43.3 years) and were less frequently female (75.4% and 68.2% vs 89.6%). Moreover, participants with elevated risk for T2D or existing T2D at baseline had higher mean weight than those who were overweight/obese without glucose impairment. Given the small sample size of individuals with “other” cardiometabolic risk, stratified analysis was not performed on this group.
Main Outcomes
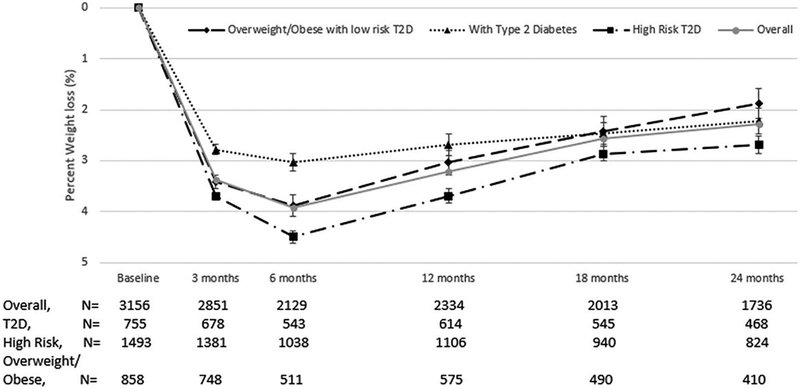
Overall, maximal mean percent weight loss among program participants was 3.9% at 6 months from baseline; at 12 and 24 months of follow-up, mean weight loss was 3.2% and 2.3%, respectively (Figure 2). Across the major cardiometabolic risk groups, unadjusted mean percent weight change at 6 months from baseline was −4.5% among those with a high risk for T2D, −3.9% for those who were overweight/obese in the absence of T2D risk, and −3.0% among those with existing T2D. Unadjusted between-group differences in mean percent weight loss were less pronounced over time.
Figure 2.
Unadjusted percent weight change from baseline, overall and by cardiometabolic risk groups. Error bars represent 95% confidence intervals. T2D, type 2 diabetes.
Overall, 31% and 29% of patients had ≥5% weight loss at 12 and 24 months from baseline, respectively (Table 3). At least 5% weight loss was observed at 12 and 24 months of follow-up among 34% and 31% of participants with high risk for T2D, 28% and 29% of those who were overweight/obese in the absence of T2D risk, and among 27% and 25% of those with existing T2D.
Table 3.
Unadjusted Weight Outcomes From Baseline Overall and by Cardiometabolic Risk Groups
| All Participants | Baseline | 3 Months | 6 Months | 12 Months | 18 Months | 24 Months |
|---|---|---|---|---|---|---|
| All participants, No. | 3156 | 2851 | 2129 | 2334 | 2013 | 1736 |
| Weight, mean ± SD, kg | 101.33 ± 23.84 | 98.10 ± 23.10 | 97.47 ± 23.47 | 97.79 ± 23.46 | 97.87 ± 23.31 | 98.38 ± 23.32 |
| Time to follow-up, mean ± SD, mo | 2.60 ± 0.46 | 5.57 ± 0.69 | 11.76 ± 1.17 | 17.92 ± 1.27 | 24.02 ± 1.29 | |
| Absolute weight change from baseline, mean (95% CI), kg | −3.45 (−3.60 to −3.30) | −4.05 (−4.33 to −3.78) | −3.31 (−3.61 to −3.00) | −2.66 (−2.97 to −2.34) | −2.41 (−2.80 to −2.02) | |
| Percent weight change from baseline, mean (95% CI) | −3.39 (−3.52 to −3.25) | −3.92 (−4.17 to −3.67) | −3.20 (−3.48 to −2.92) | −2.57 (−2.87 −2.27) | −2.27 (−2.61 to −1.92) | |
| ≥5% weight loss, No. (%) | 833 (29.22) | 769 (36.12) | 712 (30.51) | 574 (28.51) | 505 (29.09) | |
| ≥7% weight loss, No. (%) | 453 (15.89) | 516 (24.24) | 516 (22.11) | 401 (19.92) | 365 (21.03) | |
| High risk for T2D, No. | 1493 | 1381 | 1038 | 1106 | 940 | 824 |
| Weight, mean ± SD, kg | 103.22 ± 24.33 | 99.22 ± 23.40 | 97.73 ± 23.71 | 98.26 ± 23.81 | 98.24 ± 23.53 | 99.49 ± 23.86 |
| Time to follow-up, mean ± SD, mo | 2.61 ±7.52 | 5.58 ± 0.68 | 11.76 ± 1.17 | 17.93 ± 1.29 | 24.00 ± 1.33 | |
| Absolute weight change from baseline, mean (95% CI), kg | −3.81 (−4.04 to −3.60) | −4.65 (−5.08 to −4.23) | −3.79 (−4.24 to −3.34) | −2.89 (−3.34 to −2.45) | −2.85 (−3.46 to −2.25) | |
| Percent weight change from baseline, mean (95% CI) | −3.69 (−3.89 to −3.50) | −4.50 (−4.86 to −4.13) | −3.69 (−4.10 to −3.28) | −2.86 (−3.27 to −2.44) | −2.68 (−3.20 to −2.17) | |
| ≥5% weight loss, No. (%) | 445 (32.22) | 421 (40.56) | 375 (33.91) | 280 (29.79) | 260 (31.55) | |
| ≥7% weight loss, No. (%) | 252 (18.25) | 293 (28.23) | 278 (25.14) | 196 (20.85) | 187 (22.69) | |
| Overweight/obese, No. | 858 | 748 | 511 | 575 | 490 | 410 |
| Weight, mean ± SD, kg | 94.40 ± 20.55 | 91.50 ± 19.91 | 91.14 ± 20.77 | 91.20 ± 21.01 | 91.47 ± 20.67 | 90.60 ± 19.44 |
| Time to follow-up, mean ± SD, mo | 2.58 ± 0.47 | 5.55 ± 0.68 | 11.76 ± 1.24 | 18.01 ±1.40 | 24.06 ± 1.34 | |
| Absolute weight change from baseline, mean (95% CI), kg | −3.23 (−3.53 to −2.94) | −3.68 (−4.24 to −3.12) | −2.91 (−3.47 to −2.33) | −2.26 (−2.88 to −1.64) | −1.76 (−2.45 to −1.07) | |
| Percent weight change from baseline, mean (95% CI) | −3.42 (−3.71 to −3.13) | −3.88 (−4.41 to −3.35) | −3.04 (−3.61 to −2.47) | −2.42 (−3.05 to −1.78) | −1.88 (−2.59 to −1.17) | |
| ≥5% weight loss, No. (%) | 234 (31.28) | 191 (37.38) | 161 (28.00) | 129 (26.33) | 121 (29.51) | |
| ≥7% weight loss, No. (%) | 126 (16.84) | 128 (25.05) | 123 (21.39) | 104 (21.22) | 82 (20.00) | |
| Existing T2D, No. | 755 | 678 | 543 | 614 | 545 | 468 |
| Weight, mean ± SD, kg | 106.14 ± 24.23 | 103.66 ± 23.67 | 103.22 ± 23.69 | 103.57 ± 23.11 | 103.56 ± 23.86 | 103.68 ± 23.81 |
| Time to follow-up, mean ± SD, mo | 2.62 ± 0.49 | 5.57 ± 0.70 | 11.77 ± 1.09 | 17.83 ± 1.11 | 24.01 ±1.16 | |
| Absolute weight change from baseline, mean (95% CI), kg | −3.01 (−3.29 to −2.73) | −3.40 (−3.88 to −2.73) | −3.00 (−3.57 to −2.43) | −2.78 (−3.43 to −2.13) | −2.45 (−3.12 to −1.77) | |
| Percent weight change from baseline, mean (95%CI) | −2.78 (−3.03 to −2.53) | −3.03 (−3.45 to −2.61) | −2.68 (−3.17 to −2.19) | −2.46 (−3.02 to −1.90) | −2.23 (−2.81 to −1.64) | |
| ≥5% weight loss, No. (%) | 144 (21.24) | 147 (27.07) | 166 (27.04) | 154 (28.26) | 118 (25.21) | |
| ≥7% weight loss, No. (%) | 72 (10.62) | 92 (16.94) | 111 (18.08) | 96 (17.61) | 91 (19.44) |
Abbreviations: CI, confidence interval; SD, standard deviation; T2D, type 2 diabetes.
Patient Characteristics Associated With Clinically Meaningful Weight Loss
In multivariable logistic regression, non-Hispanic Asian (OR, 0.43; P < .001) and Hispanic (OR, 0.65; P < .01) participants had lower adjusted odds of ≥5% weight loss compared with NHW patients (Table 4). Each session attended was associated with a 12% increased adjusted odds of ≥5% weight loss (P < .001). A preventive visit in the 12 months prior to the program was associated with a 24% increased odds of ≥5% weight loss (P < .05). No differences in odds of clinically meaningful weight loss were observed across cardiometabolic risk groups, after adjusting for patient demographics and characteristics.
Table 4.
Association Between Patient Characteristics and at Least 5% Weight Loss
| Group-DPP Participants With 12 Months of Follow-up (N = 2334) | ||
|---|---|---|
| Characteristic | Odds Ratio (95% CI) | Adjusted Odds Ratio (95% CI) |
| Demographics | ||
| Age | 1.01 (1.00–1.02)a | 1.00 (0.98–1.01) |
| Female | 0.85 (0.69–1.05) | 0.84 (0.65–1.08) |
| Race/ethnicity | ||
| Non-Hispanic white | Reference | Reference |
| Non-Hispanic black | 0.56 (0.34–0.91)a | 0.64 (0.38–1.07) |
| Non-Hispanic Asian | 0.43 (0.27–0.67)b | 0.43 (0.26–0.71)b |
| Hispanic | 0.57 (0.42–0.77)b | 0.65 (0.47–0.91)c |
| Other/unknown | 0.85 (0.62–1.15) | 0.80 (0.57–1.14) |
| English preferred | 1.10 (0.61–1.98) | 0.87 (0.45–1.68) |
| Insurance payer | ||
| Commercial FFS/PPO | Reference | Reference |
| Commercial HMO | 1.06 (0.83–1.36) | 1.02 (0.78–1.33) |
| Medicare (FFS/HMO) | 1.15 (0.91–1.45) | 1.06 (0.78–1.47) |
| Medicaid | 1.22 (0.63–2.36) | 1.84 (0.87–3.86) |
| Other/self | 0.74 (0.38–1.44) | 0.59 (0.29–1.20) |
| Unknown | 0.96 (0.73–1.26) | 0.98 (0.64–1.48) |
| Median household income | ||
| <$50 000 | Reference | Reference |
| ≥$50 000 to <$75 000 | 1.09 (0.83–1.44) | 1.02 (0.76–1.38) |
| ≥$75 000 to <$100 000 | 1.08 (0.81–1.43) | 0.98 (0.72–1.33) |
| ≥$100 000 | 1.37 (1.03–1.83)a | 1.12 (0.82–1.54) |
| Clinical characteristics | ||
| Baseline weight | 1.00 (0.99–1.01) | 1.00 (1.00–1.01) |
| Smoking | ||
| Current | 0.94 (0.64–1.38) | 1.30 (0.85–1.96) |
| Ever | 1.05 (0.86–1.28) | 1.04 (0.83–1.30) |
| Never | Reference | Reference |
| Unknown | 0.94 (0.43–2.06) | 0.81 (0.34–1.93) |
| Comorbidities | ||
| Cardiometabolic risk groups | ||
| High risk for T2D | 1.39 (1.11–1.72)c | 1.17 (0.80–1.72) |
| Overweight/obese low T2D risk | 1.05 (0.81–1.35) | 1.04 (0.66–1.63) |
| Existing T2D | Reference | Reference |
| Other risk | 0.93 (0.44–1.95) | 1.00 (0.44–2.34) |
| Metabolic syndrome | 0.92 (0.75–1.13) | 0.97 (0.72–1.30) |
| Hypertension | 1.03 (0.86–1.22) | 1.01 (0.79–1.30) |
| Dyslipidemia | 1.19 (0.96–1.42) | 1.13 (0.90–1.40) |
| ASCVD | 1.03 (0.74–1.43) | 1.13 (0.90–1.40) |
| Depression | 0.83 (0.66–1.03) | 0.92 (0.63–1.33) |
| CCI score | 0.87 (0.68–1.12) | |
| 0 | Reference | Reference |
| 1–2 | 0.83 (0.69–1.00) | 0.96 (0.75–1.23) |
| 3–4 | 1.02 (0.70–1.51) | 1.40 (0.86–2.29) |
| 5–6 | 1.21 (0.50–2.91) | 1.65 (0.61–4.44) |
| >6 | 0.71 (0.07–6.82) | 0.98 (0.10–9.98) |
| Prescriptions | ||
| Active prescriptions | ||
| 0 | Reference | Reference |
| 1–2 | 1.15 (0.82–1.60) | 1.16 (0.81–1.67) |
| 3–4 | 0.96 (0.69–1.35) | 1.06 (0.72–1.57) |
| 5–6 | 1.03 (0.72–1.46) | 1.25 (0.83–1.90) |
| 6+ | 0.95 (0.68–1.32) | 1.29 (0.82–2.02) |
| Weight loss drug | 0.83 (0.58–1.18) | 0.87 (0.58–1.30) |
| Weight loss diabetes drug | 0.73 (0.57–0.93)c | 0.87 (0.61–1.25) |
| Other diabetes drugs | 0.70 (0.52–0.96)a | 0.70 (0.47–1.06) |
| Completed sessions | 1.12 (1.10–1.13)b | 1.12 (1.10–1.14)b |
| Missing sessions | 0.77 (0.56–1.07) | 0.98 (0.68–1.40) |
| Health care utilization | ||
| Established PCP | 0.70 (0.50–0.99)a | 0.64 (0.44–0.93)a |
| Year of index visit | ||
| 2010 | Reference | Reference |
| 2011 | 1.97 (1.01–3.85)a | 2.13 (1.05–4.34)a |
| 2012 | 2.20 (1.15–4.19)a | 2.20 (1.09–4.46)a |
| 2013 | 1.81 (0.95–3.44) | 1.68 (0.80–3.52) |
| 2014 | 1.75 (0.92–3.33) | 1.45 (0.69–3.07) |
| 2015 | 1.91 (1.01–3.61)a | 1.62 (0.77–3.39) |
| 2016 | 1.45 (0.76–2.78) | 1.24 (0.59–2.64) |
| 2017 | 1.54 (0.68–3.51) | 1.84 (0.71–4.79) |
| Season of index visit | ||
| Fall/early winter (October-December) | Reference | Reference |
| Late winter (January-February) | 0.95 (0.70–1.30) | 0.87 (0.62–1.21) |
| Spring (March-May) | 0.84 (0.62–1.15) | 0.81 (0.58–1.14) |
| Summer (June-September) | 1.01 (0.75–1.35) | 1.00 (0.72–1.37) |
| Outpatient ambulatory visits | 0.99 (0.99–1.01) | 0.99 (0.98–1.01) |
| Telephone/electronic visits | 0.99 (0.98–1.00) | 1.00 (0.99–1.01) |
| Preventive visit | 1.27 (1.05–1.52)a | 1.24 (1.00–1.55)a |
| Influenza immunization | 1.02 (0.83–1.25) | 0.89 (0.70–1.13) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CCI, Charlson comorbidity index; CI, confidence interval; FFS, fee for service; Group-DPP, group-based lifestyle program for diabetes prevention; HMO, health maintenance organization; PCP, primary care provider; PPO, preferred provider organization; T2D, type 2 diabetes.
P < .05.
P < .001.
P < .01.
Discussion
In this EHR-based retrospective study, a cohort of patients who participated in a lifestyle change program offered as part of their routine medical care across 18 clinic sites in a large health care delivery system was examined. Such behavioral interventions have primarily been studied in a clinical trial setting; thus, this study is one of the few to examine lifestyle change program utilization and outcomes in clinical practice. Moreover, to the authors’ knowledge, this study is the first to examine outcomes in this setting through 24 months. The researchers found that program participants had a range of cardiometabolic risk factors, with approximately half of all individuals having a high risk for T2D (47.3%), which is the intended target of CDC-aligned lifestyle change programs. The other half of participants were overweight/obese in the absence of T2D risk (27.2%), had evidence of T2D (23.9%), or had other cardiometabolic risk (1.6%).
Overall, program participants had a maximum mean weight loss of 3.9% at 6 months from baseline with, on average, 2.3% weight loss sustained at 24 months. More than one-quarter of participants achieved clinically meaningful (≥5%) weight loss through 24 months from baseline. Unadjusted mean percent change in weight in the short term was more pronounced for individuals with a high risk for T2D, followed by those who were overweight/obese in the absence of T2D risk and those with existing T2D, with less pronounced between-group differences in the long term. The overall trajectory of weight change observed in this study―initial, steady weight loss in the short term that plateaus, followed by recidivism in the long term―is a fairly common trend seen in other studies of weight loss interventions, including past studies of DPP.3,10,23
Findings from this study are similar to those from a recent retrospective analysis of a registry of 14 717 participants at high risk for T2D from 220 lifestyle change programs within the National DPP network, which showed mean percent weight loss of 4.2% at an average of 6 months from program initiation, with 36% attaining ≥5% weight loss.24 Long-term weight outcomes are also in the range of other retrospective studies conducted in health care settings.11,12
RCTs of translational DPP-based lifestyle interventions conducted in community and health care settings have typically shown more pronounced mean weight loss at 6 months (5.5%–7.6%) and between 12 and 15 months (5.1%–7.4%) compared with observational studies.5,7,10,25 Such differences in the magnitude of weight loss between retrospective observational studies and RCTs are not surprising, given that individuals who volunteer for trials are typically more motivated than those in the general population.26 There is a critical need to enhance the effectiveness of evidence-based interventions when translated into clinical practice, underscoring the necessity of understanding how these programs work in the real world, when, and for whom.
Most evaluations of lifestyle change programs in clinical and community settings have included participants at high risk for T2D,6,7,10,12,25,27 which, as mentioned above, is the target population for programs aligned with CDC recommendations. In this study, approximately half of the cohort did not meet these criteria. In this health care delivery system, it appears that many clinicians refer patients to the program for weight management, which is a direct result of real-world implementation of an evidence-based, structured lifestyle intervention. Notably, after adjusting for patient demographics and clinical characteristics, no differences in odds of attaining clinically meaningful weight loss were observed across cardiometabolic risk groups. Given that weight management is an important strategy for cardiometabolic risk reduction, all individuals stand to benefit from weight reduction, regardless of their underlying cardiometabolic risk.
Non-Hispanic Asian and Hispanic participants were less likely than NHW participants to have ≥5% weight loss, even after adjusting for differences in baseline weight and comorbidities. There was a trend toward lower odds of ≥5% weight loss among non-Hispanic blacks; however, this was not statistically significant, likely given the small sample size of this group. Hispanic communities in the United States bear a disproportionate burden of obesity, with a prevalence of 77% compared to 66% among NHWs, which puts them at higher risk for developing diabetes and cardiovascular disease (CVD).28 Asians, on the other hand, have a higher risk of diabetes at lower BMIs than other racial/ethnic groups.17 Mounting evidence suggests that a one-size-fits-all approach to behavioral lifestyle interventions should be reevaluated and that culturally adaptive programs are needed. Trials are under way to address gaps in effective diabetes prevention interventions, specifically for Asians29 and Hispanics.30
Over a 12-month period in this study, program participants attended, on average, 8 sessions. The number of sessions completed was positively associated with attaining clinically meaningful weight loss. Participant retention in lifestyle interventions is often challenging in settings outside of a research trial31,32 as individuals in clinical practice, including those in this analysis, are expected to pay program enrollment fees and other out-of-pocket costs associated with routine care that may limit program completion. Strategies to improve motivation for program participation may have an important impact on patient outcomes. The assessment of “readiness” for engagement in a lifestyle change program and motivation for continued participation could help clinicians better tailor treatment and improve patient activation.
The results of this study should be interpreted in the context of several limitations. This was a longitudinal analysis without a control group, and it is impossible to know if the program caused weight loss among participants; however, that program attendance was positively associated with more pronounced weight loss provides evidence of a potential dose response. Approximately 30% of participants with available follow-up had unmeasured weight at 24 months from baseline. Weight values were more frequently available for program participants who were older and with more comorbidities.
This study has several important strengths. The use of a large EHR research database leverages access to comprehensive information on patient demographics and clinical characteristics, and it permits examination of the real-world utilization of a lifestyle change program and corresponding outcomes. The study database is inclusive of a diverse patient population, which allows examination outcomes by racial/ethnic groups. To the authors’ knowledge, this study is the first to examine outcomes through 2 years of follow-up in clinical practice and to evaluate numerous factors among participants that are associated with clinically meaningful weight loss. Although this study was conducted in 18 clinic sites from a single health care delivery system in Northern California, the results from this study have high potential for generalizability to other mixed-payer, fee-for-service health care systems throughout the nation.
In summary, in a real-world health care setting, a lifestyle change program is associated, on average, with modest weight loss that is sustained through 24 months among participants with a range of cardiometabolic risk factors. More than one-quarter of participants achieve clinically meaningful weight loss, regardless of cardiometabolic risk.
Implications for Diabetes Educators
The results of this study indicate that patients with a broad range of cardiometabolic risk factors benefit from a CDC-aligned behavioral lifestyle intervention that is primarily intended for diabetes prevention. Clinicians may consider using this program to promote weight loss among individuals with elevated cardiometabolic risk, regardless of diabetes risk.
Supplementary Material
Acknowledgments:
The authors thank several groups at Sutter Health for providing valuable information on the identification lifestyle change program participants in the EHR and on the format and structure of the program at individual Sutter Health clinics: diabetes management regional leads (Karen Astrachan, RD; Amy Fox, RD; Catherine Hazlewood; Beth Schatzman, RD; and Jan Hadley, RD), lifestyle coaches, and members of the Sutter Health Diabetes Care Improvement Committee (DCIC).
Funding: Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R18DK110739. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: Ms Greenwood is a consultant for Mytonomy, Inc. No other conflicts of interest exist.
References
- 1.National Center for Health Statistics. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults Aged 20 and Over: United States, 1960–1962 Through 2013–2014. 2016, July https://www.cdc.gov/nchs/data/hestat/obesity_adult_13_14/obesity_adult_13_14.pdf. Accessed on January 16, 2019.
- 2.American Heart Association. Understand Your Risk for Diabetes, Modifiable Risk Factors for Type 2 Diabetes. 2018, January 29 http://www.heart.org/HEARTORG/Conditions/More/Diabetes/UnderstandYourRiskforDiabetes/Understand-Your-Risk-for-Diabetes_UCM_002034_Article.jsp#.WwMurO4vxaR.
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the diabetes prevention program into the community: the DEPLOY pilot study. Am J Prev Med. 2008;35(4):357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parikh P, Simon EP, Fei K, Looker H, Goytia C, Horowitz CR. Results of a pilot diabetes prevention intervention in East Harlem, New York City: Project HEED. Am J Public Health. 2010; 100(suppl 1):S232–S239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katula JA, Vitolins MZ, Rosenberger EL, et al. One-year results of a community-based translation of the Diabetes Prevention Program: Healthy-Living Partnerships to Prevent Diabetes (HELP PD) Project. Diabetes Care. 2011;34(7):1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanaya AM, Santoyo-Olsson J, Gregorich S, Grossman M, Moore T, Stewart AL. The Live Well, Be Well study: a communitybased, translational lifestyle program to lower diabetes risk factors in ethnic minority and lower-socioeconomic status adults. Am J Public Health. 2012;102(8):1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ockene IS, Tellez TL, Rosal MC, et al. Outcomes of a Latino community-based intervention for the prevention of diabetes: the Lawrence Latino Diabetes Prevention Project. Am J Public Health. 2012;102(2):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma J, Yank V, Xiao L, et al. Translating the Diabetes Prevention Program lifestyle intervention for weight loss into primary care: a randomized trial. JAMA Intern Med. 2013;173(2):113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida FA, Shetterly S, Smith-Ray RL, Estabrooks PA. Reach and effectiveness of a weight loss intervention in patients with prediabetes in Colorado. Prev Chronic Dis. 2010;7(5):A103. [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderwood KK, Hall TO, Harwell TS, et al. ; Montana Cardiovascular Disease and Diabetes Prevention Program Workgroup. Implementing a state-based cardiovascular disease and diabetes prevention program. Diabetes Care. 2010;33(12):2543–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehm CD, Marquez ME, Spurrell-Huss E, Hollingsworth N, Parsons AS. Lessons from Launching the Diabetes Prevention Program in a large integrated health care delivery system: a case study. Popul Health Manag. 2017;20(4):262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. National Diabetes Prevention Program. 2018, November 3 https://www.cdc.gov/diabetes/prevention/index.html.
- 15.Centers for Disease Control and Prevention. Centers for Disease Control and Prevention Diabetes Prevention Recognition Program: Standards and Operating Procedures. 2018, March https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf.
- 16.Bang H, Edwards AM, Bomback AS, et al. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med. 2009;151(11):775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colin Bell A, Adair LS, Popkin BM. Ethnic differences in the association between body mass index and hypertension. Am J Epidemiol. 2002;155(4):346–353. [DOI] [PubMed] [Google Scholar]
- 18.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 19.Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiscella K, Franks P, Clancy CM. Skepticism toward medical care and health care utilization. Med Care. 1998;36(2):180–189. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn G. Effect of degree of weight loss on health benefits. Obes Res. 1995;3(suppl 2):211s–216s. [DOI] [PubMed] [Google Scholar]
- 22.Kramer MK, Kriska AM, Venditti EM, et al. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37(6):505–511. [DOI] [PubMed] [Google Scholar]
- 23.Yank V, Xiao L, Wilson SR, Stafford RS, Rosas LG, Ma J. Short-term weight loss patterns, baseline predictors, and longer-term follow-up within a randomized controlled trial. Obesity (Silver Spring). 2014;22(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ely EK, Gruss SM, Luman ET, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramer MK, Vanderwood KK, Arena VC, et al. Evaluation of a Diabetes Prevention Program lifestyle intervention in older adults: a randomized controlled study in three senior/community centers of varying socioeconomic status. Diabetes Educ. 2018;44(2):118–129. [DOI] [PubMed] [Google Scholar]
- 26.Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy. 1999;4(2):112–121. [DOI] [PubMed] [Google Scholar]
- 27.Taradash J, Kramer M, Molenaar D, Arena V, Vanderwood K, Kriska AM. Recruitment for a Diabetes Prevention Program translation effort in a worksite setting. Contemp Clin Trials. 2015;41:204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daviglus ML, Talavera GA, Aviles-Santa ML, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308(17):1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber MB, Ranjani H, Staimez LR, et al. The stepwise approach to diabetes prevention: results from the D-CLIP randomized controlled trial. Diabetes Care. 2016;39(10):1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosas LG, Lv N, Xiao L, et al. Evaluation of a culturally-adapted lifestyle intervention to treat elevated cardiometabolic risk of Latino adults in primary care (Vida Sana): a randomized controlled trial. Contemp Clin Trials. 2016;48:30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardona-Morrell M, Rychetnik L, Morrell SL, Espinel PT, Bauman A. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health. 2010;10:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37(4):922–933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.