Abstract
Background
Pacemaker with remote monitoring (PRM) may be useful for silent atrial fibrillation (AF) detection. The aims of this study were to evaluate the incidence of silent AF, the role of PRM, and to determine predictors of silent AF occurrence.
Methods
Three hundred elderly patients with permanent pacemaker (PPM) were randomly assigned to the remote group (RG) or control group (CG). All patients received PPM with remote monitoring capabilities. Primary end point was AF occurrence rate and the secondary end points were time to AF detection and number of days with AF.
Results
During the average follow‐up of 15.7±7.7 months, AF episodes were detected in 21.6% (RG = 24% vs CG = 19.3%, P = 0.36]. There was no difference in the time to detect the first AF episode. However, the median time to detect AF recurrence in the RG was lower than that in the CG (54 days vs 100 days, P = 0.004). The average number of days with AF was 16.0 and 51.2 in the RG and CG, respectively (P = 0.028). Predictors of silent AF were left atrial diameter (odds ratio [OR] 1.2; 95% CI = 1.1–1.3; P < 0.001) and diastolic dysfunction (OR 4.8; 95% CI = 1.6–14.0; P = 0.005).
Conclusions
The incidence of silent AF is high in elderly patients with pacemaker; left atrial diameter and diastolic dysfunction were predictors of its occurrence. AF monitoring by means of pacemaker is a valuable tool for silent AF detection and continuous remote monitoring allows early AF recurrence detection and reduces the number of days with AF.
Keywords: atrial fibrillation, pacemaker, home monitoring, elderly
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice. Prevalence and incidence of AF are increasing, especially in the elderly population.1, 2
AF may appear benign, but it may reveal its detrimental effects many years after. AF has been associated with a twofold increase in the risk of death, irrespective of other known predictors of mortality.3 Ischemic stroke is the most important cause of morbidity and mortality in patients with AF,4, 5 and about 15% of strokes can be ascribed to a documented AF.6 In about 25% of patients who have ischemic strokes, no etiologic factor was identified,7, 8 and asymptomatic or subclinical AF may be related to those episodes.9 The CRYSTAL‐AF trial10 studied 441 patients who had had an unexplained stroke. All received at least 24 hours of standard cardiac monitoring within 90 days of the stroke, and half were then submitted to an insertable cardiac monitor implantation. By 12 months, AF was detected in 12.4% of patients in the continuous monitoring group versus 2.0% of the patients in the control group (hazard ratio, 7.3; P < 0.001).
In patients with cardiac implantable electronic devices the detection of atrial high rate episodes (AHRE) has increased the understanding of the true incidence of atrial tachyarrhythmias. Systems that allow for pacemaker remote monitoring (PRM) keep detailed information about AHRE. These episodes are thought to represent asymptomatic or silent AF, and they may be indicators of sustained AF. Remote monitoring provides continuous access to stored data, and alerts may be programmed for specific events.
The prevalence and prognostic value of silent AF have been difficult to assess.8, 9 How strict the monitoring should be remains unknown, furthermore, there is currently no consensus regarding the screening and management of silent AF. The objectives of this study were to assess the incidence of silent AF, the role of PRM and to determine predictors of silent AF occurrence, in elderly pacemaker users from a tertiary hospital.
MATERIALS AND METHODS
Study Design
This was a single center randomized study comparing the time to detect AHRE with PRM versus conventional follow‐up (as described below) in elderly patients (≥ 60 years old) with standard indications for permanent pacemaker (PPM) implantation or generator replacement.11
The exclusion criteria were a history of AF, terminal illnesses limiting survival, and the use of antithrombotic therapy or antiarrhythmic drug (AAD) class I or III.12 All patients provided written informed consent before randomization. The study protocol was approved by the Local Ethic Committee and conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
All patients received dual‐chamber PPMs (Philos II DR‐T or Cylos DR‐T models; Biotronik, Berlin, Germany) with remote monitoring capability (Home Monitoring). The Home Monitoring technology is described in detail elsewhere.13
Immediately after PPM implantation, patients were randomly assigned in a 1:1 ratio to one of the two monitoring strategies—PRM group (RG) and the control group (CG). Patients were then followed for 24 months.
The primary end points were AF occurrence rate and the time to AF detection (occurrence and recurrence). The secondary end point was the number of daily AF burden ≥10%.
Details of trial enrollment and follow‐up are shown in Figure 1.
Figure 1.
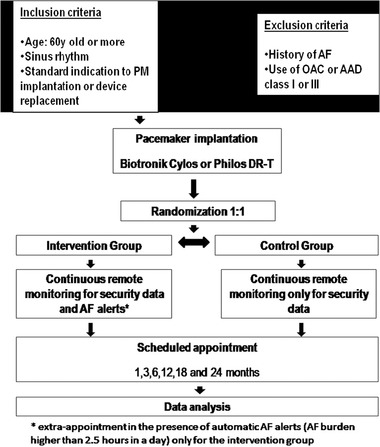
Fluxogram of the study.
AAD = antiarrhythmic drugs; AF = atrial fibrillation; OAC = oral anticoagulation; PM = pacemaker.
STUDY PROCEDURES
Baseline Assessment
The patient's medical history, physical‐examination findings, use of medications and 12‐lead ECG were recorded. The patients underwent two‐dimensional Doppler flow echocardiography. Diastolic dysfunction was defined as functional abnormalities during left ventricular relaxation/filling and was assessed integrating several techniques: analysis of mitral flow, pulmonary venous flow, tissue Doppler, color M‐mode flow propagation velocity, and left atrial volume.14 The ratio of early mitral flow velocity to early mitral annulus velocity ≥ 8 defined the presence of diastolic dysfunction. The reference values followed the American guidelines recommendations.15
Device Programming
All devices were programmed to DDD mode with lower rate of 60 pulses per minute (ppm). The automatic mode switching (AMS) detection was programmed to an atrial rate of ≥ 160 beats per minute; cross‐channel and far‐field blanking were programmed to 72 and 175 ms, respectively. Stored intracardiac electrograms (IEGMs) were analyzed for each episode, and true device‐detected AF was the designation for AHREs lasting two minutes or more and episodes in atrial tachycardia (AT) histogram higher than 250 ppm. A sensitivity and specificity analysis was performed in order to evaluate this programming for AHREs detection. The AHREs were excluded if artifacts were identified or if they lasted less than 2 minutes.
Using a device with wireless capability of transmission, the PRM system was able to receive daily information from PPM of all patients included in this study. The Home Monitoring system sends AF alerts of 10%, 25%, 75%, or 100% daily AF burden according with the programming. Specific automatic alerts were programmed in both groups to detect device dysfunctions as high (≥ 3000 Ohms) or low (< 200 Ohms) lead impedance and abnormalities in the sensitivity parameters. For RG, automatic AF alerts were programmed for episodes lasting at least 2.5 hours in a day (daily AF burden ≥ 10%), which is the minimum parameter to AF reports. We obtained the number of daily AF burden ≥ 10% by means of a retrospective analysis of stored data in the PRM systems of both groups.
Follow‐Up
For both groups, follow‐up appointments were scheduled at 30, 90, and 180 days and then every 6 months. Patients in the RG had additional appointments if automatic AF alerts were sent by PRM. The device data regarding AF occurrence were analyzed during the entire follow‐up. The physician‐investigator had exclusive access to the PRM data from the RG, and the committee members had access to all PRM data in both groups for safety.
The time to AF detection was defined as the time between the date of AF diagnosis and the last in‐office appointment. The AF diagnosis in RG was documented during additional appointment motivated by AF alerts and in the CG during routine follow‐up visit.
The therapeutic management included electrical cardioversion procedure, AAD and antithrombotic therapy, alone or associated, according to the physician discretion.
Statistical Analysis
The sample size was based on an estimated AF incidence of 15%. To detect a reduction of 5% on the AF occurrence of RG, with 80% power and type I error (two‐sided α) of 0.05, it was estimated 276 patients. Including 8% of loss to follow‐up, the same size resulted in 300 patients.
Comparison of groups was performed using t‐test or Mann–Whitney U test and categorical data were analyzed by chi‐square test according to Pearson or Fisher's exact test as appropriate. Logistic regression model was performed to determine predictors of silent AF occurrence. All tests were two‐tailed and significance level was set at 0.05. The statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, version 18.0 for Windows).
RESULTS
Patients were selected between March 2007 and January 2010, and the mean follow‐up time was 15.7 ± 7.7 months (RG = 15.8 ± 7.8 vs CG = 15.5 ± 7.8, P = 0.56). The overall average age was 75.2 years old, and female gender was more prevalent (56%). Patients received first PPM implantation in 54%, mostly due to advanced AV block (88.3%). Hypertension was common (95%), 83.3% had no cardiomyopathy, and the average left ventricular ejection fraction was 58.5%. The overall mean CHADS2 score was 1.8 ± 0.9 and 61.3% of the patients had CHADS2 scores ≥2 (Table 1).
Table 1.
Baseline Characteristics
| Total Population | Remote Group | Control Group | P Value* | |
|---|---|---|---|---|
| Patients (n) | 300 | 150 | 150 | |
| Gender, n (%) | ||||
| Male | 132 (44) | 68 (45.3) | 64 (42.6) | 0.46 |
| Female | 168 (56) | 82 (54.7) | 86 (57.4) | |
| Age (years) | 75.2 ± 7.9 | 75.6 ± 7.9 | 74.8 ± 7.8 | 0.44 |
| BMI (kg/m2) | 26.0 ± 4.2 | 26.2 ± 4.2 | 25.7 ± 4.3 | 0.91 |
| Type of procedure, n (%) | ||||
| First PPM implant | 162 (54) | 78 (52) | 84 (56) | 0.48 |
| Device replacement | 138 (46) | 72 (48) | 66 (44) | |
| PPM indication, n (%) | ||||
| SND | 35 (11.7) | 18 (10.3) | 17 (11.3) | 0.84 |
| AV block | 265 (88.3) | 132 (89.7) | 133 (88.7) | |
| Functional class (NYHA), n (%) | 1.0 | |||
| FC I | 222 (74) | 111 (74) | 111 (74) | |
| FC II | 78 (26) | 39 (26) | 39 (26) | |
| Cardiomyopathy, n (%) | ||||
| No heart disease | 250 (83.3) | 127 (84.6) | 123 (82.0) | 0.55 |
| Ischemic | 5 (1.6) | 2 (1.3) | 3 (2.0) | |
| Chagasic | 24 (8.0) | 10 (6.6) | 14 (9.3) | |
| Congenital | 1 (0.3) | 0 (0) | 1 (0.6) | |
| Hypertensive | 18 (6.0) | 10 (6.6) | 8 (5.3) | |
| Valvular | 2 (0.6) | 1 (0.6) | 1 (0.6) | |
| Echocardiogram | n = 258 | n = 127 | n = 131 | |
| LA (mm) | 39.7 ± 5.8 | 40.5 ± 6.0 | 38.9 ± 5.4 | 0.03 |
| LVDd (mm) | 50.8±7.0 | 51.6±7.1 | 50.1±6.8 | 0.09 |
| LVSd (mm) | 35.3 ± 8.8 | 36.2 ± 8.9 | 34.3 ± 8.7 | 0.14 |
| Diastolic dysfunction, n (%) | 86 (33.3) | 45 (35.4) | 41 (31.3) | 0.64 |
| LVEF (%) | 58.0 ± 13.6 | 57.8 ± 12.4 | 58.3 ± 12.9 | 0.77 |
| LVEF ≤0.35 | 24 (9.3) | 13 (10.2) | 11 (8.4) | 0.84 |
| Comorbidity, n (%) | ||||
| Hypertension | 285 (95) | 139 (92.6) | 146 (97.3) | 0.11 |
| Diabetes | 88 (29.3) | 36 (24) | 52 (34.6) | 0.06 |
| Dyslipidemia | 115 (38.3) | 62 (40) | 53 (37.1) | 0.34 |
| Hypothyroidism | 29 (9.6) | 13 (8.6) | 16 (10.6) | 0.55 |
| History of stroke | 07 (2.3) | 06 (4.0) | 01 (0.6) | 0.12 |
| CHADS2 score | 1.8 (0.9) | 1.8 (0.9) | 1.8 (0.8) | 1.0 |
| CHADS2 score ≥2 | 184 (61.3) | 89 (59.3) | 95 (63.3) | 0.09 |
| Pharmacological therapy, n (%) | ||||
| ACE inhibitor or ARA2 | 203 (76.0) | 119 (79.3) | 121 (80.6) | 0.19 |
| β‐Blocker | 95 (35.5) | 67 (44.6) | 56 (37.3) | 0.15 |
| Diuretic | 148 (55.4) | 91 (60.6) | 80 (53.3) | 0.52 |
| Statin | 102 (38.2) | 67 (40) | 56 (36.3) | 0.61 |
| ASA | 116 (43.4) | 75 (50) | 61 (40.6) | 0.02 |
n = number; BMI = body mass index; PPM = permanent pacemaker; SND = sinus node disease; AV = atrioventricular; FC = functional class; NYHA = New York Heart Association; LA = left atrium; LVDd = left ventricle end‐diastolic diameter; LVSd = left ventricle end‐systolic diameter; LVEF = left ventricular ejection fraction; ACE = angiotensin converting enzyme; ARA2 = angiotensin two receptor antagonist; ASA = acetylsalicylic acid. The data are expressed as the means ± standard deviations or numbers (%).
*P value for chi‐square or two‐sample t‐test when applicable.
The occurrence rate of AF was 21.6% (RG = 24% [36 episodes] vs CG = 19.3% [29 episodes], P = 0.360) during the entire follow‐up, yielding an annual AF incidence of 16.5%. Forty‐four (58.6%) of the 65 episodes of AF occurred in the first six months after the inclusion, being 26 (72.3%) in the RG, and 18 (62.0%) in the CG, P = 0.408.
The rate of AF recurrence was also similar between groups; 16% for the first (RG = 16.0% [24 episodes] vs CG = 16.0% [24 episodes], P = NS) and 8.3% for the second recurrence (RG = 8.0% [12 episodes] vs CG = 8.6% [13 episodes], P = 0.910).
The median time to detection the first AF episode was not different between RG and CG, 59, and 63 days, respectively, P = 0.200. However, it was significantly shorter in the RG to detection the first AF recurrence, 54 days for RG versus 100 days for CG, P = 0.004 (Fig. 2). Considering the median time to AF detection after the inclusion, it was 111 and 196 days for occurrence and recurrence, respectively (P < 0.001).
Figure 2.
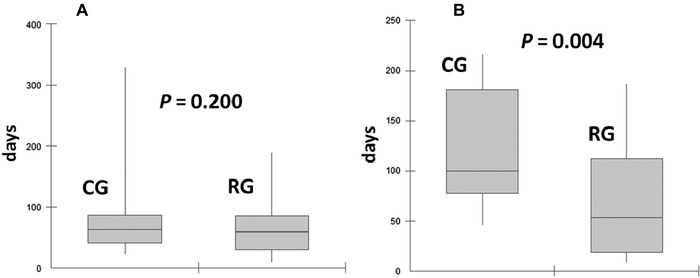
Comparison of the time to detect atrial fibrillation occurrence (A) and recurrences (B) between the two groups studied.
CG = control group; RG = remote group.
The average number of daily AF burden ≥ 10% was 16.0 (95% confidence interval [CI]: 8.9 to 23.2) in the RG and 51.2 (95% CI; 21.9–81.9) in the CG, P = 0.028 (Fig. 3). The overall AF documentation by ECG was 7%, being 10% in the RG, and 4% in the CG (P = 0.042).
Figure 3.
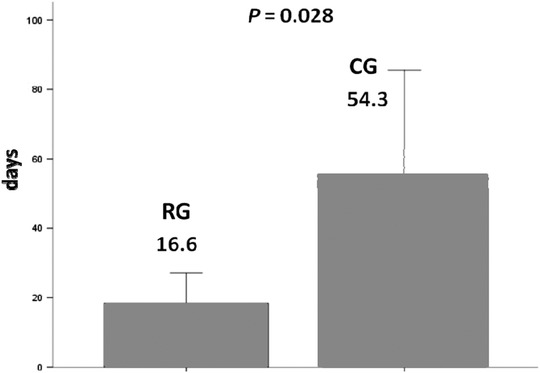
Comparison of the number of atrial fibrillation days between the two groups. There was an approximately three‐fold reduction in the remote group (RG) compared to the control group (CG).
There were 12,738 AMS activations detected during the entire follow‐up. Of 5681 stored IEGM, we excluded artifacts and high ventricular rate episodes and identified 1124 with specific AF characteristics, being 261 during at least two minutes. Criteria for true AF, including IEGM lasting two minutes or more and episodes higher than 250 beats per minute documented in AT histogram, were observed in 19 of 21 patients with AF documented by ECG (one underdiagnosed in each group), and in 44 of 179 patients without AF documented by ECG, yielding a sensibility and specificity of 90% and 84%, respectively (Fig. 4).
Figure 4.
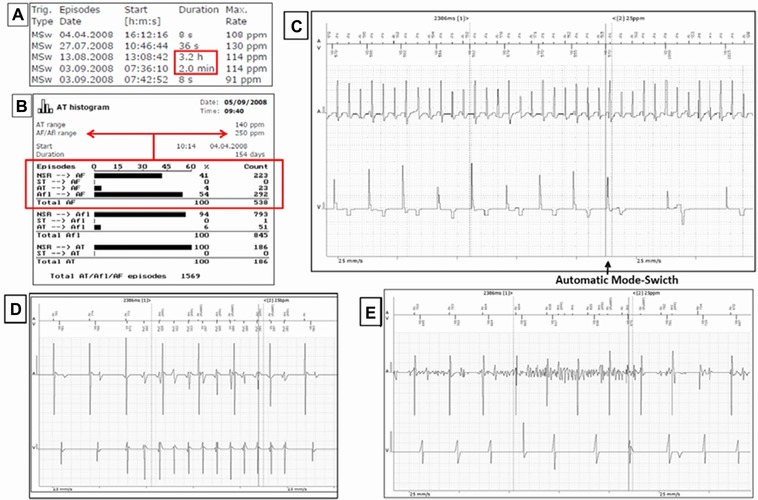
True AF criteria of stored data in pacemaker memory and reasons for inappropriate AHRE detections. (A) Stored IEGM during at least two minutes; (B) Atrial tachycardia histogram with AHRE higher than 250 ppm documented; (C) IEGM with AF characteristics; (D) High rate episode; and (E) Artifact.
There was no difference in the average cumulative atrial pacing (AP) or ventricular pacing (VP) percentages between the groups (RG: AP = 31%; VP = 82%, and CG: AP = 33%; VP = 82%). It was possible to determine the right ventricle pacing site in 167 patients and there was no difference between the groups. Right ventricular apical pacing was performed in 55 patients in the RG and in 63 patients in the CG. Right ventricular outflow tract pacing was performed in 27 patients in the RG and in 22 patients in the CG.
The overall mean CHADS2 score was 1.8 ± 0.9 and 61.3% of the patients had CHADS2 scores ≥2. There were eight patients with a history of prior stroke (RG = 7 and CG = 1) and stroke occurred in only one patient in the RG. Regarding antithrombotic therapy, acetylsalicylic acid (300 mg per day) was prescribed in 20 patients (RG = 14 vs CG = 6; P = 0.027), including eight patients with CHADS2 scores <2 and 12 patients with CHADS2 score ≥2, because warfarin was contraindicated. Warfarin was initiated in 28 patients (RG = 17 vs CG = 11; P = NS), but only 16 were still on warfarin therapy at the end of the study. The remaining 17 patients with AF did not receive antithrombotic therapy due to CHADS2 scores < 2 or patient refusal. The occurrence rates of AF and drug therapy management (antiarrhythmic and antithrombotic) are shown in Table 2. Considering AF‐related symptoms, only one patient in each group presented symptomatic AF.
Table 2.
Occurrence of AF Documented by ECG and Atrial High‐Rate Episodes Device‐Detected, Including Antiarrhythmic and Antithrombotic Therapy
| Total Population | Remote Group | Control Group | ||||
|---|---|---|---|---|---|---|
| (n = 300) | (n = 150) | (n = 150) | ||||
| Number of | Odds Ratio for | |||||
| Type of Detection of AF | Events (%) | AF Occurrence | 95% CI | P Value | ||
| AF documented by ECG | 21 (7) | 15 (10) | 6 (4) | 2.67 | 1.01‐7.07 | 0.042 |
| AHREs device‐detected | 63 (21.0) | 35 (23.3) | 28 (18.7) | 1.33 | 0.76‐2.32 | 0.321 |
| AF management | Therapeutics options applied at least once during follow‐up | |||||
| N total (n = 65) | Remote group (36) | Control group (29) | Odds Ratio for | |||
| Patients with AF | Number of patients (%) | AF management | 95% CI | P Value | ||
| No therapy | 17 (26.2) | 5 (13.8) | 12 (41.3) | |||
| Amiodarone | 14 (21.5) | 8 (22.2) | 6 (20.6) | 1.34 | 0.45‐3.97 | 0.593 |
| Antithrombotic therapy (ASA or warfarin) | 48 (73.8) | 31 (86.1) | 17 (58.6) | 2.53 | 1.26‐5.08 | 0.008 |
| Electrical cardioversion | 13 (20.0) | 8 (22.2) | 5 (17.2) | 1.63 | 0.52‐5.11 | 0.400 |
Abbreviations: ASA = acetylsalicylic acid; ECG = electrocardiogram; AF = atrial fibrillation; AHREs = atrial high‐rate episodes; CI = confidence interval.
On multivariate analysis, predictors of silent AF occurrence (Table 3) were left atrial diameter (Odds ratio, 1.20; 95% CI 1.10–1.30; P < 0.001) and presence of diastolic dysfunction (Odds ratio, 4.8; 95% CI 1.6–14.0; P = 0.005). Left atrial diameter above 39.5mm has sensitivity of 81.8% and specificity of 100% for silent AF occurrence.
Table 3.
Multivariate Logistic Regression Model for Predictors of AF Occurrence
| Odds Ratio | ||
|---|---|---|
| with 95%CI | P Value | |
| Left atrium | 1.20 (1.10 ‐ 1.30) | <0.001 |
| Diastolic dysfunction | 4.80 (1.60 ‐ 14.0) | 0.005 |
AF = atrial fibrillation; CI = confidence interval.
DISCUSSION
Major findings of this randomized trial were that in elderly patients with a dual‐chamber PPM, the time to AF occurrence did not differ between PRM versus a standard follow‐up visits, while there was a difference for the recurrence time.
The lack of difference for the time to AF occurrence between RG and CG is likely related to the moment of the AF occurrence and the scheduled follow‐up visits. Most of the first AF episodes (72.3% for the RG and 62.0% for the CG) occurred in the first six months of the inclusion, a period when the visits were more frequent in the CG (at least three visits). This is corroborated by the median time between the study inclusion and the first AF detection (111 days).
The median time to first AF recurrence was significantly shorter in the RG and the median time between the study inclusion and the first AF recurrence was 196 days, a period which reflects the biannual follow‐up and the benefit of PRM system.
Another important finding of this study was the significant reduction in the number of days with AF burden ≥ 10% in RG, probably due to standard therapy (AAD and electrical cardioversion procedure) anticipation. The number of AF days in patients with conventional follow‐up was almost three times higher than the number of AF days in the RG. AF episodes with longer duration could represent an increased risk for thromboembolic events.16 Botto et al. showed that the higher the score CHADS2 is, and the longer the AF duration, the greater the thromboembolic risk.17
Boriani et al.18 showed that AF burden was an independent predictor of ischemic stroke; moreover, among the thresholds of AF burden evaluated, one hour time was associated with the highest hazard ratio (2.11) for ischemic stroke.
The incidence of silent AF and the cutoff values of atrial rate and the episode duration varies widely among different studies. The reported annual incidence is 10–79% and the episode duration of AHREs varies from 20 seconds to 6 minutes.19 Furthermore, these episodes have already been correlated to cardiovascular events in the studies ASSERT,20 TRENDS,21 and MOST.22 PRM also allowed significantly higher proportion of patients who needed antithrombotic therapy for stroke prevention, based on CHADS2 scores. The likelihood of using an antithrombotic therapy in the RG was 2.5 times higher than in the CG.
The incidence of AF in this population was high (21.6%), considering diagnoses based on PRM, AHREs device‐detected, and episodes of AF documented by ECG. Considering only documented AF by ECG, which currently is the gold standard exam for AF diagnosis, AF incidence was 7.0%. The diagnostic capability of AF was significantly increased, due to continuous remote monitoring and stored data in PPM memory. Furthermore, the RG patients had an approximately threefold greater likelihood of having AF documentation by ECG. The earlier additional appointments motivated by AF alerts would facilitate the ECG documentation of paroxysmal AF episodes and IEGM correlation.
Ricci et al.23 reported in the first clinical experience on AF detection by Home Monitoring system that 80% of the patients had relatively few (< 30) AF days which they defined as a mode‐switch burden > 20% within 24 hours. Longer follow‐up would miss AF episodes of spontaneous reversal to sinus rhythm due to priorities criteria of PPM memory and replacement of stored IEGM AF‐related.
Orlov et al.24 showed in the A‐HIRATE study high AF incidence based on AHREs stored in the PPM of 427 elderly subjects. Patients without a history of AF showed an AF incidence of 46.2% over a follow‐up of an average of two years. In our study, 80% of patients had AV block and patients with prior AF were excluded. These patients’ characteristics explain the lower AF incidence in our study in comparison to A‐HIRATE results, which had higher number of patients with sick sinus syndrome (45%). Considering this, we believe that the AF incidence found in our study is compatible with the real occurrence of this type of arrhythmia for the profile of the studied population.
Seidl et al.25 demonstrated that appropriate device programming for atrial high‐rate episodes allows for sensitivity and specificity of 98% and 100%, respectively, in the diagnosis of arrhythmia. In our study, we observed good sensitivity and specificity, being 90% and 84%, respectively, to make diagnosis of true AF using stored data in a dual‐chamber PPM memory with a cutoff of AHRE ≥ 2 minutes and ≥ 250 beats per minute documented in AT histogram and IEGM.
It is important to notice that not every AHREs documented by pacemaker is necessarily true AF. The false‐positive detections can be found due to noise, R wave oversensing and repetitive non‐re‐entrant V‐A synchrony. Kaufman et al.,26 in a recently published study, by using a cutoff of > 6 minutes and > 190 beats per minute, showed that the rate of false‐positive AHREs is 17.3%, making physician review of IEGMs essential. For AHREs lasting >6 hours the rate of false positives is 3.3%, making physician review less crucial.
Another important finding of this study was that most patients with AF diagnoses were fully asymptomatic (99%). Stroke risk is not related to whether AF presentation is paroxysmal or permanent and most patients with paroxysmal AF had never reported typical clinical symptoms.27, 28, 29, 30, 31
The ASSERT study,20which was designed to evaluate the occurrence of ischemic stroke in a large cohort of elderly patients with PPM or implantable cardioverter‐defibrillators, showed an annual event rate of 3.78%. It was associated to the AF occurrence, detected only for three months after patients’ inclusion and in CHADS2 scores ≥ 2.
Our study did not confirm data of ASSERT study,20 but we demonstrated that the incidence of silent AF detected by the device was high in elderly patients with dual chamber PPM submitted to continuous heart rhythm monitoring, i.e., during day by day activities. Otherwise, most of patients included in this study presented CHADS2 scores ≥2, characterizing a population of moderate to high risk of stroke and emphasizing the relevance of PRM.
Ricci et al.32 using Monte Carlo methods, a special class of computer simulations, demonstrated that remote monitoring systems may reduce stroke risk by 9–18% if compared with standard in‐person visits scheduled every 6–12 months, with an absolute reduction of 0.2–0.6%. In this study, the rate of stroke occurrence was very low and precluded a statistical analysis of this clinical event. Similarly, the benefit of PRM in total mortality might have been underestimated.
The role of antithrombotic therapy on silent AF is an unresolved question and studies addressing this subject are mandatory. Unfortunately we did not intent to address this topic in this study, mainly because the limited follow‐up period and because the low rate of thromboembolic events expected. In order to answer this question we are next to start the SILENT trial—Subclinical AtrIal FibrilLation and StrokE PreveNtion Trial (https://clinicaltrials.gov/ct2/show/NCT02004509). This randomized study aims to assess the impact of antithrombotic therapy on silent AF, driven by findings of cardiac implantable electronic device (CIED) intensive monitoring, on the incidence of stroke and systemic embolism and correlate the AF episodes detected by CIED with thromboembolic events.
STUDY LIMITATIONS
The decision to analyze AF episodes of two minutes or higher may be a stringent criterion. This cutoff was chosen because in the first cases we observed a high rate of false positive findings in episodes lasting less than two minutes, mainly associated to the occurrence of noise, resulting in reduced sensibility. In addition, it was not possible to identify the right ventricular lead location in patients submitted to generator replacement, as the x‐ray is not a routine exam for patients undergoing this procedure, in our institution. Our inability to ascertain stroke risk and mortality was due to the limited follow‐up period and we are also unable to extrapolate our findings to elderly patients without PPM or to patients with left ventricular systolic dysfunction.
CONCLUSIONS
In the elderly population with a pacemaker of a tertiary hospital, the incidence of silent AF is high (16.5% per year). Higher left atrial diameter and presence of diastolic dysfunction were independent predictors of silent AF occurrence. Stored data in pacemaker memory is a valuable tool for prolonged ambulatory monitoring of AF detection in a conventional follow‐up, and continuous remote monitoring of these data might ameliorate the management of AF therapy since it allowed for early AF recurrence detection and reduction in the number of days of AF.
Clinical Implications
The use of implantable devices with remote monitoring system would definitely help establish the AF burden predictor of a higher risk of stroke. It would be especially useful for asymptomatic patients, in whom prognoses are unclear today. However, routine use of these devices in all patients has some difficulties, regarding economic and organizational issues, as well as of the patient's compliance matter. Analysis of PRM transmissions, in fact, has significant implications for the device clinic workflow, especially when the patient's compliance is poor as it may happen in elderly population.33
Acknowledgments
The authors would like to thank the financial support from the Biotronik Company Brazil and the CNPq (National Council for Scientific and Technological Development, Brazil) for the doctored scholarship granted to C.E. B. Lima.
We thank Luciene Dias de Jesus, RN, for assistance in the data collection process and the management of remote monitoring service center.
Author Contributions
Each of the nine authors has significantly contributed to the study. I, Dr Carlos Eduardo Batista de Lima, Dr Giselle de Lima Peixoto and Eng Sérgio Freitas de Siqueira were responsible for the conception of the study, data interpretation, drafting the manuscript and statistical analysis. Dr Maurício Wajngarten and Dr Rodrigo Tavares Silva coordinated the collection of clinical and electronic data. Dr Roberto Costa was responsible for the surgical procedures. Dr Ramires and Dr Kalil critically reviewed the article. All authors have read and approved the final version of the manuscript.
Conflict of Interest: None for all authors.
REFERENCES
- 1. Stewart S, Hart CL, Hole DJ, et al. Population prevalence, incidence, and predictors of atrial fibrillation in the Renfrew/Paisley study. Heart 2001;86:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 3. Stewart S, Hart CL, Hole DJ, et al. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 4. Furberg CD, Psaty BM, Manolio TA, et al. The prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol 1994;74:236–241. [DOI] [PubMed] [Google Scholar]
- 5. Wolf PA, Dawber TR, Thomas HE Jr, et al. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: The Framingham Study. Neurology 1978;28:973–977. [DOI] [PubMed] [Google Scholar]
- 6. Wolf PA, Abbot RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: The framingham study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 7. Tayal AH, Tian KM, Kelly M, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology 2008;71:1696–1701. [DOI] [PubMed] [Google Scholar]
- 8. Jabaudon D, Sztajzel J, Sievert K, et al. Usefulness of ambulatory 7‐day ECG monitoring for the detection of atrial fibrillation and flutter after acute stroke and transient ischemic attack. Stroke 2004;35:1647–1651. [DOI] [PubMed] [Google Scholar]
- 9. Sinha AM, Diener HC, Morillo CA, et al. Cryptogenic Stroke and underlying Atrial Fibrillation (CRYSTAL AF): design and rationale. Am Heart J 2010;160:36e.1‐41.e1. [DOI] [PubMed] [Google Scholar]
- 10. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 11. Martinelli Filho M, Zimerman LI, Lorga AM, et al. Guidelines for implantable electronic cardiac devices of the Brazilian society of cardiology. Arq Bras Cardiol 2007;89:e210–e238. [DOI] [PubMed] [Google Scholar]
- 12. Williams V, ed. Classification of antiarrhythmic drugs In: Sondoe E. (ed.):Symposium on Cardiac Arrhythmias. Amsterdam, Elsevier, 1970, pp. 449–501. [Google Scholar]
- 13. Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: Analysis of a worldwide database. Pacing Clin Electrophysiol 2007;30(Suppl 1):S2–S12. [DOI] [PubMed] [Google Scholar]
- 14. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–193. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 16. Darbar D, Roden DM. Symptomatic burden as an endpoint to evaluate interventions in patients with atrial fibrillation. Heart Rhythm 2005;2:544–549. [DOI] [PubMed] [Google Scholar]
- 17. Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: Crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol 2009;20:241–248. [DOI] [PubMed] [Google Scholar]
- 18. Boriani G, Glotzer TV, Santini M, et al. Device‐detected atrial fibrillation and risk for stroke: An analysis of >10 000 patients from the SOS AF project (Stroke prevention strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J 2014;35:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeCicco AE, Finkel JB, Greenspon AJ, et al. Clinical significance of atrial fibrillation detected by cardiac implantable electronic devices. Heart Rhythm 2014;11:719–724. [DOI] [PubMed] [Google Scholar]
- 20. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 21. Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: The TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 22. Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: Report of the Atrial Diagnostics Ancillary Study of the Mode Selection Trial (MOST). Circulation 2003;107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 23. Ricci RP, Russo M, Santini M. Management of atrial fibrillation ‐ what are the possibilities of early detection with home monitoring? Clin Res Cardiol 2006;95(Suppl 3):III10‐6. [DOI] [PubMed] [Google Scholar]
- 24. Orlov MV, Ghali JK, Araghi‐Niknam M, et al. Asymptomatic atrial fibrillation in pacemaker recipients:Incidence, progression, and determinants based on the atrial high rate trial. Pacing Clin Electrophysiol 2007;30:404–411. [DOI] [PubMed] [Google Scholar]
- 25. Seidl K, Meisel E, VanAgt E, et al. Is the atrial high rate episode diagnostic feature reliable in detecting paroxysmal episodes of atrial tachyarrhythmias? Pacing Clin Electrophysiol 1998;21:694–700. [DOI] [PubMed] [Google Scholar]
- 26. Kaufman ES, Israel CW, Nair GM, et al. Positive predictive value of device‐detected atrial high‐rate episodes at different rates and durations: An analysis from ASSERT. Heart Rhythm 2012;9:1241–1246. [DOI] [PubMed] [Google Scholar]
- 27. Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: Report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J 2009;31:967–975. [DOI] [PubMed] [Google Scholar]
- 28. Hart RG, Pearce LA, Rothbart RM, et al. Stroke with intermittent atrial fibrillation: Incidence and predictors during aspirin therapy. Stroke prevention in Atrial Fibrillation Investigators. J Am Coll Cardiol 2000;35:183–187. [DOI] [PubMed] [Google Scholar]
- 29. Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol 2000;4:369–382. [DOI] [PubMed] [Google Scholar]
- 30. Rizos T, Wagner A, Jenetzky E, et al. Paroxysmal atrial fibrillation is more prevalent than persistent atrial fibrillation in acute stroke and transient ischemic attack patients. Cerebrovasc Dis 2011;32:276–282. [DOI] [PubMed] [Google Scholar]
- 31. Page Rl, Wilkinson WE, Clair WK, et al. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation 1994;89:224–227. [DOI] [PubMed] [Google Scholar]
- 32. Ricci RP, Morichelli L, Gargaro A, et al. Home monitoring in patients with implantable cardiac devices: Is there a potential reduction of stroke risk? Results from a computer model tested through Monte Carlo simulations. J Cardiovasc Electrophysiol 2009;20:1244–1251. [DOI] [PubMed] [Google Scholar]
- 33. Papavasileiou LP, Forleo GB, Panattoni G, et al. Work burden with remote monitoring of implantable cardioverter defibrillator: Is it time for reimbursement policies? J Cardiovasc Med (Hagerstown) 2013;14:114–119. [DOI] [PubMed] [Google Scholar]


