Abstract
Background
Electrocardiographic (ECG) abnormalities in pulmonary embolism (PE) are increasingly reported, and mounting data have recommended that ECG plays a crucial role in the prognostic assessment of PE patient population. However, there is scarce data on the prognostic importance of fragmented QRS (fQRS) on short‐ and long‐term outcomes in patients with PE. Therefore, we aimed to investigate the prognostic role of fQRS in predicting in‐hospital and long‐term adverse outcomes in PE patients.
Methods
A total of 249 patients (155 female, 66.2%; mean age, 66.0 ± 16.0) with the diagnosis of acute PE were enrolled and followed‐up during median 24.8 months.
Results
Compared with the fQRS (−) patient group, patients with fQRS showed higher rates of in‐hospital adverse events including cardiogenic shock, the necessity of thrombolytic therapy, and in‐hospital mortality as well as long‐term all‐cause mortality. In Kaplan–Meier survival analysis, during follow‐up, all‐cause mortality occurred more frequently in the fQRS (+) group (log‐rank, P = 0.002). In multivariate Cox regression analysis, adjusted with other relevant parameters, the presence of fQRS were determined as an independent predictor of in‐hospital adverse events (HR: 2.743, 95% CI: 1.267–5.937, P = 0.003) and long‐term all‐cause mortality (HR: 3.137, 95% CI: 1.824–6.840, P = 0.001).
Conclusions
The presence of fQRS complex, as a simple and feasible ECG marker, seems to be a novel predictor of in‐hospital adverse events and long‐term all‐cause mortality in PE patient population. This parameter may utilize the identification of patients whom at higher risk for mortality and individualization of therapy.
Keywords: fragmented QRS, prognosis, acute pulmonary embolism, right ventricular dysfunction
Acute pulmonary embolism (PE) constitutes one of the leading causes of cardiovascular mortality. Despite improvements in diagnostic tools and therapeutic modalities, overall mortality is still about 12%, and patients with massive PE has a 52% mortality rate.1 Therefore, studies focus on the risk stratification to determine the patients who at higher risk and several prognostic markers have been reported such as clinical parameters, imaging of the right ventricle (RV) by echocardiogram or computed tomography (CT), and laboratory biomarkers as well as various assessment scores.2 Besides these variables, electrocardiographic (ECG) abnormalities in PE are increasingly reported, and mounting data have recommended that ECG plays a crucial role in the prognostic assessment of PE patient population.3, 4, 5
Fragmented QRS (fQRS) complexes on resting 12‐lead ECG, demonstrating the deterioration in myocardial electrical activation due to myocardial ischemia and scar,6, 7, 8 has been reported as a useful marker in prognostic evaluation of various cardiovascular pathologies including ischemic or nonischemic dilated cardiomyopathy, coronary artery disease, arrhythmogenic right ventricular cardiomyopathy, Ebstein anomaly, and Brugada syndrome.9, 10, 11, 12, 13, 14 However, there is scarce data on the prognostic importance of fQRS in short‐ and long‐term outcomes in patients with PE.
Herein, we aimed to investigate the prognostic role of fQRS in predicting in‐hospital and long‐term adverse outcomes in PE patients.
METHODS
Patient Population
A total of 249 consecutive patients diagnosed with PE by multidetector CT pulmonary angiography at our tertiary, heart‐specialized hospital between January 2009 and January 2015 were enrolled prospectively. Informed consent was obtained from all patients before enrollment. This study, consistent with the principles of the Declaration of Helsinki, was approved by the Institutional Ethics Committee.
Patients with a history or present illness of coronary heart disease, congestive heart failure, cardiomyopathy, myocarditis, severe valvular heart disease, congenital heart disease, previous PE, lung, or lobe resection were excluded.
Simplified PESI (sPESI) score was calculated in line with the recommendations of current European Society of Cardiology (ESC) guideline as burdening of the six items (age >80 years, history of cancer, history of cardiorespiratory diseases, heart rate >110 beats per minute, systolic blood pressure <100 mmHg, arterial oxygen saturation <90%), each item regarded as one point when present.2 Besides, the risk categorization as the low, intermediate, and high risk was made based on the hemodynamic status, cardiac biomarker levels, echocardiographic, and CT parameters as well as calculated sPESI score of patients according to the ESC guideline.2
Electrocardiogram Analysis and Definition of fQRS
fQRS was defined by the presence of various RSRʹ patterns with or without a Q wave and included an additional R wave (Rʹ), notching of the R wave, notching of the downstroke or upstroke of the S wave, or the presence of 1 Rʹ in more than two contiguous representing anterior (V1–V5), inferior (II, III, aVF), or lateral (I, aVL, V6) myocardial segments.8, 11 The extent of the fQRS was assessed by counting the number of ECG leads with fQRS.
The 12‐lead ECGs of patients were evaluated by two independent cardiologists blinded to the patients’ clinical outcomes. The intraobserver and interobserver reliability for detecting the presence of fQRS were found to be Kappa = 0.972 (P < 0.001) and 0.941 (P < 0.001), respectively. All patients have had repeated ECGs during follow‐up. We determined the Kappa value for intraobserver reproducibility as 0.912 (P < 0.001).
Echocardiographic Parameters
All patients were performed two‐dimensional and Doppler transthoracic echocardiography examinations within 24 hours from admission to hospital by experienced echocardiographers using 2.5–4 MHz transducers (Vivid 7, GE Medical System, Milwaukee, WI, USA). Echocardiographic parameters of RV size and function including tricuspid annular plane systolic excursion (TAPSE) were assessed with regarding current guidelines.15, 16 McConnell's sign was defined as hypokinesia of the mid‐RV free wall with preserved motion of the RV apex.17 Left ventricular (LV) ejection fraction was measured using the biplane modified Simpson's method.18
CT Pulmonary Angiography Protocol
All patients were performed 64‐section pulmonary multislice CT angiography (SOMATOM Sensation 64; Siemens, Erlangen, Germany) by using a standard CT pulmonary angiography protocol for PE with the following imaging criteria: detector width, 64 × 0.625; section thickness, 1.25 mm; rotation time, 0.5 second; 120 kVp; and 380 mAs. Images were obtained after intravenous administration of 125 mL of iopromide (Ultravist 300, Bayer Healthcare, Berlin, Germany) at a rate of 4–5 mL/s. The CT scanning was made with either a 20‐second delay or a bolus‐tracking technique after the start of the contrast medium injection, during a single inspiratory breath‐hold from the lowest hemidiaphragm to the top of the lungs.
Image Analysis and Computed Tomographic Findings
All CT pulmonary angiographic images were analyzed by a dedicated workstation and were evaluated by at least two experienced image interpreters. Readers were blinded to the patient's characteristics, hemodynamic status, and the clinical outcome. The diagnosis of acute PE was confirmed in all patients by identified partial and/or complete endoluminal filling defect in the pulmonary artery system in two consecutive CT sections. Axial images were used for interpretation and also reconstructed coronal and sagittal images were evaluated in case of axial images were inadequate for discriminating clots from adjacent soft tissue.
RV end‐diastolic diameter (RVEDD), leftward shifting of interventricular septum (IVS), and RV/LV dimension ratios were assessed from the transverse images of the diagnostic CT pulmonary angiograms of the patients with PE with regarding previously described methods.19, 20
Right Ventricular Dysfunction
RV dysfunction was regarded as present when at least one of the following signs was observed on echocardiography: RV hypokinesis, systolic paradoxical movement of the IVS, and/or RV dilation (RVEDD >30 mm or RV/LV diameter ratio >1)21 and/or on CT images: convex leftward IVS, RV enlargement (RVEDD >30 mm), and/or RV /LV dimensions ratio ≥1.22
Clinical Outcomes
Follow‐up duration was started with the admission to hospital and ended with the death or the last visit. Primary end point was designed as the occurrence of in‐hospital adverse events (cardiogenic shock, thrombolytic therapy, in‐hospital all‐cause mortality), and the secondary end point was determined as long‐term all‐cause mortality. Cardiogenic shock was diagnosed as systolic pressure <90 mmHg or systolic pressure drop ≥40 mmHg for >15 minutes without new onset arrhythmia, hypovolemia, or sepsis. In‐hospital mortality was defined as death from any cause during hospitalization. Long‐term all‐cause mortality was determined as the occurrence of death with any cause during the follow‐up period. The therapeutical decisions like necessity to thrombolytic therapy, administration of unfractionated heparin or low molecular weight heparin, the overlap to oral anticoagulants, and the duration of anticoagulant therapy as well as determination of target INR were made at the discretion of the individual cardiologist with regarding to the current guidelines.2, 23
Besides periodical INR and clinical monitoring in the hospital, all patients were scheduled for an elective 1‐, 6‐, and 12‐month clinical follow‐up. We also validated the follow‐up data with the hospital records, pharmacy databases, Turkey Ministry of Health, and Turkish National Population Register.
Statistical Analysis
Continuous variables were represented as mean ± SD, and categorical variables were reported as percentages. Comparisons of continuous variables between the two groups were analyzed with the independent samples t‐test, and chi‐square test was used for categorical variables. Intraobserver and interobserver reliability analysis using the Kappa statistic was applied to assess consistency in the determination of fQRS. Univariate and multivariable Cox proportional hazard regression were performed to evaluate the association of the variables with the occurrence of in‐hospital adverse events and long‐term all‐cause mortality, respectively. Kaplan–Meier curve analysis was used for event‐free survival in patients with or without QRS fragmentation. Statistical analyses were performed using the SPSS statistical software (Version 20.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Patients Characteristics
A total of 249 patients (155 female, 66.2%; mean age, 66.0 ± 16.0) with the diagnosis of acute PE were enrolled and followed up during median 24.8 months (range, 1–51.6 months). Baseline characteristics, ECG, echocardiographic parameters, and CT findings of the patient groups were demonstrated in Table 1.
Table 1.
Baseline Characteristics, Electrocardiographic, Echocardiographic Parameters, and CT Findings of Study Population
| All Patients | fQRS(+) | fQRS(−) | ||
|---|---|---|---|---|
| Variables | (n = 249) | (n = 39) | (n = 210) | P Value |
| Age | 66.0 ± 16.0 | 65.6 ± 15.9 | 66.1 ± 16.1 | 0.875 |
| Gender, female, n (%) | 155 (62.2%) | 26(66.7%) | 129 (61.4%) | 0.333 |
| Hypertension, n (%) | 103 (41.4%) | 13 (33.3%) | 90 (42.9%) | 0.176 |
| Hyperlipidemia, n (%) | 64 (25.7%) | 7 (17.9%) | 57 (27.1%) | 0.157 |
| Smoking, n (%) | 91 (36.5%) | 16 (41.0%) | 75 (35.7%) | 0.323 |
| Diabetes mellitus, n (%) | 86 (34.5%) | 10 (25.6%) | 76 (36.2%) | 0.137 |
| Chronic obstructive pulmonary disease | 31 (12.4%) | 6 (15.4%) | 25 (11.9%) | 0.352 |
| BMI, kg/m2 | 23.8 ± 3.1 | 23.9 ± 3.2 | 23.9 ± 3.1 | 0.256 |
| Immobilization >3 days, n (%) | 52 (20.9%) | 9 (23.1%) | 43 (20.5%) | 0.428 |
| Neoplasm, n (%) | 22 (8.8%) | 4 (10.3%) | 18 (8.6%) | 0.463 |
| Oral contraception, pregnancy, n (%) | 14 (5.6%) | 2 (5.1%) | 12 (5.7%) | 0.620 |
| Deep venous thrombosis | 84 (33.7%) | 16 (41.0%) | 68 (32.4%) | 0.193 |
| Clinical presentation | ||||
| Dispnea, n (%) | 234 (94.0%) | 36 (92.3%) | 198 (94.3%) | 0.634 |
| Angina, n (%) | 78 (31.3%) | 13(33.3%) | 65 (31.0%) | 0.768 |
| Palpitation, n (%) | 85 (34.1%) | 71 (33.8%) | 14 (35.9%) | 0.801 |
| Hemoptysis, n (%) | 8 (3.2%) | 1 (2.6%) | 7 (3.3%) | 0.636 |
| Syncope, n (%) | 22 (8.8%) | 7 (17.9%) | 15 (7.1%) | 0.038 |
| Elevated cTnI | 115 (46.2%) | 26 (66.7%) | 89 (42.4%) | 0.005 |
| Functional status | ||||
| NYHA class III–IV, n (%) | 35 (14.1%) | 10 (25.6%) | 25 (11.9%) | 0.027 |
| sPESI score ≥1, n (%) | 51 (20.5%) | 14(35.9%) | 37(17.6%) | 0.009 |
| Risk categorization | ||||
| Low risk, n (%) | 198 (79.5%) | 25(64.1%) | 173(82.4%) | 0.009 |
| Intermediate risk, n (%) | 38 (15.3%) | 11(28.2%) | 27(12.9%) | 0.014 |
| High risk, n (%) | 16 (6.4%) | 6 (15.4%) | 10(4.8%) | 0.013 |
| Acute pharmacological treatment | ||||
| Thrombolytic therapy, n (%) | 19 (7.6%) | 7 (17.9%) | 12 (5.7%) | 0.008 |
| Unfractioned heparin, n (%) | 42(16.9%) | 8(20.5%) | 34(16.2%) | 0.508 |
| Low molecular weight heparin, n (%) | 197(79.1%) | 28(71.8%) | 169(80.5%) | 0.221 |
| Electrocardiographic parameters | ||||
| Atrial fibrillation | 41 (16.7%) | 6 (15.4%) | 35 (16.9%) | 0.541 |
| Duration of PR interval | 156.3 ± 48.9 | 158.5 ± 49.3 | 153.8 ± 49.0 | 0.676 |
| Duration of QRS interval | 108.9 ± 37.3 | 112 ± 39.5 | 102.0 ± 33.8 | 0.105 |
| Duration of QTc interval | 421.0 ± 46.2 | 426.2 ± 48.7 | 415 ± 42.8 | 0.265 |
| RBBB | 46 (18.7%) | 12 (30.8%) | 34 (16.4%) | 0.034 |
| S1Q3T3 sign | 50 (20.3%) | 14 (35.9%) | 36 (17.4%) | 0.008 |
| Right axis deviation | 18 (7.2%) | 6 (15.4%) | 12 (5.7%) | 0.032 |
| Echocardiographic parameters | ||||
| LVEF,% | 55.6 ± 9.8 | 54.7 ± 9.2 | 56.3 ± 9.4 | 0.106 |
| sPAB (mmHg) | 43±21 | 44±22 | 42 ± 21 | 0.114 |
| TAPSE | 1.7 ± 0.6 | 1.6 ± 0.5 | 1.7 ± 0.6 | 0.026 |
| McConnell's sign | 23 (9.2%) | 7 (17.9%) | 16 (7.6%) | 0.041 |
| CT findings | ||||
| RVEDD (mm) | 46 ± 11 | 47 ± 12 | 45 ± 11 | 0.046 |
| RV/LV ratio | 0.93 ± 0.36 | 0.98 ± 0.38 | 0.91 ± 0.36 | 0.019 |
| Leftward shifting of the IVS | 37 (14.9%) | 11 (28.2%) | 26 (12.4%) | 0.011 |
| RV dysfunction | 43 (17.3%) | 12(30.8%) | 31 ((14.8%) | 0.015 |
Bold text indicates a statistically significant difference with a P value less than 0.05.
BMI = body mass index; CT = computed tomography; cTnI = cardiac troponin I; fQRS = fragmented QRS; IVS = interventricular septum; LV = left ventricle; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; RBBB = right bundle branch blocker; RV = right ventricle; RVEDD = right ventricular end‐diastolic diameter; sPAB = systolic pulmonary artery pressure; TAPSE = tricuspid annular plane systolic excursion.
Comparison between fQRS (+) and fQRS (−) Patient Groups
Based on the previously mentioned criteria, 39 patients (15.6%) demonstrated fQRS in ≥2 contiguous lead. fQRS were determined primarily in the V1–V2 (30 patients) and inferior leads (28 patients). QRS fragmentation was demonstrated predominantly in the R wave (29 patients).
Patients with fQRS had more frequent syncope, elevated cardiac Troponin I (cTnI), right bundle branch block (RBBB), S1Q3T3 sign, and right axis deviation in ECG. Patients with fQRS were more commonly in intermediate and high‐risk category, NYHA class III–IV functional status, sPESI ≥ 1, whereas fQRS (−) patients were at low‐risk category. In the assessment of CT findings, RVEDD and RV/LV ratio were higher and leftward shifting of the IVS was more prevalent in patients with fQRS (+) (Table 1). The RV/LV ratio was significantly correlated with the extent of fQRS (Spearman r = 0. 477, P < 0.001).
Patients with fQRS had poorer TAPSE, more frequent McConnell's sign, and RV dysfunction in echocardiography.
Clinical Outcomes
A total of 35 patients were experienced in‐hospital adverse events (cardiogenic shock occurred in 20 patients, 19 patients were administered thrombolytic therapy, and 17 patients died). During the follow‐up period, 46 patients died. Clinical outcomes were represented in Table 2.
Table 2.
Clinical Outcomes
| All Patients | fQRS(+) | fQRS(−) | OR | ||
|---|---|---|---|---|---|
| (n = 249) | (n = 39) | (n = 210) | (95% CI) | P Value | |
| In‐hospital adverse events, n (%) | 35 (14.1%) | 10 (25.6%) | 25 (11.9%) | 2.552 (1.111–5.859) | 0.023 |
| Cardiogenic shock, n (%) | 20 (8.0%) | 7(17.9%) | 13(16.2%) | 3.315 (1.229–8.938) | 0.018 |
| Thrombolytic therapy, n (%) | 19 (7.6%) | 7 (17.9%) | 12 (5.7%) | 3.609 (1.322–9.852) | 0.012 |
| In‐hospital mortality n (%) | 17 (6.8%) | 6 (15.4%) | 11 (5.2%) | 3.289 (1.139–9.501) | 0.028 |
| Long‐term all‐cause mortality, n (%) | 46 (18.5%) | 13 (33.3%) | 33 (15.7%) | 2.682 (1.251–5.749) | 0.011 |
Bold text indicates a statistically significant difference with a P value less than 0.05. CI = confidence interval; fQRS = fragmented QRS; OR = odds ratio.
The median follow‐up duration was not different between groups (22.1 ± 15.2 for fQRS (+) vs 26.3± 17.5 fQRS (−), P = 0.268). Compared with the fQRS (−) patient group, patients with fQRS showed higher rates of in‐hospital adverse events including cardiogenic shock, the necessity of thrombolytic therapy, and in‐hospital mortality as well as long‐term all‐cause mortality. Cutoff point of ≥3 leads for the fQRS was the optimal point discriminating both in‐hospital adverse event (sensitivity 51.6%, specificity 76.4%, area under the curve (AUC) = 0.648, P = 0.003) and long‐term all‐cause mortality (sensitivity 57.1%, specificity 79.3%, AUC = 0.691, P = 0.008). In Kaplan–Meier survival analysis, during follow‐up, all‐cause mortality occurred more frequently in the fQRS (+) group (log‐rank, P = 0.002, Fig. 1).
Figure 1.
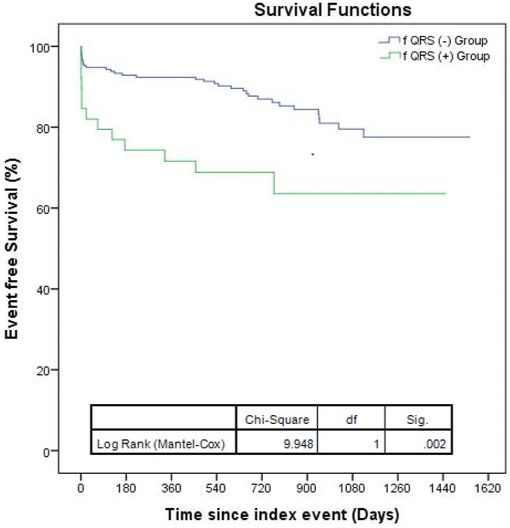
Long‐term all‐cause mortality‐free survival curves depending on the presence of fragmented QRS complexes (fQRS).
In multivariate Cox proportional hazard regression analysis, adjusted with other relevant parameters, the presence of fQRS were determined as an independent predictor of in‐hospital adverse events (HR: 2.743, 95% CI: 1.267–5.937, P = 0.003) and long‐term all‐cause mortality (HR: 3.137, 95% CI: 1.824–6.840, P = 0.001). Besides fQRS, age, elevated cTnI, NYHA class III–IV, RBBB, S1Q3T3 sign, RV/LV ratio, TAPSE, leftward shifting of IVS, and RV dysfunction were found to be independent predictors of in‐hospital adverse events (Table 3). Age, elevated cTnI, NYHA class III–IV, S1Q3T3 sign, RV/LV ratio, TAPSE, leftward shifting of IVS, and RV dysfunction were independent predictors of long‐term all‐cause mortality (Table 4).
Table 3.
Effects of Variables on In‐Hospital Adverse Events in Univariate and Multivariate Cox Regression Analysis
| Univariate Regression Analysis | Multivariate Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95 % CI | P Value | Adjusted HR | 95 % CI | P Value |
| Age | 1.648 | 1.107–1.951 | 0.007 | 1.236 | 1.174–1.302 | <0.001 |
| Elevated cTnI | 3.627 | 1.364–9.892 | 0.001 | 3.078 | 1.846–6.317 | 0.007 |
| NYHA class III–IV | 2.926 | 1.648–5.272 | 0.001 | 2.177 | 1.705–4.469 | 0.006 |
| RBBB | 1.541 | 1.128–3.637 | 0.021 | 1.306 | 1.128–2.306 | 0.036 |
| S1Q3T3 sign | 3.490 | 1.271–8.568 | 0.001 | 2.616 | 1.731–3.982 | 0.009 |
| RV/LV ratio | 3.801 | 1.455–5.071 | 0.005 | 3.370 | 1.966–7.314 | 0.018 |
| TAPSE | 3.264 | 1.942–7.044 | 0.001 | 2.726 | 1.705–9.097 | 0.001 |
| Leftward shifting of IVS | 2.918 | 1.541–6.628 | 0.006 | 2.542 | 1.680–5.594 | 0.001 |
| RV dysfuction | 3.164 | 1.148–3.237 | 0.001 | 2.973 | 1.306–5.380 | 0.001 |
| fQRS | 3.690 | 1.152–6.427 | 0.001 | 2.743 | 1.267–5.937 | 0.003 |
Bold text indicates a statistically significant difference with a P value less than 0.05.
CI = confidence interval; cTnI = cardiac troponin I; fQRS = fragmented QRS; HR = hazard ratio; IVS = interventricular septum; LV = left ventricle; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; RBBB = right bundle branch blocker; RV = right ventricle; TAPSE = tricuspid annular plane systolic excursion.
Table 4.
Effects of Variables on Long‐Term All‐Cause Mortality in Univariate and Multivariate Cox Regression Analysis
| Univariate Regression Analysis | Multivariate Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95 % CI | P Value | Adjusted HR | 95 % CI | P Value |
| Age | 1.323 | 1.041–2.007 | 0.016 | 1.127 | 1.012–1.452 | 0.032 |
| Elevated cTnI | 2.541 | 1.981–7.162 | 0.001 | 2.322 | 1.764–9.654 | 0.009 |
| NYHA class III‐IV | 1.875 | 1.344–6.472 | 0.001 | 1.653 | 1.250–2.038 | 0.011 |
| S1Q3T3 sign | 2.184 | 1.629–3.276 | 0.009 | 1.939 | 1.544–4.652 | 0.026 |
| RV/LV ratio | 3.441 | 1.874–8.655 | 0.001 | 3.111 | 1.721–9.018 | 0.001 |
| TAPSE | 3.023 | 2.745–8.180 | 0.001 | 2.875 | 2.125–9.589 | 0.001 |
| Leftward shifting of IVS | 3.368 | 1.967–5.618 | 0.003 | 2.946 | 2.249–6.832 | 0.002 |
| RV dysfuction | 3.956 | 2.645–7.884 | 0.001 | 3.607 | 2.726–7.259 | 0.001 |
| fQRS | 3.412 | 1.949–6.535 | 0.001 | 3.137 | 1.824–6.840 | 0.001 |
Bold text indicates a statistically significant difference with a P value less than 0.05.
CI = confidence interval; cTnI = cardiac troponin I; fQRS = fragmented QRS; HR = hazard ratio; IVS = interventricular septum; LV = left ventricle; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; RBBB = right bundle branch blocker; RV = right ventricle; TAPSE = tricuspid annular plane systolic excursion.
DISCUSSION
To the best of our knowledge, this is the first study demonstrating the presence of fQRS as a prognostic indicator in predicting in‐hospital complications and long‐term all‐cause mortality. There are additional significant findings to mention. (1) In patients with fQRS, RV dysfunction and elevated cTnI were more prevalent. (2) The extent of fQRS has been significantly correlated with RV/LV ratio and (3) fQRS ≥3 leads was determined as the cut‐off point discriminating patients with complicating with in‐hospital adverse events and all‐cause long‐term mortality. (4) Additional to presence of fQRS, age, elevated cTnI, NYHA class III–IV, S1Q3T3 sign, RV/LV ratio, TAPSE, leftward shifting of IVS, and RV dysfunction were found to be independent predictors of in‐hospital adverse events and long‐term all‐cause mortality.
ECG is one of the first procedures to be performed in the emergency department when a patient admitted with symptoms associated with PE. Several studies have provided mounting data suggesting the utility of common ECG abnormalities in the prognostic evaluation of patients with PE.3, 4, 5 Recent studies reported that various ECG abnormalities have been shown to be predictors of hemodynamic compromise, RV enlargement and dysfunction, elevated pulmonary artery pressure, in‐hospital adverse events, and cardiogenic shock, as well as mortality.23–28
In recent years, the presence of fQRS on a routine 12‐lead ECG has been determined as an indicator of depolarization abnormality. Fragmentation in QRS morphology emerges as a consequence of inhomogeneity in ventricular activation and myocardial conduction delay due to the myocardial ischemia, scar tissue, and fibrosis.6, 7 fQRS has been gaining attention as a prognostic indicator in several cardiovascular diseases including coronary artery disease (CAD), Brugada syndrome, ischemic and nonischemic cardiomyopathy, long‐QT syndrome, arrhythmogenic right ventricular dysplasia, and Ebstein Anomaly.9, 10, 11, 12, 13, 14 However, data are still scarce about it's in‐hospital and long‐term prognostic value in PE. Our study is the preliminary study demonstrating the prognostic importance of fQRS in predicting in‐hospital and long‐term mortality in PE. Kukla et al. reported that fQRS was more prevalent in PE patients complicated with cardiogenic shock compared with patients with no cardiogenic shock and attributed this ECG parameter as an independent predictor of cardiogenic shock in PE.27 Zhan et al. demonstrated that fQRS in V1 was present in 20% of patients with PE, whereas the prevalence was increased to approximately 95% in patients with hemodynamic decompensation.28 Consistent with these studies speculating the association of fQRS with the severity of PE, in our study, RV dysfunction, in‐hospital adverse events, the necessity for thrombolytic therapy, and cardiogenic shock were more prevalent in patients with fQRS.
On the other hand, Kukla et al. found that this parameter was not associated with in‐hospital mortality.27 Differences between our studies might explain these conflicting results. The study of Kukla et al. based on a retrospective chart review analysis and only the presence of fQRS in V1 was evaluated as a predictor of mortality.
We postulated several mechanism underlying the presence of fQRS on the surface ECG in patients with PE. As a possible explanation, the abruptly elevated right ventricular pressure overload, and right ventricular enlargement may lead to impairment in ventricular activation and delay in myocardial conduction due the subendocardial ischemia and fibrosis. Other potential mechanisms may be impaired overall perfusion of cardiac myocardium caused by the right ventricular infarction and/or the of RV dysfunction leading to decrease in the preload of the left ventricle and cellular ischemia induced by PE‐associated mediators such as catecholamines or histamine.
Increased myocardial ischemia‐/fibrosis‐related RV dysfunction may be one of the possible explanations for increased mortality in patients with fQRS. Supporting this hypothesis, TAPSE was significantly lower, and RVEDD and RV/LV ratio were significantly higher in the fQRS (+) group. Besides, the number of the leads with fQRS had significantly correlated with RV/LV ratio. The more lead involvement with fQRS in PE patients may reflect the more deteriorated RV function.
Clinical Implications
Considering the association of fQRS with worse clinical outcomes, evaluation of fQRS complex in admission ECG may utilize the identification of the patients whom at higher risk for adverse events and mortality. The role of more intensive and longer duration therapy in patients with fQRS should be studied in prospective management studies. These patients may be followed up more closely at shorter intervals. Although requiring further evaluation in prospective randomized trials, the presence of fQRS should be considered in the decision process of thrombolytic therapy.
LIMITATIONS
Our study has several limitations to mention. Prospectively designed studies on a larger scale are necessary to confirm our findings and to elucidate the prognostic importance of fQRS complexes more accurately. We did not evaluate the follow‐up ECG whether fragmentation in QRS morphology was permanent or resolved after the acute stage of PE. The assessment of the cardiac ischemia with myocardial scintigraphy and fibrosis via cardiac magnetic resonance imaging and their associations with presence and extent of fQRS complex may strengthen the results of our study.
CONCLUSIONS
In conclusion, the presence of fQRS complex, as a simple and feasible ECG marker, seems to be a novel predictor of in‐hospital adverse events and long‐term all‐cause mortality in PE patient population. This parameter may utilize the identification of patients whom at higher risk for mortality and individualization of therapy.
Ann Noninvasive Electrocardiol 2016;21(5):470–478
Funding: This research received no grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Conflicts of Interest: The authors declare that there is no conflict of interest.
REFERENCES
- 1. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: Evidence of overdiagnosis. Arch Intern Med 2011;171:831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) * Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014;35:3033–3069, 3069a–3069k. [DOI] [PubMed] [Google Scholar]
- 3. Sreeram N, Cheriex EC, Smeets JL, et al. Value of the 12‐lead electrocardiogram at hospital admission in the diagnosis of pulmonary embolism. Am J Cardiol 1994;73:298–303. [DOI] [PubMed] [Google Scholar]
- 4. Geibel A. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005;25:843–848. [DOI] [PubMed] [Google Scholar]
- 5. Digby GC, Kukla P, Zhan Z‐Q, et al. The value of electrocardiographic abnormalities in the prognosis of pulmonary embolism: A consensus paper. Ann Noninvasive Electrocardiol 2015;20:207–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gardner PI, Ursell PC, Fenoglio JJ, et al. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation 1985;72:596–611. [DOI] [PubMed] [Google Scholar]
- 7. Varriale P, Chryssos BE. The RSR’ complex not related to right bundle branch block: Diagnostic value as a sign of myocardial infarction scar. Am Heart J 1992;123:369–376. [DOI] [PubMed] [Google Scholar]
- 8. Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12‐lead ECG: A sign of myocardial scar and poor prognosis. Circ Arrhythm Electrophysiol 2008;1:258–268. [DOI] [PubMed] [Google Scholar]
- 9. Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12‐lead ECG: A predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm 2007;4:1385–1392. [DOI] [PubMed] [Google Scholar]
- 10. Das MK, Zipes DP. Fragmented QRS: A predictor of mortality and sudden cardiac death. Heart Rhythm 2009;6:S8–S14. [DOI] [PubMed] [Google Scholar]
- 11. Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve‐lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm 2010;7:74–80. [DOI] [PubMed] [Google Scholar]
- 12. Park S‐J, Chung S, On YK, et al. Fragmented QRS complex in adult patients with Ebstein anomaly and its association with arrhythmic risk and the severity of the anomaly. Circ Arrhythm Electrophysiol 2013;6:1148–1155. [DOI] [PubMed] [Google Scholar]
- 13. Canpolat U, Kabakçi G, Aytemir K, et al. Fragmented QRS complex predicts the arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol 2013;24:1260–1266. [DOI] [PubMed] [Google Scholar]
- 14. Morita H, Kusano KF, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 2008;118:1697–1704. [DOI] [PubMed] [Google Scholar]
- 15. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23:685–713; quiz 786–788. [DOI] [PubMed] [Google Scholar]
- 16. Grant ADM, Smedira NG, Starling RC, et al. Independent and incremental role of quantitative right ventricular evaluation for the prediction of right ventricular failure after left ventricular assist device implantation. J Am Coll Cardiol 2012;60:521–528. [DOI] [PubMed] [Google Scholar]
- 17. López‐Candales A, Edelman K, Candales MD. Right ventricular apical contractility in acute pulmonary embolism: The McConnell sign revisited. Echocardiography 2010;27:614–620. [DOI] [PubMed] [Google Scholar]
- 18. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two‐dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two‐Dimensional Echocardiograms. J Am Soc Echocardiogr 2:358–367. [DOI] [PubMed] [Google Scholar]
- 19. Stein PD, Beemath A, Matta F, et al. Enlarged right ventricle without shock in acute pulmonary embolism: Prognosis. Am J Med 2008;121:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stein PD, Matta F, Yaekoub AY, et al. Reconstructed 4‐chamber views compared with axial imaging for assessment of right ventricular enlargement on CT pulmonary angiograms. J Thromb Thrombolysis 2009;28:342–347. [DOI] [PubMed] [Google Scholar]
- 21. Golpe R, Pérez‐de‐Llano LA, Castro‐Añón O, et al. Right ventricle dysfunction and pulmonary hypertension in hemodynamically stable pulmonary embolism. Respir Med 2010;104:1370–1376. [DOI] [PubMed] [Google Scholar]
- 22. Henzler T, Barraza JM, Nance JW, et al. CT imaging of acute pulmonary embolism. J Cardiovasc Comput Tomogr 5:3–11. [DOI] [PubMed] [Google Scholar]
- 23. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Hear J 2008;29:2276–2315. [DOI] [PubMed] [Google Scholar]
- 24. Kukla P, Długopolski R, Krupa E, et al. Electrocardiography and prognosis of patients with acute pulmonary embolism. Cardiol J 2011;18:648–653. [DOI] [PubMed] [Google Scholar]
- 25. Escobar C, Jiménez D, Martí D, et al. Prognostic value of electrocardiographic findings in hemodynamically stable patients with acute symptomatic pulmonary embolism. Rev española Cardiol 2008;61:244–250. [PubMed] [Google Scholar]
- 26. Kosuge M, Kimura K, Ishikawa T, et al. Prognostic significance of inverted T waves in patients with acute pulmonary embolism. Circ J 2006;70:750–755. [DOI] [PubMed] [Google Scholar]
- 27. Kukla P, McIntyre WF, Fijorek K, et al. Electrocardiographic abnormalities in patients with acute pulmonary embolism complicated by cardiogenic shock. Am J Emerg Med 2014;32:507–510. [DOI] [PubMed] [Google Scholar]
- 28. Zhan Z, Wang C, Nikus KC, et al. Electrocardiogram patterns during hemodynamic instability in patients with acute pulmonary embolism. Ann Noninvasive Electrocardiol 2014;19:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]


