Abstract
Background
The risk of syncope and sudden cardiac death due to ventricular arrhythmias increased in patients with aortic stenosis (AS). Recently, it was shown that Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratio can be novel indicators for prediction of ventricular arrhythmias and mortality. We aimed to investigate the association between AS and ventricular repolarization using Tp‐e interval and Tp‐e/QT ratio.
Methods
Totally, 105 patients with AS and 60 control subjects were enrolled to this study. The severity of AS was defined by transthoracic echocardiographic examination. Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios were measured from the 12‐lead electrocardiogram.
Results
Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios were significantly increased in parallel to the severity of AS (P < 0.001, P = 0.001, and P = 0.001, respectively). Also, it was shown that Tp‐e/QTc ratio had significant positive correlation with mean aortic gradient (r = 0.192, P = 0.049). In multivariate logistic regression analysis, Tp‐e/QTc ratio and left ventricular mass were found to be independent predictors of severe AS (P = 0.03 and P = 0.04, respectively).
Conclusions
Our study showed that Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios were increased in patients with severe AS. Tp‐e/QTc ratio and left ventricular mass were found as independent predictors of severe AS.
Keywords: aortic stenosis, Tp‐e interval, Tp‐e/QT ratio
Aortic stenosis (AS) is one of the most frequent valvular disease in elder population and its incidence is increasing with age.1 Increased afterload leads to compensatory left ventricular hypertrophy (LVH) and results in deterioration of LV systolic functions and finally leads to heart failure.2 Eventually, increased LVH, elevated systolic wall stress, and impaired myocardial perfusion cause myocyte degeneration and myocardial fibrosis.3, 4, 5 Fibrosis and scar formation may increase the risk of syncope and sudden cardiac death due to ventricular arrhythmias in AS.6
Ventricular repolarization abnormalities can be reflected by QT interval and T wave on external electrocardiogram (ECG). In recent studies, Tp‐e interval, the interval between the peak and the end of the T wave, was defined as an index of total dispersion of repolarization.7, 8 Prolonged Tp‐e interval can predict ventricular arrhythmias and mortality.9, 10, 11 Nonetheless, Tp‐e interval is influenced by heart rate and body weight.12 Therefore, Tp‐e/QT ratio has been proposed to be a better marker of ventricular repolarization.12, 13
To our knowledge, no trial has evaluated the Tp‐e interval and Tp‐e/QT ratio as markers of ventricular arrhythmogenesis in patients with AS. The aim of this study was to investigate the association between AS and ventricular repolarization using Tp‐e interval and Tp‐e/QT ratio.
METHODS
Study Population
The study was conducted at two centers. Between March 2014 and March 2015, 105 patients with AS and 60 control subjects were enrolled to the study. Patients with any of the followings were excluded: significant mitral or tricuspid valvular heart disease, previous myocardial infarction, significant coronary artery disease, wall motion abnormalities with left ventricular (LV) ejection fraction below 50%, severe pulmonary disease, malignancy and complete or incomplete bundle branch block, atrial fibrillation, and paced rhythm. Baseline demographic and clinical characteristics were reviewed. The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by local ethics committee.
Echocardiography
Echocardiographic assessment was performed by using a VIVID 7 Dimension Cardiovascular Ultrasound System (Vingmed‐General Electric, Horten, Norway) with a 3.5 MHz transducer. Echocardiographic examination was performed in the left lateral decubitus position. Parasternal long‐ and short‐axis views and apical views were used as standard imaging windows. Ejection fraction was calculated by using modified Simpson method. Septal and posterior walls’ thickness, end systolic, and end diastolic dimensions were measured from parasternal long‐axis view. Aortic jet velocity was calculated by Doppler echocardiography. AS was defined as mild if mean systolic transaortic gradient was less than 25 mm Hg or jet velocity was less than 3.0 m/s, moderate if mean systolic transaortic gradient was between 25 and 40 mm Hg or jet velocity was between 3.0 and 4.0 m/s and severe if mean systolic transaortic gradient was greater than 40 mm Hg or jet velocity was greater than 4.0 m/s.14 All echocardiographic examinations were performed by an experienced cardiologist.
Electrocardiography
The 12‐lead ECG was recorded at a paper speed of 50 mm/s (Hewlett Packard, Page‐writer, Atlanta, GA, USA) in the supine position. To decrease the error measurements, all of the ECGs were scanned and transferred to a personal computer and then used for ×400% magnification by Adobe Photoshop software. ECG measurements of QT and Tp‐e intervals were performed by two cardiologists who were blinded to the patient data. Subjects with U waves on their ECGs were excluded from the study. An average value of three readings was calculated for each lead. The QT interval was measured from the beginning of the QRS complex to the end of the T wave and corrected for heart rate using the Bazett formula: cQT = QT√ (RR interval). The Tp‐e interval was defined as the interval from the peak of T wave to the end of T wave. Measurements of the Tp‐e interval were performed from precordial leads.9 The Tp‐e/QT ratio was calculated from these measurements. Interobserver and intraobserver coefficients of variation were 2.8% and 2.3%, respectively.
Statistical Analysis
In all statistical analysis, SPSS 20.0 Statistical Package Program for Windows (SPSS Inc., Chicago, IL, USA) was used. In order to test normality of distribution Kolmogorov–Smirnov test was used. Quantitative variables with a normal distribution were specified as the mean ± standard deviation and variables with nonnormal distribution were shown as median (interquartile range), categorical variables were shown as number and percentage values. Categorical variables were compared with chi‐square test. Kruskal–Wallis test or analysis of variance test was used to compare continuous variables according to severity of AS, as appropriate. Pearson correlation analysis was performed to examine the relationship between Tp‐e/QTc and mean aortic gradient. Logistic regression analysis were performed to determine the independent predictors of the severe AS. Possible confounding factors were tested in univariate regression model and confounders with a P value of < 0.1 were tested in multivariate logistic regression analysis. A P value of <0.05 was accepted as statistically significant.
RESULTS
The study population was categorized into three groups according to severity of AS as control group (n = 60), mild‐moderate AS group (n = 77), and severe AS group (n = 28). Baseline clinical characteristics and laboratory parameters of the study groups were listed in Table 1. There was no statistically significant difference between groups in terms of age, gender, hypertension, diabetes mellitus, history of CAD, smoking status, medications, and basal laboratory findings (P > 0.05). The electrocardiographic and echocardiographic findings were presented in Table 2. It was found that Tp‐e interval was increased in parallel to the severity of AS (64.5 ± 14.5 in the control group, 73.4 ± 14.3 in the mild‐moderate AS group, and 79.6 ± 13.3 ms in the severe AS group, P < 0.001). Also, Tp‐e/QT ratio (0.17 ± 0.04 in the control group, 0.20 ± 0.04 in the mild‐moderate AS group, and 0.21 ± 0.04 in the severe AS group, P < 0.001) and Tp‐e/QTc ratio were significantly increased in parallel to the severity of AS ratio (0.16 ± 0.03 in the control group, 0.18 ± 0.03 in the mild‐moderate AS group, and 0.19 ± 0.03 in the severe AS group, P < 0.001; Figure 1). The mean heart rate, QRS duration, QT, and corrected QT (QTc) intervals were similar between the groups (P > 0.05). Besides, LV ejection fractions, left ventricle end‐diastolic volume and left ventricle end‐sistolic volume were similar between three groups (P > 0.05). The maximal aortic gradients, mean aortic gradients, and LV mass were significantly higher as the degree of the AS was increased. Similarly, thickness of the interventricular septum and left atrial diameter were significantly higher in severe AS. In Pearson correlation analysis, Tp‐e/QTc ratio had a positive correlation with mean aortic gradient (r = 0.192, P = 0.049; Figure 2). Multivariate logistic regression analysis showed that Tp‐e/QTc ratio (OR, 1.158; P = 0.03) and LV mass (OR, 1.009; P = 0.04) were significantly and independently associated with the severe AS (Table 3).
Table 1.
Clinical Characteristics and Laboratory Findings of the Study Population
| Control Group | Mild‐Moderate | Severe AS | ||
|---|---|---|---|---|
| Parameters | (n = 60) | AS (n = 77) | (n = 28) | P Value |
| Age (years) | 60.8 ± 10.7 | 61.6 ± 11.8 | 64.6 ± 9.4 | 0.351 |
| Gender, male, n (%) | 30 (37.5) | 28 (36.4) | 12 (42.9) | 0.829 |
| Hypertension, n (%) | 17 (21.3) | 20 (26.0) | 10 (35.7) | 0.315 |
| Diabetes mellitus, n (%) | 5 (6.3) | 10 (13.0) | 2 (7.1) | 0.317 |
| Smoking, n (%) | 11 (13.8) | 9 (11.7) | 5 (17.9) | 0.713 |
| History of CAD, n (%) | 5 (6.3) | 4 (5.2) | 2 (7.1) | 0.922 |
| Medication, n (%) | ||||
| RAS blocker | 14 (17.5) | 17 (22.1) | 9 (32.1) | 0.267 |
| Calcium channel blocker | 7 (8.8) | 10 (13.0) | 5 (17.9) | 0.408 |
| β‐Blocker | 5 (6.3) | 5 (6.5) | 3 (10.7) | 0.708 |
| Statin | 5 (6.3) | 7 (9.1) | 4 (14.3) | 0.422 |
| Acetylsalicylic acid | 7 (8.8) | 9 (11.7) | 4 (14.3) | 0.682 |
| Hemoglobin, g/dL | 13.8 ± 1.5 | 13.8 ± 1.2 | 13.4 ± 1.7 | 0.624 |
| Platelet count ×10³/mm³ | 230.8 ± 49.7 | 243.8 ± 59.0 | 255.0 ± 78.0 | 0.392 |
| White blood cell ×10³/mm³ | 7.2 ± 2.0 | 7.6 ± 1.6 | 7.8 ± 1.6 | 0.522 |
| Glucose, mg/dL | 94.3 ± 13.0 | 108.7 ± 54.1 | 101.0 ± 25.5 | 0.157 |
| Creatinine, mg/dL | 0.8 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.363 |
| Total cholesterol, mg/dL | 195.9 ± 40.1 | 205.9 ± 48.0 | 198.8 ± 32.2 | 0.580 |
| HDL‐C, mg/dL | 52.3 ± 11.3 | 53.8 ± 21.0 | 48.4 ± 14.4 | 0.526 |
| LDL‐C, mg/dL | 114.5 ± 36.2 | 127.0 ± 42.8 | 119.0 ± 30.8 | 0.356 |
| Triglyceride, mg/dLa | 123 (40–384) | 124 (42–649) | 131 (54–270) | 0.722 |
Data were given as mean ± SD or %.
AS = aortic stenosis; CAD = coronary artery disease; HDL‐C = high‐density lipoprotein cholesterol; LDL‐C = low‐density lipoprotein cholesterol; RAS = renin–angiotensin system.
Median (minimum–maximum).
Table 2.
Electrocardiographic and Echocardiographic Findings of the Study Population
| Control Group | Mild‐Moderate | Severe AS | ||
|---|---|---|---|---|
| Parameters | (n = 60) | AS (n = 77) | (n = 28) | P Value |
| Tp‐e interval, ms | 64.5 ± 14.5 | 73.4 ± 14.3 | 79.6 ± 13.3 | <0.001 |
| QT interval, ms | 364.1 ± 32.1 | 368.6 ± 31.7 | 371.9 ± 39.2 | 0.546 |
| QTc interval, ms | 405.3 ± 38.1 | 405.1 ± 34.8 | 410.7 ± 36.8 | 0.768 |
| Tp‐e/QT ratio | 0.17 ± 0.04 | 0.20 ± 0.04 | 0.21 ± 0.04 | <0.001 |
| Tp‐e/QTc ratio | 0.16 ± 0.03 | 0.18 ± 0.03 | 0.19 ± 0.03 | <0.001 |
| Heart rate, beat/min | 75.4 ± 14.1 | 73.4 ± 12.3 | 74.0 ± 11.9 | 0.655 |
| QRS duration, ms | 96.3 ± 8.6 | 96.7 ± 7.2 | 95.8 ± 7.2 | 0.850 |
| LVEF, % | 61.7 ± 2.4 | 61.1 ± 5.3 | 60.5 ± 4.4 | 0.463 |
| LVEDV, mL | 97.2 ± 13.0 | 108.9 ± 22.2 | 107.9 ± 36.1 | 0.059 |
| LVESV, mL | 31.0 ± 7.4 | 35.7 ± 10.5 | 35.3 ± 16.0 | 0.122 |
| Interventricular septum, mm | 10.1 ± 1.4 | 11.6 ± 1.6 | 13.0 ± 2.0 | <0.001 |
| Left atrial diameter, mm | 35.0 ± 2.7 | 38.1 ± 4.0 | 39.5 ± 3.9 | <0.001 |
| LV mass, g | 161.7 ± 36.2 | 206.8 ± 51.2 | 234.4 ± 61.9 | <0.001 |
| Mean gradient, mm Hg | – | 19.5 ± 7.5 | 45.4 ± 6.1 | – |
| Maximum gradient, mm Hg | – | 33.1 ± 11.2 | 78.9 ± 10.6 | – |
Data were given as mean ± SD or %.
AS = aortic stenosis; LV, left ventricle; LVEDV = left ventricle end‐diastolic volume; LVESV = left ventricle end‐systolic volume; LVEF = left ventricular ejection fraction.
Figure 1.
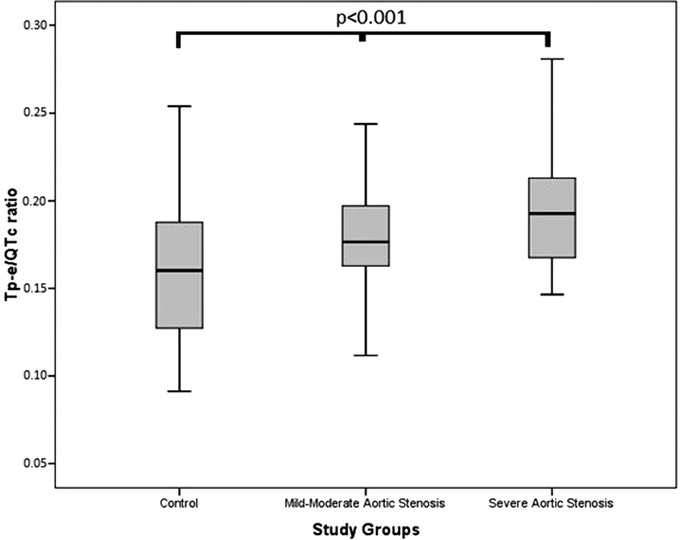
Comparison of Tp‐e/QTc ratio between study groups.
Figure 2.
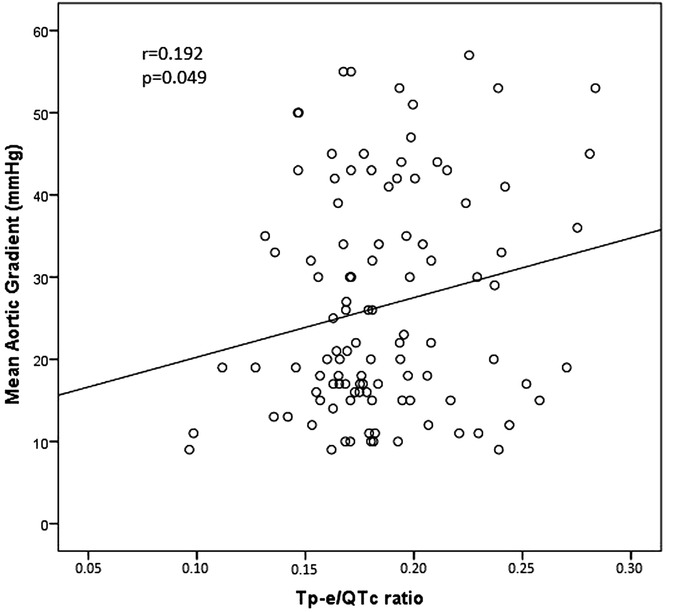
Correlation between Tp‐e/QTc ratio and mean aortic gradient.
Table 3.
Univariate and Multivariate Logistic Regression Analysis for the Prediction of Severe Aortic Stenosis
| Univariate Regression Analysis | Multivariate Regression Analysis | |||
|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| Tp‐e/QTc | 1.162 (1.042–1.295) | 0.007 | 1.158 (1.014–1.323) | 0.030 |
| LV mass | 1.013(1.005–1.022) | 0.003 | 1.009 (1.000–1.018) | 0.040 |
| LVEDV | 1.004 (0.986–1.023) | 0.657 | – | – |
| LVESV | 1.010 (0.963–1.059) | 0.681 | – | – |
| Left atrial diameter | 1.164 (1.055–1.284) | 0.002 | 1.116 (0.991–1.258) | 0.071 |
CI = confidence interval; LV = left ventricle; LVEDV = left ventricle end‐diastolic volume; LVESV = left ventricle end‐systolic volume.
DISCUSSION
In this study, we showed that Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios were prolonged in patients with AS as compared to control subjects. Our study is the first report to demonstrate the relationship between Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios and AS. We also demonstrated that Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios were also increased in parallel to the severity of AS. Also we found that Tp‐e/QTc ratio and LV mass were independent predictors of the severe AS.
Increased afterload and wall stress in left ventricle induces hypertrophy of the LV myocardium. So, myocytes enlarge and wall thickness increases to preserve LV ejection.15, 16 After a while, left ventricle fails against increased pressure afterload and eventually results in heart failure.17 Several studies showed that myocyte apoptosis and fibrosis were increased in this process.18, 19 Apoptosis and fibrosis of myocytes is related with the systolic and diastolic deterioration which eventually results in increased ventricular stiffness.20, 21 However, fibrosis makes left ventricle to become more susceptible to arrythmias by damaging electrical conduction, stimulating the development of re‐entry circuits and augmenting ventricular refractoriness and excitability of myocytes.17, 22 In a study, inverse relationship was shown between long‐term survival and the amount of myocardial fibrosis in severe AS even after aortic valve replacement.23
Ventricular arrythmias play a major role in sudden death and syncope events in patients with symptomatic AS.6 Several studies demonstrated that ventricular arrythmias are common in patients with AS. Schwartz et al.24 observed malignant arrhythmias during syncopal attacks. Klein et al.25 found that complex ventricular arrythmias were frequent in patients with AS than control subjects. In several studies, arrhythmia markers such as frequency of fragmented QRS and QT dispersion were increased significantly in patients with severe AS.26, 27, 28 Nevertheless, the Tp‐e/QT ratio was not affected by the heart rate and body weight, so it has been suggested as a more sensitive arrhythmia marker than Tp‐e or QT intervals.12 Recently, the Tp‐e interval and Tp‐e/QT ratio have been used as novel markers of increased dispersion of ventricular repolarization.12, 13 Prolonged Tp‐e interval was related with increased mortality in long QT syndrome, Brugada syndrome and in patients with acute ST‐segment elevation myocardial infarction.12 Also, several studies have demonstrated increased ventricular repolarization time in LVH.29, 30 Zhao et al.31 demonstrated that LVH was closely related with increased the QT interval, Tp‐e interval, and Tp‐e/QT ratio. Myocardial fibrosis and LVH are well‐known results of AS. Therefore, alterations in myocardial tissue may cause heterogeneity in ventricular repolarization and this may also result in significant and fatal ventricular arrhythmias in patients with AS.
In our study, we have found significant differences in Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios between patients with AS and control group. Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios were significantly higher in patients with AS than control subjects. Besides, Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios were also significantly increased in parallel to the severity of AS. According to the results of our study, we think that AS can cause some repolarization anomalies. Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios were among these marker. We can also suggest that with increased severity of AS, ventricular repolarization becomes more disturbed. Because our results support that increased aortic valve gradients were closely related with ventricular repolarization anomalies including increased Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios. Whether these anomalies can predict worse outcomes in patients with AS is not known for now and further follow‐up studies are needed.
Major limitations of our study is relatively small sample population. Second, cross‐sectional design of study and lack of follow‐up of the patients. But for now, relationship between Tp‐e interval and Tp‐e/QT ratio as markers of ventricular arrhythmias were not evaluated in patients with AS. Therefore, long‐term follow‐up and large‐scale prospective studies are needed to investigate the predictive value of the Tp‐e interval and Tp‐e/QT ratio in patients with AS.
Our study showed that Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios were increased in patients with AS. Besides, our results suggest that increased Tp‐e interval, Tp‐e/QT, and Tp‐e/QTc ratios indicates the severity of AS. In addition, we also showed that Tp‐e/QTc ratio and LV mass were independent predictors of severe AS. Thus, we believe that Tp‐e/QTc ratio may also be useful markers for the prediction of severity of AS and prognosis in these patients.
Disclosure: There are no conflict of interest.
Funding/Supporting Institutions: None.
REFERENCES
- 1. Carabello BA, Paulus WJ. Aortic stenosis. Lancet 2009;373:956–966. [DOI] [PubMed] [Google Scholar]
- 2. Carabello BA. Aortic stenosis: From pressure overload to heart failure. Heart Fail Clin 2006;2:435–442. [DOI] [PubMed] [Google Scholar]
- 3. Chambers J. The left ventricle in aortic stenosis: Evidence for the use of ace inhibitors. Heart 2006;92:420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve stenosis: Preventive or promotive of systolic dysfunction and heart failure? Eur Heart J 2005;26:1790–1796. [DOI] [PubMed] [Google Scholar]
- 5. Weber KT. From inflammation to fibrosis: A stiff stretch of highway. Hypertension 2004;43:716–719. [DOI] [PubMed] [Google Scholar]
- 6. Sorgato A, Faggiano P, Aurigemma GP, et al. Ventricular arrhythmias in adult aortic stenosis: Prevalence, mechanisms, and clinical relevance. Chest 1998;113:482–491. [DOI] [PubMed] [Google Scholar]
- 7. Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp‐Te interval and its diagnostic value. J Electrocardiol 2008;41:575–580. [DOI] [PubMed] [Google Scholar]
- 8. Antzelevitch C, Sicouri S, Di Diego JM, et al. Does T peak‐T end provide an index of transmural dispersion of repolarization? Heart Rhythm 2007;4:1114–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castro Hevia J, Antzelevitch C, Tornes Barzaga F, et al. Tpeak‐T end and T peak‐T end dispersion as risk factors for ventricular tachycardia/ventricular fibrillation in patients with the Brugada syndrome. J Am Coll Cardiol 2006;47:1828–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smetana P, Schmidt A, Zabel M, et al. Assessment of repolarization heterogeneity for prediction of mortality in cardiovascular disease: Peak to the end of the T wave interval and nondipolar repolarization components. J Electrocardiol 2011;44:301–308. [DOI] [PubMed] [Google Scholar]
- 11. Erikssen G, Liestol K, Gullestad L, et al. The terminal part of the QT interval (T peak to T end): A predictor of mortality after acute myocardial infarction. Ann Noninvasive Electrocardiol 2012;17:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta P, Patel C, Patel H, et al. T(p‐e)/QT ratio as an index of arrhythmogenesis. J Electrocardiol 2008;41:567–574. [DOI] [PubMed] [Google Scholar]
- 13. Zhao X, Xie Z, Chu Y, et al. Association between Tp‐e/QT ratio and prognosis in patients undergoing primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction. Clin Cardiol 2012;35:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American College of C, American Heart Association Task Force on Practice G, Society of Cardiovascular A , Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the society of cardiovascular anesthesiologists endorsed by the society for cardiovascular angiography and interventions and the society of thoracic surgeons. J Am Coll Cardiol 2006;48:e1–148. [DOI] [PubMed] [Google Scholar]
- 15. Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest 1975;56:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carabello BA. The relationship of left ventricular geometry and hypertrophy to left ventricular function in valvular heart disease. J Heart Valve Dis 1995;4(Suppl 2):S132–S138; discussion S138–S139. [PubMed] [Google Scholar]
- 17. Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis: A disease of the valve and the myocardium. J Am Coll Cardiol 2012;60:1854–1863. [DOI] [PubMed] [Google Scholar]
- 18. Bishopric NH, Andreka P, Slepak T, et al. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr Opin Pharmacol 2001;1:141–150. [DOI] [PubMed] [Google Scholar]
- 19. Dweck MR, Joshi S, Murigu T, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011;58:1271–1279. [DOI] [PubMed] [Google Scholar]
- 20. Martos R, Baugh J, Ledwidge M, et al. Diastolic heart failure: Evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation 2007;115:888–895. [DOI] [PubMed] [Google Scholar]
- 21. Gonzalez A, Fortuno MA, Querejeta R, et al. Cardiomyocyte apoptosis in hypertensive cardiomyopathy. Cardiovasc Res 2003;59:549–562. [DOI] [PubMed] [Google Scholar]
- 22. Nerheim P, Krishnan SC, Olshansky B, et al. Apoptosis in the genesis of cardiac rhythm disorders. Cardiol Clin 2001;19:155–163. [DOI] [PubMed] [Google Scholar]
- 23. Milano AD, Faggian G, Dodonov M, et al. Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. J Thorac Cardiovasc Surg 2012;144:830–837. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz LS, Goldfischer J, Sprague GJ, et al. Syncope and sudden death in aortic stenosis. Am J Cardiol 1969;23:647–658. [DOI] [PubMed] [Google Scholar]
- 25. Klein RC. Ventricular arrhythmias in aortic valve disease: Analysis of 102 patients. Am J Cardiol 1984;53:1079–1083. [DOI] [PubMed] [Google Scholar]
- 26. Acikgoz E, Yaman B, Acikgoz SK, et al. Fragmented QRS can predict severity of aortic stenosis. Ann Noninvasive Electrocardiol 2015;20:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Agac MT, Korkmaz L, Bektas H, et al. Increased frequency of fragmented QRS in patients with severe aortic valve stenosis. Med Princ Pract 2014;23:66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kosar F, Hisar I, Durmaz T, et al. QTc dispersion measurement for risk of syncope in patients with aortic stenosis. Angiology 2001;52:259–265. [DOI] [PubMed] [Google Scholar]
- 29. Guo D, Young L, Patel C, et al. Calcium‐activated chloride current contributes to action potential alternations in left ventricular hypertrophy rabbit. Am J Physiol Heart Circ Physiol 2008;295:H97–H104. [DOI] [PubMed] [Google Scholar]
- 30. Salles GF, Cardoso CR, Leocadio SM, et al. Recent ventricular repolarization markers in resistant hypertension: Are they different from the traditional QT interval? Am J Hypertens 2008;21:47–53. [DOI] [PubMed] [Google Scholar]
- 31. Zhao Z, Yuan Z, Ji Y, et al. Left ventricular hypertrophy amplifies the QT, and Tp‐e intervals and the Tp‐e/QT ratio of left chest EKG. J Biomed Res 2010;24:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


