Abstract
Background
Cannabis has a long history of medicinal use. Cannabis‐based medications (cannabinoids) are based on its active element, delta‐9‐tetrahydrocannabinol (THC), and have been approved for medical purposes. Cannabinoids may be a useful therapeutic option for people with chemotherapy‐induced nausea and vomiting that respond poorly to commonly used anti‐emetic agents (anti‐sickness drugs). However, unpleasant adverse effects may limit their widespread use.
Objectives
To evaluate the effectiveness and tolerability of cannabis‐based medications for chemotherapy‐induced nausea and vomiting in adults with cancer.
Search methods
We identified studies by searching the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO and LILACS from inception to January 2015. We also searched reference lists of reviews and included studies. We did not restrict the search by language of publication.
Selection criteria
We included randomised controlled trials (RCTs) that compared a cannabis‐based medication with either placebo or with a conventional anti‐emetic in adults receiving chemotherapy.
Data collection and analysis
At least two review authors independently conducted eligibility and risk of bias assessment, and extracted data. We grouped studies based on control groups for meta‐analyses conducted using random effects. We expressed efficacy and tolerability outcomes as risk ratio (RR) with 95% confidence intervals (CI).
Main results
We included 23 RCTs. Most were of cross‐over design, on adults undergoing a variety of chemotherapeutic regimens ranging from moderate to high emetic potential for a variety of cancers. The majority of the studies were at risk of bias due to either lack of allocation concealment or attrition. Trials were conducted between 1975 and 1991. No trials involved comparison with newer anti‐emetic drugs such as ondansetron.
Comparison with placebo People had more chance of reporting complete absence of vomiting (3 trials; 168 participants; RR 5.7; 95% CI 2.6 to 12.6; low quality evidence) and complete absence of nausea and vomiting (3 trials; 288 participants; RR 2.9; 95% CI 1.8 to 4.7; moderate quality evidence) when they received cannabinoids compared with placebo. The percentage of variability in effect estimates that was due to heterogeneity rather than chance was not important (I2 = 0% in both analyses).
People had more chance of withdrawing due to an adverse event (2 trials; 276 participants; RR 6.9; 95% CI 1.96 to 24; I2 = 0%; very low quality evidence) and less chance of withdrawing due to lack of efficacy when they received cannabinoids, compared with placebo (1 trial; 228 participants; RR 0.05; 95% CI 0.0 to 0.89; low quality evidence). In addition, people had more chance of 'feeling high' when they received cannabinoids compared with placebo (3 trials; 137 participants; RR 31; 95% CI 6.4 to 152; I2 = 0%).
People reported a preference for cannabinoids rather than placebo (2 trials; 256 participants; RR 4.8; 95% CI 1.7 to 13; low quality evidence).
Comparison with other anti‐emetics There was no evidence of a difference between cannabinoids and prochlorperazine in the proportion of participants reporting no nausea (5 trials; 258 participants; RR 1.5; 95% CI 0.67 to 3.2; I2 = 63%; low quality evidence), no vomiting (4 trials; 209 participants; RR 1.11; 95% CI 0.86 to 1.44; I2 = 0%; moderate quality evidence), or complete absence of nausea and vomiting (4 trials; 414 participants; RR 2.0; 95% CI 0.74 to 5.4; I2 = 60%; low quality evidence). Sensitivity analysis where the two parallel group trials were pooled after removal of the five cross‐over trials showed no difference (RR 1.1; 95% CI 0.70 to 1.7) with no heterogeneity (I2 = 0%).
People had more chance of withdrawing due to an adverse event (5 trials; 664 participants; RR 3.9; 95% CI 1.3 to 12; I2 = 17%; low quality evidence), due to lack of efficacy (1 trial; 42 participants; RR 3.5; 95% CI 1.4 to 8.9; very low quality evidence) and for any reason (1 trial; 42 participants; RR 3.5; 95% CI 1.4 to 8.9; low quality evidence) when they received cannabinoids compared with prochlorperazine.
People had more chance of reporting dizziness (7 trials; 675 participants; RR 2.4; 95% CI 1.8 to 3.1; I2 = 12%), dysphoria (3 trials; 192 participants; RR 7.2; 95% CI 1.3 to 39; I2 = 0%), euphoria (2 trials; 280 participants; RR 18; 95% CI 2.4 to 133; I2 = 0%), 'feeling high' (4 trials; 389 participants; RR 6.2; 95% CI 3.5 to 11; I2 = 0%) and sedation (8 trials; 947 participants; RR 1.4; 95% CI 1.2 to 1.8; I2 = 31%), with significantly more participants reporting the incidence of these adverse events with cannabinoids compared with prochlorperazine.
People reported a preference for cannabinoids rather than prochlorperazine (7 trials; 695 participants; RR 3.3; 95% CI 2.2 to 4.8; I2 = 51%; low quality evidence).
In comparisons with metoclopramide, domperidone and chlorpromazine, there was weaker evidence, based on fewer trials and participants, for higher incidence of dizziness with cannabinoids.
Two trials with 141 participants compared an anti‐emetic drug alone with a cannabinoid added to the anti‐emetic drug. There was no evidence of differences between groups; however, the majority of the analyses were based on one small trial with few events.
Quality of the evidence The trials were generally at low to moderate risk of bias in terms of how they were designed and do not reflect current chemotherapy and anti‐emetic treatment regimens. Furthermore, the quality of evidence arising from meta‐analyses was graded as low for the majority of the outcomes analysed, indicating that we are not very confident in our ability to say how well the medications worked. Further research is likely to have an important impact on the results.
Authors' conclusions
Cannabis‐based medications may be useful for treating refractory chemotherapy‐induced nausea and vomiting. However, methodological limitations of the trials limit our conclusions and further research reflecting current chemotherapy regimens and newer anti‐emetic drugs is likely to modify these conclusions.
Plain language summary
Cannabis‐based medicine for nausea and vomiting in people treated with chemotherapy for cancer
Background As many as three‐quarters of people who receive chemotherapy experience nausea (feeling sick) and vomiting (being sick), which many find distressing. While conventional anti‐sickness medicines are effective, they do not work for everyone, all of the time. Therapeutic drugs based on the active ingredient of cannabis, known as THC (delta‐9‐tetrahydrocannabinol), have been approved for use as anti‐sickness medicines in some countries.
Review question This review evaluated how well cannabis‐based medicines work for treating nausea and vomiting due to chemotherapy treatment in people with cancer, and what the side effects were.
Main findings This review of 23 randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) found that fewer people who received cannabis‐based medicines experienced nausea and vomiting than people who received placebo (a pretend medicine). The proportion of people who experienced nausea and vomiting who received cannabis‐based medicines was similar to conventional anti‐nausea medicines. However, more people experienced side effects such as 'feeling high', dizziness, sedation (feeling relaxed or sleepy) and dysphoria (feeling uneasy or dissatisfied) and left the study due to the side effects with cannabis‐based medicines, compared with either placebo or other anti‐nausea medicines. In trials where people received cannabis‐based medicines and conventional medicines in turn, overall people preferred the cannabis‐based medicines.
Quality of the evidence The trials were of generally of low to moderate quality and reflected chemotherapy treatments and anti‐sickness medicines that were around in the 1980s and 1990s. Also, the results from combining studies on the whole were of low quality. This means that we are not very confident in our ability to say how well the anti‐sickness medicines worked, and further research reflecting modern treatment approaches is likely to have an important impact on the results.
Cannabis‐based medicines may be useful for treating chemotherapy‐induced nausea and vomiting that responds poorly to commonly used anti‐sickness medicines.
Summary of findings
Background
Description of the condition
Nausea and vomiting are considered the most stressful adverse effects of chemotherapy by people with cancer (Barowski 1984; de Boer‐Dennert 1997; Russo 2014). Up to 75% of all people with cancer experience chemotherapy‐related nausea and vomiting (Schwartzberg 2007), which can lead to depression, anxiety and a feeling of helplessness, lower quality of life and may affect chemotherapy adherence (Dodds 1985; Janelsins 2013; Wilcox 1982).
Guidelines that inform standard protocols and algorithms ensure best practice in managing chemotherapy‐induced nausea and vomiting (Basch 2011; NCCN 2014; Roila 2010). However, standardised care and clinical decision‐making occurs within the context of individualised care, where focus on a person's preference is key to reducing chemotherapy‐related stress in people with cancer. People's preference for cancer treatment is illustrated by several studies that report people's preferences for specific chemotherapy regimens based on quality of life (reduced treatment toxicity), rather than treatment efficacy (increased predicted survival) (Beusterien 2014; Dubey 2005; Kuchuk 2013; Sun 2002). Therefore, it is important to consider use of all approved anti‐emetics that treat chemotherapy‐induced nausea and vomiting, where people may have a preference for one or another type of treatment.
During the 1990s, serotonin (5‐HT3) receptor antagonists, combined with dexamethasone, became the gold standard in the prevention of vomiting caused by chemotherapy (Gralla 1999; MASCC 1998). Episodes of chemotherapy‐induced nausea and vomiting are classified by distinct clinical phases: acute ‐ within the first 24 hours of treatment; delayed ‐ following the first 24 hours of treatment and anticipatory ‐ a learned response where refractory nausea and vomiting have been experienced during previous chemotherapy cycles, which results in nausea and vomiting prior to a subsequent treatment cycle (Roila 2010). Nowadays, the anti‐emetics indicated for chemotherapy with high emesis‐inducing potential are 5‐HT3 receptor antagonists, dexamethasone and aprepitant given during the acute emetic phase (Basch 2011; Gralla 2013; NCCN 2014; Olver 2004). However, if there is failure to respond, or there is an increase in vomiting, this cannot be corrected by increasing the dose or frequency of administration of the prophylactic anti‐emetics (5‐HT3 receptor antagonists, dexamethasone and aprepitant). People who experience refractory nausea and vomiting (i.e. people who do not respond to first‐line prophylactic anti‐emetics) can have additional anti‐emetics added to their existing prophylactic anti‐emetic regimen, such as a dopamine antagonist (metoclopramide, domperidone), a phenothiazine (prochlorperazine or levomepromazine), an antihistamine (cyclizine) or a butyrophenone (haloperidol) anti‐emetic (Gralla 1999; Gralla 2013). Benzodiazepines (lorazepam) can also be added to the prophylactic anti‐emetic regimen for refractory people, particularly those who are anxious or experience anticipatory nausea and vomiting (Gralla 1999). Dexamethasone is one of the most effective anti‐emetics for delayed nausea and vomiting, so people experiencing delayed refractory emesis can be prescribed an extended course of dexamethasone on a reducing dosage (Gralla 1999; Huang 2004; Ioannidis 2000). More recently, there have been reports of olanzapine being an effective adjunctive treatment for refractory nausea and vomiting (Gralla 2013). A second‐generation 5HT3 receptor antagonist, palonosetron, is effective in refractory nausea and vomiting to substitute for a first‐generation 5HT3 receptor antagonist (Gralla 2013). In addition, if people are unable to tolerate oral 5HT3 receptor antagonists, other formulations can be considered such as a 24‐hour granisetron transdermal patch, an orally disintegrating ondansetron melt, or ondansetron oral film (Gralla 2013). Consideration should also be made for other formulations of adjunctive anti‐emetics, such as buccal or rectal formulations (Gralla 2013).
According to Walsh 2003, cannabinoids, the active agents derived from cannabis (marijuana), may be considered for controlling nausea and vomiting as fourth‐line agents. They have been recommended in international anti‐emetic guidelines for the prevention of chemotherapy‐induced nausea and vomiting (Gralla 1999). Cannabinoids are thought to work through different mechanisms to other agents given for nausea and vomiting (see: How the intervention might work) and may be effective in people with cancer who respond poorly to commonly used agents (Machado Rocha 2008).
Description of the intervention
Cannabis has been used for medicinal purposes throughout history (Karniol 2001). It was listed on the American pharmacopoeia until 1944 (Bonnie 1974), when it was removed due to political pressure and was banned in the USA (Walsh 2003). Although cannabis has not been re‐listed on the American pharmacopoeia, in 1986 the Food and Drug Administration (FDA) authorised the use of its active element, delta‐9‐tetrahydrocannabinol (delta‐9‐THC), for medical purposes (Walsh 2003), to treat the adverse effects of nausea and vomiting in people with cancer receiving chemotherapy (Gralla 1999).
Currently, there are two synthetic delta‐9‐THC (cannabinoid) agents that have been evaluated in clinical trials that are approved for the treatment of nausea and vomiting in people with cancer treated with chemotherapy. These are oral formulations of trans(+)‐3‐(1,1‐dimethylheptyl)‐6,6a,7,8,10,10a‐hexahydro‐1‐hydroxy‐6,6‐dimethyl‐9H‐dibenzo(b,d),pyran‐9‐one, nabilone, and l(6aR‐trans)‐6a,7,8,10a‐tetrahydro‐6,6,9‐trimethyl‐3‐pentyl‐6H‐dibenzo[b,d]pyran‐1‐ol, dronabinol.
How the intervention might work
Cannabinoids affect the user by interacting with various receptors in different areas of the brain (Grotenhermen 2002). To date, two types of cannabinoid receptors have been identified, termed CB1 and CB2. Two substances naturally occurring in the brain that bind to and activate CB1 receptors are anandamide (Devane 1992) and 2‐arachidonoylglycerol (2‐AG) (Mechoulam 1995; Sugiura 1995). The cannabinoid receptors, and other naturally occurring substances that bind to them, are collectively termed the 'endocannabinoid system' (Rodríguez de Fonseca 2005). The blockage of CB1 cannabinoid receptors induces vomiting, suggesting the existence of cannabinoid receptors within the areas of the brain related to nausea and vomiting. This also suggests that the delta‐9‐THC anti‐emetic activity may be due to stimulation of the CB1 receptor (Darmani 2001).
Why it is important to do this review
A systematic review of randomised controlled trials (RCTs) published up to the year 2000 concluded that cannabinoids may be useful for controlling chemotherapy‐induced nausea and vomiting, but that harmful adverse effects may limit their widespread use (Tramer 2001). This meta‐analysis pooled placebo‐controlled and active controlled trials together. Furthermore, a more recently published systematic review came to a similar conclusion regarding effectiveness, but did not report on the adverse effects (Machado Rocha 2008). Cannabinoids are currently rarely used in clinical practice, and the publication of a systematic review of cannabinoids in highly emetic chemotherapy will provide an evidence base for their use in people with refractory nausea and vomiting.
Objectives
To evaluate the effectiveness and tolerability of cannabis‐based medications for chemotherapy‐induced nausea and vomiting in adults with cancer.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of cross‐over or parallel group design with active or placebo control groups, or both.
Types of participants
Adults aged 18 years and over presenting with any type of cancer and receiving chemotherapeutic treatment, independent of gender and clinical setting. The chemotherapeutic regimens include drugs with low, moderate or high emetic potential.
We excluded children and young people aged under 18 years, since prevention and treatment of chemotherapy‐induced nausea and vomiting, including use of cannabinoids, has been reported in this population in another Cochrane Review (Phillips 2010).
For the purpose of this review, chemotherapeutic treatments were those containing cytotoxic systemic anti‐cancer treatments.
Two review authors (VL and NS) independently classified chemotherapeutic regimens, containing one or more chemotherapy agents as low, moderate, moderate to high, or high emetic potential using both American Society of Clinical Oncology (ASCO) guidelines (Basch 2011) and MASCC (Multinational Association of Supportive Care in Cancer)/European Society for Medical Oncology (ESMO) guidelines (Roila 2010). We resolved differences in assessment by discussion.
Types of interventions
Experimental arm: licensed pharmacological interventions based on cannabinoids derived from cannabis: nabilone and dronabinol used either as monotherapy or adjunct to conventional dopamine antagonists.
Control arm: placebo or conventional dopamine antagonists.
Types of outcome measures
Primary outcomes
Complete control of nausea and vomiting (absence of episodes of nausea and vomiting without use of rescue medication) in the acute phase (within 24 hours of treatment with chemotherapy) and in the delayed phase (after 24 hours' treatment with chemotherapy) of nausea and vomiting.
Complete control of vomiting (absence of episodes of vomiting without use of rescue medication) in the acute and delayed phases of nausea and vomiting.
Complete control of nausea (absence of episodes of nausea without use of rescue medication) in the acute and delayed phases of nausea and vomiting.
Secondary outcomes
Withdrawal due to adverse effects of anti‐emetic.
Withdrawal due to any anti‐emetic‐related reason.
Withdrawal due to lack of anti‐emetic efficacy.
Cross‐over studies only: participant preference for one or other of the interventions (cannabis or control).
Incidence of particular adverse effects: 'feeling high', sedation, euphoria, dizziness, heightened sense of anxiety or agitation (dysphoria), depression, hallucinations, paranoia, hypotension, focal dystonia, extrapyramidal effects and oculogyric crisis.
Search methods for identification of studies
We sought papers in all languages and carried out translations wherever necessary.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2015, Issue 1), MEDLINE accessed via Ovid (from 1966 to January week 3 2015), EMBASE accessed via Ovid (from 1980 to January week 3 2015), PsycINFO accessed via Ovid (from inception to January week 2 2015) and LILACS (from inception to January 2015). Appendix 1, Appendix 2, Appendix 3, Appendix 4, and Appendix 5 show the search strategies.
All relevant articles were identified on PubMed and, using the 'related articles' feature, we carried out a further search for newly published articles.
Searching other resources
Unpublished and grey literature
We searched metaRegister (www.controlled-trials.com/rct), Physicians Data Query (www.nci.nih.gov), wwwclinicaltrials.gov, and www.cancer.gov/clinicaltrials for ongoing trials. We searched for conference proceedings and abstracts through ZETOC (zetoc.mimas.ac.uk) and WorldCat Dissertations.
Handsearching
We examined bibliographical references of all the relevant studies in detail in order to find studies not identified in the electronic search, and handsearched key textbooks and previous systematic reviews and reports of conferences (i.e. ESMO and ASCO).
Data collection and analysis
Selection of studies
We downloaded all the titles and abstracts retrieved by electronic searching to a reference management database; we removed duplicates and three review authors (LS, FA, SB) independently examined the remaining references. We excluded those studies that clearly did not meet the inclusion criteria and we obtained copies of the full text of potentially relevant references. Three review authors (LS, FA, SB) independently assessed the eligibility of the retrieved papers. The review authors were not blinded to the authors' names, institutions and journals of publication. We resolved disagreements by discussion and documented the reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
For the included studies, two review authors (FA, LS) independently abstracted data on characteristics of study participants (inclusion criteria, age, gender, type of cancer and stage of disease, co‐morbidities, co‐interventions and chemotherapy regimens); dose, frequency, route of administration and duration of experimental and control interventions; risk of bias (see Assessment of risk of bias in included studies); outcomes (see Types of outcome measures) and deviations from the protocol onto a data abstraction form specially designed for the review and checked by a third author (SB). We resolved disagreements by discussion or by appeal.
For dichotomous outcomes (such as number of people with chemotherapy‐induced nausea and vomiting per treatment group that did not present with symptoms of nausea and vomiting, described as absence of episodes of nausea and vomiting, to the end of the period of study; or withdrawals), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed in order to estimate a risk ratio (RR).
Wherever possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which participants were analysed in the groups to which they were assigned. For cross‐over studies, we extracted information on the number of cross‐over periods, duration of washout periods and whether a paired design had been taken into consideration in the analysis.
We notes the time points at which outcomes were collected and reported.
Unit of analysis
For cross‐over studies, we extracted the number of events as the numerator and the number analysed as the denominator for each treatment period.
Assessment of risk of bias in included studies
We assessed the risk of bias in included RCTs using the Cochrane's 'Risk of bias' tool (Higgins 2011). This included assessment of:
method used for generating the randomisation sequence allocation of participants to the treatment arms;
allocation concealment;
blinding (of participants, healthcare providers and outcome assessors);
reporting of incomplete outcome data (studies were considered at high risk of bias if more than 80% of people were assessed for primary outcomes): proportion of losses to follow‐up and association with treatment arms, reasons for drop‐out and association of drop‐outs with treatment arms;
selective reporting of outcomes;
any other sources of bias that were pre‐defined as carry‐over effects and unbiased data available for analysis for cross‐over trials.
Three review authors independently applied the 'Risk of bias' tool and resolved differences by discussion. We summarised results in both a 'Risk of bias' graph and a 'Risk of bias' summary. We interpreted results of meta‐analyses in light of the findings with respect to risk of bias.
Measures of treatment effect
For dichotomous outcomes, we calculated the RR and its respective 95% confidence interval (CI). We incorporated cross‐over trials in the meta‐analyses using reported summary effect estimates. Where the carry‐over effects were evident for a particular study, then we only used the data for the first period for the meta‐analysis.
Unit of analysis issues
None expected.
Dealing with missing data
We did not impute missing outcome data for any of the outcomes. If contact details could be obtained, we contacted trial authors and requested missing data.
Assessment of heterogeneity
We assessed the heterogeneity between the trials by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that could not be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). We interpreted the I2 value according to recommendations in the Cochrane Handbook for Systematic Reviews of Interventions as follows (Higgins 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Where there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for this.
Assessment of reporting biases
We examined funnel plots corresponding to meta‐analysis of the primary outcome if there were at least 10 trials included in the meta‐analysis to assess the potential for small‐study effects such as publication bias.
Data synthesis
Where we judged the trials sufficiently similar, we pooled their results in a meta‐analysis. For dichotomous outcomes, we combined the RR for each study. We used random‐effects models with inverse variance weighting for all meta‐analyses due to the clinical and methodological diversity of the studies (see Characteristics of included studies table).
If trials had multiple treatment groups, we divided the 'shared' comparison group into the number of treatment groups and treated comparisons between each treatment group and the split comparison group as independent comparisons.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses for the primary outcome if sufficient trials were available:
history of cannabis use, naive users versus prior users of cannabis;
history of exposure to chemotherapy, chemotherapy naive versus prior chemotherapy treatment;
type of cannabinoid agent, nabilone versus dronabinol.
Sensitivity analysis
We carried out sensitivity analyses for the primary outcome, if sufficient trials were available, excluding trials at high risk of bias and trials of a cross‐over design. We also analysed the influence of the following factors on estimates of treatment effect:
repeating the analysis excluding trials where chemotherapeutic regimens had low or low‐moderate emetic potential, or the emetic potential was unclassifiable;
repeating the analysis excluding trials where the primary outcome data were gathered after more than 24 hours of chemotherapeutic treatment.
Results
Description of studies
Results of the search
The search identified 441 records of which 135 were potentially eligible. We obtained hard copies of the full article of these articles for further consideration and excluded 112 (Figure 1). We identified no unpublished data.
1.
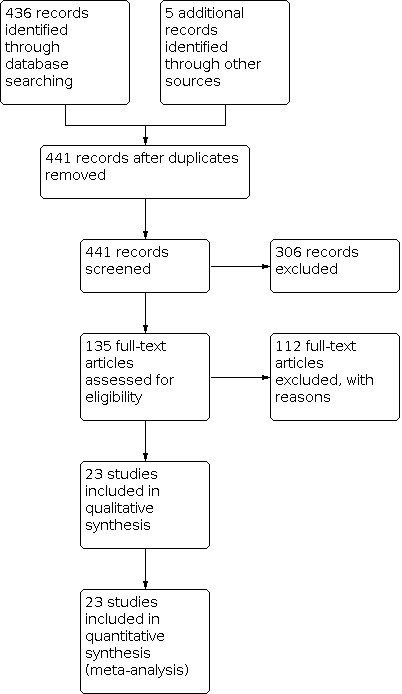
Identification and selection of randomised controlled trials for review inclusion.
Included studies
Of the 23 included RCTs, the majority (19) were of cross‐over design with four that were of parallel group design (Frytak 1979; Gralla 1984; Lane 1991; Pomeroy 1986).
The RCTs included people with a variety of cancers undergoing different chemotherapy regimens ranging from moderate to high anti‐emetic potential, except for one of low emetic potential (Chang 1979a); five were unclassifiable as reporting of chemotherapy regimen was unclear (Kleinman 1983; Lane 1991; Levitt 1982; Sallan 1975a; Ungerleider 1982). Four trials were conducted on participants who were cannabis naive (Ahmedzai 1983; Frytak 1979; Johansson 1982; Lane 1991), one where 88% of participants were naive (Chang 1981), and one where 27% of participants were naive (Chang 1979a). One study excluded current users of cannabis (McCabe 1988), and in the other trials previous exposure to cannabinoids was unclear.
Nine RCTs compared cannabinoids given as monotherapy compared with placebo (Chang 1979a; Chang 1981; Frytak 1979; Jones 1982; Kluin‐Neleman 1979; Levitt 1982; McCabe 1988; Sallan 1975a; Wada 1982), with another anti‐emetic agent (prochlorperazine) in 11 RCTs (Ahmedzai 1983; Einhorn 1981; Frytak 1979; Herman 1979; Johansson 1982; Lane 1991; McCabe 1988; Niiranen 1985; Orr 1981; Steele 1980; Ungerleider 1982), metoclopramide in two RCTs (Crawford 1986; Gralla 1984), domperidone in one RCT (Pomeroy 1986), and chlorpromazine in one RCT (George 1983). Cannabinoids were also given as co‐therapy with another anti‐emetic agent compared with an anti‐emetic agent alone in two RCTs (Kleinman 1983; Lane 1991). Two different cannabis‐based medications were tested: nabilone in 12 RCTs (Ahmedzai 1983; Crawford 1986; Einhorn 1981; George 1983; Herman 1979; Johansson 1982; Jones 1982; Levitt 1982; Niiranen 1985; Pomeroy 1986; Steele 1980; Wada 1982), and dronabinol in 11 RCTs (Chang 1979a; Chang 1981; Frytak 1979; Gralla 1984; Kleinman 1983; Kluin‐Neleman 1979; Lane 1991; McCabe 1988; Orr 1981; Sallan 1975a; Ungerleider 1982).
Dosing schedules varied across trials. Nabilone when given as monotherapy was administered most commonly as a fixed dose of 2 mg twice daily with lower doses administered when given as co‐therapy. Dronabinol was mainly given at doses according to body surface area and ranged from 10 mg/m2 twice daily to 15 mg/m2 six times daily. Both were given as an oral formulations. In two trials, oral dronabinol was replaced with cannabis‐based cigarettes if the participants vomited (Chang 1979a; Chang 1981).
The majority of the nausea or vomiting (or both) outcomes were reported for those that occurred within a 24‐hour period. However, for some trials, it was unclear when outcomes were assessed and they may have been reported for a longer time‐period (Herman 1979; Johansson 1982; Jones 1982; Kluin‐Neleman 1979; Lane 1991; Levitt 1982). Trials were conducted between 1975 and 1991.
Excluded studies
We excluded 112 studies for reasons described in the Characteristics of excluded studies table. The main reasons were due to not being a primary study (i.e. a review, editorial or letter) (64) or were a non‐randomised single‐arm study (eight). RCTs were excluded due to not being an eligible treatment group (six); comparison (six) or a relevant outcome (one); recruited children (three); only presenting preliminary (three) or subsidiary results (one); having no extractable data (eight) or a duplicate of an existing study (10). Two were unobtainable.
Risk of bias in included studies
The trials were of variable quality ranging from low to moderate (Figure 2; Figure 3).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Six trials adequately reported how the randomisation sequence was generated (Chang 1979a; Chang 1981; Frytak 1979; George 1983; Gralla 1984; Ungerleider 1982); the remaining 17 trials were unclear. Concealment of allocation was adequate in five trials (Einhorn 1981; Frytak 1979; George 1983; Gralla 1984; Herman 1979), and unclear in the remaining 18 trials.
Blinding
The majority of the trials were described as double‐blind, which was implemented by using identical tablets. Eight were reported as double‐blind, but it was unclear how this was achieved (Crawford 1986; Johansson 1982; Jones 1982; Lane 1991; Levitt 1982; Steele 1980; Ungerleider 1982; Wada 1982), and one study made no attempt at blinding (McCabe 1988).
Incomplete outcome data
Most trials were prone to attrition bias with only 9/23 trials judged as low risk of bias.
Selective reporting
All of the trials reported on the incidence of nausea or vomiting (or both); however, not all contributed to the meta‐analyses. We were unable to include data for trials if they only reported results for nausea and vomiting as mean frequency of episodes, rather than the proportion of participants with and without nausea or vomiting (or both). While a reduction in severity of nausea or a reduction in vomiting episodes (or both) may be considered a worthwhile outcome for people with chemotherapy‐induced nausea and vomiting, in these included trials, nausea severity was not measured with a validated instrument and episodes of vomiting were not analysed using standard methods for such (count) data. Therefore, we have not reported these data.
Other potential sources of bias
A large proportion of the trials were of cross‐over design. We assumed that the washout period was sufficient and there were no carry‐over effects of treatment due to the gap between chemotherapy treatment cycles, which would typically be around three weeks. The main potential source of bias was due to lack of information reported on whether a paired analysis was performed or not, and it was unclear if the groups were balanced at baseline.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Cannabinoids compared with placebo for chemotherapy‐induced nausea and vomiting.
| Cannabinoids compared with placebo for chemotherapy‐induced nausea and vomiting | ||||||
|
Patient or population: people with chemotherapy‐induced nausea and vomiting Intervention: cannabinoids Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Cannabinoids | |||||
|
Absence of nausea (follow‐up) |
3 per 100 | 6 per 100 (1 to 63) |
RR 2.0 (0.2 to 21) | 96 (2) | ⊕⊕⊝⊝ low3,5 | RR > 1 indicates treatment favours cannabinoids |
|
Absence of vomiting (follow‐up) |
6 per 100 | 34 per 100 (16 to 76) |
RR 5.7 (2.6 to 12.6) | 168 (3) | ⊕⊕⊝⊝ low3,5 | RR > 1 indicates treatment favours cannabinoids |
|
Absence of nausea and vomiting (follow‐up) |
11 per 100 | 32 per 100 (20 to 52) |
RR 2.9 (1.8 to 4.7) | 288 (3) | ⊕⊕⊕⊝ moderate3 | RR > 1 indicates treatment favours cannabinoids |
|
Participant preference (follow‐up) |
Low‐risk value2 | RR 4.8 (1.7 to 13) | 256 (2) | ⊕⊕⊝⊝ low3,4 | RR > 1 indicates treatment favours cannabinoids | |
| 8 per 100 | 38 per 100 (14 to 104) |
|||||
| High‐risk value2 | ||||||
| 22 per 100 | 106 (37 to 286) |
|||||
|
Withdrawal any reason (follow‐up) |
10 per 1000 | 3 per 1000 (0.1 to 7) |
RR 0.31 (0.01 to 7) | 33 (1) | ⊕⊝⊝⊝ very low1,3,5 | RR < 1 indicates treatment favours cannabinoids |
|
Withdrawal due to adverse event (follow‐up) |
80 per 1000 | 4 per 1000 (0.0 to 72) |
RR 6.9 (1.96 to 24) | 276 (2) | ⊕⊝⊝⊝ very low1,3,5 |
RR < 1 indicates treatment favours cannabinoids |
| *The assumed risk for all outcomes is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Sparse data.
2 The low‐ and high‐risk values are the two extreme proportions of people with a preference for one drug over another.
3 Limitations in the design (cross‐over study) and high attrition.
4 Unexplained heterogeneity.
5 Imprecision.
Summary of findings 2. Cannabinoids compared with other anti‐emetic agent for chemotherapy‐induced nausea and vomiting.
| Cannabinoids compared with other anti‐emetic agent for chemotherapy‐induced nausea and vomiting | |||||||
|
Patient or population: people with chemotherapy‐induced nausea and vomiting Intervention: cannabinoids Comparison: other anti‐emetic agent |
|||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Other anti‐emetic agent | Cannabinoids | ||||||
|
Absence of nausea (follow‐up) |
37 per 100 | 56 per 100 (25 to 118) |
RR 1.46 (0.67 to 3.15) | 258 (5) | ⊕⊕⊝⊝ low3,4 | RR > 1 indicates treatment favours cannabinoids | |
|
Absence of vomiting (follow‐up) |
Low‐risk value2 | RR 1.1 (0.86 to 1.4) | 209 (4) | ⊕⊕⊕⊝ moderate3 | RR > 1 indicates treatment favours cannabinoids | ||
| 10 per 1 000 | 11 per 1 000 (9 to 14) |
||||||
| High‐risk value2 | |||||||
| 70 per 100 | 77 per 100 (60 to 98) |
||||||
|
Absence of nausea and vomiting (follow‐up) |
Low‐risk value2 | RR 2.0 (0.74 to 5.4) | 414 (4) | ⊕⊕⊝⊝ low3,4 | RR > 1 indicates treatment favours cannabinoids | ||
| 1 per 100 | 2 per 100 (1 to 5) |
||||||
| High‐risk value2 | |||||||
| 42 per 100 | 84 per 100 (31 to 227) |
||||||
|
Participant preference (follow‐up) |
23 per 100 | 64 per 100 (44 to 92) |
RR 2.8 (1.9 to 4.0) | 799 (9) | ⊕⊕⊝⊝ low3,4 | RR > 1 indicates treatment favours cannabinoids | |
|
Withdrawal any reason (follow‐up) |
19 per 100 | 67 per 100 (27 to 171) |
RR 3.5 (1.4 to 9.0) | 42 (1) | ⊕⊕⊝⊝ low1,3 | RR < 1 indicates treatment favours cannabinoids | |
|
Withdrawal due to lack of efficacy (follow‐up) |
20 per 100 | 19 per 100 (1 to 420) |
RR 0.97 (0.04 to 21) | 118 (2) |
⊕⊝⊝⊝ very low1,3,4 | RR < 1 indicates treatment favours cannabinoids | |
|
Withdrawal due to adverse event (follow‐up) |
3 per 100 | 10 per 100 (4 to 24) |
RR 3.2 (1.3 to 8.0) | 740 (6) | ⊕⊕⊝⊝ low3,5 | RR < 1 indicates treatment favours cannabinoids | |
| *The assumed risk for all outcomes is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||||
1 Sparse data.
2 The low‐ and high‐risk values are the two extreme proportions of people with a preference for one drug over another.
3 Limitations in the design (cross‐over study) and high attrition.
4 Unexplained heterogeneity.
5 Imprecision.
Summary of findings 3. Cannabinoid plus other anti‐emetic agent compared with other anti‐emetic monotherapy for chemotherapy‐induced nausea and vomiting.
| Cannabinoid plus other anti‐emetic agent compared with other anti‐emetic monotherapy for chemotherapy‐induced nausea and vomiting | ||||||
|
Patient or population: people with chemotherapy‐induced nausea and vomiting Intervention: cannabinoid plus other anti‐emetic agent Comparison: anti‐emetic monotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Anti‐emetic monotherapy | Cannabinoid plus other anti‐emetic agent | |||||
|
Absence of nausea (follow‐up) |
1 per 100 | 10 per 100 (0 to 183) |
RR 10 (0.61 to 183) | 37 (1) | ⊕⊝⊝⊝ very low1,2,3 | RR > 1 indicates treatment favours cannabinoids |
|
Absence of vomiting (follow‐up) |
29 per 100 | 44 per 100 (20 to 90) |
RR 1.5 (0.69 to 3.1) | 89 (2) | ⊕⊕⊝⊝ low1,2 | RR > 1 indicates treatment favours cannabinoids |
|
Absence of nausea and vomiting (follow‐up) |
30 per 100 | 48 per 100 (20 to 108) |
RR 1.6 (0.68 to 3.6) | 37 (1) | ⊕⊕⊝⊝ low1,2 | RR > 1 indicates treatment favours cannabinoids |
|
Withdrawal any reason (follow‐up) |
20 per 100 | 26 per 100 (8 to 84) |
RR 1.3 (0.41 to 4.2) | 41 (1) | ⊕⊕⊝⊝ low1,2 | RR < 1 indicates treatment favours cannabinoids |
|
Withdrawal due to adverse event (follow‐up) |
1 per 100 | 7 per 100 (1 to 55) |
RR 7.0 (0.88 to 55) | 105 (2) | ⊕⊝⊝⊝ very low1,2,3 | RR < 1 indicates treatment favours cannabinoids |
|
Withdrawal due to lack of efficacy (follow‐up) |
20 per 100 | 2 per 100 (0 to 40) |
RR 0.12 (0.01 to 2.0) | 41 (1) | ⊕⊕⊝⊝ low1,2 | RR < 1 indicates treatment favours cannabinoids |
| *The assumed risk for all outcomes is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Sparse data.
2 Limitations in the design (cross‐over study) and high attrition.
3 Imprecision.
Cannabinoids versus placebo
Nine trials with 819 participants compared cannabinoids with placebo (Chang 1979a; Chang 1981; Frytak 1979; Jones 1982; Kluin‐Neleman 1979; Levitt 1982; Orr 1981; Sallan 1975a; Wada 1982), although not all trials contributed data for each outcome.
Primary outcome ‐ anti‐emetic efficacy
Two trials involving 96 participants showed no evidence of a difference between groups in the proportion of participants reporting complete absence of nausea with cannabinoids compared with placebo (RR 2.0; 95% CI 0.19 to 21; Analysis 1.1).
1.1. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 1: Absence of nausea
Three trials involving 168 participants showed that people had more chance of reporting complete absence of vomiting when they received cannabinoids compared with when they received placebo (RR 5.7; 95% CI 2.6 to 13). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance was not important (I2 = 0%, Tau2 = 0.0, Chi2 test for heterogeneity P value = 0.33; Analysis 1.2).
1.2. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 2: Absence of vomiting
Three trials involving 288 participants showed that people had more chance of reporting complete absence of nausea and vomiting when they received cannabinoids compared with placebo (RR 2.9; 95% CI 1.8 to 4.7). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance was not important (I2 = 0%, Tau2 = 0.0, Chi2 test for heterogeneity P value = 0.50; Analysis 1.3).
1.3. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 3: Absence of nausea and vomiting
Subgroup analyses, comparing results in trials with cannabis‐naive people to trials where participants either had previous experience with cannabis or where previous use was unclear, showed no evidence of a difference between the two subgroups (P value = 0.4) with respect to absence of nausea and vomiting.
Secondary outcome ‐ participant preference
Two trials involving 256 participants showed that people had more chance of reporting a preference for cannabinoids compared with placebo (RR 4.8; 95% CI 1.7 to 13) with substantial heterogeneity (I2 = 71%, Tau2 = 0.43, Chi2 test for heterogeneity P value = 0.06; Analysis 1.9).
1.9. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 9: Participant preference
Secondary outcomes ‐ tolerability and adverse events
One trial involving 33 participants showed no evidence of a difference between groups in the proportion of participants withdrawing for any reason (RR 0.31; 95% CI 0.01 to 7.21; Analysis 1.10).
1.10. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 10: Withdrawal for any reason
Participants had more chance of withdrawing due to an adverse event when they received cannabinoids compared with placebo (2 trials; 226 participants; RR 6.9; 95% CI 2.0 to 24; Analysis 1.11), and less chance of withdrawing due to lack of efficacy (1 trial; 228 participants; RR 0.05; 95% CI 0.0 to 0.89; Analysis 1.12).
1.11. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 11: Withdrawal due to adverse event
1.12. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 12: Withdrawal due to lack of efficacy
Participants had more chance of reporting 'feeling high' (3 trials; 137 participants; RR 31; 95% CI 6.4 to 152). The percentage of the variability in effect estimates that is due to heterogeneity rather than chance was not important (I2 = 0%, Tau2 = 0.0, Chi2 test for heterogeneity P value = 0.95; Analysis 1.6).
1.6. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 6: 'Feeling high'
There was no evidence of a difference between groups in the proportion of participants reporting depression (1 trial; 16 participants; RR 3.8; 95% CI 0.18 to 80; Analysis 1.4), dysphoria (2 trials; 96 participants; RR 9.0; 95% CI 0.50 to 161; Analysis 1.5), paranoia (1 trial; 64 participants; RR 3.0; 95% CI 0.13 to 71; Analysis 1.7), or sedation (2 trials; 139 participants; RR 4.5; 95% CI 0.35 to 58; Analysis 1.8) with substantial heterogeneity (I2 = 72%, Tau2 = 2.65, Chi2 test for heterogeneity P value = 0.06).
1.4. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 4: Depression
1.5. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 5: Dysphoria
1.7. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 7: Paranoia
1.8. Analysis.

Comparison 1: Cannabinoid versus placebo, Outcome 8: Sedation
The CIs for the estimates shown above are wide reflecting the uncertainty of these estimates.
Cannabinoids versus prochlorperazine
Nine trials with 1221 participants compared cannabinoids with prochlorperazine (Ahmedzai 1983; Frytak 1979; Herman 1979; Johansson 1982; Lane 1991; McCabe 1988; Niiranen 1985; Steele 1980; Ungerleider 1982), although not all trials contributed data for each outcome.
Primary outcome ‐ anti‐emetic efficacy
Five trials involving 258 participants showed no evidence of a difference between groups in the proportion of participants reporting no nausea (RR 1.5; 95% CI 0.67 to 3.2) with substantial heterogeneity (I2 = 58%, Tau2 = 0.33, Chi2 test for heterogeneity P value = 0.05; Analysis 2.1).
2.1. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 1: Absence of nausea
Four trials involving 209 participants showed no evidence of a difference between groups in the proportion of participants reporting no vomiting (RR 1.1; 95% CI 0.86 to 1.4). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance was not important (I2 = 0%, Tau2 = 0.0, Chi2 test for heterogeneity P value = 0.53; Analysis 2.3).
2.3. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 3: Absence of vomiting
Four trials involving 414 participants showed no evidence of a difference between groups in the proportion of participants reporting absence of nausea and vomiting (RR 2.0; 95% CI 0.74 to 5.4) with substantial heterogeneity (I2 = 60%, Tau2 = 0.51, Chi2 test for heterogeneity P value = 0.06; Analysis 2.5). Sensitivity analysis, where the two parallel group trials were pooled after removal of the five cross‐over trials, had an RR of 1.1 (95% CI 0.70 to 1.7) with no heterogeneity(I2 = 0%, Tau2 = 0.0, Chi2 test for heterogeneity P value = 0.56).
2.5. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 5: Absence of nausea and vomiting
Subgroup analyses ‐ comparing results in trials with cannabis‐naive people to trials where participants either had previous experience with cannabis or where previous use was unclear, showed no evidence of a difference between the two subgroups with respect to absence of nausea (P value = 0.11), but a difference between the subgroups for absence of nausea and vomiting with a smaller effect in people with no previous cannabis use (P value = 0.007). We were unable to conduct a subgroup analysis for absence of vomiting as all trials were of people who were cannabis naive (Analysis 2.6).
2.6. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 6: Absence of nausea and vomiting (subgroup analysis 1)
In addition, there was no evidence of a difference between subgroups comprised of different cannabinoid medications for absence of nausea (P value = 0.54), absence of vomiting (P value = 0.60) or absence of nausea and vomiting (P value = 0.10). The subgroup analyses did not explain the source of heterogeneity. There were insufficient data to perform other subgroup analyses listed in methods of analysis.
Secondary outcome ‐ participant preference
Seven trials involving 695 participants showed participants had more chance of reporting a preference for cannabinoids compared with prochlorperazine (RR 3.2; 95% CI 2.2 to 4.7) with substantial heterogeneity (I2 = 53%, Tau2 = 0.13, Chi2 test for heterogeneity P value = 0.05; Analysis 2.17).
2.17. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 17: Participant preference
Secondary outcomes ‐ tolerability and adverse events
Based on one trial with 42 participants, participants had more chance of withdrawing for any reason (RR 3.5; 95% CI 1.4 to 8.9; Analysis 2.18), and due to lack of anti‐emetic efficacy (RR 3.5; 95% CI 1.4 to 8.9; Analysis 2.20) when they received cannabinoids compared with prochlorperazine.
2.18. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 18: Withdrawal for any reason
2.20. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 20: Withdrawal due to lack of efficacy
Five trials with 664 participants showed participants had more chance of withdrawing due to an adverse event when they received cannabinoids compared with prochlorperazine (RR 3.9; 95% CI 1.3 to 12) with unimportant heterogeneity(I2 = 17%, Tau2 = 0.31, Chi2 test for heterogeneity P value = 0.31; Analysis 2.19).
2.19. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 19: Withdrawal due to adverse event
Participants had more chance of reporting the following adverse events when they received cannabinoids compared with prochlorperazine: dizziness (7 trials; 675 participants; RR 2.4; 95% CI 1.8 to 3.1; unimportant heterogeneity: I2 = 12%, Tau2 = 0.02, Chi2 test for heterogeneity P value = 0.34; Analysis 2.8), dysphoria (3 trials; 192 participants; RR 7.2; 95% CI 1.3 to 39; unimportant heterogeneity: I2 = 0%, Tau2 = 0.0, Chi2 test for heterogeneity P value = 0.75; Analysis 2.9), euphoria (2 trials; 280 participants; RR 18; 95% CI 2.4 to 133; unimportant heterogeneity: I2 = 0%, Tau2 = 0.00, Chi2 test for heterogeneity P value = 0.47; Analysis 2.10), 'feeling high' (4 trials; 389 participants; RR 6.2; 95% CI 3.5 to 11; unimportant heterogeneity: I2 = 0%, Tau2 = 0.0, Chi2 test for heterogeneity P value = 0.75; Analysis 2.11), and sedation (8 trials; 947 participants; RR 1.4; 95% CI 1.2 to 1.8; moderate heterogeneity: I2 = 31%, Tau2 = 0.02, Chi2 test for heterogeneity P value = 0.18; Analysis 2.15).
2.8. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 8: Dizziness
2.9. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 9: Dysphoria
2.10. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 10: Euphoria
2.11. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 11: 'Feeling high'
2.15. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 15: Sedation
There was no evidence of a difference between groups in the proportion of participants reporting depression (3 trials; 317 participants; RR 0.81; 95% CI 0.51 to 1.3; unimportant heterogeneity: I2 = 0%, Tau2 = 0.0, Chi2 test for heterogeneity P value = 0.47; Analysis 2.16), hallucinations (2 trials; 144 participants; RR 5.4; 95% CI 0.66 to 44; unimportant heterogeneity: I2 = 0%, Tau2 = 0.0, Chi2 test for heterogeneity P value = 0.80; Analysis 2.12), postural hypotension (3 trials; 305 participants; RR 1.2; 95% CI 0.52 to 2.9; moderate heterogeneity: I2 = 41%, Tau2 = 0.29, Chi2 test for heterogeneity P value = 0.18; Analysis 2.13), or paranoia (1 trial; 42 participants; RR 3.0; 95% CI 0.13 to 70; Analysis 2.14).
2.16. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 16: Depression
2.12. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 12: Hallucinations
2.13. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 13: Postural hypotension
2.14. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 14: Paranoia
Cannabinoid versus metoclopramide
Two trials with 57 participants compared cannabinoid with metoclopramide (Crawford 1986; Gralla 1984), although both trials did not contribute data for each outcome.
Primary outcome ‐ anti‐emetic efficacy
Neither trial reported data for the proportion of participants with absence of nausea or vomiting (or both) (Crawford 1986; Gralla 1984).
Secondary outcome ‐ participant preference
One trial involving 64 participants showed no evidence of a difference between groups in the proportion of participants reporting a preference for cannabinoids (RR 1.2; 95% CI 0.61 to 2.4; Analysis 2.17).
Secondary outcomes ‐ tolerability and adverse events
Neither trial reported withdrawals.
Participants had more chance of reporting dizziness (1 trial, 30 participants; RR 12; 95% CI 1.8 to 81; Analysis 2.8), and postural hypotension (1 trial, 30 participants; RR 17; 95% CI 1.1 to 270; Analysis 2.13) when they received cannabinoids compared with metoclopramide. The CIs for these estimates were very wide reflecting the uncertainty of these estimates.
There was no evidence of a difference between groups in the proportion of participants reporting 'feeling high' (1 trial, 30 participants; RR 3.0; 95% CI 0.35 to 26; Analysis 2.11), or sedation (1 trial; 30 participants; RR 0.93; 95% CI 0.73 to 1.2; Analysis 2.15).The CIs for these estimates were very wide reflecting the uncertainty of these estimates. There were no dystonic reactions in either treatment group.
Cannabinoids versus domperidone
One trial with 38 participants compared cannabinoids versus domperidone (Pomeroy 1986).
Primary outcome ‐ anti‐emetic efficacy
The trial did not report data for the proportion of participants with absence of nausea or vomiting (or both).
Secondary outcome ‐ participant preference
The trial did not report data for participant preference.
Secondary outcomes ‐ tolerability and adverse events
There was no evidence of a difference between groups in the proportion of participants withdrawing due to lack of efficacy (RR 0.14; 95% CI 0.01 to 2.7; Analysis 2.20) or withdrawal due to an adverse event (RR 0.14; 95% CI 0.01 to 2.7; Analysis 1.11), with both estimates based on very low event rates.
Participants had more chance of reporting dizziness when they received cannabinoids compared with domperidone (RR 2.8; 95% CI 1.1 to 7.1; Analysis 2.8).
There was no evidence of a difference between groups in the proportion of participants reporting euphoria (RR 5.0; 95% CI 0.26 to 98; Analysis 2.10), postural hypotension (RR 4.0; 95% CI 0.49 to 33; Analysis 2.13) or sedation (RR 1.2; 95% CI 0.66 to 2.3; Analysis 2.15).
Cannabinoids versus chlorpromazine
One trial with 20 participants compared cannabinoids with chlorpromazine (George 1983).
Primary outcome ‐ anti‐emetic efficacy
The trial did not report data for anti‐emetic efficacy.
Secondary outcome ‐ participant preference
There was no evidence of a difference between groups in participants' preferences for treatment with cannabinoids or chlorpromazine (RR 2.0; 95% CI 0.83 to 4.8; Analysis 2.17).
Secondary outcomes ‐ tolerability and adverse events
The trial did not report data for withdrawals.
There was no evidence of a difference between groups in the proportion of participants reporting euphoria (RR 3.0; 95% CI 0.13 to 70; Analysis 2.10), postural hypotension (RR 7.0; 95% CI 0.95 to 52; Analysis 2.13), or sedation (RR 1.7; 95% CI 0.85 to 3.4; Analysis 2.15), with few events giving rise to wide CIs around the point estimates.
Cannabinoid plus other anti‐emetic agent versus other anti‐emetic agent monotherapy
Two trials with 105 participants compared cannabinoid plus other anti‐emetic agent with other anti‐emetic agent monotherapy (Kleinman 1983; Lane 1991), although neither trial contributed data for all outcomes. The majority of the analyses were based on one small trial with few events (Lane 1991).
Primary outcome ‐ anti‐emetic efficacy
There was no evidence of a difference between groups in the proportion of participants reporting no nausea (RR 11; 95% CI 0.61 to 182; Analysis 3.1).
3.1. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 1: Absence of nausea
There was no evidence of a difference between groups in the proportion of participants reporting no vomiting (RR 1.5; 95% CI 0.69 to 3.1; Analysis 3.2).
3.2. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 2: Absence of vomiting
There was no evidence of a difference between groups in the proportion of participants reporting no nausea or vomiting (RR 1.6; 95% CI 0.68 to 3.6; Analysis 3.3).
3.3. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 3: Absence of nausea and vomiting
Secondary outcome ‐ participant preference
The trials did not report data for participant preference.
Secondary outcomes ‐ tolerability and adverse events
There was no evidence of a difference between groups in the proportion of participants withdrawing due to any reason (RR 1.3; 95% CI 0.41 to 4.2; Analysis 3.9).
3.9. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 9: Withdrawal for any reason
There was no evidence of a difference between groups in the proportion of participants withdrawing due to an adverse event (RR 7.0; 95% CI 0.88 to 55; Analysis 3.10).
3.10. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 10: Withdrawal due to adverse event
There was no evidence of a difference between groups in the proportion of participants withdrawing due to lack of efficacy (RR 0.12; 95% CI 0.01 to 2.0; Analysis 3.11).
3.11. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 11: Withdrawal due to lack of efficacy
There was no evidence of a difference between groups in the proportion of participants reporting depression (no participants in either group; Analysis 3.4), dizziness (RR 2.1; 95% CI 0.21 to 21; Analysis 3.5), dysphoria (RR 7.3; 95% CI 0.40 to 134; Analysis 3.6), paranoia (RR 5.2; 95% CI 0.27 to 103; Analysis 3.7), or sedation (RR 1.8; 95% CI 0.48 to 6.4; Analysis 3.8).
3.4. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 4: Depression
3.5. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 5: Dizziness
3.6. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 6: Dysphoria
3.7. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 7: Paranoia
3.8. Analysis.

Comparison 3: Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy, Outcome 8: Sedation
Discussion
Summary of main results
The included trials showed that cannabinoids were more effective than placebo and were similar to conventional anti‐emetics for treating chemotherapy‐induced nausea and vomiting. However, despite causing more adverse events than placebo, overall there was weak evidence that people receiving chemotherapy for cancer preferred cannabinoids to placebo with stronger evidence that people preferred them to other anti‐emetics.
Cannabinoids were highly effective. When compared with placebo, participants who received cannabinoids were five times as likely to report complete absence of vomiting, and three times as likely to report complete absence of nausea and vomiting. Although, some participants were six times more likely to withdraw from the study due to an adverse event with cannabinoids, other participants were more likely to withdraw due to lack of efficacy with placebo. Adverse events associated with cannabinoids were reported, however, the only one with evidence of a difference between cannabinoids and placebo was 'feeling high'. Overall, there was weak evidence that participants preferred cannabinoids to placebo.
When cannabinoids were compared with conventional anti‐emetic drugs, there was no evidence of a difference for nausea, vomiting, or nausea and vomiting. The majority of the data for these analyses were from comparison with prochlorperazine. However, participants were three or four times more likely to withdraw due to an adverse event with cannabinoids than prochlorperazine. Dizziness, dysphoria, 'feeling high' and sedation were all more likely with cannabinoids. Dizziness in particular was more likely with cannabinoids compared with metoclopramide and domperidone. Overall, there was evidence that participants preferred cannabinoids to conventional anti‐emetics; however, the majority of the trials were of prochlorperazine.
There may be an additional benefit of administering a cannabinoid with another anti‐emetic agent. These benefits include reduced nausea, vomiting, and nausea and vomiting. Adverse events were similar to those for comparisons with anti‐emetics given as monotherapy, but there were insufficient data to make firm conclusions.
Overall completeness and applicability of evidence
The trials included in this review were on adults with a wide variety of cancers undergoing a wide range of chemotherapy regimens. Many of the trials included participants who were refractory to conventional anti‐emetic medications. The synthetic cannabis‐based compounds were given orally and were either dronabinol or nabilone. The most informative RCTs were the ones that compared a cannabis‐based medication with a conventional anti‐emetic, rather than placebo. These trials showed that cannabis‐based medications had similar anti‐emetic effects compared with prochlorperazine and metoclopramide.
Nowadays, people receiving moderate to highly emetogenic chemotherapy regimens will be prescribed combination prophylactic anti‐emetic regimens including a 5‐HT3 antagonist and steroid, and perhaps also include a neurokinin‐1 (NK‐1) inhibitor for very highly emetogenic regimens (NCCN 2015). In the event of a person experiencing breakthrough or refractory, acute chemotherapy‐induced nausea and vomiting, an additional agent from a different pharmacological class of anti‐emetics would be recommended, such as metoclopramide, prochlorperazine or lorazepam (NCCN 2015). Cannabis‐based anti‐emetics offer an alternative additional anti‐emetic agent for breakthrough or refractory acute chemotherapy‐induced nausea and vomiting. Since there is a lack of studies that compare the use of cannabinoids to 5‐HT3 antagonists and NK‐1 inhibitors, this review found no evidence to support the use of cannabinoids in place of current prophylactic combination anti‐emetic regimens.
Quality of the evidence
Overall, the trials were of variable quality (very low to moderate by Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach). Strengths included the use of blinding by using double‐dummy preparations by the majority of the trials. However, it is possible that the trials were at risk of observer bias, due to the characteristic adverse effect profile of cannabinoids. The risk of bias from selective reporting of the primary outcome was low. The majority of the trials were unclear with respect to methods used to generate randomisation sequence and whether randomisation was concealed, so may be at risk of selection bias. A major weakness lies in the fact that a large proportion of the trials were of cross‐over design, and we were unable to adjust the data to take into account the paired data, which will result in narrower CIs around effect estimates. Another weakness was high risk of bias from attrition from the trials. This was largely due to participants being excluded from analyses in the cross‐over trials if they did not complete all cross‐over periods. The summary of findings are shown in Table 1; Table 2; and Table 3. The quality of the evidence for most outcomes was generally of low quality. The main reasons were due to risk of bias, imprecise results due to few studies or few events (or both) and unexplained heterogeneity. The impact of the downgrading decisions means that further research is likely to influence the confidence in our estimates of effects and may change the estimates.
Potential biases in the review process
Some trials only reported episodes of nausea and vomiting, rather than the proportion of participants with no nausea and vomiting, therefore we did not include these results in meta‐analyses. We also analysed dichotomous outcomes from the cross‐over studies without adjusting the analyses, which potentially gives rise to more precise (narrower CIs) estimates of effect.
In order to avoid publication bias, we searched for ongoing trials in clinical trial registry databases; however, we identified no further trials.
Agreements and disagreements with other studies or reviews
Our findings are in broad agreement with previously published systematic reviews (Machado Rocha 2008; Tramer 2001). We have updated and extended these earlier reviews by pooling placebo‐controlled trials separately from trials with active comparison groups, and where cannabis was given as co‐therapy with another anti‐emetic, and reporting on tolerability as well as efficacy outcomes.
Authors' conclusions
Implications for practice.
The widespread use of cannabis‐based medicines for management of nausea and vomiting with chemotherapy is unlikely due to the adverse effects they cause. However, cannabinoids are a useful adjunctive treatment to consider for people on moderately or highly emetic chemotherapy that are refractory to other anti‐emetic treatments, when all other options of therapy have been tried. Consideration needs to be made of the adverse effect profile of the cannabinoids, and how the adverse effects may be exacerbated with other concurrent anti‐emetic treatments, as well as the age of the person. This systematic review will be valuable evidence for clinicians and future development of international guidelines to summarise the evidence available.
Implications for research.
Adequate study design is important for anti‐emetic studies, ideally using a double‐blind trial design that is stratified for known prognostic factors, such as gender, age, alcohol intake, previous experience of chemotherapy, emetic potential of chemotherapy and a person's susceptibility to motion sickness (De Mulder 1992; Olver 1992a; Olver 1992b; Pater 1984). It is preferable for people to be chemotherapy naive and receiving the same chemotherapy regimens, or, if that is not possible, to receive those of the same emetogenicity as classified by international guidelines. Uniform anti‐emetic regimens should be used, when comparing an adjunctive anti‐emetic being added to the regimen in one arm (Rhodes 1984). Studies that compare the use of newer anti‐emetics that have efficacy for treating refractory nausea and vomiting (olanzapine and palonosetron) with cannabinoids would also be informative. It is difficult to compare anti‐emetic studies (Martin 1992), due to the variation in anti‐emetic doses, routes of administration, time periods of assessment of nausea and vomiting, assessment of episodes of nausea and vomiting, and any additional anti‐emetics that may have been administered. It also needs to be clear whether acute or delayed (or both) nausea and vomiting is being assessed, and there is also a variation in the definitions of complete response across studies, which impacts on comparing studies (Pater 1984). In the original anti‐emetic trials, assessment of nausea and vomiting has been inconsistent where no reliable and valid measures have been used, which also impacts on their analysis and interpretation (Pater 1984; Rhodes 1984).
While cross‐over trials are attractive to evaluate this type of therapy, they are susceptible to loss of participants if not all cross‐over to the second and subsequent phases of the trial. Following recommendations of the CONSORT (Consolidated Standards of Reporting Trials) statement for cross‐over studies would improve interpretation of such studies.
What's new
| Date | Event | Description |
|---|---|---|
| 11 October 2021 | Amended | Most recent search date 11 October 2021. No new studies identified for inclusion. |
History
Protocol first published: Issue 11, 2011 Review first published: Issue 11, 2015
| Date | Event | Description |
|---|---|---|
| 27 November 2019 | Amended | A search for studies on 14 November 2019 has identified 2 potentially relevant studies (see 'Characteristics of studies awaiting classification'). These studies have not yet been incorporated into this Cochrane Review. |
Acknowledgements
We thank Jo Morrison for clinical and editorial advice on the protocol, Jane Hayes for designing the search strategy and running the searches in all databases for us, Sarah Howcutt for translation of one article from French (George 1983), and Dax Steins for translation of one article from Dutch (Kluin‐Nelemans 1981a). Finally, we thank Clare Jess and Gail Quinn for their contribution to the editorial process.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Antineoplastic Agents] explode all trees #2 MeSH descriptor: [Antineoplastic Combined Chemotherapy Protocols] explode all trees #3 chemotherap* #4 #1 or #2 or #3 #5 MeSH descriptor: [Nausea] explode all trees #6 MeSH descriptor: [Vomiting] explode all trees #7 nause* or vomit* #8 emesis* or emetic* or antiemetic* or emetogenic* #9 #5 or #6 or #7 or #8 #10 MeSH descriptor: [Cannabinoids] explode all trees #11 MeSH descriptor: [Cannabis] explode all trees #12 cannab* #13 dronabinol #14 nabilone #15 tetrahydrocannabinol #16 cesamet #17 delta‐9‐THC #18 delta‐9‐tetrahydrocannabinol #19 marinol #20 #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 #21 #4 and #9 and #20
Appendix 2. MEDLINE search strategy
1 exp Antineoplastic Agents/ 2 exp Antineoplastic Combined Chemotherapy Protocols/ 3 chemotherap*.mp. 4 1 or 2 or 3 5 exp Nausea/ 6 exp Vomiting/ 7 (nause* or vomit*).mp. 8 (emesis* or emetic* or antiemetic* or emetogenic*).mp. 9 5 or 6 or 7 or 8 10 exp Cannabinoids/ 11 exp Cannabis/ 12 cannab*.mp. 13 marinol.mp. 14 dronabinol.mp. 15 nabilone.mp. 16 tetrahydrocannabinol.mp. 17 cesamet.mp. 18 delta‐9‐THC.mp. 19 delta‐9‐tetrahydrocannabinol.mp. 20 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21 randomized controlled trial.pt. 22 controlled clinial trial.pt. 23 randomized.ab. 24 placebo.ab. 25 drug therapy.fs. 26 randomly.ab. 27 trial.ab. 28 groups.ab. 29 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30 4 and 9 and 20 and 29
Key: mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier
Appendix 3. EMBASE search strategy
1 exp chemotherapy/ 2 exp antineoplastic agent/ 3 chemotherap*.mp. 4 1 and 2 and 3 5 exp "nausea and vomiting"/ 6 (nause* or vomit*).mp. 7 (emesis* or emetic* or antiemetic* or emetogenic*).mp. 8 5 or 6 or 7 9 exp cannabinoid/ 10 cannabis/ 11 cannab*.mp. 12 marinol.mp. 13 dronabinol.mp. 14 nabilone.mp. 15 tetrahydrocannabinol.mp. 16 cesamet.mp. 17 delta‐9‐THC.mp. 18 delta‐9‐tetrahydrocannabinol.mp. 19 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20 4 and 8 and 19 21 crossover procedure/ 22 double‐blind procedure/ 23 randomized controlled trial/ 24 single‐blind procedure/ 25 random*.mp. 26 factorial*.mp. 27 (crossover* or cross over* or cross‐over*).mp. 28 placebo*.mp. 29 (double* adj blind*).mp. 30 (singl* adj blind*).mp. 31 assign*.mp. 32 allocat*.mp. 33 volunteer*.mp. 34 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 35 20 and 34
Key: [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 4. PsycInfo search strategy
1 antineoplastic drugs/ 2 chemotherapy/ 3 chemotherap*.mp. 4 1 or 2 or 3 5 nausea/ 6 vomiting/ 7 nause*.mp. 8 vomit*.mp. 9 (emesis or emetic* or antiemetic* or emetogenic*).mp 10 5 or 6 or 7 or 8 or 9 11 exp cannabinoids/ 12 exp cannabis/ 13 cannab*.mp. 14 marinol.mp. 15 dronabinol.mp. 16 nabilone.mp. 17 tetrahydrocannabinol.mp. 18 cesamet.mp. 19 delta‐9‐THC.mp. 20 delta‐9‐tetrahydrocannabinol.mp. 21 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 22 4 and 10 and 21
key: [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
Appendix 5. LILACS search strategy
((MH:D02.455.848.090$ OR MH:B01.650.940.800.575.100.175.500 OR cannab$ OR marinol OR dronabinol OR nabilone OR tetrahydrocannabinol OR cesamet OR delta‐9‐THC OR delta‐9‐tetrahydrocannabinol) AND (MH:nausea or MH:vomiting OR MH:emetics OR MH:antiemetics OR nausea$ OR vomit$ OR emesis OR emetic$ OR emetogenic$ OR antiemetic$) AND (MH:D27.505.954.248$ OR MH:E02.183.750.500 OR MH:E02319.077.500 OR MH:E02.319.310.037 OR chemotherap$))
Data and analyses
Comparison 1. Cannabinoid versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Absence of nausea | 2 | 96 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.19, 20.97] |
| 1.2 Absence of vomiting | 3 | 168 | Risk Ratio (IV, Random, 95% CI) | 5.69 [2.56, 12.64] |
| 1.2.1 Nabilone | 1 | 72 | Risk Ratio (IV, Random, 95% CI) | 7.25 [2.84, 18.52] |
| 1.2.2 Dronabinol | 2 | 96 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.65, 13.76] |
| 1.3 Absence of nausea and vomiting | 3 | 288 | Risk Ratio (IV, Random, 95% CI) | 2.86 [1.76, 4.65] |
| 1.3.1 Cannabis naive | 1 | 75 | Risk Ratio (IV, Random, 95% CI) | 2.23 [1.04, 4.78] |
| 1.3.2 Prior cannabis use | 2 | 213 | Risk Ratio (IV, Random, 95% CI) | 3.40 [1.80, 6.39] |
| 1.4 Depression | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.5 Dysphoria | 2 | 96 | Risk Ratio (IV, Random, 95% CI) | 9.00 [0.50, 160.59] |
| 1.6 'Feeling high' | 3 | 137 | Risk Ratio (IV, Random, 95% CI) | 31.10 [6.37, 151.85] |
| 1.7 Paranoia | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.8 Sedation | 2 | 139 | Risk Ratio (IV, Random, 95% CI) | 4.47 [0.35, 57.81] |
| 1.9 Participant preference | 2 | 256 | Risk Ratio (IV, Random, 95% CI) | 4.82 [1.74, 13.36] |
| 1.10 Withdrawal for any reason | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.11 Withdrawal due to adverse event | 2 | 276 | Risk Ratio (IV, Random, 95% CI) | 6.85 [1.96, 23.99] |
| 1.12 Withdrawal due to lack of efficacy | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 2. Cannabinoid versus other anti‐emetic agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Absence of nausea | 5 | 258 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.67, 3.15] |
| 2.1.1 Prochlorperazine | 5 | 258 | Risk Ratio (IV, Random, 95% CI) | 1.46 [0.67, 3.15] |
| 2.2 Absence of nausea (subgroup analysis 2) | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 Nabilone | 3 | 141 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.33, 6.03] |
| 2.2.2 Dronabinol | 2 | 117 | Risk Ratio (IV, Random, 95% CI) | 2.38 [0.21, 26.91] |
| 2.3 Absence of vomiting | 4 | 209 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.86, 1.44] |
| 2.3.1 Prochlorperazine | 4 | 209 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.86, 1.44] |
| 2.4 Absence of vomiting (subgroup analysis 2) | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.4.1 Nabilone | 2 | 93 | Risk Ratio (IV, Random, 95% CI) | 1.55 [0.39, 6.24] |
| 2.4.2 Dronabinol | 2 | 116 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.64, 1.71] |
| 2.5 Absence of nausea and vomiting | 4 | 414 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.74, 5.38] |
| 2.5.1 Prochlorperazine | 4 | 414 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.74, 5.38] |
| 2.6 Absence of nausea and vomiting (subgroup analysis 1) | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.6.1 Cannabis naive | 2 | 116 | Risk Ratio (IV, Random, 95% CI) | 1.10 [0.70, 1.72] |
| 2.6.2 Prior cannabis use | 2 | 298 | Risk Ratio (IV, Random, 95% CI) | 17.98 [2.44, 132.43] |
| 2.7 Absence of nausea and vomiting (subgroup analysis 2) | 4 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.7.1 Nabilone | 1 | 226 | Risk Ratio (IV, Random, 95% CI) | 17.00 [0.99, 291.06] |
| 2.7.2 Dronabinol | 3 | 188 | Risk Ratio (IV, Random, 95% CI) | 1.44 [0.62, 3.31] |
| 2.8 Dizziness | 9 | 743 | Risk Ratio (IV, Random, 95% CI) | 2.54 [1.91, 3.37] |
| 2.8.1 Domperidone | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 2.75 [1.06, 7.12] |
| 2.8.2 Prochlorperazine | 7 | 675 | Risk Ratio (IV, Random, 95% CI) | 2.36 [1.82, 3.07] |
| 2.8.3 Metoclopramide | 1 | 30 | Risk Ratio (IV, Random, 95% CI) | 12.00 [1.78, 81.06] |
| 2.9 Dysphoria | 3 | 192 | Risk Ratio (IV, Random, 95% CI) | 7.17 [1.33, 38.84] |
| 2.9.1 Prochloperazine | 3 | 192 | Risk Ratio (IV, Random, 95% CI) | 7.17 [1.33, 38.84] |
| 2.10 Euphoria | 4 | 358 | Risk Ratio (IV, Random, 95% CI) | 8.89 [2.05, 38.63] |
| 2.10.1 Domperidone | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 5.00 [0.26, 97.70] |
| 2.10.2 Prochlorperazine | 2 | 280 | Risk Ratio (IV, Random, 95% CI) | 17.97 [2.42, 133.37] |
| 2.10.3 Chlorpromazine | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.13, 69.52] |
| 2.11 'Feeling high' | 5 | 419 | Risk Ratio (IV, Random, 95% CI) | 5.90 [3.42, 10.17] |
| 2.11.1 Prochlorperazine | 4 | 389 | Risk Ratio (IV, Random, 95% CI) | 6.18 [3.52, 10.85] |
| 2.11.2 Metoclopramide | 1 | 30 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.35, 25.68] |
| 2.12 Hallucinations | 2 | 144 | Risk Ratio (IV, Random, 95% CI) | 5.39 [0.66, 43.68] |
| 2.12.1 Prochlorperazine | 2 | 144 | Risk Ratio (IV, Random, 95% CI) | 5.39 [0.66, 43.68] |
| 2.13 Postural hypotension | 6 | 413 | Risk Ratio (IV, Random, 95% CI) | 2.40 [0.88, 6.53] |
| 2.13.1 Domperidone | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 4.00 [0.49, 32.57] |
| 2.13.2 Prochlorperazine | 3 | 305 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.52, 2.89] |
| 2.13.3 Metoclopramide | 1 | 30 | Risk Ratio (IV, Random, 95% CI) | 17.00 [1.07, 270.41] |
| 2.13.4 Chlorpromazine | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 7.00 [0.95, 51.80] |
| 2.14 Paranoia | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.14.1 Prochlorperazine | 1 | 42 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.13, 69.70] |
| 2.15 Sedation | 11 | 1055 | Risk Ratio (IV, Random, 95% CI) | 1.33 [1.08, 1.64] |
| 2.15.1 Domperidone | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 1.22 [0.66, 2.25] |
| 2.15.2 Prochlorperazine | 8 | 947 | Risk Ratio (IV, Random, 95% CI) | 1.44 [1.18, 1.76] |
| 2.15.3 Metoclopramide | 1 | 30 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.73, 1.18] |
| 2.15.4 Chlorpromazine | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 1.71 [0.85, 3.44] |
| 2.16 Depression | 3 | 317 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.51, 1.28] |
| 2.16.1 Prochlorperazine | 3 | 317 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.51, 1.28] |
| 2.17 Participant preference | 9 | 799 | Risk Ratio (IV, Random, 95% CI) | 2.76 [1.88, 4.03] |
| 2.17.1 Prochlorperazine | 7 | 695 | Risk Ratio (IV, Random, 95% CI) | 3.24 [2.23, 4.72] |
| 2.17.2 Metoclopramide | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 1.20 [0.61, 2.37] |
| 2.17.3 Chlorpromazine | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 2.00 [0.83, 4.81] |
| 2.18 Withdrawal for any reason | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.18.1 Prochlorperazine | 1 | 42 | Risk Ratio (IV, Random, 95% CI) | 3.50 [1.38, 8.89] |
| 2.19 Withdrawal due to adverse event | 6 | 740 | Risk Ratio (IV, Random, 95% CI) | 3.16 [1.26, 7.93] |
| 2.19.1 Domperidone | 1 | 76 | Risk Ratio (IV, Random, 95% CI) | 3.00 [0.13, 71.40] |
| 2.19.2 Prochlorperazine | 5 | 664 | Risk Ratio (IV, Random, 95% CI) | 3.90 [1.25, 12.20] |
| 2.20 Withdrawal due to lack of efficacy | 2 | 118 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.04, 20.93] |
| 2.20.1 Domperidone | 1 | 76 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.01, 2.67] |
| 2.20.2 Prochlorperazine | 1 | 42 | Risk Ratio (IV, Random, 95% CI) | 3.50 [1.38, 8.89] |
2.2. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 2: Absence of nausea (subgroup analysis 2)
2.4. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 4: Absence of vomiting (subgroup analysis 2)
2.7. Analysis.

Comparison 2: Cannabinoid versus other anti‐emetic agent, Outcome 7: Absence of nausea and vomiting (subgroup analysis 2)
Comparison 3. Cannabinoid plus other antiemetic agent versus other antiemetic monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Absence of nausea | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.2 Absence of vomiting | 2 | 89 | Risk Ratio (IV, Random, 95% CI) | 1.47 [0.69, 3.13] |
| 3.3 Absence of nausea and vomiting | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.4 Depression | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.5 Dizziness | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.6 Dysphoria | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.7 Paranoia | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.8 Sedation | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.9 Withdrawal for any reason | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.10 Withdrawal due to adverse event | 2 | 105 | Risk Ratio (IV, Random, 95% CI) | 6.97 [0.88, 55.19] |
| 3.11 Withdrawal due to lack of efficacy | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmedzai 1983.
| Study characteristics | ||
| Methods | Randomised, double‐blinded, 2‐period cross‐over study | |
| Participants | 34 people (19 (56%) men/15 (44%) women), median age 58 years. All cannabis naive Tumour types: small cell bronchial carcinoma Chemotherapy regimen: 2 x 21‐day cycles. Cyclophosphamide 1 g/m2, doxorubicin 40 mg/m2 and etoposide (VP‐16) 100 mg/m2 day 1; etoposide 100 mg/m2 days 2 and 3; vincristine 2 mg with methotrexate 50 mg/m2 day 10 followed by folinic acid rescue. Cyclophosphamide and doxorubicin given IV bolus; VP‐16 IV over 1‐2 hours Chemotherapy emetogenicity: High |
|
| Interventions | Nabilone 2 mg orally twice daily x 3 days, n = 34 Prochlorperazine 10 mg orally 3 times daily x 3 days, n = 34 |
|
| Outcomes | Episodes and frequency of nausea and vomiting day 1; withdrawal due to adverse effects; withdrawals due to death; participant preference due to adverse effects; incidence of feeling high, euphoria, postural dizziness, dysphoria | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/34 (24%) participants withdrew |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Approximately 10 days' washout period. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double dummy tablet" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" and dummy tablet used |
Chang 1979a.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 3‐period cross‐over, placebo‐controlled trial | |
| Participants | 15 people (10/15 (67%) men/5/15 (33%) women) aged 15‐49 years (median = 24 years). 4/15 (27%) participants were cannabis naive Tumour type: osteogenic sarcoma Chemotherapy regimens: methotrexate 250 mg/kg with leucovorin calcium rescue every 3 weeks for 18 months Chemotherapy emetogenicity: low |
|
| Interventions | Dronabinol 10 mg/m2 orally every 3 hours for total 5 doses (Phase I), n = 15. If participant vomited during this period oral dose was replaced with THC cigarette for remaining doses Placebo, n = 15 |
|
| Outcomes | Episodes of nausea and vomiting on day of therapy; frequency and severity of nausea; episodes of sedation, euphoria, dizziness, depression, paranoia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Order of THC‐placebo administration was randomized into three paired trials" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 58/77 (75%) participants received THC, 39/53 (74%) participants received placebo |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Low risk | Groups balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical gelatin capsules with sesame oil". "Identical cigarettes, the odour and taste of a lit placebo cigarette were identical to those of cannabis cigarette" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
Chang 1981.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 3‐period cross‐over trial | |
| Participants | 8 people (6/8 (75%) men/2/8 (25%) women) aged 17‐58 years (median = 41 years), 7/8 (88%) participants were cannabis naive Tumour types: resected soft tissue sarcoma Chemotherapy regimen: adjuvant doxorubicin and cyclophosphamide every 4 weeks until a total cumulative doxorubicin dose of 500‐550 mg/m2 Doxorubicin (70 mg/m2)and cyclophosphamide (700 mg/m2) were given at constant doses for all participants Chemotherapy emetogenicity: high |
|
| Interventions | Dronabinol 10 mg/m2 orally every 3 hours for total 5 doses, if vomited then participant given marijuana cigarettes 900 mg, containing THC 1.93% (approximately 17.4 mg), n = 8 Placebo, n = 8 |
|
| Outcomes | Episodes of nausea and vomiting on day of therapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Order of THC‐placebo administration was randomized into paired trials" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17/27 (63%) participants received THC, 16/27 (59%) participants received placebo |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Paired analysis was performed. Unclear if groups balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical gelatin capsules with sesame oil. Identical cigarettes, the odour and taste of a lit placebo cigarette were identical to those of cannabis cigarette" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind". "Neither patients nor nursing staff was [sic] informed which drug was administered" |
Crawford 1986.
| Study characteristics | ||
| Methods | Randomised, 2‐period cross‐over study | |
| Participants | 32 people Tumour type: adenocarcinoma of the ovary or germ cell tumours. Chemotherapy regimen: cisplatin 100 mg/m2, cyclophosphamide and doxorubicin (for people with adenocarcinoma of ovary), cisplatin 120 mg/m2, methotrexate and vincristine (for people with germ cell tumours). No information on doses reported Chemotherapy emetogenicity: high |
|
| Interventions | Nabilone 1 mg orally every 8 hours, n = 32 Metoclopramide 1 mg/kg IV every 3 hours, n = 32 |
|
| Outcomes | Episodes of vomiting during 24 hours, nausea, dizziness, euphoria and drowsiness | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/32 (22%) participants received the 4 planned treatment and only 37/64 (58%) participants received 1 or 2 treatment episodes of nabilone and 39/64 (61%) participants received 1 or 2 treatment episodes of metochlopramide |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | High risk | Assumed washout period sufficient. Paired analysis was not performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
Einhorn 1981.
| Study characteristics | ||
| Methods | Randomised, prospective, double‐blind, 2‐period cross‐over study | |
| Participants | 100 people aged 15‐74 years, mean = 28 years Tumour type; sarcoma (1 person), Hodgkin's disease (2 people), lymphoma (4 people), bladder carcinoma (3 people), testicular carcinoma (70 people) Chemotherapy regimens: doxorubicin hydrochloride and cyclophosphamide (1 person), nitrogen mustard, vincristine, prednisone and procarbazine (2 people), cyclophosphamide, doxorubicin hydrochloride, vincristine and prednisone (4 people), cisplatin, doxorubicin hydrochloride and 5‐fluorouracil (3 people), cisplatin, vinblastine and bleomycin (45 people), cisplatin, vinblastine, bleomycin and doxorubicin hydrochloride (25 people). No information on doses reported Chemotherapy emetogenicity: high |
|
| Interventions | Nabilone 2 mg, orally every 6 hours, n = 100 Prochlorperazine 10 mg, orally every 6 hours, n = 100 |
|
| Outcomes | Episodes of nausea and vomiting during 24 hours of therapy; frequency of vomiting; withdrawal due to adverse effects; withdrawal due to early death and change of chemotherapy; episodes of 'feeling high', depression, hallucination, paranoia, hypotension | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "Identical capsules used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 80/100 (80%) participants received nabilone, 80/100 (80%) participants received prochlorperazine |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind", identical capsules used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind", identical capsules used |
Frytak 1979.
| Study characteristics | ||
| Methods | Randomised, double‐blind, parallel group trial | |
| Participants | 116 people, median age = 61 years. All cannabis naive. THC n = 38 (22 men/16 women), prochlorperazine n = 41 (21 men/20 women), placebo n = 37 (27 men/10 women) Tumour types: colorectal cancer (28 people), gastric cancer (7 people), liver cancer (2 people), miscellaneous (1 person), gastric surgery (5 people), hepatic metastasis (20 people) Chemotherapy regimens: 5‐fluorouracil and semustine or 5‐fluorouracil and semustine plus triazinate, razoxane, doxorubicin or vincristine. 5‐fluorouracil 300‐350 mg/m2 IV for 5 days. Semustine 110‐175 mg/m2 day 1 only Chemotherapy emetogenicity: moderate |
|
| Interventions | Dronabinol 15 mg on day 1, 2 hours prior to chemotherapy, then 2 and 8 hours after initiation of chemotherapy. Then 3 times daily x 3 days orally, n = 38 Prochlorperazine 10 mg on day 1, 2 hours prior to chemotherapy, then 2 and 8 hours after initiation of chemotherapy. Then 3 times daily x 3 days orally, n = 41 Placebo n = 37 |
|
| Outcomes | Episodes of nausea and vomiting during 24 hours, sedation, feeling high; withdrawal due to intolerable central nervous system toxicity or excessive vomiting | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Drugs dispensed in individual packets identified by code number |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1/117 (0.8%) participants withdrew. After day 1, 10/38 (26%) participants withdrew in THC group, 5/41 (12%) participants withdrew in prochlorperazine group, 3/37 (8%) participants withdrew in placebo group |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Low risk | Groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical opaque gelatin capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
George 1983.
| Study characteristics | ||
| Methods | Randomized double‐blind 2‐period cross‐over study | |
| Participants | 20 people, mean age 54.1 years Tumour type: advanced gynaecological cancer who vomited during the first chemotherapy treatment Chemotherapy regimen: cis‐platinum (50 mg/m2) with hydration. Vomited during the first treatment. Doxorubicin (40 mg/m2), cyclophosphamide (600 mg/m2) and cis‐platinum (11 people); cyclophosphamide 600 mg and cis‐platinum (3 people); cis‐platinum (6 people) Chemotherapy emetogenicity: high |
|
| Interventions | Nabilone 1 mg 24 hours before chemotherapy then 1 mg 3 times daily orally Chlorpromazine 12.5 mg IM before chemotherapy with additional dose if requested |
|
| Outcomes | Number of vomiting episodes in 24 hours, participant preference, adverse events | |
| Notes | Translated from French | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated by lottery |
| Allocation concealment (selection bias) | Low risk | Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All people were included in the analysis |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Low risk | There was no evident difference caused by the order of administration of the drugs |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo, double‐dummy tablets used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as double‐blind |
Gralla 1984.
| Study characteristics | ||
| Methods | Randomised, double‐blinded parallel group trial | |
| Participants | 31 people (23 men/ 5 women). THC n = 15 (13 men/2 women), aged 39‐72 years (median = 58 years); metoclopramide n = 16 (11 men/5 women), aged 45‐70 years (median = 58 years) Tumour types: bronchogenic carcinoma (12 people), oesophageal carcinoma (2 people), head and neck carcinoma head and neck carcinoma (1 person) Chemotherapy regimens: all receiving first course of cisplatin 120 mg/m2 IV Chemotherapy emetogenicity: high |
|
| Interventions | Dronabinol 10 mg/m2 1.5 hours prior to chemotherapy, then at 1.5, 4.5, 7.5 and 10.5 hours after chemotherapy orally, n = 15 Metoclopramide, 2 mg/kg 30 minutes prior to chemotherapy, then 1.5, 3.5, 5.5 and 8.5 hours after chemotherapy IV, n = 16 |
|
| Outcomes | Episodes of nausea and vomiting during 24 hours, sedation, dizziness, orthostatic hypotension, feeling high | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Paired design in which one patient in every pair was randomly assigned to each treatment" |
| Allocation concealment (selection bias) | Low risk | "Identical vials and capsules used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15/15 (100%) participants received THC, 15/16 (94%) participants received metoclopramide |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical vials and capsules used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
Herman 1979.
| Study characteristics | ||
| Methods | Randomised, double‐blinded, 2‐period cross‐over study | |
| Participants | 152 people (126 men/26 women) aged 15‐74 years (median = 33 years) Tumour type: testicular carcinoma (70 people), non‐Hodgkin's disease (12 people), Hodgkin's disease (11 people) Chemotherapy regimen: cisplatin daily for 5 days, vinblastine and bleomycin (70 people); cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP 12 people); nitrogen mustard (mechlorethamine?), vincristine, procarbazine and prednisone (MOPP 11 people); other regimens included dactinomycin, dacarbazine, 5‐fluorouracil, melphalan and nitrosourea compounds. No information on doses reported Chemotherapy emetogenicity: high |
|
| Interventions | Nabilone 2 mg, every 8 hours orally, n = 152 Prochlorperazine 10 mg, every 8 hours orally, n = 152 |
|
| Outcomes | Episodes of nausea and vomiting daily during chemotherapy; withdrawal due to adverse effects; episodes of somnolence, dizziness, depression, euphoria, preference | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | "Drugs packaged in identical containers marked only with a number code" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 113/152 (74%) participants received nabilone, 113/152 (74%) participants received prochlorperazine |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical containers marked only with a number code" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" and "identical containers marked only with a number code" |
Johansson 1982.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 2‐period cross‐over study | |
| Participants | 27 people aged 18‐70 years Tumour types: cervical cancer (2 people), cancer of fallopian tubes (2 people), ovarian cancer (13 people), testicular cancer (2 people), head and neck cancer (1 person), bronchus cancer (1 person), histiocytoma (1 person), fibrosarcoma (1 person), oligodendroma (1 person), lymphoma (2 people) Chemotherapy regimens: doxorubicin 40 mg/m2, cyclophosphamide 500 mg/m2 and cisplatinum 50 mg/m2 (11 people) in combination with vinblastine, vincristine or ftorafur (tegfur‐uracil). Cyclophosphamide 750‐1000 mg/m2 and cisplatinum 75 mg/m2 when given as sole agents Chemotherapy emetogenicity: high |
|
| Interventions | Nabilone 2 mg twice daily x 4 days orally, n = 27 Prochlorperazine 10 mg twice daily x 4 days orally, n = 27 |
|
| Outcomes | Episodes of nausea and vomiting assessed daily and reported for follow‐up at end of anti‐emetic therapy; withdrawal due to lack of efficacy; withdrawal due to hypotension, vertigo and headache; participant preference; episodes of drowsiness, dizziness, depression, hypotension | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18/27 (67%) participants received nabilone, 18/27 (67%) participants received prochlorperazine |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
Jones 1982.
| Study characteristics | ||
| Methods | Prospective, randomised, double‐blind, 2‐period cross‐over trial | |
| Participants | 54 people; aged 20‐37 years (n = 9), 38‐57 years (n = 23), > 58 years (n = 22) Tumour types: breast cancer (15 people), lymphoma (12 people), ovarian cancer (8 people), lung cancer (7 people), melanoma (3 people), testicular cancer (2 people), miscellaneous (7 people) Chemotherapy regimens: adriamycin‐based regimens (25 people), cisplatinum‐based regimens (14 people), other combinations (12 people). No information on doses reported Chemotherapy emetogenicity: high |
|
| Interventions | Nabilone 2 mg every 12 hours orally, n = 54 Placebo, n = 54 |
|
| Outcomes | Episodes of nausea and vomiting unclear time period of results unclear; withdrawal due to severe nausea and vomiting; episodes of drowsiness, euphoria, hallucination, hypotension | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24/54 (44%) participants received nabilone, 24/54 (44%) participants received placebo |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
Kleinman 1983.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 4‐period cross‐over study | |
| Participants | 16 people (9 men/7 women) aged 18‐53 years (median = 38 years) Tumour types: not reported Chemotherapy regimens: "Cancer chemotherapy known to cause acute gastrointestinal toxicity" Chemotherapy emetogenicity: unable to classify |
|
| Interventions | Prochlorperazine 10 mg + dronabinol 15 mg x 2 courses orally, n = 16 Prochlorperazine + placebo orally, n = 16 |
|
| Outcomes | Episodes of nausea and vomiting 24 hours after chemotherapy, euphoria, sedation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 28/32 (87.5%) participants received prochlorperazine + THC, 24/32 (75%) participants received prochlorperazine + placebo (overall 52/64 (81%) participants received either of the 2 courses) |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind" and "identical capsules used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind and identical capsules used" |
Kluin‐Neleman 1979.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 2‐period cross‐over study | |
| Participants | 11 people (10 men/1 woman) aged 21‐53 years Tumour types: Hodgkin's or non‐Hodgkin's lymphoma Chemotherapy regimens: mitoxine 6 mg/m2 (maximum 10 mg), vincristine 1.4 mg/m2 (maximum 2 mg) IV on days 1 and 8. Procarbazine 100 mg/m2 and prednisone 40 mg/m2 oral days 1‐14 for 6 cycles with intervals of 2 weeks Chemotherapy emetogenicity: high |
|
| Interventions | Dronabinol 10 mg/m2 orally, n = 11 Placebo, n = 11 |
|
| Outcomes | Episodes of nausea and vomiting at end of day of therapy, feeling high, dizziness, hallucinations | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17 participants received THC, 11 participants received placebo |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Washout period 2 weeks. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical gelatin capsules were used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" and "identical gelatin capsules used" |
Lane 1991.
| Study characteristics | ||
| Methods | Randomised, double‐blind, parallel group study | |
| Participants | Dronabinol n = 21 (10 men/11 women) aged 20‐68 years (median = 47 years), prochlorperazine n = 21 (10 men/11 women) aged 22‐64 years (median = 49 years), dronabinol plus prochlorperazine n = 20 (9 men/11 women) aged 25‐65 years (median = 55.5 years). Total n = 62 (29 men/33 women) aged 20‐68 years (median = 52 years) All cannabis naive Tumour types: breast cancer (24 people), colon cancer (3 people), lung cancer (8 people), lymphoma (17 people), miscellaneous (10 people) Chemotherapy regimens: cyclophosphamide and doxorubicin (26 people), 5‐fluorouracil (14 people), vincristine (13 people), etoposide (10 people), No information on doses reported. 48/62 participants received chemotherapy with high emetogenic potential Chemotherapy emetogenicity: unable to classify |
|
| Interventions | Dronabinol 10 mg every 6 hours orally, n = 21 Prochlorperazine 10 mg every 6 hours orally, n = 21 Dronabinol 10 mg + prochlorperazine 10 mg orally, n = 20 |
|
| Outcomes | Episodes of nausea and vomiting during chemotherapy treatment; withdrawal due to adverse effects; episodes of somnolence, dizziness, paranoid reaction, depression | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17/21 (81%) participants received dronabinol, 20/21 (95%) participants received prochlorperazine, 17/20 (85%) participants received dronabinol + prochlorperazine |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Low risk | Groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
Levitt 1982.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 2‐period cross‐over study | |
| Participants | 58 people aged 17‐78 years Tumour types: lung cancer (21 people), ovarian cancer (11 people), breast cancer (10 people), other cancers (16 people) Chemotherapy regimens: combinations of doxorubicin, bleomycin, cisplatinum, cyclophosphamide, dactinomycin, melphalan, mitomycin C, methotrexate, vincristine, etoposide, 5‐fluorouracil. No information on doses reported Chemotherapy emetogenicity: unable to classify |
|
| Interventions | Nabilone, n = 58 Placebo, n = 58 |
|
| Outcomes | Episodes of nausea and vomiting time of assessment unclear, frequency and severity of nausea, withdrawal due to lack of efficacy, adverse effects, episodes of drowsiness | |
| Notes | Dose and duration not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36/58 (62%) participants received nabilone, 36/58 (62%) participants received placebo |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
McCabe 1988.
| Study characteristics | ||
| Methods | Randomised, 2‐period cross‐over trial | |
| Participants | 36 (9 men/27 women) aged 18‐69 years (median = 48 years) Tumour types: breast cancer (11 people), haematological malignancies (9 people), sarcomas (6 people), gastrointestinal malignancies (5 people), melanoma (2 people), ovarian cancer (2 people), testicular cancer (1 person) Chemotherapy regimens: doxorubicin (13 people), cyclophosphamide, methotrexate and 5‐fluorouracil (7 people), nitrogen mustard, vincristine, procarbazine and prednisone (7 people), platinum combinations (4 people), DTIC (2 people), 5‐fluorouracil combinations (2 people), 5‐azacytadine (1 person). No information on doses reported Chemotherapy emetogenicity: moderate to high |
|
| Interventions | Dronabinol 15 mg/m2 1 hour prior to chemotherapy, then every 4 hours for 24 hours after chemotherapy orally, n = 36 Prochlorperazine 10 mg 1 hour prior to chemotherapy, then every 4 hours for 24 hours after chemotherapy orally, n = 36 |
|
| Outcomes | Episodes of nausea and vomiting during 24 hours, feeling high, dizziness, dysphoria, hallucination, paranoia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All people were analysed |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Blinding not achieved" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study not blinded |
Niiranen 1985.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 2‐period cross‐over study | |
| Participants | 32 people (20 men/4 women) aged 48‐78 years, mean = 61 years Tumour type: lung cancer Chemotherapy regimen: cyclophosphamide 1.2 g/m2 day 1, etoposide 150 mg/m2 IV day 1, 250 mg/m2 orally day 3, and vincristine 1.3 mg/m2 days 1 and 8 (5 people); cyclophosphamide 400 mg/m2, adriamycin 40 mg/m2, cisplatinum 40 mg/m2 every 28 days (8 people); cisplatinum 90 mg/m2 day 1 and vindesine 3 mg/m2 5 x weekly then twice monthly every 28 days (2 people); cisplatinum 90 mg/m2 day 1 and etoposide 50 mg/m2 days 1‐5 every 28 days (9 people); cisplatinum 60 mg/m2 day 1 and etoposide 150 mg/m2 IV day 1 and 200 mg/m2 orally day 3 every 28 days (1 person) Chemotherapy emetogenicity: high |
|
| Interventions | Nabilone 1 mg orally night before chemotherapy, 1 hour before chemotherapy and every 12 hours up to 24 hours as required orally, n = 32 Prochlorperazine 7.5 mg orally night before chemotherapy, 1 hour before chemotherapy and every 12 hours up to 24 hours as required orally, n = 32 |
|
| Outcomes | Episodes, frequency and severity of nausea and vomiting at 24 hours; withdrawal due to adverse effects; participant preference; episodes of drowsiness, hallucination, hypotension | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24/32 (75%) participants received nabilone, 24/32 (75%) participants received prochlorperazine |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical appearing capsules used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" and "identical appearing capsules used" |
Orr 1981.
| Study characteristics | ||
| Methods | Randomised double‐blind 2‐period cross‐over | |
| Participants | 79 people (28 men/51 women) aged 22‐71 years, mean = 46 years Tumour type: variety of neoplasms Chemotherapy regimen: doxorubicin, cyclophosphamide, 5‐fluorouracil (with methotrexate), nitrogen mustard, imidazole carboxamide, nitrosaurea and cytosine arabinoside. No information on doses reported Chemotherapy emetogenicity: high (5‐fluorouracil + methotrexate low risk but only 3/55 people) |
|
| Interventions | Dronabinol 7 mg/m2 every 4 hours x 4 doses orally, n = 79 Prochlorperazine 7 mg every 4 hours x 4 doses orally, n = 79 |
|
| Outcomes | Nausea 24 hours post treatment and adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 55/79 (69%) participants in both groups analysed |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical capsule used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | States "double‐blind" |
Pomeroy 1986.
| Study characteristics | ||
| Methods | Randomised, double‐blind parallel group trial | |
| Participants | 38 people (23 men/15 women) aged 21‐66 years (median = 42 years) Tumour types: ovarian cancer (11 people), testicular cancer (9 people), bronchus carcinoma (8 people), non‐Hodgkin's lymphoma (3 people), Hodgkin's disease (2 people), sarcoma (2 people), breast cancer (1 person), melanoma (1 person), nephroblastoma (1 person) Chemotherapy regimens: cisplatin (10 people); cisplatin and treosulphan (7 people); cisplatin, vincristine, methotrexate and bleomycin (4 people), cisplatin, actinomycin D and etoposide (2 people); cisplatin, vinblastine and bleomycin (2 people); cisplatin and vindesine (1 person); adriamycin, bleomycin, vincristine and DTIC (2 people); adriamycin, vincristine and cyclophosphamide (2 people); adriamycin, vincristine, cyclophosphamide and prednisone (2 people); adriamycin, vincristine and etoposide (1 person); ifosfamide (2 people); vincristine, methotrexate and 5‐fluorouracil (1 person); vindesine, DTIC and 1‐(2‐chloroethyl)3‐cyclohexyl‐1‐nitrosurea (CCNU) (1 person). No information on doses reported Chemotherapy emetogenicity: high |
|
| Interventions | Nabilone 1 mg 3 times daily x 2 cycles orally, n = 19 Domperidone 20 mg 3 times daily x 2 cycles orally, n = 19 |
|
| Outcomes | Episodes of vomiting daily, withdrawal due to adverse effects, lack of efficacy, episodes of drowsiness, dizziness, hypotension, euphoria | |
| Notes | 2 cycles of chemotherapy evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32/38 (84%) participants received nabilone, 33/38 (87%) participants received domperidone |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical capsules used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" and "identical capsules used" |
Sallan 1975a.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 2‐period cross‐over study | |
| Participants | 22 people (10 men/12 women) aged 18‐76 years (median = 29.5 years) Tumour types: variety of neoplasms Chemotherapy regimen: adriamycin, 5‐azacytidine, nitrogen mustard, imidazole carboxamide, procarbazine, high‐dose cyclophosphamide or high‐dose methotrexate or combinations. No information on doses reported Chemotherapy emetogenicity: unable to classify |
|
| Interventions | Dronabinol 15 mg, later changed to 10 mg/m2, every 4 hours x 3 doses orally, n = 33 Placebo, n = 33 |
|
| Outcomes | Episodes of nausea and vomiting on day after treatment, withdrawal due to adverse effects, episodes of feeling high, somnolence, paranoia, hallucination | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20/33 (61%) participants received THC, 22/33 (67%) participants received placebo |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical gelatin capsules used" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" and "identical gelatin capsules used" |
Steele 1980.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 2‐period cross‐over study | |
| Participants | 55 people aged 19‐65 years Tumour types: not reported Chemotherapy regimen: high‐dose cis‐dichlorodiammineplatinum 120 mg/m2 ± vindesine 3 mg/m2 every 4‐6 weeks; low‐dose cis‐dichlorodiammineplatinum 60 mg/m2 ± vindesine 3 mg/m2, every 4‐6 weeks; low‐dose cis‐dichlorodiammineplatinum 60 mg/m2 ± adriamycin 45 mg/m2 every 3‐4 weeks; mechlorethamine 6 mg/m2 + vincristine 1.4 mg/m2 + procarbazine orally x 14 days 100 mg/m2 every 4 weeks days 1‐8; streptozotocin 500 mg/m2 every week; actinomycin D 1 mg/m2 ± vinblastine 4 mg/m2 + chlorambucil orally x 14 days 4 mg/m2 or 0.15 mg/kg every 3‐4 weeks; DTIC 800 mg/m2 ± cyclophosphamide orally x 14 days 100 mg/m2 every 4 weeks. All drugs IV unless otherwise stated Chemotherapy emetogenicity: high |
|
| Interventions | Nabilone 2 mg every 12 hours x 3‐5 doses orally, n = 55 Prochlorperazine 10 mg every 12 hours x 3‐5 doses orally, n = 55 |
|
| Outcomes | Episodes of nausea and vomiting during 24 hours; withdrawal due to adverse effects; lack of efficacy, episodes of somnolence, dizziness, feeling high, postural hypotension, dysphoria, hallucination | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 37/55 (67%) participants received nabilone, 37/55 (67%) participants received prochlorperazine |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
Ungerleider 1982.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 2‐period cross‐over study | |
| Participants | 214 people (107 men/107 women) aged 18‐82 years (median = 47 years) Tumour types: "wide variety of neoplasms" Chemotherapy regimens: antibiotics (70 people), nitrosoureas (21 people), alkylating agents (119 people), antimetabolites (82 people), vinca‐alkaloids (60 people), hormones (13 people), miscellaneous (33 people) and combinations. Rated as high for 66% of people, moderate for 27% of people or low for 7% of people emetic potential Chemotherapy emetogenicity: unable to classify ‐ unknown combinations |
|
| Interventions | Dronabinol 7.5 mg for < 1.4/m2, 10 mg for 1.4‐1.8 m2 or 12.5 mg for > 1.8 m2 orally, n = 214 Prochlorperazine 10 mg 1 hour prior to chemotherapy, then every 4 hours x 4 doses per day x all chemotherapy days orally, n = 214 |
|
| Outcomes | Episodes of nausea and vomiting during 24 hours; withdrawal due to adverse effects; episodes of sedation, depression, feeling high | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 172/214 (80%) participants received THC, 181/214 (85%) received prochlorperazine |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Low risk | Washout period 1‐3 weeks. Paired analysis was performed. Groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
Wada 1982.
| Study characteristics | ||
| Methods | Randomised, double‐blind, 2‐period cross‐over trial | |
| Participants | 114 people (47 men/67 women) aged 18‐81 years (median = 57 years) Tumour types: lung cancer (23 people), breast cancer (18 people), ovarian cancer (16 people), lymphoma (including Hodgkin's) (12 people), colonic cancer (7 people), prostatic cancer (5 people), adenocarcinoma (5 people), bladder cancer (3 people), melanoma (3 people), pancreatic cancer (3 people), oesophageal cancer (3 people), stomach cancer (3 people), sarcoma (2 people), testicular cancer (2 people), others (9 people) Chemotherapy regimens: adriamycin (66 people), carmustine (2 people), bleomycin (7 people), cisplatinum (40 people), cytoxan (46 people), dactinomycin (1 person), DTIC (7 people), 5‐fluorouracil (29 people), mustine (4 people), MCCNU (6 people), melphalan (1 person), methotrexate (14 people), mitomycin (17 person), procarbazine (7 people), streptozotocin (1 person), tamoxifen (1 person), vinblastine (5 person), vincristine (16 people), VP‐16 (1 person) |
|
| Interventions | Nabilone 2 mg night prior and 1‐3 hours before chemotherapy and then every 12 hours orally, n = 114 Placebo, n = 114 |
|
| Outcomes | Episodes of nausea and vomiting during 24 hours; withdrawal due to adverse effects; lack of efficacy; progressive disease; death; participant preference; episodes of dizziness, drowsiness, euphoria, dysphoria, hypotension, hallucination | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 84/114 (74%) participants completed both the courses, 92/114 (81%) participants were evaluable for efficacy |
| Selective reporting (reporting bias) | Low risk | Data reported for primary outcome |
| Other bias | Unclear risk | Assumed washout period sufficient. Unclear if paired analysis was performed. Unclear if groups were balanced at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as "double‐blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding assumed as study reported as "double‐blind" |
DTIC: 5‐(3,3‐dimethyl‐1‐triazeno)‐imidazole‐4‐carboxamide; HN2: ; IM: intramuscular; IV: intravenous; MCCNU: methyl lomustine; n: number; THC: delta‐9‐tetrahydrocannabinol.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aapro 1981 | Not a primary study ‐ editorial |
| Allan 1987 | Not a primary study ‐ review |
| Anderson 1981 | Not a primary study ‐ review |
| Artim 1983 | Participants received chemotherapy and radiotherapy |
| Bateman 1982 | Not a primary study ‐ letter |
| Ben 2006 | Not a primary study ‐ review |
| Biedrzycki 2007 | Not a primary study ‐ conference presentation |
| Broder 1982 | Lacks data ‐ abstract of preliminary findings, participant age and characteristics not reported |
| Carey 1983 | Not a primary study ‐ review |
| Chan 1987 | Randomised controlled trial involving children |
| Chang 1979b | Duplicate of Chang 1979a |
| Citron 1985 | Cross route comparison of intramuscular versus oral cannabinoid |
| Cocchetto 1981 | Not a primary study ‐ review |
| Colls 1980a | Letter ‐ lacks detail on study methods, participant groups, control intervention and results |
| Colls 1980b | Did not report data for primary outcome, measurement of nausea and vomiting using a non‐validated measure. No details on participants reported |
| Cone 1982 | Not randomised ‐ single‐arm study |
| Costa 2007 | Not a primary study ‐ review |
| Cotter 2009 | Not a primary study ‐ review |
| Cronin 1981 | Not randomised ‐ single‐arm cross‐over study |
| Croxford 2003 | Not a primary study ‐ review |
| Cunningham 1985 | Control group was cannabinoid monotherapy and not conventional anti‐emetic |
| Cunningham 1988 | Sub‐therapeutic dose of prochlorperazine used |
| Dalzell 1986 | Randomised controlled trial involving children |
| Darmani 2010 | Not a primary study ‐ review |
| Davis 2007 | Not a primary study ‐ review |
| Davis 2008 | Not a primary study ‐ review |
| Devine 1987 | Not randomised ‐ single‐arm cross‐over study |
| Dodds 1985 | Not a primary study ‐ review from thesis |
| Dow 1984 | Not a primary study ‐ letter |
| Duran 2010 | Not an approved formulation of delta‐9‐tetrahydrocannabinol |
| Ekert 1979 | Randomised controlled trial involved children not adults |
| Ettinger 2007 | Not a primary study ‐ clinical practice guidelines |
| Feyer 2011 | Not a primary study ‐ review |
| Fiore 1984 | Not a primary study ‐ review |
| Fox 1979 | Not a primary study ‐ letter |
| Galal 2009 | Not a primary study ‐ review |
| Gallego 1984 | Not a primary study ‐ review |
| Gerhartz 1983 | Not randomised ‐ single‐arm study |
| Gerra 2010 | Not a primary study ‐ review |
| Goodman 1997 | Not a primary study ‐ review |
| Gorter 1999 | Not randomised |
| Grunberg 1989 | Not a primary study ‐ review |
| Guzman 2003 | Not a primary study ‐ review |
| Heim 1984 | Evaluates a non‐approved formulation of delta‐9‐tetrahydrocannabinol |
| Herrstedt 1998 | Not a primary study ‐ review |
| Herrstedt 2008 | Not a primary study ‐ review |
| Higi 1982 | Pilot study |
| Hiller 1984 | Not a primary study ‐ review |
| Hutcheon 1983 | Evaluates a non‐approved formulation of delta‐9‐tetrahydrocannabinol |
| Jordan 2007 | Not a primary study ‐ review |
| Jordan 2011 | Not a primary study ‐ review guideline |
| Kearsley 1985 | Not a primary study ‐ review |
| Kenny 1982 | Non‐randomised single‐arm study |
| Kluin‐Nelemans 1981a | Duplicate to included |
| Kluin‐Nelemans 1981b | Not randomised. Abstract with scant details of methods reported |
| Krasnow 1991 | Not a primary study ‐ review |
| Kreutz 2007 | Not a primary study ‐ review |
| Lane 1989 | Duplicate Lane 1991 |
| Lane 1990 | Duplicate data. Single‐centre study included in Lane 1991 |
| Laszlo 1982 | Not a primary study ‐ review |
| Levitt 1981 | Evaluates ophthalmological outcomes. Nausea and vomiting not evaluated |
| Levitt 1984 | Cross‐route comparison of oral versus smoked cannabis |
| Lohr 2008 | Not a primary study ‐ review |
| Long 1982 | Preliminary data presented |
| Machado 2008 | Not a primary study ‐ systematic review and meta‐analysis |
| Mechoulam 1978 | Not a primary study ‐ drug development |
| Mechoulam 1999 | Not a primary study ‐ review |
| Mechoulam 2001 | Not a primary study ‐ review |
| Meiri 2007 | Not acute nausea and vomiting but evaluating delayed nausea and vomiting |
| Murakami 1986 | Not a primary study ‐ review |
| Musty 2001 | Not a primary study ‐ review |
| Nagy 1978 | Scanty data in an abstract ‐ no extractable data |
| Navari 2009a | Not a primary study ‐ review |
| Navari 2009b | Not a primary study ‐ review |
| Niederle 1986 | Evaluates a non‐eligible anti‐emetic (alizapride) |
| Niiranen 1987 | Control group was cannabinoid monotherapy and not conventional anti‐emetic |
| Nyman 1982 | Not a primary study ‐ review |
| Orr 1980 | Duplicate of Orr 1981 |
| Penta 1981 | Not a primary study ‐ review |
| Perwitasari 2011 | Not a primary study ‐ review |
| Phillips 2010 | Not a primary study ‐ review |
| Plasse 1991 | Not a primary study ‐ expert opinion |
| Porta 2002 | Not a primary study ‐ review |
| Poster 1981 | Not a primary study ‐ review |
| Reynolds 2002 | Not a primary study ‐ letter |
| Sallan 1975b | Duplicate study of Sallan 1975a |
| Sallan 1980 | Participants aged 9‐70 years; number or percent of children included not reported |
| Schuette 1985 | Duplicate study reported in Niederle 1986 |
| Sheidler 1984 | Evaluates a non‐approved formulation of delta‐9‐tetrahydrocannabinol |
| Slatkin 2007 | Not a primary study ‐ review |
| Smith 2007 | Not a primary study ‐ review |
| Stambaugh 1982 | Cross‐route comparison of intramuscular versus oral cannabinoid |
| Stambaugh 1984 | Evaluates a non‐approved formulation of delta‐9‐tetrahydrocannabinol |
| Steele 1979 | Duplicate study reported in Steele 1980 |
| Stewart 1990 | Not a primary study ‐ review |
| Stuart 1982 | Not randomised ‐ single‐arm study |
| Stuart‐Harris 1983 | Not randomised |
| Sweet 1980 | Not a primary study ‐ letter |
| Toth 2008 | Not a primary study ‐ review |
| Tramer 2001 | Not a primary study ‐ review |
| Ungerleider 1985 | Sub‐group analysis of study reported in Ungerleider 1982 |
| Venner 1986 | Preliminary results ‐ ongoing study |
| Vincent 1983 | Not a primary study ‐ review |
| Voth 1997 | Not a primary study ‐ review |
| Wang 2008 | Not a primary study ‐ review |
| Ward 1985 | Not a primary study ‐ drug evaluation |
| Ware 2008 | Not a primary study ‐ review |
| Zuardi 2008 | Not a primary study ‐ review |
Characteristics of studies awaiting classification [ordered by study ID]
Citron 1983.
| Methods | Double‐blind, randomised, crossover study |
| Participants | People reciveing chemotherapy |
| Interventions | IM levonantradol, a synthetic cannabinoid, given at a dose of 1 mg every 4 hours versus oral delta‐9‐tetrahydrocannabinol (THC) given at a dose of 15 mg every 4 hours |
| Outcomes | Nausea, emetic episodes |
| Notes |
Earhart 1983.
| Methods | RCT |
| Participants | Cancer patients receiving cisplatin chemotherapy |
| Interventions | Evonantradol versus prochlorperazine as parenteral antiemetics |
| Outcomes | Nausea and vomiting |
| Notes |
Harden‐Harrison 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Jhangiani 2005.
| Methods | Double‐blind, placebo‐controlled study |
| Participants | Patients receiving moderately to highly emetogenic chemotherapy |
| Interventions | Dronabinol alone or in combination with ondansetron versus ondansetron alone |
| Outcomes | Nausea and vomiting intensity |
| Notes |
Mersiades A, 2018.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Neidhart 1981.
| Methods | A prospective, randomized and double‐blinded trial |
| Participants | All patients are receiving chemotherapeutic agents known to induce severe vomiting |
| Interventions | Effects of delta‐9‐tetrahydrocannabinol and haloperidol |
| Outcomes | Nausea and vomiting |
| Notes |
Differences between protocol and review
Based on peer review feedback of a draft of the review and inclusion of clinical experts on the review team, we made a number of post‐protocol amendments.
'Types of Participants’ have changed from "people to "Adults aged 18 years and over".
We removed the plan for a subgroup analysis "by emetic potential of the chemotherapy agent, high versus low emetogenic potential" and added a new subgroup analysis "by history of exposure to chemotherapy, chemotherapy naive versus prior chemotherapy treatment".T
he primary outcomes we stated in the protocol are listed in the bullet points below. However, we were unable to analyse data for frequency and severity of nausea or vomiting (or both) due to use of non‐valid and reliable measures, and inappropriate analysis of results reported in the primary studies. We focused on the proportion of people with cancer with complete absence of nausea or vomiting or both in common with other systematic reviews of treatments for nausea and vomiting.
Absence of episodes of nausea and vomiting.
Frequency of nausea and vomiting.
Severity of nausea.
We also stated that we would only extract data for the outcome 'participant preference' for the first cross‐over period only (erroneously), and, due to none of the trials reporting this, we extracted responses for the entire study period.
We added three additional adverse effects as secondary outcomes: focal dystonia, extrapyramidal effects and oculogyric crisis.
We did not contact pharmaceutical companies for data on file.
Methods for future updates
Data extraction and management
For continuous outcomes (severity of nausea measured using a validated symptom scale), we will extract the final value and standard deviation of the outcome of interest and the number of participants assessed in each treatment arm at the end of follow‐up in order to estimate the mean difference between treatment arms (or standardised mean difference if measured on different scales) and its standard error.
Data for frequency of nausea or vomiting, or both, may be reported in a number of ways. For data presented as counts (number of nausea or vomiting (or both) episodes), we will extract the number of events and person‐time at risk, if presented, in order to calculate a nausea and vomiting rate per treatment group. For data presented as continuous data, we will extract the mean number of events (nausea or vomiting (or both) episodes) in each treatment group. For data presented as categorical data (number of participants who experience at least five events), we will proceed as described above for dichotomous data.
Data collection and analysis
If the results are statistically significant, we will calculate the number needed to treat for an additional beneficial outcome (NNTB) or additional harmful outcome (NNTH). For continuous outcomes, we will calculate the difference in means between treatment arms at the end of follow‐up. We will consider the magnitude of the effect of an intervention as at least moderate if the 'effect size' is superior to 0.5 (Cohen 1988). For outcomes reported as rates, we will calculate the rate ratio.
Wherever the data are missing or only imputed data are reported, we will contact the trial authors and request the data on the outcomes only among the participants who were assessed.
Where the trials have multiple treatment groups, we will divide the 'shared' comparison group into the number of treatment groups and treat comparisons between each treatment group and the split comparison group as independent comparisons.
Unit of analysis
In future updates it may be possible to:
obtain data from study authors for each treatment period or summary statistics of the degree of agreement between each person's responses, or both;
adjust the analyses for the dichotomous outcomes to take into account the paired data.
Contributions of authors
Lesley Smith: write protocol, screened studies for inclusion, extracted and analysed data, write final review.
Fredric Azariah: screened searches, screened studies for inclusion, extracted data, contributed to drafts of the review.
Verna Lavender: classified chemotherapy regimens, assessed nausea and vomiting measurements, contributed to drafts of the review.
Nicola Stoner: classified chemotherapy regimens, assessed nausea and vomiting measurements, contributed to drafts of the review.
Silvana Bettiol: screened searches, screened studies for inclusion, extracted data, contributed to drafts of the review.
Sources of support
Internal sources
-
Cochrane, UK
Gynaecological Cancer Review Group
External sources
No sources of support provided
Declarations of interest
The authors have no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Ahmedzai 1983 {published data only}
- Ahmedzai S, Carlyle DL, Calder IT, Moran F. Anti-emetic efficacy and toxicity of nabilone, a synthetic cannabinoid, in lung cancer chemotherapy. British Journal of Cancer 1983;48(5):657-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 1979a {published data only}
- Chang AE, Shiling DJ, Stillman RC. Delta-9-tetrahydrocannabinol as an antiemetic in cancer patients receiving high-dose methotrexate. A prospective, randomized evaluation. Annals of Internal Medicine 1979;91(6):819-24. [DOI] [PubMed] [Google Scholar]
Chang 1981 {published data only}
- Chang AE, Shiling DJ, Stillman RC. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer 1981;47(7):1746-51. [DOI] [PubMed] [Google Scholar]
Crawford 1986 {published data only}
- Crawford SM, Buckman R. Nabilone and metoclopramide in the treatment of nausea and vomiting due to cisplatinum: a double blind study. Medical Oncology and Tumor Pharmacotherapy 1986;3(1):39-42. [DOI] [PubMed] [Google Scholar]
Einhorn 1981 {published data only}
- Einhorn LH, Nagy C, Furnas B, Williams SD. Nabilone: an effective antiemetic in patients receiving cancer chemotherapy. Journal of Clinical Pharmacology 1981;21(8-9 Suppl):64S-9S. [DOI] [PubMed] [Google Scholar]
Frytak 1979 {published data only}
- Frytak S, Moertel CG, O'Fallon JR. Delta-9-tetrahydrocannabinol as an antiemetic for patients receiving cancer chemotherapy. A comparison with prochlorperazine and a placebo. Annals of Internal Medicine 1979;91(6):825-30. [DOI] [PubMed] [Google Scholar]
George 1983 {published data only}
- George M, Pejovic MH, Thuaire M, Kramar A, Wolff JP. Randomized comparative trial of a new anti-emetic: nabilone, in cancer patients treated with cisplatin [French]. Biomedicine & Pharmacotherapy 1983;37(1):24-7. [PubMed] [Google Scholar]
Gralla 1984 {published data only}
- Gralla RJ Tyson LB Bordin LA. Antiemetic therapy: a review of recent studies and a report of a random assignment trial comparing metoclopramide with delta-9-tetrahydrocannabinol. Cancer Treatment Reports 1984;68(1):163-72. [PubMed] [Google Scholar]
Herman 1979 {published data only}
Johansson 1982 {published data only}
- Johansson R, Kilkku P, Groenroos M. A double-blind, controlled trial of nabilone vs. prochlorperazine for refractory emesis induced by cancer chemotherapy. Cancer Treatment Reviews 1982;9(Suppl B):25-33. [DOI] [PubMed] [Google Scholar]
Jones 1982 {published data only}
- Jones SE, Durant JR, Greco FA, Robertone A. A multi-institutional phase III study of nabilone vs. placebo in chemotherapy-induced nausea and vomiting. Cancer Treatment Reviews 1982;9(Suppl B):45-8. [DOI] [PubMed] [Google Scholar]
Kleinman 1983 {published data only}
- Kleinman S, Weitzman SA, Cassem N, Andrews E. Double blind trial of delta-9-tetrahydrocannabinol (THC) versus placebo as an adjunct to prochlorperazine for chemotherapy-induced vomiting. Current Therapeutic Research - Clinical and Experimental 1983;33(61):1014-7. [Google Scholar]
Kluin‐Neleman 1979 {published data only}
- Kluin-Neleman JC, Neleman FA, Meuwissen OJ, Maes RA. Delta 9-tetrahydrocannabinol (THC) as an antiemetic in patients treated with cancer chemotherapy; a double-blind cross-over trial against placebo. Veterinary & Human Toxicology 1979;21(5):338-40. [PubMed] [Google Scholar]
Lane 1991 {published data only}
- Lane M, Vogel CL, Ferguson J, Krasnow S, Saiers JL, Hamm J, et al. Dronabinol and prochlorperazine in combination for treatment of cancer chemotherapy-induced nausea and vomiting. Journal of Pain and Symptom Management 1991;6(6):352-9. [DOI] [PubMed] [Google Scholar]
Levitt 1982 {published data only}
- Levitt M. Nabilone vs. placebo in the treatment of chemotherapy-induced nausea and vomiting in cancer patients. Cancer Treatment Reviews. 1982;9(Suppl B):49-53. [DOI] [PubMed] [Google Scholar]
McCabe 1988 {published data only}
- McCabe M, Smith FP, Macdonald JS, Woolley PV, Goldberg D, Schein PS. Efficacy of tetrahydrocannabinol in patients refractory to standard antiemetic therapy. Investigational New Drugs 1988;6(3):243-6. [DOI] [PubMed] [Google Scholar]
Niiranen 1985 {published data only}
- Niiranen A, Mattson K. A cross-over comparison of nabilone and prochlorperazine for emesis induced by cancer chemotherapy. American Journal of Clinical Oncology: Cancer Clinical Trials 1985;8(4):336-40. [DOI] [PubMed] [Google Scholar]
Orr 1981 {published data only}
- Orr LE, McKernan JF. Antiemetic effect of delta 9-tetrahydrocannabinol in chemotherapy-associated nausea and emesis as compared to placebo and compazine. Journal of Clinical Pharmacology 1981;21(8-9 Suppl):76S-80S. [DOI] [PubMed] [Google Scholar]
Pomeroy 1986 {published data only}
- Pomeroy M, Fennelly JJ, Towers M. Prospective randomized double-blind trial of nabilone versus domperidone in the treatment of cytotoxic-induced emesis. Cancer Chemotherapy and Pharmacology 1986;17(3):285-8. [DOI] [PubMed] [Google Scholar]
Sallan 1975a {published data only}
Steele 1980 {published data only}
- Steele N, Gralla RJ, Braun Jr DW, Young CW. Double-blind comparison of the antiemetic effects of nabilone and prochlorperazine on chemotherapy-induced emesis. Cancer Treatment Reports 1980;64(2-3):219-24. [PubMed] [Google Scholar]
Ungerleider 1982 {published data only}
- Ungerleider JT, Andrysiak T, Fairbanks L, Goodnight J, Sarna G, Jamison K. Cannabis and cancer chemotherapy: a comparison of oral delta-9-THC and prochlorperazine. Cancer 1982;50(4):636-45. [DOI] [PubMed] [Google Scholar]
Wada 1982 {published data only}
- Wada JK, Bogdon DL, Gunnell JC. Double-blind, randomized, crossover trial of nabilone vs. placebo in cancer chemotherapy. Cancer Treatment Reviews 1982;9(Suppl B):39-44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aapro 1981 {published data only}
- Aapro MS. Prevention of chemotherapy-induced nausea and vomiting in patients with cancer. Arizona Medicine 1981;38(11):843-5. [PubMed] [Google Scholar]
Allan 1987 {published data only}
- Allan SG. Mechanisms and management of chemotherapy-induced nausea and vomiting. Blood Reviews 1987;1(1):50-7. [DOI] [PubMed] [Google Scholar]
Anderson 1981 {published data only}
- Anderson PO, McGuire GG. Delta-9-tetrahydrocannabinol as an antiemetic. American Journal of Hospital Pharmacy 1981;38(5):639-46. [PubMed] [Google Scholar]
Artim 1983 {published data only}
- Artim R, Dibella N. Tetrahydrocannabinol plus prochlorperazine for refractory nausea and vomiting. In: Proceedings of the 19th annual meeting of the American Society of Clinical Oncology. Vol. 2. 1983:85.
Bateman 1982 {published data only}
- Bateman DN, Rawlins MD. Therapeutic potential of cannabinoids. British Medical Journal Clinical Research Ed 1982;284(6324):1211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ben 2006 {published data only}
- Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. Journal of Ethnopharmacology 2006;105(1-2):1-25. [DOI] [PubMed] [Google Scholar]
Biedrzycki 2007 {published data only}
- Biedrzycki BA. Evolving paradigms in chemotherapy-induced nausea and vomiting and symptom management for the breast cancer patient. ONS Connect 2007;22(8 suppl):9-10. [PubMed] [Google Scholar]
Broder 1982 {published data only}
- Broder LE, Lean NL, Hilsenbeck SG. A randomized blinded clinical trial comparing delta-9-tetrahydrocannabinol (THC) and hydroxizine (HZ) as antiemetics (AE) for cancer chemotherapy (CT). Proceedings of the American Association for Cancer Research 1982;23:514. [Google Scholar]
Carey 1983 {published data only}
- Carey MP, Burish TG, Brenner DE. Delta-9-tetrahydrocannabinol in cancer chemotherapy: research problems and issues. Annals of Internal Medicine 1983;99(1):106-14. [DOI] [PubMed] [Google Scholar]
Chan 1987 {published data only}
- Chan HSL, Correia JA, MacLeod SM. Nabilone versus prochlorperazine for control of cancer chemotherapy-induced emesis in children: a double-blind, crossover trial. Pediatrics 1987;79(6):946-52. [PubMed] [Google Scholar]
Chang 1979b {published data only}
- Chang AE, Shiling DJ, Stillman RC. A prospective randomized trial of delta-9-tetrahydrocannabinol (THC) as an antiemetic in patients receiving high dose methotrexate (MTX). American Society of Clinical Oncology 1979:No-C-357.
Citron 1985 {published data only}
- Citron ML Herman TS Vreeland F. Antiemetic efficacy of levonantradol compared to delta-9-tetrahydrocannabinol for chemotherapy-induced nausea and vomiting. Cancer Treatment Reports 1985;69(1):109-12. [PubMed] [Google Scholar]
Cocchetto 1981 {published data only}
- Cocchetto DM, Cook LF, Cato AE. A critical review of the safety and antiemetic efficacy of delta-9- tetrahydrocannabinol. Drug Intelligence and Clinical Pharmacy 1981;15(11):867-75. [DOI] [PubMed] [Google Scholar]
Colls 1980a {published data only}
Colls 1980b {published data only}
- Colls BM, Ferry DG, Gray AJ. The antiemetic activity of tetrahydrocannabinol versus metoclopramide and thiethylperazine in patients undergoing cancer chemotherapy. New Zealand Medical Journal 1980;91(662):449-51. [PubMed] [Google Scholar]
Cone 1982 {published data only}
- Cone LA, Greene DS, Helm NA. Use of nabilone in the treatment of chemotherapy-induced vomiting in an outpatient setting. Cancer Treatment Reviews 1982;9(Suppl B):63-70. [DOI] [PubMed] [Google Scholar]
Costa 2007 {published data only}
- Costa B. On the pharmacological properties of 9-tetrahydrocannabinol (THC). Chemistry and Biodiversity 2007;4(8):1664-77. [DOI] [PubMed] [Google Scholar]
Cotter 2009 {published data only}
Cronin 1981 {published data only}
- Cronin CM, Sallan SE, Gelber R, Lucas VS, Laszlo J. Antiemetic effect of intramuscular levonantradol in patients receiving anticancer chemotherapy. Journal of Clinical Pharmacology 1981;21(8-9 Suppl):43S-50S. [DOI] [PubMed] [Google Scholar]
Croxford 2003 {published data only}
- Croxford JL. Therapeutic potential of cannabinoids in CNS disease. CNS Drugs 2003;17:179-202. [DOI] [PubMed] [Google Scholar]
Cunningham 1985 {published data only}
- Cunningham D, Forrest GJ, Soukop M. Nabilone and prochlorperazine: a useful combination for emesis induced by cytotoxic drugs. British Medical Journal 1985;291(6499):864-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunningham 1988 {published data only}
- Cunningham D, Bradley CJ, Forrest GJ, Hutcheon AW, Adams L, Sneddon M, et al. A randomized trial of oral nabilone and prochlorperazine compared to intravenous metoclopramide and dexamethasone in the treatment of nausea and vomiting induced by chemotherapy regimens containing cisplatin or cisplatin analogues. European Journal of Cancer and Clinical Oncology 1988;24(4):685-9. [DOI] [PubMed] [Google Scholar]
Dalzell 1986 {published data only}
- Dalzell AM, Bartlett H, Lilleyman JS. Nabilone: an alternative antiemetic for cancer chemotherapy. Archives of Disease in Childhood 1986;61(5):502-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Darmani 2010 {published data only}
- Darmani NA. Cannabinoid-induced hyperemesis: a conundrum-from clinical recognition to basic science mechanisms. Pharmaceuticals 2010;3(7):2163-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2007 {published data only}
- Davis M, Maida V, Daeninck P, Pergolizzi J. The emerging role of cannabinoid neuromodulators in symptom management. Supportive Care in Cancer 2007;15(1):63-71. [DOI] [PubMed] [Google Scholar]
Davis 2008 {published data only}
- Davis MP. Oral nabilone capsules in the treatment of chemotherapy-induced nausea and vomiting and pain. Expert Opinion on Investigational Drugs 2008;17(1):85-95. [DOI] [PubMed] [Google Scholar]
Devine 1987 {published data only}
- Devine ML, Dow GJ, Greenberg BR, Holstein DW, Icaza L, Jue PY, et al. Adverse reactions to delta-9-tetrahydrocannabinol given as an antiemetic in a multicenter study. Clinical Pharmacy 1987;6(4):319-22. [PubMed] [Google Scholar]
Dodds 1985 {published data only}
- Dodds LJ. The control of cancer chemotherapy-induced nausea and vomiting. Journal of Clinical Hospital Pharmacy 1985;10(2):143-66. [DOI] [PubMed] [Google Scholar]
Dow 1984 {published data only}
- Dow GJ, Meyers FH, Stanton W, Devine ML. Serious reactions to oral delta-9-tetrahydrocannabinol in cancer chemotherapy patients. Clinical Pharmacy 1984;3(1):14. [PubMed] [Google Scholar]
Duran 2010 {published data only}
- Duran M, Perez E, Abanades S, Vidal X, Saura C, Majem M, et al. Preliminary efficacy and safety of an oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. British Journal of Clinical Pharmacology 2010;70(5):656-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekert 1979 {published data only}
- Ekert H, Waters KD, Jurk IH, Mobilia J, Loughnan P. Amelioration of cancer chemotherapy-induced nausea and vomiting by delta-9-tetrahydrocannabinol. Medical Journal of Australia 1979;2(12):657-9. [PubMed] [Google Scholar]
Ettinger 2007 {published data only}
- Ettinger DS, Bierman PJ, Bradbury B, Comish CC, Ellis G, Ignoffo RJ, et al. Antiemesis: clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 2007;5(1):12-33. [DOI] [PubMed] [Google Scholar]
Feyer 2011 {published data only}
- Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Annals of Oncology 2011;22(1):30-8. [DOI] [PubMed] [Google Scholar]
Fiore 1984 {published data only}
- Fiore JJ, Gralla RJ. Pharmacologic treatment of chemotherapy-induced nausea and vomiting. Cancer Investigation 1984;2(5):351-61. [DOI] [PubMed] [Google Scholar]
Fox 1979 {published data only}
- Fox FJ, Baker JJ, Lokey JL. Nabilone as an antiemetic. New England Journal of Medicine 1979;301(13):728. [DOI] [PubMed] [Google Scholar]
Galal 2009 {published data only}
- Galal AM, Slade D, Gul W, El-Alfy AT, Ferreira D, Elsohly MA. Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications. Recent Patents on CNS Drug Discovery 2009;4(2):112-36. [DOI] [PubMed] [Google Scholar]
Gallego 1984 {published data only}
- Gallego Fernandez C, Ferrandiz Gosalbez JR. Nausea and vomiting induced by cancer chemotherapy. Review of Cannabis and its antiemetic effects. Farmacia Clinica 1984;1(9):51-62. [Google Scholar]
Gerhartz 1983 {published data only}
- Gerhartz HH, Binsack T, Hiller E. Levonantradol for the treatment of chemotherapy-induced nausea and vomiting. Klinische Wochenschrift 1983;61(14):719-21. [DOI] [PubMed] [Google Scholar]
Gerra 2010 {published data only}
- Gerra G, Zaimovic A, Gerra ML, Ciccocioppo R, Cippitelli A, Serpelloni G, et al. Pharmacology and toxicology of Cannabis derivatives and endocannabinoid agonists. Recent Patents on CNS Drug Discovery 2010;5(1):46-52. [DOI] [PubMed] [Google Scholar]
Goodman 1997 {published data only}
- Goodman M. Risk factors and antiemetic management of chemotherapy-induced nausea and vomiting. Oncology Nursing Forum 1997;24(7 Suppl):20-32. [PubMed] [Google Scholar]
Gorter 1999 {published data only}
- Gorter RW. Cancer cachexia and cannabinoids. Forschende Komplementarmedizin 1999;6(Suppl 3):21-2. [DOI] [PubMed] [Google Scholar]
Grunberg 1989 {published data only}
- Grunberg SM. Advances in the management of nausea and vomiting induced by non-cisplatin containing chemotherapeutic regimens. Blood Reviews 1989;3(4):216-21. [DOI] [PubMed] [Google Scholar]
Guzman 2003 {published data only}
- Guzman M. Cannabinoids: potential anticancer agents. Cancer 2003;3(10):745-55. [DOI] [PubMed] [Google Scholar]
Heim 1984 {published data only}
- Heim ME, Queisser W, Altenburg HP. Randomized crossover study of the antiemetic activity of levonantradol and metoclopramide in cancer patients receiving chemotherapy. Cancer Chemotherapy and Pharmacology 1984;13(2):123-5. [DOI] [PubMed] [Google Scholar]
Herrstedt 1998 {published data only}
- Herrstedt J, Aapro MS, Smyth JF, Del Favero A. Corticosteroids, dopamine antagonists and other drugs. Supportive Care in Cancer 1998;6(3):204-14. [DOI] [PubMed] [Google Scholar]
Herrstedt 2008 {published data only}
- Herrstedt J. Antiemetics: an update and the MASCC guidelines applied in clinical practice. Nature Clinical Practice Oncology 2008;5(1):32-43. [DOI] [PubMed] [Google Scholar]
Higi 1982 {published data only}
- Higi M, Niederle N, Bremer K, Schmitt G, Schmidt CG, Seeber S. Levonantradol in the treatment of nausea and vomiting caused by cytostatic drugs (author's translation) clinical studies [German]. Deutsche Medizinische Wochenschrift 1982;107(3):1232-4. [DOI] [PubMed] [Google Scholar]
Hiller 1984 {published data only}
- Hiller E, Gerhartz H. New aspects in the antiemetic therapy of cytostatic drug-induced vomiting [German]. Klinische Wochenschrift 1984;62(10):441-5. [DOI] [PubMed] [Google Scholar]
Hutcheon 1983 {published data only}
- Hutcheon AW, Palmer JBD, Soukop M. A randomised multicentre single blind comparison of cannabinoid anti-emetic (levonantradol) with chlorpromazine in patients receiving their first cytotoxic chemotherapy. European Journal of Cancer and Clinical Oncology 1983;19(8):1087-90. [DOI] [PubMed] [Google Scholar]
Jordan 2007 {published data only}
- Jordan K, Schmoll HJ, Aapro MS. Comparative activity of antiemetic drugs. Critical Reviews in Oncology-Hematology 2007;61(2):162-75. [DOI] [PubMed] [Google Scholar]
Jordan 2011 {published data only}
- Jordan K, Roila F, Molassiotis A, Maranzano E, Clark-Snow RA, Feyer P, et al. Antiemetics in children receiving chemotherapy. MASCC/ESMO guideline update 2009. Supportive Care in Cancer 2011;19(Suppl 1):S37-42. [DOI] [PubMed] [Google Scholar]
Kearsley 1985 {published data only}
- Kearsley JH, Tattersall MH. Recent advances in the prevention or reduction of cytotoxic-induced emesis. Medical Journal of Australia 1985;143(8):341-6. [DOI] [PubMed] [Google Scholar]
Kenny 1982 {published data only}
- Kenny JB, Wilkinson PM. Levonantradol effectiveness in cancer patients resistant to conventional anti-emetics. Clinical Oncology 1982;8(4):335-9. [PubMed] [Google Scholar]
Kluin‐Nelemans 1981a {published data only}
- Kluin-Nelemans JC, Meuwissen Th OJA, Nelemans FA, Maes RAA. Tetrahydrocannabinol as antiemetic in patients treated with cytostatics: double blind crossover trial against placebo. Nederlands Tijdschrift voor Geneeskunde 1981;125(2):900-1. [Google Scholar]
Kluin‐Nelemans 1981b {published data only}
- Kluin-Nelemans JC, Meuwissen Th OJA, Nelemans FA, Maes RAA. 9-Tetrahydrocannabinol (THC) as an anti-emetic in patients treated with cancer chemotherapy. A double-blind cross-over trial against placebo. Netherlands Journal of Medicine 1981;24(2):90. [Google Scholar]
Krasnow 1991 {published data only}
- Krasnow SH. New directions in managing chemotherapy-related emesis. Oncology 1991;5(9 Suppl):19-24. [PubMed] [Google Scholar]
Kreutz 2007 {published data only}
- Kreutz S. The magic plant as an antiemetic. Cannabinoids for treatment of nausea and vomiting [German]. Pharmazie in Unserer Zeit 2007;36(5):389-92. [DOI] [PubMed] [Google Scholar]
Lane 1989 {published data only}
- Lane M, Vogel CL, Ferguson J, Krasnow S, Saiers JH, Hamm J, et al. Dronabinol and prochlorperazine in combination are better than either single agent alone for treatment of chemotherapy-induced nausea and vomiting. Proceedings of the American Society of Clinical Oncology 1989;8:326, Abstract 1269.
Lane 1990 {published data only}
- Lane M, Smith FE, Sullivan RA, Plasse TF. Dronabinol and prochlorperazine alone and in combination as antiemetic agents for cancer chemotherapy. American Journal of Clinical Oncology: Cancer Clinical Trials 1990;13(6):480-4. [DOI] [PubMed] [Google Scholar]
Laszlo 1982 {published data only}
- Laszlo J. Treatment of nausea and vomiting caused by cancer chemotherapy. Cancer Treatment Reviews 1982;9(Suppl B):3-9. [DOI] [PubMed] [Google Scholar]
Levitt 1981 {published data only}
- Levitt M, Wilson A, Bowman D, Kemel S, Krepart G, Marks V, et al. Physiologic observations in a controlled clinical trial of the antiemetic effectiveness of 5, 10, and 15 mg of delta 9-tetrahydrocannabinol in cancer chemotherapy. Ophthalmologic implications. Journal of Clinical Pharmacology 1981;21(8-9 Suppl):103S-9S. [DOI] [PubMed] [Google Scholar]
Levitt 1984 {published data only}
- Levitt M, Faiman C, Hawks R, Wilson A. Randomized double blind comparison of delta-9-tetrahydrocannabinol and marijuana as chemotherapy antiemetics [abstract]. Proceedings - Annual Meeting of American Society of Clinical Oncology 1984;3:91, Abstract C-354.
Lohr 2008 {published data only}
- Lohr L. Chemotherapy-induced nausea and vomiting. Cancer Journal 2008;14(2):85-93. [DOI] [PubMed] [Google Scholar]
Long 1982 {published data only}
- Long A, Mioduszewski JNR. A randomized double-blind cross-over comparison of the antiemetic activity of levonantradol and prochlorperazine. Proceedings of the American Society of Clinical Oncology 1982;1:C-220. [Google Scholar]
Machado 2008 {published data only}
- Machado Rocha FC, Stefano SC, De Cassia Haiek R, Rosa Oliveira LM, Da Silveira DX. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. European Journal of Cancer Care 2008;17(5):431-43. [DOI] [PubMed] [Google Scholar]
Mechoulam 1978 {published data only}
- Mechoulam R, Carlini EA. Toward drugs derived from cannabis. Naturwissenschaften 1978;65(4):174-9. [DOI] [PubMed] [Google Scholar]
Mechoulam 1999 {published data only}
- Mechoulam R. Recent advantages in cannabinoid research. Forschende Komplementarmedizin 1999;6(Suppl 3):16-20. [DOI] [PubMed] [Google Scholar]
Mechoulam 2001 {published data only}
- Mechoulam R, Hanus L. The cannabinoids: an overview. Therapeutic implications in vomiting and nausea after cancer chemotherapy, in appetite promotion, in multiple sclerosis and in neuroprotection. Pain Research and Management 2001;6(2):67-73. [DOI] [PubMed] [Google Scholar]
Meiri 2007 {published data only}
- Meiri E, Jhangiani H, Vredenburgh JJ, Barbato LM, Carter FJ, Yang HM, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Current Medical Research and Opinion 2007;23(3):533-43. [DOI] [PubMed] [Google Scholar]
Murakami 1986 {published data only}
- Murakami M, Ota K. Recent advances in the management of chemotherapy-induced emesis [Japanese]. Japanese Journal of Cancer Chemotherapy 1986;13(3):401-11. [PubMed] [Google Scholar]
Musty 2001 {published data only}
- Musty RE, Rossi R. Effects of smoked cannabis and oral DELTA9-tetrahydrocannabinol on nausea and emesis after cancer chemotherapy: a review of state clinical trials. Journal of Cannabis Therapeutics 2001;1(1):29.
Nagy 1978 {published data only}
- Nagy CM, Furnas BA, Einhorn LH, Bond WH. Nabilone (N) anti-emetic crossover study in cancer chemotherapy patients. American Association for Cancer Research and American Society of Clinical Oncology 1978;19:30. [Google Scholar]
Navari 2009a {published data only}
- Navari RM. Pharmacological management of chemotherapy-induced nausea and vomiting. Drugs 2009;69(5):515-33. [DOI] [PubMed] [Google Scholar]
Navari 2009b {published data only}
- Navari RM. Antiemetic control: toward a new standard of care for emetogenic chemotherapy. Expert Opinion on Pharmacotherapy 2009;10(4):629-44. [DOI] [PubMed] [Google Scholar]
Niederle 1986 {published data only}
- Niederle N, Schutte J, Schmidt CG. Crossover comparison of the antiemetic efficacy of nabilone and alizapride in patients with nonseminomatous testicular cancer receiving cisplatin therapy. Klinische Wochenschrift 1986;64(8):362-5. [DOI] [PubMed] [Google Scholar]
Niiranen 1987 {published data only}
- Niiranen A, Mattson K. Antiemetic efficacy of nabilone and dexamethasone: a randomized study of patients with lung cancer receiving chemotherapy. American Journal of Clinical Oncology 1987;10(4):325-9. [DOI] [PubMed] [Google Scholar]
Nyman 1982 {published data only}
- Nyman JV, Fritz WL. A review of the antiemetic use of delta-9-tetrahydrocannabinol and prescribing requirements. Arizona Medicine 1982;39(1):38-43. [PubMed] [Google Scholar]
Orr 1980 {published data only}
- Orr LE, McKernan JF, Bloome B. Antiemetic effect of tetrahydrocannabinol. Compared with placebo and prochlorperazine in chemotherapy-associated nausea and emesis. Archives of Internal Medicine 1980;140(11):1431-3. [DOI] [PubMed] [Google Scholar]
Penta 1981 {published data only}
- Penta JS, Poster DS, Bruno S, Macdonald JS. Clinical trials with antiemetic agents in cancer patients receiving chemotherapy. Journal of Clinical Pharmacology 1981;21(8-9):11S-22S. [DOI] [PubMed] [Google Scholar]
Perwitasari 2011 {published data only}
- Perwitasari DA, Gelderblom H, Atthobari J, Mustofa M, Dwiprahasto I, Nortier JWR, et al. Anti-emetic drugs in oncology: pharmacology and individualization by pharmacogenetics. International Journal of Clinical Pharmacy 2011;33(1):33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2010 {published data only}
- Phillips RS, Gopaul S, Gibson F, Houghton E, Craig JV, Light K, et al. Antiemetic medication for prevention and treatment of chemotherapy induced nausea and vomiting in childhood. Cochrane Database of Systematic Reviews 2010, Issue 9. Art. No: CD007786. [DOI: 10.1002/14651858.CD007786.pub2] [DOI] [PubMed] [Google Scholar]
Plasse 1991 {published data only}
- Plasse TF, Gorter RW, Krasnow SH, Lane M, Shepard KV, Wadleigh RG. Recent clinical experience with dronabinol. Pharmacology, Biochemistry and Behavior 1991;40(3):695-700. [DOI] [PubMed] [Google Scholar]
Porta 2002 {published data only}
- Porta I, Sales J, Schoenenberger I, Arnaiz JA. Cannabinoids, a myth or a scientific reality? Medicina Paliativa 2002;9(3):129-33. [Google Scholar]
Poster 1981 {published data only}
- Poster DS, Penta JS, Bruno S, Macdonald JS. Delta 9-tetrahydrocannabinol in clinical oncology. JAMA 1981;245(20):2047-51. [PubMed] [Google Scholar]
Reynolds 2002 {published data only}
- Reynolds R. Comparative efficacy of dronabinol and megestrol acetate. Journal of Clinical Oncology 2002;20(12):2912-3; author reply 2913. [DOI] [PubMed] [Google Scholar]
Sallan 1975b {published data only}
- Sallan S, Zinberg N, Frei E. Oral delta 9 tetrahydrocannabinol (THC) in the prevention of vomiting (V) associated with cancer chemotherapy (CC). Annual Meeting, proceedings of the American Association for Cancer Research 1975;16:575.
Sallan 1980 {published data only}
- Sallan SE, Cronin C, Zelen M, Zinberg NE. Antiemetics in patients receiving chemotherapy for cancer. A randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. New England Journal of Medicine 1980;302(3):135-8. [DOI] [PubMed] [Google Scholar]
Schuette 1985 {published data only}
- Schuette J, Niederle N, Krischke W. Randomized crossover trial comparing the antiemetic efficacy of nabilone versus alizapride in patients (pts) with nonseminomatous testicular cancer (NSTC) receiving low-dose cisplatin therapy. Proceedings of the American Association for Cancer Research 1985;26:665. [Google Scholar]
Sheidler 1984 {published data only}
- Sheidler VR, Ettinger DS, Diasio RB, Enterline JP, Brown MD. Double-blind multiple-dose crossover study of the antiemetic effect of intramuscular levonantradol compared to prochlorperazine. Journal of Clinical Pharmacology 1984;24(4):155-9. [DOI] [PubMed] [Google Scholar]
Slatkin 2007 {published data only}
- Slatkin NE. Cannabinoids in the treatment of chemotherapy-induced nausea and vomiting: beyond prevention of acute emesis. Journal of Supportive Oncology 2007;5(Suppl 3):1-9. [PubMed] [Google Scholar]
Smith 2007 {published data only}
- Smith PF. Will medicinal cannabinoids prove to be useful clinically? Current Drug Therapy 2007;2(2):143-50. [Google Scholar]
Stambaugh 1982 {published data only}
- Stambaugh JE, McAdams J, Vreeland F. A phase II randomized trial of the antiemetic activity of levonantradol (CP-50,556) in cancer patients receiving chemotherapy. Proceedings of the American Society of Clinical Oncology 1982;1:C-240. [Google Scholar]
Stambaugh 1984 {published data only}
- Stambaugh Jr JE, McAdams J, Vreeland F. Dose ranging evaluation of the antiemetic efficacy and toxicity of intramuscular levonantradol in cancer subjects with chemotherapy-induced emesis. Journal of Clinical Pharmacology 1984;24(11-12):480-5. [DOI] [PubMed] [Google Scholar]
Steele 1979 {published data only}
- Steele N, Braun D, O'Hehir M, Young C. Double-blind comparison of the antiemetic effects of nabilone and prochlorperazine on chemotherapy-induced emesis. Cancer Treatment Reports 1979;64(2-3):219-24. [PubMed]
Stewart 1990 {published data only}
- Stewart DJ. Cancer therapy, vomiting, and antiemetics. Canadian Journal of Physiology & Pharmacology 1990;68(2):304-13. [DOI] [PubMed] [Google Scholar]
Stuart 1982 {published data only}
- Stuart JFB, Welsh Sangster G, Scullion M, Cash H, Kaye SB, Calman KC. The anti-emetic potential of oral levonantradol in patients receiving cancer chemotherapy. British Journal of Cancer 1982;46:492.
Stuart‐Harris 1983 {published data only}
- Stuart-Harris RC, Mooney CA, Smith IE. Levonantradol: a synthetic cannabinoid in the treatment of severe chemotherapy-induced nausea and vomiting resistant to conventional anti-emetic therapy. Clinical Oncology 1983;9(2):143-6. [PubMed] [Google Scholar]
Sweet 1980 {published data only}
- Sweet DL. Marijuana for drug-induced nausea and vomiting. JAMA 1980;243(12):1265. [DOI] [PubMed] [Google Scholar]
Toth 2008 {published data only}
- Toth J, Szanto J. Prophylaxis of chemotherapy-induced vomiting and nausea [Hungarian]. Magyar Onkologia 2008;52(4):391-4. [DOI] [PubMed] [Google Scholar]
Tramer 2001 {published data only}
- Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. BMJ 2001;323(7303):16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ungerleider 1985 {published data only}
- Ungerleider JT, Sarna G, Fairbanks LA, Goodnight J, Andrysiak T, Jamison K. THC or compazine for the cancer chemotherapy patient - the UCLA study. Part II: patient drug preference. American Journal of Clinical Oncology 1985;8(2):142-7. [DOI] [PubMed] [Google Scholar]
Venner 1986 {published data only}
- Venner P, Bruera E, Drebit D, Shankowsky H. Intensive treatment scheduling of nabilone plus dexamethasone (DM) vs metoclopramide plus DM in cisplatin-induced emesis. Proceedings of the American Society of Clinical Oncology 1986;5:253. [Google Scholar]
Vincent 1983 {published data only}
- Vincent BJ, McQuiston DJ, Einhorn LH. Review of cannabinoids and their antiemetic effectiveness. Drugs 1983;25(Suppl 1):52-62. [DOI] [PubMed] [Google Scholar]
Voth 1997 {published data only}
- Voth EA, Schwartz RH. Medicinal applications of delta-9-tetrahydrocannabinol and marijuana. Annals of Internal Medicine 1997;126(10):791-8. [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. Canadian Medical Association Journal 2008;178(13):1669-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 1985 {published data only}
- Ward A, Holmes B. Nabilone. A preliminary review of its pharmacological properties and therapeutic use. Drugs 1985;30(2):127-44. [DOI] [PubMed] [Google Scholar]
Ware 2008 {published data only}
- Ware MA, Daeninck P, Maida V. A review of nabilone in the treatment of chemotherapy-induced nausea and vomiting. Therapeutics and Clinical Risk Management 2008;4(1):99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zuardi 2008 {published data only}
- Zuardi AW. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Revista Brasileira de Psiquiatria 2008;30(3):271-80. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Citron 1983 {published data only}
- Citron ML, Herman TS, Fossieck BE, Krasnow SH, Vreeland F, Harwood S, et al. Double-blind, randomised, crossover study of the antiemetic effect of levonantradol versus tetrahydrocannabinol. Proceedings of the American Association of Cancer Research 1983;24:165. [Google Scholar]
Earhart 1983 {published data only}
- Earhart RH, Koeller JM. Levonantradol vs prochlorperazine as parenteral antiemetics in cancer patients receiving cisplatin chemotherapy. Proceedings of the American Association for Cancer Research. 1983;24:163. [Google Scholar]
Harden‐Harrison 2012 {published data only}
- Harden-Harrison MM, Munsell MF, Fisch MJ, Ule UJ, Saccaro S, Onitilo A, Metzner-Sadurski JK, Giguere JK, Grunberg SM. Dronabinol for the prevention of nausea from cyclophosphamide and/or adriamycin. Supportive Care in Cancer 2012;20:S209-S210. [Google Scholar]
Jhangiani 2005 {published data only}
- Jhangiani H, Vredenburgh J, Barbato L, Yang H, Yang H M, Baranowski V, et al. Dronabinol or ondansetron alone and combined for delayed chemotherapy-induced nausea and vomiting (CINV). Abstracts for the 47th Annual Meeting of the American Society of Hematology; 2005 Dec 10-13; Atlanta, Georgia 2005;106:477.
Mersiades A, 2018 {published data only}
- Mersiades A, Tognela A, Haber PS, Stockler M, Lintzeris N, Simes J, McGregor I, Olver I, Allsop DJ, Gedye C, Kirby A, Morton RL, Briscoe K, Fox P, Aghmesheh M, Wong N, Bhardwaj A, Tran AD, Hahn C, Grimison P. Pilot and definitive randomised double-blind placebocontrolled trials evaluating an oral cannabinoid-rich THC/CBD cannabis extract for secondary prevention of chemotherapyinduced nausea and vomiting (CINV). Asia-pacific journal of clinical oncology 2017;13:67-68. [Google Scholar]
Neidhart 1981 {published data only}
- Neidhart JA, Gagen MM, Wilson HE, Young DC. Comparative trial of the antiemetic effects of THC and haloperidol. Journal of Clinical Pharmacology 1981;21(8-9 Suppl):38S-42S. [DOI] [PubMed] [Google Scholar]
Additional references
Barowski 1984
- Barowski MT. Advances in anti-emetic therapy. Cancer Treatment Reviews 1984;11:237-56. [DOI] [PubMed] [Google Scholar]
Basch 2011
- Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. Journal of Clinical Oncology 2011;29(31):4189-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beusterien 2014
- Beusterien K, Grinspan J, Kuchuk I, Mazzarello S, Dent S, Gertler S, et al. Use of conjoint analysis to assess breast cancer patient preferences for chemotherapy side effects. Oncologist 2014;19(2):127-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonnie 1974
- Bonnie RJ, Whitebread CH. The Marijuana Conviction: a History of Marijuana Prohibition in the United States. Charlottesville, VA: University Press of Virginia: The Lindesmith Center, 1974. [Google Scholar]
Cohen 1988
- Cohen, J. Statistical power analysis for the behavioural sciences. 2nd edition. New Jersey: Lawrence Erlbaum, 1988. [Google Scholar]
Darmani 2001
- Darmani NA. Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB (1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology 2001;24:198-203. [DOI] [PubMed] [Google Scholar]
de Boer‐Dennert 1997
- Boer-Dennert M, Wit R, Schmitz PI, Djontono J, v Beurden V, Stoter G, et al. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. British Journal of Cancer 1997;76(8):1055-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: BMJ, 2001:285-312. [Google Scholar]
De Mulder 1992
- De Mulder PHM. Measurement of success: parameters of efficacy. British Journal of Cancer 1992;66(Suppl XIX):S68. [PMC free article] [PubMed] [Google Scholar]
Devane 1992
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992;258:1946-9. [DOI] [PubMed] [Google Scholar]
Dubey 2005
- Dubey S, Brown RL, Esmond SL, Bowers BJ, Healy JM, Schiller JH. Patient preferences in choosing chemotherapy regimens for advanced non-small cell lung cancer. Journal of Supportive Oncology 2005;3(2):149-54. [PubMed] [Google Scholar]
Gralla 1999
- Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, et al. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. Journal of Clinical Oncology 1999;17(9):2971-94. [DOI] [PubMed] [Google Scholar]
Gralla 2013
- Gralla RJ, Roila F, Tonato M, Herstedt J. MASCC antiemetic guidelines, 2013. www.mascc.org/antiemetic-guidelines (accessed 29 October 2015).
Grotenhermen 2002
- Grotenhermen F. Effects of cannabis and the cannabinoids. In: Grotenhermen F, Russo E, editors(s). Pharmacology, Toxicology and Therapeutic Potential. 1st edition. New York: The Haworth Press, Inc., 2002:55-65. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Huang 2004
- Huang JQ, Zheng GF, Chan GC, Karlberg J, Lam SK, Wong BC. Efficacy of current antiemetic treatments for preventing delayed chemotherapy-induced nausea and vomiting: a meta-analysis of randomized controlled trials. Clinical Research and Regulatory Affairs 2004;21(3-4):191-212. [Google Scholar]
Ioannidis 2000
- Ioannidis JP, Hesketh PJ, Lau J. Contribution of dexamethasone to control of chemotherapy-induced nausea and vomiting: a meta-analysis of randomized evidence. Journal of Clinical Oncology 2000;18:3409-22. [DOI] [PubMed] [Google Scholar]
Janelsins 2013
- Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opinion on Pharmacotherapy 2013;14(6):757-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karniol 2001
- Karniol IG. Cannabis sativa and derivatives. In: Seibel SD, Toscano JRA, editors(s). Dependência de Drogas. 1st edition. Rio de Janeiro: Atheneu, 2001:131-42. [Google Scholar]
Kuchuk 2013
- Kuchuk I, Bouganim N, Beusterien K, Grinspan J, Vandermeer L, Gertler S, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Breast Cancer Research and Treatment 2013;142(1):107. [DOI] [PubMed] [Google Scholar]
Machado Rocha 2008
- Machado Rocha FC, Stefano SC, De Cássia Haiek R, Rosa Oliveira LMQ, da Silveira DX. Therapeutic use of Cannabis sativa on chemotherapy-induced nausea and vomiting among cancer patients: systematic review and meta-analysis. European Journal of Cancer Care 2008;17(5):431-43. [DOI] [PubMed] [Google Scholar]
Martin 1992
- Martin M. Myths and realities of antiemetic treatment. British Journal of Cancer 1992;66(Suppl XIX):S46-51. [PMC free article] [PubMed] [Google Scholar]
MASCC 1998
- Multinational Association of Supportive Care in Cancer (MASCC). Prevention of chemotherapy- and radiotherapy-induced emesis: results of Perugia Consensus Conference. Antiemetic Subcommittee of the Multinational Association of Supportive Care in Cancer (MASCC). Annals of Oncology 1998;9(8):811-9. [PubMed] [Google Scholar]
Mechoulam 1995
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical Pharmacology 1995;50:83-90. [DOI] [PubMed] [Google Scholar]
NCCN 2014
- National Comprehensive Cancer Network (NCCN). National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) Antiemesis Version 2, 2014. www.nccn.org/professionals/physician_gls/f_guidelines.asp, last retrieved 09.11.15.
NCCN 2015
- National Comprehensive Cancer Network (NCCN). National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Antiemesis Version 2. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf, last retrieved 09.11.15.
Olver 1992a
- Olver IN. Methodology in anti-emetic trials. Oncology 1992;49:269-72. [DOI] [PubMed] [Google Scholar]
Olver 1992b
- Olver IN. Antiemetic study design: desirable objectives, stratifications and analyses. British Journal of Cancer 1992;66(Suppl XIX):S30-4. [PMC free article] [PubMed] [Google Scholar]
Olver 2004
- Olver I. Two decades of progress in treating chemotherapy induced emesis. Cancer Forum 2004;28:140-3. [Google Scholar]
Pater 1984
- Pater JL, Willan AR. Methodologic issues in trials of antiemetics. Journal of Clinical Oncology 1984;2(5):484-7. [DOI] [PubMed] [Google Scholar]
Rhodes 1984
- Rhodes VA, Watson PM, Johnson MH. Development of reliable and valid measures of nausea and vomiting. Cancer Nursing 1984;7(1):33-41. [PubMed] [Google Scholar]
Rodríguez de Fonseca 2005
- Rodríguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: physiology and pharmacology. Alcohol and Alcoholism 2005;40(1):2-14. [DOI] [PubMed] [Google Scholar]
Roila 2010
- Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Annals of Oncology 2010;21(5):232-43. [DOI] [PubMed] [Google Scholar]
Russo 2014
- Russo S, Cinausero M, Gerratana L, Bozza C, Iacono D, Driol P, et al. Factors affecting patient's perception of anticancer treatments side-effects: an observational study. Expert Opinion on Drug Safety 2014;13(2):139-50. [DOI] [PubMed] [Google Scholar]
Schwartzberg 2007
- Schwartzberg LS. Chemotherapy-induced nausea and vomiting: clinician and patient perspective. Journal of Support Oncology 2007;5:5-12. [PubMed] [Google Scholar]
Sugiura 1995
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochemical and Biophysical Research Communications 1995;215:89-97. [DOI] [PubMed] [Google Scholar]
Sun 2002
- Sun CC, Bodurka DC, Donato ML, Rubenstein EB, Borden CL, Basen-Engquist K, et al. Patient preferences regarding side effects of chemotherapy for ovarian cancer: do they change over time? Gynecologic Oncology 2002;87(1):118-28. [DOI] [PubMed] [Google Scholar]
Walsh 2003
- Walsh D, Nelson KA, Mahmoud FA. Established and potential therapeutic applications of cannabinoids in oncology. Supportive Care in Cancer 2003;11:137-43. [DOI] [PubMed] [Google Scholar]
Wilcox 1982
- Wilcox PM, Fetting JH, Nettesheim KM, Abeloff MD. Anticipatory vomiting in women receiving cyclophosphamide, methotrexate, and 5-FU (CMF) adjuvant chemotherapy for breast carcinoma. Cancer Treatment Reports 1982;66:1601-4. [PubMed] [Google Scholar]


