Abstract
Background
Control of sympathetic hyperactivity is pivotal for treatment of heart failure (HF) in patients with coronary artery disease (CAD). Our earlier studies demonstrated that the auricular pulsed electrical stimulation of the vagus nerve (VNS) beneficially affected condition of CAD patients with HF. The aim of our study was to evaluate changes in heart rate (HR) and the levels of heat shock proteins in peripheral blood lymphocytes in patients with CAD in the course of VNS.
Methods
The study comprised 70 individuals aged 50–68 years with chronic coronary insufficiency, severe left ventricular dysfunction, and NYHA functional class (FC) III–IV HF. Main group included 63 patients who received VNS course (group 1). Control patients (n = 7) received sham therapy (group 2).
Results
According to the results of 6‐minute walk test and 24‐hour ECG monitoring, administration of VNS improved clinical condition of 58 of 63 patients, decreased HF FC, and attenuated HR. Clinical condition in sham therapy group did not change. Immunoenzyme method demonstrated that hsp70 and hsp60 contents in peripheral blood lymphocyte lysate increased by 58% and 48% (P < 0.05), respectively, in patients who initially had HR < 80 bpm. The hsp70 level significantly increased and hsp60 level remained unchanged in patients with initial HR > 80 bpm.
Conclusions
Correction of autonomous nervous status by VNS attenuated HR and improved functional state of the heart in CAD patients. Cardiotropic effect of VNS was the most pronounced in patients with preserved endogenous stress‐limiting systems associated with hsp60 and/or hsp70.
Keywords: autonomous nervous system; coronary artery disease; heart failure; heart rate, heat shock proteins; vagus nerve stimulation
Hyperactivation of the sympathetic nervous system (SNS) is an important pathophysiologic factor determining chronic course of ischemic heart failure (HF).1, 2 Correction of SNS hyperactivity is an essential component of treatment of HF in patients with coronary artery disease (CAD).2, 3, 4 However, pharmacological correction of the inotropic myocardial response may be challenging to achieve in patients with severe HF.5 Pharmacological downregulation of SNS is also not always feasible. These considerations provide rationale for the development of new nonpharmacological approaches for treatment of patients with HF.
The function of the autonomic nervous system can be modulated by nonpharmacological methods. The autonomic regulation status is usually estimated based on heart rate (HR), HR variability, and QT duration. Among these parameters, resting HR is of high significance. 6, 7, 8, 9, 10, 11, 12, 13 Resting HR is inversely related to the lifespan. Increase in this parameter is associated with greater risk of death.6, 7, 8 HR modulation is both a sign and a result of change in balance of the autonomic nervous system.
HR may be controlled via vagus nerve stimulation (VNS). Vagus nerve nuclei can be stimulated through the sensory nerve endings of the auricular branch of the vagus nerve (r. auricularis n. vagi) located beneath the skin of the internal surface of the auricles 14, 15 Previously, we demonstrated that the course of the auricle pulsed electrical stimulation of the vagus nerve nuclei improves condition of patients with nonischemic HF.16, 17
Improvement of the myocardial function is accompanied by positive changes in the metabolic parameters of the myocardium. In particular, evidence suggests that vagus nerve nuclei stimulation can affect the cellular resistance factors such as heat shock protein hsp70. In the presence of pathological process, organism preserves ability to additionally activate endogenous defense factors that include heat shock proteins.18 However, indications and details for the procedure of the vagus nerve nuclei stimulation in different groups of patients with HF require further study and elaboration.
The aim of our study was to evaluate changes in HR and the levels of heat shock proteins in peripheral blood lymphocytes in patients with chronic coronary insufficiency and severe left ventricular (LV) dysfunction in the course of nonpharmacological correction of hypersympathicotonia.
METHODS
Study Population
The study comprised a total of 70 individuals aged 50 to 68 years with chronic coronary insufficiency and severe LV dysfunction. According to NYHA classification, patients had functional class III‐IV HF. At a time of enrolment into the study, all patients had stable clinical condition with unchanged symptoms of chronic heart failure (CHF) for at least 30 days. Their HR was over 60 beats per minute (bpm) and LV ejection fraction was less than 40%. Patients were free of LV dyssynchrony and valvular heart disease. All patients received optimal therapy for CHF unchanged for at least 30 days prior to the study and during the entire follow‐up period. Pharmacological therapy included inhibitors of angiotensin‐converting enzyme, β‐adrenoblockers, diuretics, nitrates, and cardiac glycosides. Patients were randomized into two groups with comparable clinical characteristics (Table 1). Main group (group 1) comprised 63 patients who were administered with the course of the pulsed electrical stimulation of r. auricularis n. vagi. Patients of control group (group 2, n = 7) received sham treatment. Randomization of patients into groups of different sizes was based on the following considerations: (1) primary statistical hypothesis consisted in the presence of differences between patients’ initial condition and the condition after the course of vagus nerve electrical stimulation in group 1; (2) we did not do intergroup comparisons though we compared subgroups 1a and 1b formed from group 1; (3) group 2 was assigned to check whether sham treatment modulated physiological condition of patients and, therefore, we compared their initial condition with posttreatment condition which did not affect statistical power of the conclusions based on the comparisons of parameters in group 1; (4) ethical committee recommended us to limit the size of group 2 to minimize time and efforts for patients and physicians without affecting statistical power of comparisons in main group.
Table 1.
Clinical Characteristics of Control and Main Group of Patients with Heart Failure
| Patient Groups | |||
|---|---|---|---|
| Indicator | Control (n = 7) | VNS (n = 63) | P |
| Age (years) | 59.571 ± 12.447 | 55.446 ± 11.971 | NS |
| NYHA FC III HF, n | 7 | 60 | NS |
| NYHA FC IV HF, n | 0 | 3 | NS |
| Duration of heart failure (years) | 8.142 ± 1.864 | 6.539 ± 2.123 | NS |
| HR (bpm) | 76.000 ± 9.521 | 74.968 ± 11.967 | NS |
| Office SBP (mmHg) | 120.285 ± 9.013 | 121.571 ± 14.658 | NS |
| Office DBP (mmHg) | 77.285 ± 4.715 | 77.746 ± 8.978 | NS |
| AMI, n (%) | 3 (42.8) | 21 (33.3) | NS |
| Dyspnea, n (%) | 7 (100) | 63 (100) | NS |
| Edema, n (%) | 1 (14.2) | 19 (30.1) | NS |
| Ascite, n (%) | 0 | 3 (4.7) | NS |
| Hydrothorax, n (%) | 1 (14.2) | 2 (3.1) | NS |
| Hydropericardium, n (%) | 1 (14.2) | 1 (1.5) | NS |
| Fatigue, n (%) | 7 (100) | 63 (100) | NS |
VNS = electrical stimulation of the auricular branch of the vagus nerve; HR = heart rate; bpm = beats per minute; SBP = systolic blood pressure; DBP = diastolic blood pressure; FC = functional class; HF = heart failure; AMI = acute myocardial infarction; NS = statistically nonsignificant differences between groups.
All clinical instrumental examinations were performed at the stage of enrolment of patients into the study and one day after the completion of the course of auricle vagal nerve electrical stimulation.
Assessment of HR
HR increase is one of the most apparent signs of sympathetic hyperactivity.19, 20 HR was measured by 24‐hour Holter ECG monitoring (MARS PC, GE Medical Systems Information Technologies, Wauwatosa, WI, USA) at a time of enrolment of patients into the study and one day after the completion of the course of r. auricularis n. vagi electrical stimulation.
Six‐Minute Walk Test
Six‐minute walk test (6MWT) enabled reliable evaluation of physical activity and exercise tolerance in patients with CHF before and after treatment.21 According to instructions, patients were asked to walk as quickly as they can for six‐minutes along the corridor. Before and after six minutes of walking, HR and arterial blood pressure (ABP) were measured. Covered distance was used to determine the CHF functional class based on a standard scale (108). Self‐measurements of HR and ABP were taken in a sitting position in a chair with support to the back and the arms in a quiet room with automatic tonometer. Two measurements with two‐minute interval between them were taken both before and after six‐minute walk. Results of every measurement were stored in the device and controlled by physician. Average values were used for the analysis.22, 23
The Technique of Vagal Nerve Electrical Stimulation
To correct the status of autonomic nervous system in patients, we performed indirect electrical stimulation of the vagus nerve nuclei as described earlier.16, 17, 24
Stimulation was delivered by low frequency bipolar electrical pulses through the leads attached to internal surfaces of the auricles (Fig. 1) in the proximity of sensory endings of r. auricularis n. vagi.14, 15
Figure 1.
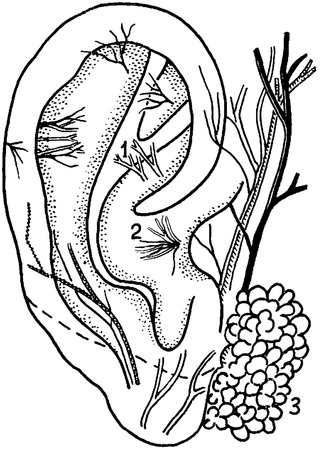
Afferent nerve endings of cranial nerves on a surface of an auricle, (1) n. facialis; (2) r. auricularis n. vagi; and (3) parotid salivary gland.
Electrical stimulation of the auricular branch of the vagus nerve was delivered as a course of 15 procedures with stepwise increases in current strength from 0.05 mA to 0.15 mA and in duration of stimulation from 5 s to 30 min. Amperages and times were as follows: 0.05 mA for 1 min (procedure 1), 0.05 mA for 5 min (procedure 2 and 3), 0.08 mA for 10 min (procedures 4 and 5), 0.1 mA for 15 min (procedures 6 and 7), 0.12 mA for 20 min (procedure 8 and 9), and 0.15 mA for 30 min (procedure 10 to 15). Sham‐controlled patients received treatment in the same environmental conditions and the electrodes were applied at the same area, but without delivering the electric pulses.
The Presence of Stress Proteins
The presence of hsp60 and hsp70 proteins was evaluated by immunoenzymatic method 25 with ELISA Kits (USA) according to manufacturer's protocol. Hsp60 and hsp70 contents were tested in the lysate of lymphocytes isolated from peripheral blood of patients.26, 27 Blood samples were obtained at the time of enrolment of patients into the study and one day after the last procedure of VNS.
Statistical Analysis
Checking the hypothesis for Gaussian distribution of data by Kolmogorov‐Smirnov test with Lilliefors correction and Shapiro‐Wilk normality tests discarded this hypothesis. Therefore, statistical significance of the results was evaluated by the Mann‐Whitney‐Wilcoxon test. Values were considered statistically significant when P was <0.05. Results are presented as M ± SD (M is arithmetic mean; SD is standard deviation), median, and the lower and the upper quartiles.
The work was carried out according to Declaration of Helsinki developed by the World Medical Association. Protocol of the study and the text of a patient's informed consent were approved by the local ethics committee of the authors’ institute.
RESULTS
The study did not reveal any changes in clinical condition of patients from the sham‐treated group. Table 2 demonstrates that the results of 6MWT did not significantly change after treatment.
Table 2.
Results of Six‐Minute Walk Test in Control Group before and after Sham Treatment
| Parameter | M ± SD | ME | Min‐Max | LQ‐UQ |
|---|---|---|---|---|
| 6MWT before sham treatment (m) | 211.4 ± 40.1 | 200.0 | 160.0–270.0 | 175.0–250.0 |
| 6MWT after sham treatment (m) | 213.6 ± 38.5 | 205.0 | 155.0–270.0 | 190.0–250.0 |
6MWT = six‐minute walk test distance.
On the opposite, in main group, electrical stimulation of the auricular branch of the vagus nerve resulted in a significant increase in 6MWT distance (Table 3). The course of electrical stimulation improved clinical condition in 58 of 63 patients. Functional class of HF decreased from IV to III in 3 patients, from III to II in 52 patients, and from III to I in 3 patients. DyspnEA and signs of fatigue decreased in 45 and 49 patients, respectively. Alleviation of edema occurred in 13 of 17 patients. Nobody of patients had ascites, hydrothorax or hydropericardium.
Table 3.
Results of Six‐Minute Walk Test in Main Group before and after the Course of Transcutaneous Electrical Stimulation of the Auricular Branch of the Vagus Nerve
| Parameter | M ± SD | ME | Min‐Max | LQ‐UQ |
|---|---|---|---|---|
| 6MWT before VNS (m) | 265.4 ± 38.9 | 275.0 | 175.0–300.0 | 255.0–290.0 |
| 6MWT after VNS (m) | 376.4 ± 39.4 | 385.0 | 290.0–435.0 | 350.0–395.0 |
6MWT = six‐minute walk test distance; VNS= electrical stimulation of the auricular branch of the vagus nerve.
In main group, 24‐hour Holter ECG monitoring was performed in 44 patients who responded with improvement of clinical condition after the course of electrical stimulation. In this cohort, 32 patients initially had HR less than 80 bpm (subgroup 1a); after the electric stimulation, HR fall was documented in 26 of them (Fig. 2). HR was initially over 80 bpm in other 12 patients (subgroup 1b); HR decreased only in 8 of them after the course of electrical stimulation. The mean HR values are presented in Figures 2 and 3. Tables 4 and 5 compare HR variability parameters.
Figure 2.
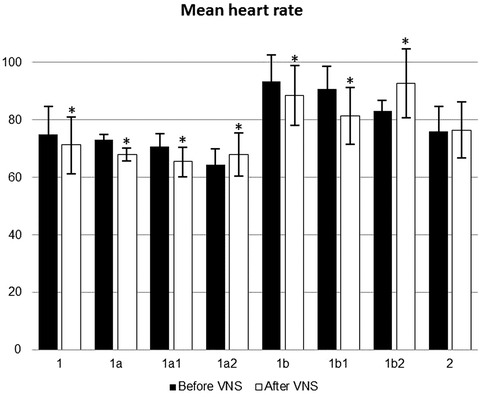
Mean values of heart rate in patients before and after the course of vagus nerve stimulation or sham treatment. *P < 0.05 compared with initial pretreatment values. 1: patients received VNS treatment. 1a: patients from group 1 whose pretreatment heart rate was ≤80 bpm. 1a1: patients from subgroup 1a whose heart rate decreased after VNS course. 1a2: patients from subgroup 1a whose heart rate did not change after VNS course. 1b: patients from group 1 whose pretreatment heart rate was >80 bpm. 1b1: patients from subgroup 1a whose heart rate decreased after VNS course. 1b2: patients from subgroup 1a whose heart rate did not change after VNS course. 2: patients received sham treatment.
Figure 3.
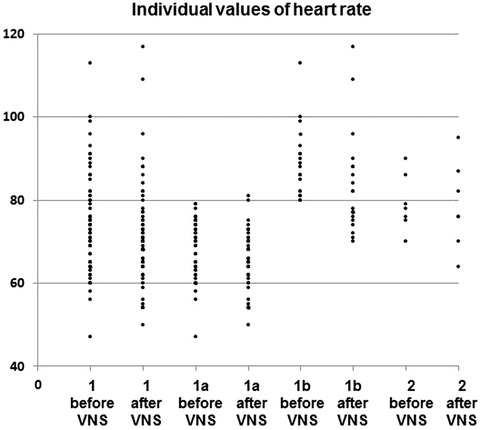
Individual points for mean heart rate before and after VNS. Group 1: patients received VNS treatment. Subgroup 1a: patients from group 1 whose pretreatment heart rate was ≤ 80 bpm. 1b: patients from group 1 whose pretreatment heart rate was >80 bpm. Subgroup 2: patients received sham treatment.
Table 4.
Comparison of the Initial Standard Deviations of all NN Intervals between Subgroups 1a and 1b (Mann‐Whitney U Test)
| Subgroups | SDNN (ms) |
|---|---|
| Subgroup 1a (n = 32) | 107.500 ± 30.456 |
| Subgroup 1b (n = 12) | 81.666 ± 28.262* |
SDNN = standard deviation of all NN intervals.
*Statistically significant difference (P < 0.05).
Table 5.
Comparison of the Standard Deviations of all NN Intervals between Patients from 1a and 1b Subgroups with and without VNS‐Induced Heart Rate Decrease (Mann‐Whitney U test)
| SDNN (ms) | ||
|---|---|---|
| HR Decreased | HR Did not Decrease | |
| Subgroup 1a | 101.230 ± 30.284 (n = 26) | 134.667 ± 8.687* (n = 6) |
| Subgroup 1b | 63.666 ± 23.534# (n = 8) | 104.5 ± 24.826*# (n = 4) |
SDNN = standard deviation of all NN intervals; HR = heart rate.
*Statistically significant differences for comparisons between patients whose heart rate decreased and did not decrease within each subgroup (P < 0.05). #Statistically significant differences for intergroup comparisons (P < 0.05).
Table 6 shows that control and main groups did not differ in the initial contents of hsp60 and hsp70 proteins. Contents of hsp60 and hsp70 proteins did not significantly change after sham treatment. In main group, vagus nerve electrical stimulation exerted different effect on these proteins. In subgroup 1a, hsp70 and hsp60 significantly decreased by 58% and 48%, respectively (P<0.05). In subgroup 1b, the electrical stimulation induced significant increase in hsp70 content only (P < 0.05) though the magnitude of this increase (by 85%) was significantly higher than in subgroup 1a (P < 0.05). Content of hsp60 protein did not significantly change after the treatment course in subgroup 1b. However, it is worthy of note that initial hsp60 content in this group was 1.6 times higher than in patients of subgroup 1a. In samples obtained from patients who did not respond with HR decrease to the VNS, treatment‐induced changes in the stress proteins were statistically insignificant (data not shown).
Table 6.
The Levels of Stress Proteins hsp60 and hsp70 in Peripheral Blood Lymphocytes before and after the Course of Transcutaneous Electrical Stimulation of the Auricular Branch of the Vagus Nerve
| Main Group (Group 1) | ||||||
|---|---|---|---|---|---|---|
| Control Group (Group 2), n = 5 | Subgroup 1a | Subgroup 2 | ||||
| Before Sham | After Sham | HR <80 bpm, n = 28 | HR >80 bpm, n = 8 | |||
| Parameters | Treatment | Treatment | Before VNS | After VNS | Before VNS | After VNS |
| Hsp60 | 131.5 ± 34.3 | 133.8 ± 34.0 | 103.9 ± 75.1 | 154.3 ± 92.6* | 166.5 ± 97.2 | 165.0 ± 168.7 |
| Hsp70 | 9.4 ± 6.7 | 14.3 ± 12. 3 | 12.9 ± 5.8 | 20.5 ± 9.1* | 12.0 ± 6.8 | 22.4 ± 22.0* |
VNS = electrical stimulation of the auricular branch of the vagus nerve; HR = heart rate; bpm = beats per minute; hsp = heat shock protein.
*Statistically significant differences between heat shock protein levels before and after treatment (P < 0.05).
DISCUSSION
Treatment of patients with HF may require suppression of hypersympathicotonia 3, 19 which is an important element of pathogenesis of CHF. 1, 2 Main sign of hypersympathicotonia is elevated resting HR. Value of resting HR is of high significance. Indeed, an increasing body of evidence suggests that HR is inversely related to the lifespan. In patients stratified by resting HR, HR increase is associated with greater risk of death. This correlation may be due to high basal metabolic rate and cardiovascular‐related mortality risk. 6, 7, 8 Accelerated resting HR represents an independent predictor of noncardiovascular mortality in both genders, and of cardiovascular mortality in men, independent of age and the presence of hypertension in a large French population.9, 10 Resting HR and blood pressure proportionally raise the risk for diabetes mellitus in healthy men and women. 11 In Western societies, sympathetic overactivity and, correspondingly, high HR is frequently found in a distinct subgroup of subjects, who exhibit the characteristic features of the insulin resistance syndrome.8 Among CAD patients with hypertension, resting HR predicts adverse outcomes, and on‐treatment resting HR values are more predictive than baseline values;13 the adverse effects of fast HR and high BP are independent of each other.12
Decline in HR is associated with favorable findings on cardiovascular health and with observed long term declines in cardiovascular mortality.28 Slowing HR could potentially decrease the progression of atherosclerosis by reducing the local pro‐atherosclerotic vascular environment. This effect may be involved in beneficial effects of HR lowering agents in prevention of CAD.29 HR is considered a therapeutic target in HF.30 However, according to European Guidelines on cardiovascular disease prevention.31 “No trial of heart rate lowering for CVD prevention in a healthy population has been conducted to date; therefore, pharmacological lowering of HR in primary prevention cannot be recommended.” Moreover, pharmacological correction of elevated HR in patients with severe HF is sometimes impossible to achieve.
These considerations provided rationale for the use of transcutaneous electrical stimulation of the auricular branch of the vagus nerve in order to lower HR and alleviate symptoms of CHF in the presence of standard therapy. The course of electrical stimulation enabled to decrease HF functional class by one grade in 58 of 63 patients and to improve 6MWT results. Our results agree with data of other studies.32, 33, 34 Authors of these works suggest that the VNS can improve the quality of life and LV function in patients with CHF and severe systolic LV dysfunction. In these works, an attenuation of hypersympatheticotonia was achieved through the feed‐back mechanism of increase in parasympathetic activity. For this purpose, direct stimulation of the vagus nerve was achieved through implantation of an electronic device. In our study, vagus nerve was stimulated transcutaneously through the sensory endings of the auricular branch of the vagus nerve (ramus auricularis nervi vagi). 14, 15
We believe that significant improvement of 6MWT and decrease in functional class of HF after the VNS were caused by establishing new balance between sympathetic and parasympathetic innervation and lowering HR. A change in HR is considered the most obvious and clinically significant indicator and outcome of shift in the autonomic nervous system balance. 20, 35
Elevated HR occurs due to an increased sympathetic and decreased parasympathetic tone. This altered balance of the autonomic nervous system tone could explain and increase in events with the increased HR. Blood flow changes, associated with high HR, contribute to the formation of the atherosclerotic lesion and the occurrence of the cardiovascular event.36 Williams with coworkers proposed hypothesis of lipoprotein‐induced relationships of coronary heart disease with resting HR.37 HR may be also associated with status of body hemostasis associated with particular metabolic conditions expressed in serum triglycerides levels.38
It is still unclear what upper limit of HR should be considered normal. In patients with CAD and left‐ventricular systolic dysfunction, HR of 70 bpm or greater is considered elevated and identifies those at increased risk of cardiovascular outcomes.39, 40 Other study suggests that the relative risk for cardiovascular death increases when HR > 60 bpm. More specifically, the relative risks for cardiovascular death in groups with HR 60–80, 80–100, and >100 bpm are 1.35 (1.01 to 1.80), 1.44 (1.04 to 2.00), and 2.18 (1.37–3.47), respectively, when compared with patients with HR <60 bpm. 9 Resting HR of 60–80 bpm is considered normal.41 Based on this assumption, we divided our patients into two subgroups: HR ≤ 80 (subgroup 1a) and >80 bpm (subgroup 1b). Decrease in HR was documented in majority of our patients after the course of transcutaneous electrical stimulation of the auricular branch of the vagus nerve. It occurred in both subgroup 1a and 1b. The fact that patients of subgroup 1a more often experienced HR decrease after the treatment can be explained by their better initial balance of autonomic innervation. After transcutaneous vagus nerve electrical stimulation, HR decrease was absent in sham‐treated patients, but was present in main group on unchanged pharmacological therapy for CHF. In our opinion, this fact suggests that transcutaneous vagus nerve electrical stimulation changes autonomic regulation of the heart. These changes are evidently aimed at maintaining functional self‐consistency of the myocardium through establishing new balance between sympathetic and parasympathetic innervation.
According to existing concept, in the course of the correction of autonomic status in patients with CHF, more favorable functional conditions for the myocardium are achieved through an additional activation of the endogenous stress‐limiting systems, in particular, associated with heat shock proteins.42 In our study, contents of hsp60 and hsp70 remained unchanged in sham‐treated group. On the contrary, in main group, we observed the diversification of the effect. Indeed, subgroup 1a had an increase in hsp60 and hsp70 contents by 1.5 times and more. Patients of subgroup 1b had an increase in hsp70 content only. These results agree with data regarding nature of heat shock proteins. Proteins of hsp70 family are cytoplasmic proteins whereas hsp60 are localized in mitochondria.43, 44, 45 It is possible that mitochondrial localization of hsp60 underlies the observed pattern of changes in heat shock protein contents, in particular, high initial content of hsp60 in subgroup 1b. Indeed, in case of severe long‐term tachycardia, energy metabolism of the cells is under stress.46 In the presence of HF, this is an additional factor negatively affecting mitochondria as main energy factories of the cell.47, 48 Perhaps, when HR is higher than 80 bpm, endogenous stress‐limiting mechanisms, associated with hsp60, are close to exhaustion. Such interpretation of the obtained results agrees well with data of other studies showing decreased or unchanged levels of heat shock proteins in peripheral blood lymphocytes in patients with postinfarction cardiosclerosis. These data suggest about exhaustion of adaptive capabilities which is an unfavorable prognostic factor.26, 27
Therefore, based on the obtained results, we conclude that correction of the autonomic nervous system status through the course of the transcutaneous electrical stimulation of the auricular branch of the vagus nerve efficaciously improves cardiac function and is associated with reduction in HR and an increased expression of heat shock proteins hsp60 and/or hsp70 in patients with HF of high functional classes. Cardiotropic effect of the course is more efficacious in patients with the preserved reserve of the endogenous stress‐limiting systems associated with hsp60 and/or hsp70 proteins.
Ann Noninvasive Electrocardiol 2016;21(6):548–556
Conflict of interest: The authors declare that there are no conflicts of interest.
Financial support: This study was performed as a budgetary‐funded research project.
REFERENCES
- 1. Olshansky B, Sabbah HN, Hauptman PJ, et al. Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation 2008;118:863–871. [DOI] [PubMed] [Google Scholar]
- 2. Singh RB, Demeester F, Wilczynska A. The tsim tsoum approaches for prevention of cardiovascular disease. Cardiol Res Pract 2010;824938–824940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Floras JS. Sympathetic nervous system activation in human heart failure. J Am Coll Cardiol 2009;54:375–385. [DOI] [PubMed] [Google Scholar]
- 4. Watson AMD, Hood SG, Ramchandra R, et al. Increased cardiac sympathetic nerve activity in heart failure is not due to desensitization of the arterial baroreflex. Am J Physiol Heart 2007;293:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith ER, Kacker SR, Raskin A, et al. Central propranolol and pindolol, but not atenolol nor metoprolol, inhibit sexual behavior in male rats. Physiol Behav 1996;59:241‐246. [DOI] [PubMed] [Google Scholar]
- 6. Zhang GQ, Zhang W. Heart rate, lifespan, and mortality risk. Ageing Res Rev 2009;8:52–60. [DOI] [PubMed] [Google Scholar]
- 7. Levine HJ. Rest heart rate and life expectancy. J Am Coll Cardiol 1997;30:1104–1106. [DOI] [PubMed] [Google Scholar]
- 8. Palatini P, Julius S. Association of tachycardia with morbidity and mortality: Pathophysiological considerations. J Hum Hypertens 1997;11( Suppl 1):S19–27. [PubMed] [Google Scholar]
- 9. Benetos A, Rudnichi A, Thomas F, et al. Influence of heart rate on mortality in a French population: Role of age, gender, and blood pressure. Hypertension 1999;33:44–52. [DOI] [PubMed] [Google Scholar]
- 10. Reil JC, Böhm M. BEAUTIFUL results—The slower, the better? Lancet 2008;372:779–780. [DOI] [PubMed] [Google Scholar]
- 11. Shigetoh Y, Adachi H, Yamagishi S, et al. Higher heart rate may predispose to obesity and diabetes mellitus: 20‐year prospective study in a general population. Am J Hypertens 2009;22:151–155. [DOI] [PubMed] [Google Scholar]
- 12. Nagaya T, Yoshida H, Takahashi H, et al. Resting heart rate and blood pressure, independent of each other, proportionally raise the risk for type‐2 diabetes mellitus. Int J Epidemiol 2010;39:215–222. [DOI] [PubMed] [Google Scholar]
- 13. Kolloch R, Legler UF, Champion A, et al. Impact of resting heart rate on outcomes in hypertensive patients with coronary artery disease: Findings from the INternational VErapamil‐SR/trandolapril STudy (INVEST). Eur Heart J 2008;29:1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khlopov NA, Sharafislamov FS, Rubakova LC. Anatomo‐Topographical Basis of Acupuncture. Moscow, Nauka, 1988. [Google Scholar]
- 15. YaS Pyesikov, SYa Rybalko. Atlas of Clinical Auriculotherapy. Moscow, Medicina, 1990. [Google Scholar]
- 16. Popov SV, Afanasiev SA, Kurlov IO, et al. Drug‐free correction of the tone of the autonomic nervous system in the management of cardiac arrhythmia in coronary artery disease. International Journal of Biomedicine 2013;3:74–77. [Google Scholar]
- 17. Zamotrinsky A, Afanasiev S, Karpov RS, et al. Effects of electrostimulation of the vagus afferent endings in patients with coronary artery disease. Coron Artery Dis 1997;8:551–557. [PubMed] [Google Scholar]
- 18. Afanasev SA, Pavlyukova EN, ShD Ahmedov, et al. Initiation of stress protein synthesis in the myocardium of coronary patients. Bull Exp Biol Med 2004;137:368–370. [DOI] [PubMed] [Google Scholar]
- 19. Mitoff PR, Gam D, Ivanov J, et al. Cardiac‐specific sympathetic activation in men and women with and without heart failure .Heart 2011;97:382–387. [DOI] [PubMed] [Google Scholar]
- 20. Wang W, Xie JX, Liu L, et al. Agreement comparison between home and clinic blood pressure measurements in 200 Chinese participants. Blood Press Monit 2011;16:277–281. [DOI] [PubMed] [Google Scholar]
- 21. VYu Mareev, Ageev FT, Arutyunov GP, et al. National recommendations of the Society of Heart Failure Specialists, Russian Cardiology Society, and the Russian Scientific Medical Society of Therapists for diagnosis and treatment of CHF (forth revision). Serdechnaya Nedostatochnost 2013;14:379–472. [Google Scholar]
- 22. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press 2014;23:3–16. [DOI] [PubMed] [Google Scholar]
- 23. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93(5):1043–1065. [PubMed] [Google Scholar]
- 24. Afanasev SA, Pisklova AV, Filipov GP. The experience of non‐pharmacological correction of autonomic disorders in children. Klinicheskaya Meditsina 2004;7:69–71. [PubMed] [Google Scholar]
- 25. Afanasev SA, Falaleeva LP, Rebrova TYu, et al. The influence of stress proteins on berievement mesenchymal stem cells from bone marrow after intramyocardial transplantation on the background of postinfarction remodeling of the heart. Cell Technologies in Biology and Medicine 2008;3:123–127. [DOI] [PubMed] [Google Scholar]
- 26. Drapkina OM. Synthesis of heat shock proteins in patients with postinfarction cardiosclerosis. Klinicheskaya Meditsina 2004. Z;9:25–28. [PubMed] [Google Scholar]
- 27. Ivashkin VT, Zadoroznaya OO. Clinical characteristics of stress proteins in peripheral blood lymphocytes in patients with myocardial infarction. Klinicheskaya Meditsina 2001;2:33–37. [PubMed] [Google Scholar]
- 28. Black A, Murray L, Cardwell C, et al. Secular trends in heart rate in young adults, 1949 to 2004: Analyses of cross sectional studies. Heart 2006;92:468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giannoglou GD, Chatzizisis YS, Zamboulis C, et al. Elevated heart rate and atherosclerosis: An overview of the pathogenetic mechanisms. Int J Cardiol 2008;126:302–312. [DOI] [PubMed] [Google Scholar]
- 30. Kjekshus J, Gullestad L. Heart rate as a therapeutic target in heart failure. Eur Heart J 1999;1(Suppl H):H64–H69. [Google Scholar]
- 31. Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 32. De Ferrari GM, Crijns HJ, Borggrefe M, et al. CardioFit Multicenter Trial Investigators. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur Heart J 2011;32:847–855. [DOI] [PubMed] [Google Scholar]
- 33. Klein HU, Ferrari GM. Vagus nerve stimulation: A new approach to reduce heart failure. Cardiol J 2010;17:638–644. [PubMed] [Google Scholar]
- 34. Sabbah HN. Electrical vagus nerve stimulation for the treatment of chronic heart failure. Cleve Clin J Med 2011;78(Suppl 1):24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Routledge FS, Campbell TS, McFetridge‐Durdle JA, et al. Improvements in heart rate variability with exercise therapy. Can J Cardiol 2010;26:303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palatini P, Benetos A, Julius S. Impact of increased heart rate on clinical outcomes in hypertension: Implications for antihypertensive drug therapy. Drugs 2006;66:133–144. [DOI] [PubMed] [Google Scholar]
- 37. Williams PT, Haskell WL, Vranizan KM, et al. Associations of resting heart rate with concentrations of lipoprotein subfractions in sedentary men. Circulation 1985;71:441–449. [DOI] [PubMed] [Google Scholar]
- 38. Silva de Paula R, Antelmi I, Vincenzi MA, et al. Influence of age, gender, and serum triglycerides on heart rate in a cohort of asymptomatic individuals without heart disease. Int J Cardiol 2005;105:152–158. [DOI] [PubMed] [Google Scholar]
- 39. Fox K, Ford I, Steg PG, et al. Ivabradine for patients with stable coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): A randomised, double‐blind, placebo‐controlled trial. Lancet 2008;372:807–816. [DOI] [PubMed] [Google Scholar]
- 40. Fox K, Ford I, Steg PG, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): A subgroup analysis of a randomised controlled trial. Lancet 2008;372:817–821. [DOI] [PubMed] [Google Scholar]
- 41. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: Full text: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114:257–354. [DOI] [PubMed] [Google Scholar]
- 42. Kregel KC. Invited Review: Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J Applied Physiol 2002;92:2177–2186. [DOI] [PubMed] [Google Scholar]
- 43. Cechetto JD, Soltys BJ, Gupta RS. Localization of mitochondrial 60‐kD heat shock chaperonin protein (Hsp60) in pituitary growth hormone secretory granules and pancreatic zymogen granules. J Histochem Cytochem 2000;48:45–56. [DOI] [PubMed] [Google Scholar]
- 44. Sammut IA, Harrison JC. Cardiac mitochondrial complex activity is enhanced by heat shock proteins. Clin Exp Pharmacol Physiol 2003;30:110–115. [DOI] [PubMed] [Google Scholar]
- 45. Melling CW, Thorp DB, Milne KJ, Krause MP, Noble EG. Exercise‐mediated regulation of Hsp70 expression following aerobic exercise training. Am J Physiol Heart Circ Physiol 2007;293:3692–3698. [DOI] [PubMed] [Google Scholar]
- 46. Kondrat'ev BY, Ugdyzhekova DS, Antonchenko IV, Aleev VV, Popov SV, Afanas'ev SA. Metabolic alterations in rat myocardium in experimental acute atrial fibrillation. Bull Exp Biol Med 2005;140:397–399. [DOI] [PubMed] [Google Scholar]
- 47. Ferrari R. The role of mitochondria in ischemic heart disease. J Cardiovasc Pharmacol 1996;28 Suppl 1:S1–S10. [DOI] [PubMed] [Google Scholar]
- 48. Zaobornyj T, Valdez LB. Heart mitochondrial nitric oxide synthase: A strategic enzyme in the regulation of cellular bioenergetics. Vitam Horm 2014;96:29–58. [DOI] [PubMed] [Google Scholar]


