Abstract
Background
During atrial fibrillation (AF), conventional electrophysiological techniques for assessment of refractory period or conduction velocity of the atrioventricular (AV) node cannot be used. We aimed at evaluating changes in AV nodal properties during administration of tecadenoson and esmolol using a novel ECG‐based method.
Methods
Fourteen patients (age 58 ± 8 years, 10 men) with AF were randomly assigned to either 75 or 300 μg intravenous tecadenoson. After tecadenoson wash‐out, patients received esmolol continuously (100 μg/kg per min for 10 mins, then 50 μg/kg per min for 50 mins). Atrial fibrillatory rate (AFR) and heart rate (HR) were assessed in 15‐min segments. Using the novel method, we assessed the absolute refractory periods of the slow and fast pathways (aRPs and aRPf) of the AV node to produce an estimate of the functional refractory period.
Results
During esmolol infusion, AFR and HR were significantly decreased and the absolute refractory period was significantly prolonged in both pathways (aRPs: 387 ± 73 vs 409 ± 62 ms, P < 0.05; aRPf: 490 ± 80 vs 529 ± 58 ms, P < 0.05). During both tecadenoson doses, HR decreased significantly and AFR was unchanged. Both aRPs and aRPf were prolonged for a 75 μg dose (aRPs: 322 ± 97 vs 476 ± 75 ms, P < 0.05; aRPf: 456 ± 102 vs 512 ± 55 ms, P < 0.05) whereas a trend toward prolongation was observed for a 300 μg dose.
Conclusions
The estimated parameters reflect expected changes in AV nodal properties, i.e., slower conduction through the AV node for tecadenoson and prolongation of the AV node refractory period for esmolol. Thus, the proposed approach may be used to assess drug effects on the AV node in AF patients.
Keywords: atrioventricular node, atrial fibrillation, functional refractory period, tecadenoson, esmolol
Electrophysiological effects of antiarrhythmic drugs on the atrioventricular (AV) node can be assessed invasively during sinus rhythm at different stages of drug development and in clinical settings. For example, assessment is made to compare response to pharmacological interventions,1 to assess dose‐dependent effect,2 and to compare administration method (i.e., oral vs i.v.3). However, these aspects cannot be assessed in patients with atrial fibrillation (AF) since an atrial pacing protocol cannot be applied. Yet, it is highly desirable to assess the effect of a drug on AV nodal electrophysiology during AF, especially for antiarrhythmic compounds aimed at rate‐control and tested in clinical trials during the initial phases of drug development. Today, the easily measurable effect on heart rate (HR) is usually subject to assessment, whereas the effect on the AV node, reflected, e.g., by the refractory period, is not assessed. HR irregularity has been proposed as a means to assess the effect of drugs in AF patients.4, 5 The atrial fibrillatory rate (AFR), being the inverse measure of AF cycle length,6 has been found useful for monitoring drug effects.7
The relationship between atrial and ventricular rate during AF has recently been investigated in two studies, suggesting that the length of the predominant RR intervals is related to AFR,8 and that a higher degree of RR irregularity is associated with faster AFR.9 Both these studies explored the relationship between the two rates in statistical terms, and thus made no attempt to describe AV nodal properties.
We have recently proposed a novel method for noninvasive assessment of AV nodal function in AF patients.10, 11 Based on information on atrial and ventricular rates, the method assesses AV nodal function by estimating parameters that are indirect measures of the refractory periods of the two AV nodal pathways, the probability of an impulse not passing through the fast pathway, and the prolongation of the refractory periods due to, e.g., concealed conduction.
The present study employs the novel method for investigating changes in AV nodal properties during administration of tecadenoson and esmolol. The hypothesis to be explored is that the indirect estimates of AV nodal refractory periods reflect the overall changes observed in AV nodal properties, as reported in studies on drug development performed during sinus rhythm. In particular, tecadenoson prolongs the effective refractory period of the AV node12 and slows down its conduction.13 Similarly, esmolol prolongs refractoriness and conduction time in both pathways during AV nodal reentrant tachycardia.14
METHODS
Patients
Patient data were collected in a phase II, open‐label, sequential‐group, dose‐escalation trial of tecadenoson administered i.v. alone and in combination with esmolol. The study protocol is accessible via http://www.clinicaltrials.gov/ct2/show/study/NCT00713401. The phase II trial assessed the tolerability and safety of a range of i.v. bolus doses of tecadenoson administered alone to patients with AF. By study protocol, patients with AF in need of rate‐control treatment, but otherwise clinically stable, were randomly assigned to receive different doses of i.v. tecadenoson. During the protocol, esmolol was also infused and maintained (100 μg/kg per min for 10 mins, then 50 μg/kg per min for 50 mins).
In this study, the two subgroups of patients were analyzed who received the minimum and maximum doses, i.e., 75 and 300 μg i.v. tecadenoson. Each subgroup had 7 patients whose clinical characteristics are shown in Table 1.
Table 1.
Demographic Characteristics and Cardiovascular History in the Study Population
| Variable | Group 75 | Group 300 |
|---|---|---|
| Age (years) | 57 ± 9 | 58 ± 8 |
| Gender (male/female) | 6/1 | 4/3 |
| AF duration (months) | 14 (1–168) | 60 (0.5–122) |
| BMI | 27.4 ± 2.8 | 26.9 ± 3.5 |
| Heart failure | 3 | 4 |
| Diabetes | 0 | 0 |
| Hypertension | 6 | 4 |
| Dyslipidemia | 3 | 4 |
| Previous MI | 0 | 0 |
AF = atrial fibrillation; BMI = body mass index; MI = myocardial infarction.
Any concomitant antiarrhythmic therapy (including AV nodal blocking agents) were temporarily discontinued from no later than 8:00 pm on the day prior to studying drug dosing until completion of the last dose period assessment. Blood samples for determining plasma levels of antiarrhythmics and AV nodal blocking agents were collected prior to the tecadenoson bolus.
The study complied with the Declaration of Helsinki, the research protocol was approved by the ethics committee, and informed consent was obtained from all subjects.
The standard 12‐lead ECG was recorded. The effect of tecadenoson and esmolol was analyzed separately by comparing the preinfusion segment (i.e., baseline) to the postinfusion segment, both having 15‐min duration.
Tecadenoson (CVT‐510) is a selective A1‐adenosine receptor agonist with an immediate onset of action (less than 1 min) and a half‐life of approximately 30 mins. Tecadenoson was developed specifically to exploit the A1‐adenosine receptor‐mediated effect of slowing conduction through the AV node,13, 15 while avoiding the effects mediated by the A2 and A3 receptors (e.g., vasodilation and bronchospasm as observed with adenosine).13, 16 Esmolol is a short‐acting beta‐blocker with a distribution half‐life of 2 mins and an elimination half‐life after i.v. infusion of approximately 9 mins. The major action of esmolol is on the sinus node: it prolongs the basic sinus cycle length but has no significant effect on intrinsic automaticity as reflected by the corrected sinus node recovery time and sinoatrial conduction. The direct effects of esmolol on AV nodal function are reflected by effects on conduction and refractoriness.14
AV Nodal Function
We have recently proposed a method to noninvasively estimate five parameters characterizing the AV node in patients with AF, namely, the refractory periods of the two AV nodal pathways, the probability of an impulse not passing through the fast pathway, and the prolongation of the refractory periods.10, 11 The AV node is treated as a lumped structure that accounts for both temporal and spatial summation of the electrical activity of the cells. Atrial impulses are treated as if they arrive randomly to the AV node, with a mean arrival rate proportional to AFR. The AFR is determined from the f‐wave pattern of the ECG (lead V1), extracted with spatiotemporal QRST cancellation17 and subjected to spectral analysis using a noise‐resistant method.18
Impulses arriving to the AV node are assumed to produce ventricular activations unless blocked by a refractory AV node. The slow and the fast AV nodal pathways are characterized by their absolute refractory period (aRP) and relative refractory period (rRP); slow or fast pathway is indicated by appending the letter s or f. All atrial impulses arriving to the AV node before the end of the aRP are blocked, whereas no impulses arriving after the end of the maximally prolonged refractory period (aRP+rRP) are blocked. The definition of aRP includes both the effective refractory period of the AV node and the AV conduction interval, and therefore aRP may serve as an indirect estimate of the functional refractory period. The relative refractory period accounts for both relative refractoriness and concealed conduction. The probability of an impulse to pass through the slow pathway is denoted with α.
The block diagram in Figure 1 shows how the ECG signal is processed to produce the RR series and the AFR, i.e., the two quantities that constitute the complete basis for estimation of the AV node parameters. A brief description of the method for estimating the above‐mentioned five parameters is found in the Appendix; more details are found in References 10 and.11
Figure 1.
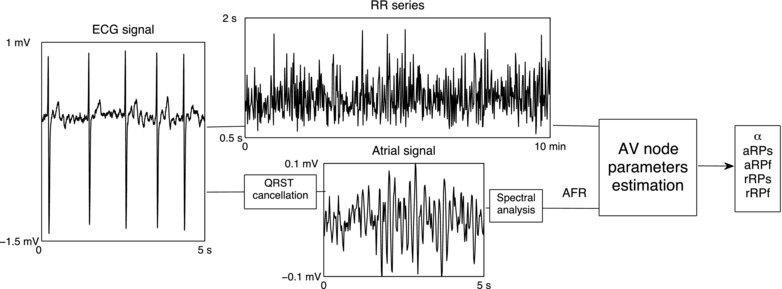
Block diagram of the method for estimating AV node parameters. The ECG signal is processed to produce (i) the RR series and (ii) the AFR (determined by spectral analysis of the atrial signal extracted by QRST cancellation). The RR series and the AFR form together the basis for estimation of the AV node parameters. AFR = atrial fibrillatory rate; aRPs = absolute refractory period of the slow pathway; aRPf = absolute refractory period of the fast pathway; rRPs = relative refractory period of the slow pathway; rRPf = relative refractory period of the fast pathway.
The method is illustrated in Figure 2 by a number of RR interval histograms associated with different AV nodal properties. The histograms correspond to increasing probability of the atrial impulse passing through the slow pathway (the probabilities are 0, 0.25, and 0.5).
Figure 2.
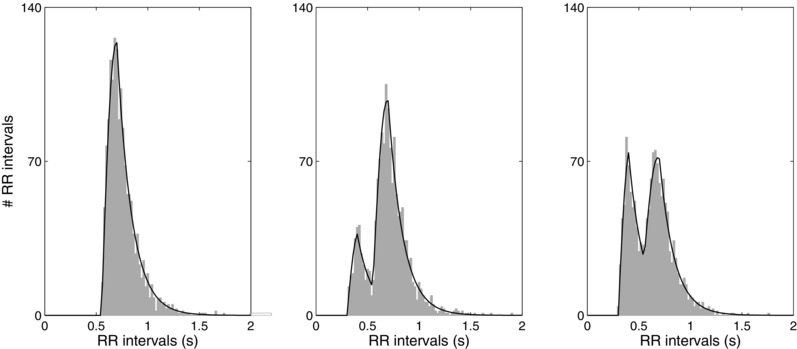
Collection of RR intervals histograms and fitted statistical models (solid line), which reflect different parameter settings. The histograms derive from data with increasing probability of an atrial impulse to pass through the slow pathway (probabilities 0, 0.25, and 0.5); the other AV nodal parameters are held constant.
RESULTS
Esmolol Effect
Table 2 shows the effect of esmolol on HR, AFR, and aRP for the whole population, as the esmolol dose was the same and the patients of the two subgroups did not differ in terms of demographic characteristics and cardiovascular history. As expected, AFR and HR were significantly decreased by esmolol. aRP was significantly prolonged in both pathways, whereas rRP was not significantly different between baseline and esmolol. The analysis of individual patients revealed that one patient had a much larger rRP than all the other patients; this particular patient had very long RR intervals, with 15% of all RR intervals longer than 1 s.
Table 2.
Esmolol Effect: Median (25th–75th percentile)
| Parameter | Baseline | Esmolol |
|---|---|---|
| Atrial and ventricular rate during AF | ||
| AFR (fpm) | 421 (404–450) | 420 (393–445)* |
| HR (bpm) | 85 (79–95) | 83 (77–93)* |
| Estimated AV node parameters | ||
| aRPs (ms) | 365 (331–444) | 403 (356–469)* |
| aRPf (ms) | 478 (435–553) | 525 (482–561)* |
| rRPs (ms) | 232 (86–595) | 229 (108–356) |
| rRPf (ms) | 287 (250–442) | 323 (184–599) |
*P < 0.05.
The fast pathway was the most frequently used in all patients since the probability α was lower than 0.5, with a median value of 0.35 at baseline and 0.36 during esmolol, and an interquartile range of (0.21–0.42) and (0.27–0.43), respectively.
Tecadenoson Effect
Figure 3 illustrates the effect of tecadenoson on the RR interval histogram and the refractory periods of a patient receiving a dose of 300 μg. The top panel shows the refractory periods of the slow and fast pathways at baseline and after tecadenoson. A significant prolongation in both refractory periods can be observed. It is worth noting that aRPf (represented by the filled part of the marker) is larger than aRPs and therefore the fast pathway is more often used. Prolongation of the refractory periods causes the histogram to be right‐shifted, see the bottom panel of Figure 3. This prolongation is also manifested in the whole population since HR decreased significantly with both doses, AFR was unchanged, and the absolute refractory periods were prolonged, see Table 3.
Figure 3.
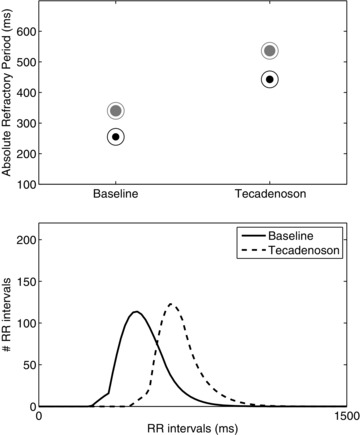
Example of results from a patient from the group with 300 μg dose. Top panel: refractory periods of the slow (black circles) and fast (gray circles) pathways in the protocol phases: the filled part of the marker is proportional to the probability of atrial impulses to pass through that pathway. Bottom panel: the estimated PDFs from the different protocol phases.
Table 3.
Tecadenoson Effect: Median (25th–75th Percentile)
| Group Taking Dose 75 | Group Taking Dose 300 | |||
|---|---|---|---|---|
| Parameter | Baseline | Tecadenoson | Baseline | Tecadenoson |
| Atrial and ventricular rate during AF | ||||
| AFR (fpm) | 434 (417–467) | 430 (422–464) | 407 (371–419) | 408(377—420) |
| HR (bpm) | 95 (86–107) | 87 (82–98)* | 95 (83–101) | 87 (81–95)* |
| Estimated AV node parameters | ||||
| aRPs (ms) | 304 (252–366) | 366 (348–381)* | 283 (239–317) | 326 (248–435) |
| aRPf (ms) | 435 (395–551) | 515 (460–550)* | 422 (353–451) | 485 (426–522) |
| rRPs (ms) | 324 (143–543) | 284 (103–380) | 277 (102–459) | 171 (128–383) |
| rRPf (ms) | 291 (171–513) | 250 (157–813) | 168 (130–233) | 225 (157–303) |
*P < 0.05.
Figure 4 shows the normalized mean RR, AFR, and the absolute refractory periods of the slow and fast pathways for the two subgroups. The values are normalized to baseline. For both doses, the mean RR increased significantly after administration, while AFR remained almost unchanged. Analogously with both doses, aRP and aRP increased after administration, the effect of the 300 μg dose being the more pronounced. It is noted that tecadenoson affects the absolute refractory periods much more than the HR.
Figure 4.
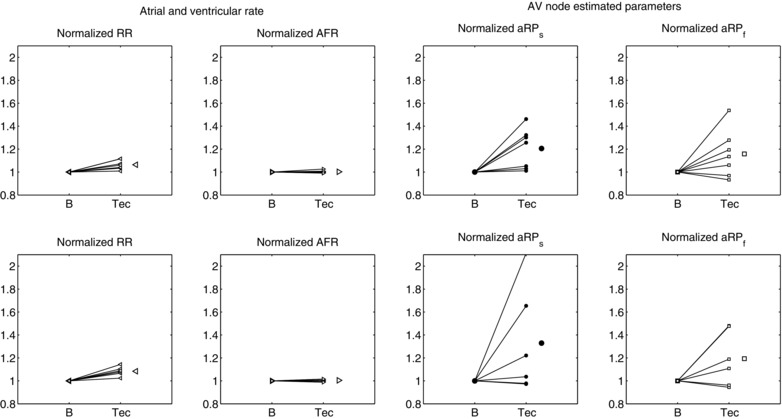
Normalized heart rate (HR), AF rate (AFR), and absolute refractory period of the slow (aRP) and fast (aRP) pathways in the two groups of patients (top row dose 75, bottom row dose 300). In each plot, the single patient response and the average response are shown. *P < 0.05.
DISCUSSION
Noninvasive estimation of the functional refractory period of the AV node during AF has been attempted in the past by estimating it as the shortest RR interval,19, 20 the 5th percentile of the RR series,21 or the lower envelope of the RR Poincaré plot.22 Talajic et al.20 showed in dogs that the minimum RR, determined during AF, correlated well statistically with the functional refractory period determined during sinus rhythm, and therefore used this value as a surrogate measurement of the functional refractory period. Hayano et al.22 used the 1.0‐s intercept of the lower envelope and the degree of scatter above the envelope as surrogate measurements of AV node refractoriness and concealed AV conduction, respectively. In contrast to the 5th percentile, the lower envelope method has not been evaluated on electrophysiological data.
In the present study, our recently proposed method10, 11 has been considered for noninvasive estimation of AV nodal properties using ECG data recorded during administration of the selective A1‐adenosine receptor agonist tecadenoson and the beta‐blocker esmolol. To the best of our knowledge, the effect of these two drugs on the refractory periods has never before been assessed noninvasively in patients with AF. Since an atrial pacing protocol cannot be applied during AF, a comparison of refractory periods obtained invasively and noninvasively is not feasible. Instead, a more qualitative approach has been pursued in which we assess whether the estimated parameters reflect expected drug‐induced changes in AV nodal properties.
The present results support the hypothesis that indirect estimates of refractory periods reflect changes in AV nodal properties similar to those reported on in earlier studies on drug development performed during sinus rhythm. The parameter aRP, including both the effective refractory period of the AV node and its conduction interval, was prolonged for both tecadenoson and esmolol. This prolongation is in line with a slower conduction through the AV node for tecadenoson and a prolongation of AV node refractory period for esmolol.
The refractory period of the pathways was estimated during esmolol infusion, with results that are in agreement with a previous study by Philippon et al.,14 although their patients were in sinus rhythm whereas ours were in AF. During an invasive electrophysiological procedure, they measured the effect of esmolol on refractoriness and conduction time for both pathways in patients with AV nodal reentrant tachycardia. They found that the anterograde effective refractory period of the fast pathway increased from 381 ± 75 ms at baseline to 453 ± 92 ms during the infusion of esmolol (P = 0.003), and the anterograde effective refractory period of the slow pathway increased from 289 ± 26 ms to 310 ± 17 ms (P = 0.005). These increases agree with our results since the estimated aRP, being the combination of the refractory period of the AV node and the AV conduction time, can serve as an estimate of the functional refractory period. It should be underlined that the results of previous studies were limited to patients in sinus rhythm; no invasive study has been published with the aim to measure AV nodal refractory period in AF patients.
In previous studies, esmolol did not affect atrial function in patients with sinus rhythm,23 or AFR in patients with induced AF.24 On the contrary, we found a significant decrease in AFR, suggesting that esmolol acts on the atrial level as well as on AV nodal properties.
Tecadenoson was developed specifically to exploit the A1‐adenosine receptor‐mediated effect of slowing conduction through the AV node.13, 15 Therefore, invasive studies have reported on the effect of tecadenoson on the AV conduction, i.e., the A‐H interval.13, 16 Tecadenoson also prolongs AV node effective refractory period.12 These results agree with ours as we found an increased aRP of both pathways, suggesting a prolongation of the effective refractory period, AV conduction or both. In addition, the present analysis shows that tecadenoson affects HR but not AFR. This means that the decrease in HR can be attributed to tecadenoson effects on AV node.
In addition to refractory periods, the method assesses the probability of impulses to pass through each pathway. Therefore, when α is very close to zero, it suggests that propagation of atrial pulses through AV node occurs via a single pathway. In this study, the median alpha was 0.37, being the interquartile range 0.28–0.37. Five patients had α<0.15, which is likely a reflection of the lack of dual AV physiology in those patients.
In conclusion, noninvasive evaluation of AV nodal electrophysiology during AF has been explored. The preliminary results show that the parameter estimates obtained with our novel method reflect the expected changes in AV nodal properties, i.e., a slower conduction through the AV node for tecadenoson and a prolongation of AV node refractory period for esmolol. The method may therefore be suitable for assessing the drug effect on AV nodal electrophysiology during AF, especially for antiarrhythmic compounds aimed at rate‐control during AF being tested in clinical trials during initial clinical phases of drug development.
Study Limitations
The ultimate validation of the noninvasively obtained measures of AV nodal conduction properties and refractoriness can only be made using programmed stimulation, which, however, is not possible during AF. A validation through a pacing protocol after ablation for AF may be possible, but very questionable, as pacing may induce AF again. However, our indirect estimates of AV nodal properties are in line with data from previous electrophysiological studies performed in patients during sinus rhythm and therefore support the clinical validity of the method.
Acknowledgments
The authors thank Luiz Belardinelli, Gilaed Sciences Inc., Palo Alto, CA, for permission to use the data and valuable discussions.
The main ideas behind the method are briefly described in the following. The foundation of the method is a mathematical model in which atrial impulses are assumed to arrive randomly to the AV node according to a Poisson process with mean arrival rate proportional to AFR. The slow and fast pathways are characterized by their absolute (aRP) and relative (rRP) refractory periods, aRPs, rRPs and aRPf, rRPf, respectively. Impulses result in ventricular activations unless blocked by a refractory AV node. For both pathways, the following is assumed: all atrial impulses arriving to the AV node before the end of aRP are blocked, then follows an interval with linearly increasing likelihood of penetration into the AV node. Finally, no impulses are blocked if they arrive after the end of rRP. The mathematical characterization of refractoriness of the slow pathway is thus defined by
| (1) |
where t denotes the time elapsed since the preceding ventricular activation. The refractoriness of the fast pathway is also described by (1), but aRPf and rRPs replacing aRPs and rRPs.
The probability of an atrial impulse to pass through the slow pathway is equal to α, and accordingly the fast pathway is used with probability (1–α). With the assumption that the AV conduction interval is incorporated into the aRP, ventricular activations will occur immediately after a nonblocked atrial impulse. As a result, ventricular activations occur according to an inhomogeneous Poisson process, i.e., the rate which characterizes the Poisson process is not constant but changes over time, and the distribution of the RR intervals is an exponential function which depends on the refractory periods and the AFR.
With these assumptions, the model accounts for successive RR intervals which are statistically independent. However, since this property is not fully valid for observed RR intervals, a simple linear dependence of the refractory periods to the previous RR interval is used to preprocess the RR interval series so that possible interdependence of successive RR intervals is reduced.22
All model parameters, except AFR, are determined by maximum likelihood estimation, i.e., the model parameters aRPs, aRPf, rRPs, rRPf, and α are estimated by jointly maximizing the log‐likelihood function with respect to θ = [aRPs, aRPf, rRPs, rRPf, α]. The multiswarm particle swarm optimization is used to optimize the log‐likelihood function.7, 8
Conflicts of interest: The authors report no conflicts of interest.
Funding: The CVT‐4129 trial was funded by Gilaed Sciences, but no financial support was provided for the present study. Support was provided by The Swedish Research Council (#2012‐3509) and funds available through governmental support of clinical research within the Swedish National Health Care Service (grant #2011/1816) and The Swedish Heart‐Lung Foundation (grant #20110537).
REFERENCES
- 1. Stark G, Schwarzl I, Stark U, et al. Rate‐dependent effects of ajmaline and propafenone on atrioventricular conduction. Eur J Pharmacol 1996;310:29–35. [DOI] [PubMed] [Google Scholar]
- 2. Han H, Hewett K, McKay C, et al. Different effects of flec‐ainide on atrioventricular conduction properties in the adult and immature rabbit heart. Cardiovasc Drugs Ther 1997;11:767–776. [DOI] [PubMed] [Google Scholar]
- 3. Gambhir D, Bhargava M, Nair M, et al. Comparison of electrophysiologic effects and efficacy of single‐dose intra‐venous and long‐term oral amiodarone therapy in patients with AV nodal reentrant tachycardia. Indian Heart J 1996;48:133–137. [PubMed] [Google Scholar]
- 4. Hsieh MH, Chen SA, Wen ZC, et al. Effects of antiarrhythmic drugs on variability of ventricular rate and exercise performance in chronic atrial fibrillation complicated with ventricular arrhythmias. Int J Cardiol 1998;64:37–45. [DOI] [PubMed] [Google Scholar]
- 5. Incze A, Frigy A, Cotoi S. The efficacy of sublingual verapamil in controlling rapid ventricular rate in chronic atrial fibrillation. Rom J Intern Med 1998;36:219–225. [PubMed] [Google Scholar]
- 6. Holm M, Pehrson S, Ingemansson M, et al. Non‐invasive assessment of the atrial cycle length during atrial fibrillation in man: Introducing, validating and illustrating a new ECG method. Cardiovasc Res 1998;38:69–81. [DOI] [PubMed] [Google Scholar]
- 7. Husser D, Stridh M, Sörnmo L, et al. Analysis of the surface electrocardiogram for monitoring and predicting antiarrhythmic drug effects in atrial fibrillation. Cardiovasc Drugs Ther 2004;18:377–386. [DOI] [PubMed] [Google Scholar]
- 8. Climent AM, Guillem MS, Husser D, et al. Role of atrial rate as a factor modulating ventricular response during atrial fibrillation. Pacing Clin Electrophysiol 2010;33:1–8. [DOI] [PubMed] [Google Scholar]
- 9. Corino VDA, Cygankiewicz I, Mainardi LT, et al. Association between atrial fibrillatory rate and heart rate variability in patients with atrial fibrillation and congestive heart failure. Ann Noninvasive Electrocardiol 2013;18:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corino VDA, Sandberg F, Mainardi LT, et al. An atrioventricular node model for analysis of the ventricular response during atrial fibrillation. IEEE Trans Biomed Eng 2011;58:3386–3395. [DOI] [PubMed] [Google Scholar]
- 11. Corino VDA, Sandberg F, Mainardi LT, et al. Atrioventricular nodal function during atrial fibrillation: Model building and robust estimation. Biomed Signal Proc Control 2013;8:1017–1025. [Google Scholar]
- 12. Mor M, Shalev A, Dror S, et al. INO‐8875, a highly selective A1 adenosine receptor agonist: Evaluation of chronotropic, dromotropic, and hemodynamic effects in rats. J Pharmacol Exp Ther 2013;344:59–67. [DOI] [PubMed] [Google Scholar]
- 13. Prystowsky E, Niazi I, Curtis AA. Termination of paroxysmal supraventricular tachycardia by tecadenoson (CVT‐510), a novel A1‐ adenosine receptor agonist. J Am Coll Cardiol 2003;42:1098–1102. [DOI] [PubMed] [Google Scholar]
- 14. Philippon F, Plumb V, Kay G. Differential effect of esmolol on the fast and slow AV nodal pathways in patients with AV nodal reentrant tachycardia. J Cardiovasc Electrophysiol 1994;5:810–817. [DOI] [PubMed] [Google Scholar]
- 15. Snowdy S, Liang H, Blackburn EA. A comparison of an A1‐adenosine receptor agonist (tecadenoson CVT‐510) with diltiazem for slowing of AV nodal conduction in guinea pig. Br J Pharmacol 1999;126:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lerman B, Ellenbogen K, Kadish AEA. Electrophysiologic effects of a novel selective adenosine a1 agonist (CVT‐510) on atrioventricular nodal conduction in humans. J Cardiovasc Pharmacol Ther 2001;6:237–245. [DOI] [PubMed] [Google Scholar]
- 17. Stridh M, Sörnmo L. Spatiotemporal QRST cancellation techniques for analysis of atrial fibrillation. IEEE Trans Biomed Eng 2001;48:105–111. [DOI] [PubMed] [Google Scholar]
- 18. Sandberg F, Stridh M, Sörnmo L. Frequency tracking of atrial fibrillation using hidden Markov models. IEEE Trans Biomed Eng 2008;55:502–511. [DOI] [PubMed] [Google Scholar]
- 19. Billette J, Nadeau RA, Roberge F. Relation between the minimum RR interval during atrial fibrillation and the functional refractory period of the AV junction. Cardiovasc Res 1974;8:347–351. [DOI] [PubMed] [Google Scholar]
- 20. Talajic M, Nayebpour M, Jing W, et al. Frequency‐dependent effects of diltiazem on the atrioventricular node during experimental atrial fibrillation. Circulation 1989;80:380–389. [DOI] [PubMed] [Google Scholar]
- 21. Khand AU, Rankin AC, Cleland JG, et al. The assessment of autonomic function in chronic atrial fibrillation: Description of a non‐invasive technique based on circadian rhythm of atrioventricular nodal functional refractory period. Europace 2006;8:927–934. [DOI] [PubMed] [Google Scholar]
- 22. Hayano J, Sakata S, Okada A, et al. Circadian rhythms of atrioventricular conduction properties in chronic atrial fibrillation with and without heart failure–relation between mean heart rate and measures of heart rate variability. J Am Coll Cardiol 1998;31:158–166. [DOI] [PubMed] [Google Scholar]
- 23. Greenspan AM, Spielman SR, Horowitz LN, et al. Electrophysiology of esmolol. Am J Cardiol 1985;56:19F–26F. [DOI] [PubMed] [Google Scholar]
- 24. Sticherling C, Tada H, Hsu W, et al. Effects of diltiazem and esmolol on cycle length and spontaneous conversion of atrial fibrillation. J Cardiovasc Pharmacol Ther 2002;7:81–88. [DOI] [PubMed] [Google Scholar]


