Abstract
Background
Fragmented QRS (fQRS) complex is an electrocardiographic pattern which reflects myocardial scarring. We aimed to investigate the relationship between the presence of fragmented QRS (fQRS) on electrocardiogram (ECG) and plasma galectin‐3 levels in patients with heart failure (HF) and severely decreased left ventricular ejection fraction (LVEF ≤ 35%).
Methods
We prospectively enrolled 125 symptomatic HF patients (NYHA class II‐III) with severely reduced LVEF (≤35%). fQRS was identified in ECG. Galectin‐3 and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels were measured. Patients were divided into two groups based on the presence (n = 40) or absence (n = 85) of a fQRS on ECG.
Results
Majority of patients were male (87.70%), and mean age was 65.1 ± 11.6. Galectin‐3 and NT‐proBNP levels were found to be significantly higher in the fQRS (+) group compared with the fQRS (−) group (NT‐proBNP 5,362 ± 701 pg/ml vs. 4,452 ± 698 pg/ml; p < 0.001, galectin‐3 607 ± 89.8 pg/ml vs. 509.4 ± 63.5 pg/ml; p < 0.001). Multivariate analyses revealed galectin‐3 and NT‐proBNP levels are the presence of fQRS on ECG (p < 0.001 and p < 0.001, respectively). The area under the curve using the galectin‐3 level for fQRS was 0.819.
Conclusions
fQRS and serum galectin‐3 levels are associated with myocardial fibrosis and are associated with poor prognosis in heart failure. In our study, a positive correlation was found between serum galectin‐3 levels and fQRS on ECG.
Keywords: galectin‐3, heart failure, NT‐proBNP, NYHA
1. INTRODUCTION
Heart failure (HF) is a highly prevalent disease in the general population which requires repeat hospitalization and causes significant morbidity and mortality (Ponikowski et al., 2016; Yancy, Jessup, & Bozkurt, 2017). As a consequence of this serious outcomes, HF results in a high economic burden for healthcare systems. Therefore, early recognition of poor outcome predictors is essential for patient management. Mortality occurs commonly due to pump failure or arrhythmogenic episodes (Goldstein & Hjalmarson, 1999; Narang, Clelandf, & Erhardt, 1996). Whether ischemic or nonischemic origin, patients with HF have more myocardial fibrosis than healthy subjects which is a substrate for arrhythmias and negative remodeling which gradually decreases left ventricular functions (Bernardo, Weeks, Pretorius, & McMullen, 2010; Piek, Boer, & Sillje, 2016; Weeks & McMullen, 2011). ECG is a part of routine evaluation of patients with HF (Ponikowski et al., 2016). Recently, a novel ECG parameter called fragmented QRS (fQRS) is demonstrated to be related to poor prognosis in patients with HF (Ozcan et al., 2013; Sha et al., 2011). Prognostic utility of fQRS has also been demonstrated in Brugada syndrome and arrhythmogenic right ventricular dysplasia (ARVD; Hiroshi et al., 2008; Peters, Trummel, & Koehler, 2008). fQRS reflects a generalized pathology in myocardial depolarization which is believed to be caused by disrupted electrical conduction in diseased or fibrotic myocardium (Das, Khan, Jacob, Kumar, & Mahenthiran, 2006). The association between fQRS and myocardial fibrosis detected by cardiac imaging has previously been demonstrated (Das et al., 2008).
Besides ECG, biomarkers have an essential role during diagnosis, follow‐up, and treatment of HF (Zethelius et al., 2008). Galectin‐3, a novel biomarker, is demonstrated to be associated with all‐cause mortality in general population, as well as revascularization and mortality after myocardial infarction in patients with ischemic heart disease. Galectin‐3 is also established to be useful in the diagnosis and prediction of prognosis in HF patients (De Boer et al., 2011). Galectin‐3 plays a critical role in fibroblast activation and is found to be associated with the development of cardiac hypertrophy, fibrosis, and remodeling in numerous studies (Dumic, Dabelic, & Flogel, 2006; Ochieng, Furtak, & Lukyanov, 2004). Also, increased levels of Galectin‐3 are clearly associated with increased myocardial fibrosis and cardiac arrhythmias (Hiroshi et al., 2008; Peters et al., 2008; Sha et al., 2011). Furthermore, the correlation between galectin‐3 and myocardial fibrosis has been demonstrated using cardiac magnetic resonance imaging (MRI; Vergaro et al., 2015).
The relationship between fQRS and galectin‐3 has not previously been investigated in patients with HF. We aimed to investigate the relationship between galectin‐3 levels and fQRS in patients with HF and severely decreased LV ejection fraction (LVEF ≤ %35).
2. METHODS
2.1. Study population
We prospectively enrolled 125 symptomatic HF patients (NYHA class II‐III) with severely reduced LVEF (≤%35) and between January 2015 and October 2017. Patients were treated with evidence‐based guideline‐recommended medical therapy by an experienced cardiologist team in a specific HF outpatient clinic (McMurray, Adamopoulos, Anker, & Auricchio, 2012; Ponikowski et al., 2016; Yancy et al., 2017). All patients were older than 18 years, and written informed consent was obtained. The study complies with the Declaration of Helsinki, and the trial protocol was approved by the local ethics committee. Exclusion criteria were as follows: (a) chronic inflammatory diseases which can involve myocardium, (b) presence of significant valvular disease, (c) history of recent acute coronary syndrome, (d) malignancy, and (e) ECG demonstration of depolarization disorders such as Brugada syndrome, Wolff‐Parkinson‐White syndrome, or paced rhythms. Baseline demographic data including age, gender, functional status in terms of NYHA class, hypertension, diabetes mellitus, smoking habits, and potential etiological factors for the development of HF, such as coronary artery disease, pregnancy, previous cardiotoxic drug exposure, and chronic renal failure were gathered from patient files. Patients were considered to be ischemic in origin if significant coronary artery disease was demonstrated or patients underwent coronary revascularization. The NYHA functional classification was used to describe patients’ symptoms and exercise capacity (Little, Brown, & Co, 1994). Patient's urea, creatinine, electrolytes, fasting glucose, serum albumin, troponin T, and C‐reactive protein, galectin‐3, and NT‐proBNP were recorded from patient's files.
2.2. Echocardiography
Transthoracic echocardiography (TTE) studies were carried out using a Philips iE33 echocardiography machine and X5 transducer (Philips Healthcare) with the patient in the left lateral decubitus position. The standard evaluation included M‐mode, 2‐dimensional, and Doppler studies according to the recommendations of the American Society of Echocardiography (ASE; Lang et al., 2015). Left ventricular ejection fraction (EF) was calculated from apical 4‐chamber views by manually tracing end‐diastolic and end‐systolic endocardial borders, using Simpson's method (Schiller et al., 1979).
2.3. Electrocardiography
A 12‐lead ECG (filter range 0.5 Hz–150 Hz, AC filter 60 Hz, 25 mm/s, 10 mm/mV) was obtained from all patients during clinical visits and analyzed by two independent, experienced cardiologist who were blinded to study design and patients’ clinical data. The presence of fQRS was decided using the following criteria: (a) RSR’ patterns with or without Q waves in two continuous derivations (QRS time <120 ms), (b) in the presence of an additional R wave (R’ wave) or notching within the S wave, and (c) in the presence of more than 1 R waves without typical bundle branch block (Das et al., 2006). The interobserver concordance rate in detection of fQRS was 96%. In case of disagreement, the final diagnosis was achieved by mutual agreement. The intraobserver concordance rate was 97%.
2.4. Galectin‐3 and NT‐proBNP analysis
Venous blood samples were collected from each patient and were centrifuged at 3000 rpm for 15 min after clotting occurred in the serum tubes. After centrifugation, samples were stored at −80 C until analysis. Serum galectin‐3 levels were measured by a commercially available Enzyme‐linked Immunosorbent Assay Kit (Bioassay Technology Laboratory) according to the manufacturer's protocol. Its detection limit was 5 pg/ml → 2,000 pg/ml. Biochemical analyses were made by clinicians who were blinded to clinical information and to ensure accurate measurements all of the samples were analyzed in duplicates. Plasma NT‐pro B‐type natriuretic peptide (NT‐proBNP) was also measured by an Enzyme‐linked Immunosorbent Assay Kit (Bioassay Technology Laboratory).
2.5. Statistical analyses
All statistical tests were conducted using the Statistical Package for the Social Sciences 19.0 for Windows (SPSS Inc). The Kolmogorov–Smirnov test was used to analyze the normality of the data. Continuous data were expressed as mean ± SD, and categorical data were expressed as percentages. The relationships among parameters were assessed using Pearson's or Spearman's correlation analysis as appropriate. Student's t test or Mann–Whitney U test was used to compare unpaired samples as needed. Univariate and multivariate logistic regression analyses were used to identify independent variables. Independent variables in univariate analysis were NT‐proBNP, galectin‐3, NYHA class, presence of atrial fibrillation (AF), age, and LVEF. These variables were utilized in multivariate logistic regression analysis with the stepwise method. The results of univariate and multivariate regression analyses were presented as odds ratio with 95% confidence interval (CI). The predictive ability of galectin‐3 ve NT‐proBNP for the presence of fQRS was evaluated using receiver operating characteristic (ROC) analysis. The results were expressed as relative risk (RR) and 95% CI. Significance was assumed at a 2‐sided p < 0.05.
3. RESULTS
Table 1 demonstrates demographical and clinical characteristics of study population. Majority of patients were male (n = 87.70%), and mean age was 65.1 ± 11.6. The patients were categorized into an fQRS (+) group (n = 40) and fQRS (−) group (n = 85) based on the presence of fQRS on ECG. A comparison of the two groups showed no difference in terms of hypertension, diabetes mellitus, coronary artery disease, atrial fibrillation, creatinine, GFR, sodium, potassium, hemoglobin, albumin, CRP, and troponin. There was a statistically significant difference with respect to galectin‐3 and NT‐proBNP between patients with fQRS (+) in comparison with patients fQRS (−) (NT‐proBNP 5,362 ± 701 pg/ml vs. 4,452 ± 698 pg/ml; p < 0.001, galectin‐3 607 ± 89.8 pg/ml vs. 509.4 ± 63.5 pg/ml; p < 0.001; Figure 1). Moreover, galectin‐3 and NT‐proBNP levels were significantly higher in patients with AF compared with those in sinus rhythm (NT‐proBNP 5,142 ± 723 pg/ml vs. 4,460 ± 762 pg/ml; p < 0.001, galectin‐3 559.6 ± 93.8 pg/ml vs. 527.2 ± 77.5 pg/ml; p = 0.037), respectively (Table 1).
Table 1.
Baseline demographic and clinical characteristics of fQRS (+) and fQRS (−) groups
| fQRS(+), n = 40 | fQRS(−), n = 85 | p | |
|---|---|---|---|
| Age, years (mean ± SD) | 66 ± 10.4 | 66.1 ± 12.1 | 0.966 |
| Male, n (%) | 27 (68) | 60 (70) | 0.726 |
| Body mass index, (kg/m2) | 29.9 ± 6.3 | 29.4 ± 6.5 | 0.720 |
| NYHA II, n (%) | 25 (63) | 56 (66) | 0.712 |
| NYHA III, n (%) | 15 (37) | 29 (34) | 0.841 |
| Smoking, n (%) | 14 (35) | 35 (41) | 0.509 |
| Ischemic heart disease, n (%) | 24 (60) | 54 (63) | 0.704 |
| Hypertension, n (%) | 27 (68) | 55 (65) | 0.759 |
| Diabetes mellitus, n (%) | 21 (53) | 38 (45) | 0.415 |
| Atrial fibrillation, n (%) | 18 (45) | 43 (51) | 0.432 |
| Beta blocker, n (%) | 30 (75) | 68 (80) | 0.526 |
| ACE‐I/ARB, n (%) | 28 (70) | 59 (69) | 0.947 |
| Spironolactone, n (%) | 19 (48) | 32 (38) | 0.296 |
| Digoxin, n (%) | 6 (15) | 23 (27) | 0.136 |
| LVEF (%) | 0.27 ± 0.07 | 0.29 ± 0.06 | 0.319 |
| Left atrium diameter (mm) | 47.5 ± 5.7 | 47.1 ± 6.2 | 0.722 |
| PASP (mmHg) | 45.7 ± 9.3 | 45.8 ± 10.6 | 0.946 |
| Left ventricle mass index (g/m2) | 145 ± 32.1 | 149.8 ± 39.1 | 0.495 |
| Creatinine, mg/dl | 1.18 ± 0.36 | 1.29 ± 0.56 | 0.282 |
| GFR, ml/min/1.73 m2 | 73.4 ± 26 | 65.6 ± 22.1 | 0.086 |
| Sodium, mEq/L | 138.1 ± 5.3 | 138.6 ± 5.1 | 0.658 |
| Potassium, mEq/L | 4.49 ± 0.57 | 4.59 ± 0.63 | 0.409 |
| Hemoglobin, g/dl | 12.3 ± 2.2 | 12.4 ± 2.19 | 0.954 |
| Glucose, mg/dl | 144.4 ± 45.9 | 152.4 ± 47.8 | 0.384 |
| Albumin, g/dl | 3.9 ± 0.6 | 4 ± 0.5 | 0.626 |
| CRP (mg/dl) | 33.2 ± 11.7 | 31.4 ± 10.4 | 0.394 |
| Troponin (mg/dl) | 0.117 ± 0.047 | 0.105 ± 0.037 | 0.096 |
| NT‐pro‐BNP (pg/ml) | 5,362 ± 701 | 4,452 ± 698 | <0.001 |
| Galectin‐3(pg/ml) | 607 ± 89.8 | 509.4 ± 63.5 | <0.001 |
Abbreviations: ACE‐I, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; CRP, C‐reaktif protein; GFR, glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure.
Figure 1.
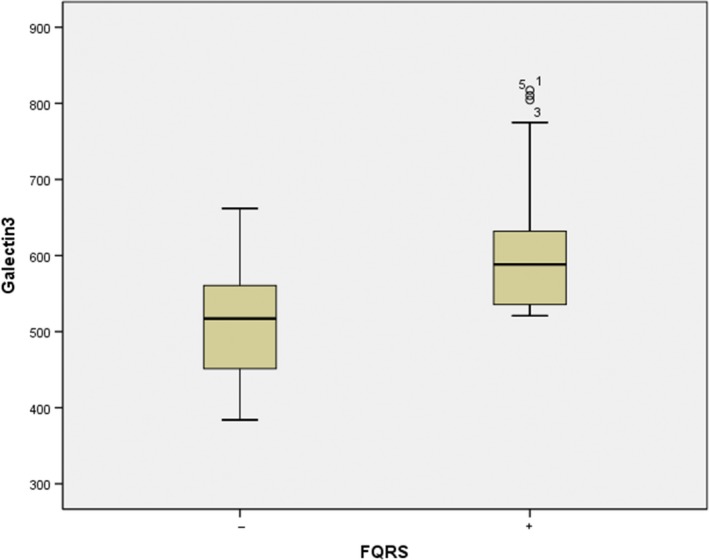
Comparison of galectin‐3 levels in fQRS (+) and fQRS (−) groups
Pearson's correlation test was performed to investigate the relationship between NT‐proBNP and galectin‐3, which was found to be statistically significant (p < 0.001, r = −0.622; Figure 2). Logistic regression using univariate and multivariate methods were performed to analyze parameters which may influence the development of fQRS. NT‐proBNP, galectin‐3, NYHA functional class, AF, age, and LVEF were included in univariate logistic regression analysis, and NT‐proBNP and galectin‐3 were found to be statistically significant for development of fQRS. NT‐proBNP and galectin‐3 were also analyzed using multivariate methods and found to be presence of fQRS on ECG (NT‐proBNP: OR = 1.003, p < 0.001; galectin‐3: OR = 1.020, p < 0.001; Table 2).
Figure 2.
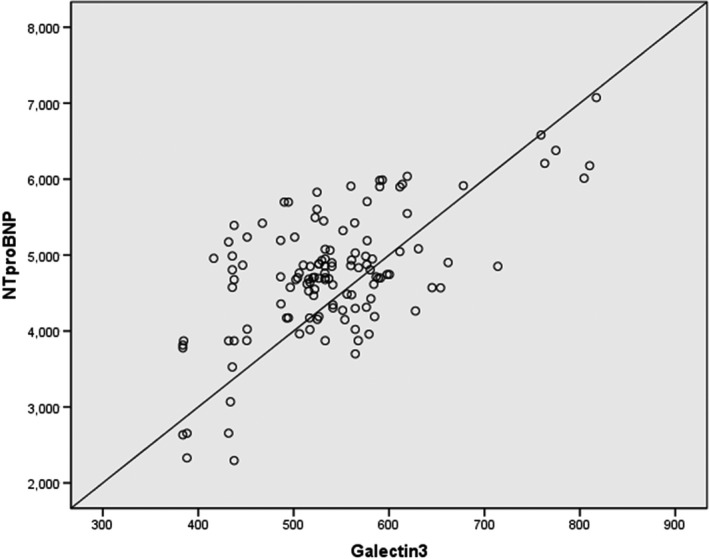
Spearman's correlation analysis of NT‐proBNP and galectin‐3 levels
Table 2.
Univariate and multivariate logistic regression analyses for predictors of fQRS
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| NT‐proBNP | 1.003 | 1.001–1.004 | 0.001 | 1.003 | 1.001–1.004 | 0.001 |
| Galectin‐3 | 1.020 | 1.009–1.032 | 0.001 | 1.020 | 1.009–1.032 | 0.001 |
| NYHA | 1.448 | 0.496–4.227 | 0.498 | |||
| AF | 3.288 | 0.948–11.406 | 0.061 | |||
| Age | 1.004 | 0.957–1.054 | 0.869 | |||
| LVEF | 0.968 | 0.957–1.054 | 0.479 | |||
Abbreviations: AF, atrial fibrillation; CI, confidence interval; EF, ejection fraction; fQRS, fragmented QRS complex; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; OR, odds ratio.
Sensitivity and specificity of NT‐proBNP and galectin‐3 levels, which were found to be statistically significant for fQRS, were calculated in ROC analyses. The blue line in Figure 3 represents galectin‐3 levels, and area under the curve (AUC) was measured, with value of 0.81 (0.74–0.89, p < 0.001). Meanwhile, the green line represents NT‐proBNP levels, with an AUC of 0.80 (0.72–0.88, p < 0.001). A serum galectin‐3 level of 537.52 pg/ml was defined as the optimal cutoff points fQRS. The best performing value of serum galectin‐3 (537.52 pg/mL) to presence of fQRS on ECG was associated with 75% sensitivity and 68% specificity. The area under the curve (AUC) of the serum galectin‐3 levels for fQRS was 0.819 (95% CI 0.745–0.893; p < 0.001; Figure 3).
Figure 3.
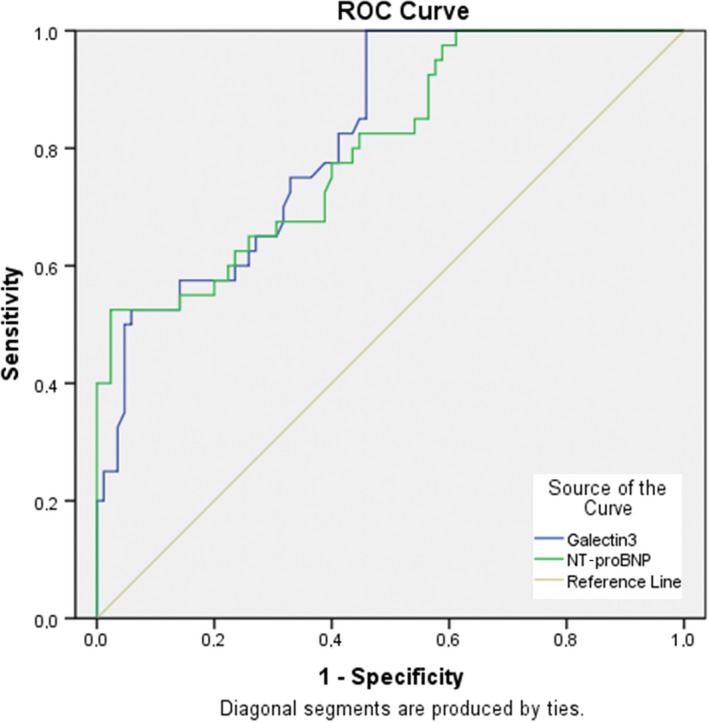
ROC curve analysis. Diagnostic value of galectin‐3 and NT‐proBNP for fQRS
4. DISCUSSION
In this study, we investigated the relationship between galectin‐3 levels and fQRS in patients with heart failure with reduced left ventricular ejection fraction. Principal findings of our study are as follows: (a) in patients with fQRS, galectin‐3 and NT‐proBNP levels were higher compared with those without fQRS (NT‐proBNP 5,362 ± 701 vs. 4,452 ± 698; p < 0.001, galectin‐3 607 ± 89.8 vs. 509.4 ± 63.5; p < 0.001), (b) logistic regression analyses revealed galectin‐3 and NT‐proBNP levels are the presence of fQRS on ECG (p < 0.001 and p < 0.001, respectively).
Despite current advancements in the treatment of HF, the prognosis still remains poor (Yancy et al., 2017). Among patients with HF, leading cause of death is sudden cardiac death due to ventricular arrhythmias in patients with NYHA class II‐III (%64) and pump failure in NYHA class IV patients (%33; Goldstein & Hjalmarson, 1999). There is an ongoing need for better prognostic markers in HF patients, for which markers of myocardial fibrosis seem to be good candidates. Previously, it has been demonstrated that the amount of fibrosis in cardiac tissue is related to slowed electrical conduction and increased the arrhythmogenic substrate. Myocardial ischemia or scar is known to be a poor prognosis marker in HF patients, with associated increase in mortality due to ventricular arrhythmias and pump failure (Das et al., 2007; Varriale & Chryssos, 1992). In dilated cardiomyopathy patients, amount of fractionated potentials seem to be increased which is thought to result from myocardial fiber disorientation (Abrahamsson et al., 2009). Increased interstitial fibrosis separates myocardial bundles, which impairs transverse conduction but lets longitudinal conduction virtually undisturbed (Spach et al., 1982). Various studies consistently associated fibrosis with either atrial or ventricular arrhythmias: fibrosis may be associated with all possible pathological arrhythmia mechanisms like triggered activity, reentry, and automaticity (Manabe, Shindo, & Nagai, 2002; Miragoli, Salvarani, & Rohr, 2007; Weber, 2004).
Myocardial fibrosis has a significant influence on impulse propagation in heart, hence, on surface ECG. Typically, ECGs became fractionated when myocardial fibers, collagen fibers, and connection tissue intermingled (Gardner, Ursell, Fenoglio, & Wit, 1985). The pathophysiologic mechanism of fQRS on surface ECG is delayed depolarization of myocardial tissue due to electrically inactive scar tissue. Fragmented QRS complex (fQRS) from a 12‐lead ECG was shown to be associated with myocardial fibrosis with a sensitivity and specificity of 40% and 80%, respectively (Konno et al., 2015). The consequence of this heterogeneous activation is multiple spikes in any part of the QRS complex (Sinha et al., 2016). Literature includes strong evidence about the relationship between the presence of fQRS, a reflector of myocardial scarring, and poor prognosis in patients with HF. The explanation of this association still needs to be clarified. Fragmented QRS is associated with increased mortality as fQRS is correlated with the size of the infarct area and the presence of myocardial fibrosis (Lorgis et al., 2014). Rattanawong et al. showed that fQRS could be an important tool for risk assessment in patients with Brugada syndrome and hypertrophic cardiomyopathy (HCM; Rattanawong, Riangwiwat, Kanitsoraphan, et al., 2018; Rattanawong, Riangwiwat, Prasitlumkum, et al., 2018). Gulsen et al. showed that fragmented QRS could predict mortality in aortic stenosis patients after transcatheter aortic valve replacement (TAVR) procedure (Gulsen et al., 2019). Kanitsoraphan et al. showed that baseline fQRS is associated with increased all‐cause mortality in patients with HF reduced LVEF (Kanitsoraphan et al., 2019). Attachaipanich et al. showed that the presence of fQRS complex on admission ECG was found to be an independent predictor of in‐hospital life‐threatening arrhythmic events in ST‐segment elevation myocardial infarction (STEMI) patients (Attachaipanich & Krittayaphong, 2019).
Galectin‐3 is a relatively novel biomarker which is associated with myocardial fibrosis (Ochieng et al., 2004). Secretion of galectin‐3 is predominantly occurring during monocyte differentiation, and galectin‐3 plays an important role during neutrophil activation and adhesion, chemoattraction of further monocytes, opsonization of apoptotic neutrophils, and activation of mast cells (Henderson & Sethi, 2009; Liu et al., 1995). Although the primary purpose of inflammation is tissue healing, chronic and prolonged inflammation results in tissue damage and ultimately fibrosis. In the tissue level, fibrosis, acute inflammation, and chronic inflammation are present at the same time (Berk, Fujiwara, & Lehoux, 2007). Sharma et al demonstrated that even without occult heart failure, galectin‐3 overexpression in macrophages is detectable during the early stages of myocardial dysfunction. They also demonstrated that continuous infusion of galectin‐3 in mice triggers cardiac fibroblast proliferation and collagen deposition and eventually results in ventricular dysfunction (Sharma et al., 2004). Similar to Sharma et al, Lin et al reported that overexpression of galectin‐3 in macrophage cells enhance the synthesis of the fibrosis‐related factor in fibroblasts (Lin et al., 2014). Furthermore, Liu et al demonstrated that overexpression of galectin‐3 in myocardium causes macrophage and mast cell infiltration and consequently interstitial and perivascular fibrosis (Liu et al., 1995). Previous reports demonstrated that galectin‐3 is a strong predictor of adverse events in patients with HF. In patients with chronic HF, elevated levels of galectin‐3 are associated with higher NYHA class and worse prognosis including repeat and longer hospitalization and mortality (De Boer et al., 2011). Galectin‐3 is a marker of organ fibrosis, including cardiac fibrosis (De Boer, Daniels, Maisel, & Januzzi, 2015; Yu et al., 2013). HF is a multi‐organ syndrome, and other organs could also contribute to increased Gal‐3 levels in HF. Galectin‐3 and soluble suppression of tumorigenicity 2 (sST2) are the only novel HF biomarkers that are included in the recent ACC/AHA HF guidelines, but their clinical utility still needs to be demonstrated (Yancy et al., 2017). In addition, galectin‐3 is related to various arrhythmias: Peters S et al. demonstrated the arrhythmogenic value of galectin‐3 in patients with arrhythmogenic right ventricular dysplasia (ARVD). They concluded that galectin‐3 levels were higher in patients with previous VT/VF compared with those without VT/VF in ARVD patients (Peters et al., 2008). Similarly, previous studies demonstrated the value of galectin‐3 for myocardial fibrosis in patients with AF (Fashanu et al., 2017). These results are concordant with our findings: we demonstrated that galectin‐3 levels are higher in patients with HF who are complicated with AF (Galectin‐3 559.6 ± 93.8 pg/ml vs. 527.2 ± 77.5 pg/ml; p = 0.037).
In this study, we investigated the relationship between fQRS which is believed to be associated with myocardial scar and galectin‐3 as a marker of myocardial fibrosis. To the best of our knowledge, this is the first study over this subject. Galectin‐3 levels were higher in patients with fQRS in comparison with patients without fQRS. Univariate and multivariate regression analyses revealed galectin‐3 and NT‐proBNP levels are the presence of fQRS on ECG. ROC analyses revealed that galectin‐3 levels with cutoff value as 532.52 pg/ml have 75% sensitivity and 68% specificity in detecting fQRS. In a previous study, there was a relationship between the presence of fQRS and NT‐proBNP levels in STEMI patients (Zhao, Zhang, Hou, & Yu, 2018). Likewise, galectin‐3 and NT‐proBNP levels were significantly related the presence of fQRS in our study.
Galectin‐3 levels have been found to be associated with the risk of development of HF following acute coronary syndromes, and an association between galectin‐3 and NT‐proBNP levels were demonstrated (Grandin et al., 2012). In our study, we were able to demonstrate galectin‐3 levels to be correlated with NT‐proBNP levels (p < 0.001, r = −0.622; Figure 2).
5. CONCLUSION
It is previously reported that fQRS is a potential noninvasive and reproducible method for risk stratification of patients with HF (Das & Zipes, 2009). This study has shown that the presence of fQRS on ECG, which is a recognized poor prognostic indicator in heart failure patients, is related to levels of galectin‐3, which is known to be an indicator of myocardial fibrosis.
5.1. Limitations
The major limitation of our study is its single‐center nature which may have led to significant referral bias. We also did not include patients with NYHA I and IV symptoms which may have influenced our results. We did not perform analyses regarding the number of leads in which fQRS developed limiting our ability to study the relationship between magnitude of fQRS and the biomarkers. Myocardial fibrosis is commonly identified using cardiac MRI; however, cardiac MRI has limited availability in developing countries and other low‐resource settings. In addition to the high cost of cardiac MRI, the images must be interpreted by a specialist. Therefore, cardiac MRI could not be performed.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Barman HA, Durmaz E, Atici A, et al. The relationship between galectin‐3 levels and fragmented QRS (fQRS) in patients with heart failure with reduced left ventricular ejection fraction. Ann Noninvasive Electrocardiol. 2019;24:e12671 10.1111/anec.12671
REFERENCES
- Abrahamsson, P. , Dobson, J. , Granger, C. B. , McMurray, J. J. , Michelson, E. L. , Pfeffer, M. , … Swedberg, K. (2009). Impact of hospitalization for acute coronary events on subsequent mortality in patients with chronic heart failure. European Heart Journal, 30(3), 338–345. 10.1093/eurheartj/ehn503 [DOI] [PubMed] [Google Scholar]
- Attachaipanich, T. , & Krittayaphong, R. (2019). Fragmented QRS as a predictor of in‐hospital life‐threatening arrhythmic complications in ST‐elevation myocardial infarction patients. Annals of Noninvasive Electrocardiology, 24(1), e12593– 10.1111/anec.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk, B. C. , Fujiwara, K. , & Lehoux, S. (2007). ECM remodeling in hypertensive heart disease. Journal of Clinical Investigation, 117(3), 568–575. 10.1172/JCI31044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo, B. C. , Weeks, K. L. , Pretorius, L. , & McMullen, J. R. (2010). Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacology & Therapeutics, 128, 191–227. 10.1016/j.pharmthera.2010.04.005 [DOI] [PubMed] [Google Scholar]
- Das, M. K. , Khan, B. , Jacob, S. , Kumar, A. , & Mahenthiran, J. (2006). Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation, 113, 2495–2501. [DOI] [PubMed] [Google Scholar]
- Das, M. K. , Saha, C. , El Masry, H. , Peng, J. , Dandamudi, G. , Mahenthiran, J. , … Zipes, D. P. (2007). Fragmented QRS on a 12‐lead ECG: A predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 4(11), 1385–1392. 10.1016/j.hrthm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Das, M. K. , Suradi, H. , Maskoun, W. , Michael, M. A. , Shen, C. , Peng, J. , … Mahenthiran, J. (2008). Fragmented wide QRS on a 12‐lead ECG: A sign of myocardial scar and poor prognosis. Circulation Arrhythmia and Electrophysiology, 1, 258–268. [DOI] [PubMed] [Google Scholar]
- Das, M. K. , & Zipes, D. P. (2009). Fragmented QRS: A predictor of mortality and sudden cardiac death. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 6(3 Suppl), S8–14. 10.1016/j.hrthm.2008.10.019. Epub 2008 Oct 17. [DOI] [PubMed] [Google Scholar]
- de Boer, R. A. , Daniels, L. B. , Maisel, A. S. , & Januzzi, J. L. (2015). State of the art: Newer biomarkers in heart failure. European Journal of Heart Failure, 17, 559–569. 10.1002/ejhf.273 [DOI] [PubMed] [Google Scholar]
- De Boer, R. A. , Lok, D. J. A. , Jaarsma, T. , Van Der Meer, P. , Voors, A. A. , Hillege, H. L. , et al. (2011). Predictive value of plasma galectin‐3 levels in heart failure with reduced and preserved ejection fraction. Annals of Medicine, 43, 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumic, J. , Dabelic, S. , & Flogel, M. (2006). Galectin‐3: An open‐ended story. Biochimica Et Biophysica Acta, 1760, 616–635. 10.1016/j.bbagen.2005.12.020 [DOI] [PubMed] [Google Scholar]
- Fashanu, O. E. , Norby, F. L. , Aguilar, D. , Ballantyne, C. M. , Hoogeveen, R. C. , Chen, L. Y. , … Folsom, A. R. (2017). Galectin‐3 and incidence of atrial fibrillation: The atherosclerosis risk in communities (ARIC) study. American Heart Journal, 192, 19–25. 10.1016/j.ahj.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner, P. I. , Ursell, P. C. , Fenoglio, J. J. Jr , & Wit, A. L. (1985). Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation, 72, 596–611. 10.1161/01.CIR.72.3.596 [DOI] [PubMed] [Google Scholar]
- Goldstein, S. , & Hjalmarson, A. (1999). The mortality effect of metoprolol CR/XL in patients with heart failure: Results of the MERIT‐HF Trial. Clinical Cardiology, 22(Suppl 5), V30–V35. [PubMed] [Google Scholar]
- Grandin, E. W. , Jarolim, P. , Murphy, S. A. , Ritterova, L. , Cannon, C. P. , Braunwald, E. , & Morrow, D. A. (2012). Galectin‐3 and the development of heart failure after acute coronary syndrome: Pilot experience from PROVE IT‐TIMI 22. Clinical Chemistry, 58(1), 267–273. 10.1373/clinchem.2011.174359 [DOI] [PubMed] [Google Scholar]
- Gulsen, K. , Ince, O. , Kum, G. , Ozkalayci, F. , Sahin, I. , & Okuyan, E. (2019). Could fragmented QRS predict mortality in aortic stenosis patients after transcatheter aortic valve replacement? Annals of Noninvasive Electrocardiology, 24(2), e12618– 10.1111/anec.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, N. C. , & Sethi, T. (2009). The regulation of inflammation by galectin‐3. Immunological Reviews, 230(1), 160–171. 10.1111/j.1600-065X.2009.00794.x [DOI] [PubMed] [Google Scholar]
- Kanitsoraphan, C. , Rattanawong, P. , Mekraksakit, P. , Chongsathidkiet, P. , Riangwiwat, T. , Kanjanahattakij, N. , … Thavaraputta, S. (2019). Baseline fragmented QRS is associated with increased all‐cause mortality in heart failure with reduced ejection fraction: A systematic review and meta‐analysis. Annals of Noninvasive Electrocardiology, 24(2), e12597– 10.1111/anec.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konno, T. , Hayashi, K. , Fujino, N. , Oka, R. , Nomura, A. , Nagata, Y. , … Yamagishi, M. (2015). Electrocardiographic QRS fragmentation as a marker for myocardial fibrosis in hypertrophic cardiomyopathy. Journal of Cardiovascular Electrophysiology, 26(10), 1081–1087. 10.1111/jce.12742 [DOI] [PubMed] [Google Scholar]
- Lang, R. M. , Badano, L. P. , Mor‐Avi, V. , Afilalo, J. , Armstrong, A. , Ernande, L. , … Voigt, J.‐U. (2015). Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Journal of the American Society of Echocardiography, 28(1), 1–39.e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- Lin, Y.‐H. , Chou, C.‐H. , Wu, X.‐M. , Chang, Y.‐Y. , Hung, C.‐S. , Chen, Y.‐H. , … Wu, K.‐D. (2014). Aldosterone induced galectin‐3 secretion in vitro and in vivo: From cells to humans. PLoS ONE, 9(9), e95254 10.1371/journal.pone.0095254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, Brown & Co . (1994). Co.The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed. (pp. 253–256). Boston, MA: Little, Brown & Co. [Google Scholar]
- Liu, F. T. , Hsu, D. K. , Zuberi, R. I. , Kuwabara, I. , Chi, E. Y. , & Henderson W. R. Jr. (1995). Expression and function of galectin‐3, a beta‐galactoside‐binding lectin, in human monocytes and macrophages. American Journal of Pathology, 147(4):1016–1028. [PMC free article] [PubMed] [Google Scholar]
- Lorgis, L. , Cochet, A. , Chevallier, O. , Angue, M. , Gudjoncik, A. , Lalande, A. , … Cottin, Y. (2014). Relationship between fragmented QRS and no‐reflow, infarct size, and peri‐infarct zone assessed using cardiac magnetic resonance in patients with myocardial infarction. Canadian Journal of Cardiology, 30(2), 204–210. 10.1016/j.cjca.2013.11.026 [DOI] [PubMed] [Google Scholar]
- Manabe, I. , Shindo, T. , & Nagai, R. (2002). Gene expression in fibroblasts and fibrosis: Involvement in cardiac hypertrophy. Circulation Research, 91, 1103–1113. 10.1161/01.RES.0000046452.67724.B8 [DOI] [PubMed] [Google Scholar]
- McMurray, J. J. , Adamopoulos, S. , Anker, S. D. , Auricchio, A. , et al. (2012). ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. European Heart Journal, 33(14), 1787–1847. 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- Miragoli, M. , Salvarani, Nicolò , & Rohr, S. (2007). Myofibroblasts induce ectopic activity in cardiac tissue. Circulation Research, 12, 101(8), 755–758. 10.1161/CIRCRESAHA.107.160549 [DOI] [PubMed] [Google Scholar]
- Morita, H. , Kusano, K. F. , Miura, D. , Nagase, S. , Nakamura, K. , Morita, S. T. , … Wu, J. (2008). Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation, 118, 1697–1704. 10.1161/CIRCULATIONAHA.108.770917 [DOI] [PubMed] [Google Scholar]
- Narang, R. , Clelandf, J. G. F. , & Erhardt, L. (1996). Mode of death in chronic heart failure. European Heart Journal, 17, 1390–2140. [DOI] [PubMed] [Google Scholar]
- Ochieng, J. , Furtak, V. , & Lukyanov, P. (2004). Extracellular functions of galectin‐3. Glycoconjugate Journal, 19, 527–535. 10.1023/B:GLYC.0000014082.99675.2f [DOI] [PubMed] [Google Scholar]
- Ozcan, S. , Cakmak, H. A. , Ikitimur, B. , Yurtseven, E. , Stavileci, B. , Tufekcioglu, E. Y. , & Enar, R. (2013). The prognostic significance of narrow fragmented QRS on admission electrocardiogram in patients hospitalized for decompensated systolic heart failure. Clinical Cardiology, 36(9), 560–564. 10.1002/clc.22158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, S. , Trummel, M. , & Koehler, B. (2008). QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia‐cardiomyopathy. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 5, 1417–1421. 10.1016/j.hrthm.2008.07.012 [DOI] [PubMed] [Google Scholar]
- Piek, A. , de Boer, R. A. , & Sillje, H. H. (2016). The fibrosis‐cell death axis in heart failure. Heart Failure Reviews, 21, 199–211. 10.1007/s10741-016-9536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponikowski, P. , Voors, A. A. , Anker, S. D. , Bueno, H. , Cleland, J. G. F. , Coats, A. J. S. , … van der Meer, P. (2016). 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diag‐ nosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. European Heart Journal, 37, 2129–2200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- Rattanawong, P. , Riangwiwat, T. , Kanitsoraphan, C. , Chongsathidkiet, P. , Kanjanahattakij, N. , Vutthikraivit, W. , & Chung, E. H. (2018). Baseline fragmented QRS increases the risk of major arrhythmic events in hypertrophic cardiomyopathy: Systematic review and meta‐analysis. Annals of Noninvasive Electrocardiology, 23(4), e12533– 10.1111/anec.12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattanawong, P. , Riangwiwat, T. , Prasitlumkum, N. , Limpruttidham, N. , Kanjanahattakij, N. , Chongsathidkiet, P. , … Chung E. H. (2018). Baseline fragmented QRS increases the risk of major arrhythmic events in Brugada syndrome: Systematic review and meta‐analysis. Annals of Noninvasive Electrocardiology, 23(2), e12507– 10.1111/anec.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller, N. B. , Acquatella, H. , Ports, T. A. , Drew, D. , Goerke, J. , Ringertz, H. , … Parmley, W. W. (1979). Left ventricular volume from paired biplane two‐dimensional echocardiography. Circulation, 60, 547e55 10.1161/01.CIR.60.3.547 [DOI] [PubMed] [Google Scholar]
- Sha, J. , Zhang, S. , Tang, M. , Chen, K. , Zhao, X. , & Wang, F. (2011). Fragmented QRS is associated with all‐cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Annals of Noninvasive Electrocardiology, 16, 270–275. 10.1111/j.1542-474X.2011.00442.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, U. C. , Pokharel, S. , van Brakel, T. J. , van Berlo, J. H. , Cleutjens, J. P. M. , Schroen, B. , … Pinto, Y. M. (2004). Galectin‐3 marks activated macrophages in failure‐prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation, 110, 3121–3128. 10.1161/01.CIR.0000147181.65298.4D [DOI] [PubMed] [Google Scholar]
- Sinha, S. K. , Bhagat, K. , Asif, M. , Singh, K. , Sachan, M. , Mishra, V. , … Pandey, U. (2016). Fragmented QRS as a marker of electrical dyssynchrony to predict inter‐ventricular conduction defect by subsequent echocardiographic assessment in symptomatic patients of non‐isch‐ emic dilated cardiomyopathy. Cardiology Research, 7(4), 140–145. 10.14740/cr495w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach, M. S. , Miller, W. T. , Dolber, P. C. , Kootsey, J. M. , Sommer, J. R. , & Mosher, C. E. Jr . (1982). The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circulation Research, 50, 175–191. 10.1161/01.RES.50.2.175 [DOI] [PubMed] [Google Scholar]
- Varriale, P. , & Chryssos, B. E. (1992). The RSR' complex not related to right bundle branch block: Diagnostic value as a sign of myocardial infarction scar. American Heart Journal, 123(2), 369–376. 10.1016/0002-8703(92)90648-F [DOI] [PubMed] [Google Scholar]
- Vergaro, G. , Del Franco, A. , Giannoni, A. , Prontera, C. , Ripoli, A. , Barison, A. , … Emdin, M. (2015). Galectin‐3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. International Journal of Cardiology, 1(184), 96–100. 10.1016/j.ijcard.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Weber, K. T. (2004). Fibrosis in hypertensive heart disease: Focus on cardiac fibroblasts. Journal of Hypertension, 22, 47–50. 10.1097/00004872-200401000-00011 [DOI] [PubMed] [Google Scholar]
- Weeks, K. L. , & McMullen, J. R. (2011). The athlete's heart vs. the failing heart: Can signaling explain the two distinct outcomes? Physiology (Bethesda), 26, 97–105. [DOI] [PubMed] [Google Scholar]
- Yancy, C. W. , Jessup, M. , Bozkurt, B. , et al. (2017). 2017 ACC/ AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Journal of Cardiac Failure, 23, 628–651. [DOI] [PubMed] [Google Scholar]
- Yu, L. , Ruifrok, W. P. T. , Meissner, M. , Bos, E. M. , van Goor, H. , Sanjabi, B. , … de Boer, R. A. (2013). Genetic and pharmacological inhibition of galectin‐3 prevents car‐ diac remodeling by interfering with myocardial fibrogenesis. Circulation: Heart Failure, 6, 107–117. 10.1161/CIRCHEARTFAILURE.112.971168 [DOI] [PubMed] [Google Scholar]
- Zethelius, B. , Berglund, L. , Sundstrom, J. , Ingelsson, E. , Basu, S. , Larsson, A. , et al. (2008). Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. New England Journal of Medicine, 358, 2107–2116. [DOI] [PubMed] [Google Scholar]
- Zhao, Q. , Zhang, R. , Hou, J. , & Yu, B. (2018). Relationship between Fragmented QRS and NT‐proBNP in patients with ST elevation myocardial infarction who underwent primary percutaneous coronary intervention. Acta Cardiol Sin., 34(1), 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]


