Abstract
Background
Fragmented QRS (fQRS) is an indicator of nonhomogeneous ventricular activity caused by myocardial fibrosis. Aortic stenosis (AS) is known to be a cause of myocardial fibrosis. We aimed to investigate the relationship of fQRS with severity of AS, echocardiographic, and electrocardiographic findings, and development of atrial fibrillation and manifest heart failure in AS patients.
Methods
One hundred four patients with moderate and severe AS were recruited for the study. Patients with mitral or tricuspid stenosis, previous myocardial infarction, segmental wall motion abnormality or left ventricular ejection fraction (LVEF) below 50% and patients with complete‐incomplete BBB and pacemaker rhythm were excluded.
Results
Mean age of the patients was 69 ± 14.8 and 73.1% had fQRS. Patients with fQRS had lower LVEF, higher mean QRS duration, intrinsic deflection, Cornell voltage, Romhilt‐Estes Score, systolic pulmonary artery pressure, mean and peak systolic transaortic gradients and left atrium diameter. Manifest heart failure was more frequent in patients with fQRS. In stepwise multivariate logistic regression analyze, manifest heart failure, peak systolic transaortic gradient, LVEF, intrinsic deflection, strain pattern and Cornell voltage were independently associated with fQRS. Strain pattern and fQRS were found as independent predictors of severe AS.
Conclusions
fQRS is independently associated with the severity of AS while traditional LVH criteria, except strain pattern, are not. fQRS may be better than traditional ECG criteria of LVH and echocardiographic LVH as an indicator of myocardial fibrosis in AS. Thus, fQRS may have a role in determining the severity and prognosis of AS.
Keywords: aortic valve stenosis, fragmented QRS, myocardial fibrosis, heart failure
Fragmented QRS (fQRS) is a relatively novel indicator of nonhomogeneous ventricular activity caused by myocardial scar.1 Fragmented QRS is simply defined as additional spikes within QRS complexes, such as a RSR, without a typical bundle‐branch block pattern.2 Previous studies revealed that fQRS is related with depolarization anomalies and fibrosis in patients with ischemic cardiomyopathy.3, 4, 5 It was also associated with increased mortality and arrhythmic events in Brugada syndrome and arrhythmogenic right ventricular dysplasia.6, 7, 8
Several studies have investigated the relationship of fQRS with valvular heart disease. It was shown that severity of mitral stenosis, myocardial dysfunction, and pulmonary hypertension were associated with fQRS in mitral stenosis.9 Aortic stenosis (AS) is the most frequent type of valvular heart disease in Europe and North America.10 As a response to pressure overload, a marked remodeling process including fibrosis and myocyte degeneration occurs in AS.11 However, there are not any studies investigating the relationship of echocardiographic and clinical findings of AS with the newly proposed fibrosis marker fQRS. Therefore, we aimed to investigate the relationship of fQRS with severity of AS, echocardiographic findings, electrocardiographic findings, and development of atrial fibrillation and manifest heart failure in AS patients.
METHODS
The study population included patients who were admitted to the cardiology outpatient clinics for any reasons and had moderate or severe AS. Patients who have mitral or tricuspid stenosis, previous myocardial infarction, documented coronary artery stenosis greater than 50%, nonischemic dilated cardiomyopathy, segmental wall motion abnormality or left ventricular ejection fraction (LVEF) below 50% in echocardiography and patients with complete or incomplete BBB and pacemaker rhythm on ECG were excluded from the study.
A transthoracic echocardiography was performed in all subjects (Vivid 7, GE Vingmed Ultrasound AS, Horten, Norway). All patients underwent a comprehensive examination, including M‐mode, two‐dimensional and Doppler echocardiography.
Septal and posterior walls’ thicknesses end systolic and end diastolic dimensions are measured from parasternal long‐axis view. Ejection fraction was calculated by modified Simpson's single plane method using left ventricular end systolic and end diastolic volumes from apical four‐chamber view. Aortic jet velocity was calculated by Doppler echocardiography. Valve anatomy was evaluated by two‐dimensional echocardiography. Aortic stenosis was accepted as mild if mean systolic transaortic gradient is less than 20 mmHg or jet velocity is less than 3.0 m/s, moderate if mean systolic transaortic gradient is between 20 mmHg and 40 mmHg or jet velocity is between 3.0 and 4.0 m/s, and severe if mean systolic transaortic gradient is more than 40 mmHg or jet velocity greater than 4.0 m/s. All examinations were performed by an experienced cardiologist who had no knowledge of the patients’ clinical information.
A 12‐lead electrocardiogram with standard chest and limb leads was used to evaluate the presence of fQRS. The paper speed and amplitude were set to 25 mm/s and 10 mm/mV, respectively. The low‐frequency cutoff (high‐pass filter) was set at 0.16 Hz and high‐frequency cut off (low‐pass filters) was set at 100 Hz. All ECGs were interpreted by two cardiologists who had no knowledge of the patients’ clinical information. Fragmented QRS was defined as the QRS complexes with the presence of an additional R wave (R’) or notching in the nadir of the R wave or the S wave, or the presence of >1 R’ in two contiguous leads, corresponding to a major coronary territory (Fig. 1). Some electrocardiographic criteria, namely QRS voltage, intrinsic deflection, LV strain pattern, Cornell voltage and Romhilt‐Estes score were used and compared with fQRS to evaluate severity of aortic stenosis. Intrinsic deflection was measured from the beginning of the QRS complex to the peak of the R wave. LV strain pattern was defined as the presence of down‐sloping convex ST segment with at least 0.5 mm ST depression and concomitant inverted asymmetrical T wave opposite to QRS axis in any of the leads V4‐6, DI, or aVL. Cornell voltage was defined as summation of R‐wave voltage on aVL and S‐wave voltage on V3. Romhilt‐Estes Score was calculated as described elsewhere.12
Figure 1.
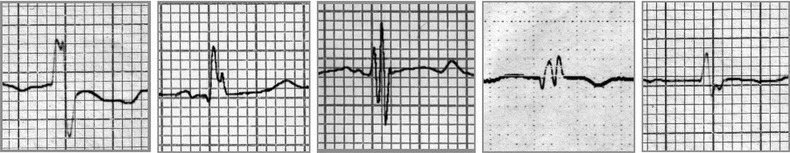
Examples of fragmented QRS.
Demographic information, comorbidities, medications, symptoms, and medical histories of the patients were obtained from patients and hospital archives. Manifest heart failure is defined as current NYHA III and IV heart failure or history of hospitalization with a diagnosis of decompensated heart failure due to the AS, and includes both low output and congestive states.
The SPSS statistical software (SPSS 17.0 for Windows, Inc., Chicago, IL, USA) was used for data analysis. Quantitative data were presented as mean ± standard deviation or median and interquartile range, and categorical variables presented as percentages. After testing data for normal distribution by using Shapiro‐Wilk test, Student's t‐test or Mann‐Whitney U test were used to compare continuous variables. Chi‐square test or Fisher's exact test, as appropriate, were used to identify statistically significant differences for categorical variables. Logistic regression analysis was used to examine an association between fragmented QRS, aortic stenosis, and other variables. Variables with a P value of <0.05 in univariate logistic regression analysis were included in a multivariate logistic regression model. Statistical significance was defined as P < 0.05.
RESULTS
Of 104 consecutive patients, 54 (51.9%) were male and 50 (48.1%) were female, with moderate and severe AS were recruited for the study. Mean age of the patients was 69 ± 14.8. Among the participants, 70 (67.3%) had hypertension, 32 (30.8%) had diabetes mellitus, and 30 (28.8%) had hyperlipidemia.
Seventy six (73.1%) patients had fQRS on their ECGs. Compared to patients without fQRS, patients with fQRS had lower LVEF, higher mean QRS duration, intrinsic deflection, Cornell voltage, Romhilt‐Estes Score, systolic pulmonary artery pressure, mean systolic transaortic gradient, peak systolic transaortic gradient, and left atrium diameter. Left ventricular strain pattern was more frequent in fQRS group. Septal and posterior left ventricular wall thicknesses of the groups were similar. While manifest heart failure was more frequent in patients with fQRS, frequency of atrial fibrillation did not differ significantly between groups (Table 1).
Table 1.
Clinical and Echocardiographic Characteristics of Patients with and without Fragmented QRS
| Patients without fQRS (n = 28) | Patients with fQRS (n = 76) | P Value | |
|---|---|---|---|
| Age (years)a | 68.1 ± 11.4 | 69.3 ± 15.9 | 0.71 |
| Male gender | 18 (64.3%) | 32 (42.1%) | 0.045 |
| Hypertension | 1 (57.1%) | 54 (71.1%) | 0.18 |
| Diabetes mellitus | 10 (35.7%) | 22 (28.9%) | 0.507 |
| Hyperlipidemia | 10 (35.7%) | 20 (26.3%) | 0.348 |
| Smoking | 2 (7.1%) | 14 (18.4%) | 0.225 |
| QRS durationa | 83.9 ± 8.7 | 89.3 ± 10.9 | 0.021 |
| Intrinsic deflectiona | 37.1 ± 4.6 | 43.4 ± 9.5 | 0.001 |
| Cornell voltagea | 1.48 ± 0.40 | 1.98 ± 0.64 | <0.001 |
| Romhilt‐Estes scorea | 2.14 ± 1.91 | 4.55 ± 2.08 | <0.001 |
| LV strain pattern | 6 (21.4%) | 42 (55.2%) | 0.002 |
| Left axis deviation | 8 (28.5%) | 32 (42.1%) | 0.208 |
| LVEF%a | 65.1 ± 3.6 | 63.3 ± 4.5 | 0.037 |
| Septum (mm)a | 13.6 ± 1.4 | 14.3 ± 2.6 | 0.15 |
| Posterior wall (mm)a | 12.5 ± 1.2 | 12.9 ± 1.9 | 0.30 |
| sPAP (mmHg)a | 30.7 ± 5.3 | 43.2 ± 19.8 | <0.001 |
| Mean gradient (mmHg)b | 24.5 (11) | 38.0 (17) | <0.001 |
| Peak gradient (mmHg)b | 45.0 (17) | 61.5 (25) | <0.001 |
| Left atrium (mm)b | 40.5 (5) | 44.0 (9) | 0.01 |
| Heart failure | 2 (7.1%) | 28 (36.8%) | 0.003 |
| Atrial fibrillation | 4 (14.3%) | 20 (26.3%) | 0.20 |
LV = left ventricle; LVEF = left ventricular ejection fraction; sPAP = systolic pulmonary artery pressure.
aPresented as mean ± standard deviation, bPresented as median and interquartile range.
In stepwise multivariate logistic regression analyze, peak systolic transaortic gradient and LVEF were independently associated with fQRS. Furthermore, the presence of fQRS was independently predictive of manifest heart failure (Table 2). Strain pattern and fQRS were independent predictors of severe aortic stenosis in another stepwise multivariate logistic regression model that evaluates association of severe aortic stenosis with electrocardiographic parameters. (Table 3).
Table 2.
Association of Fragmented QRS with Clinical and Echocardiographic Characteristics
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | P Value | OR (95% CI) | P Value |
| LVEF | 0.898 (0.800–1.008) | 0.067 | ‐ | |
| Septum | 1.162 (0.945–1.429) | 0.156 | ‐ | |
| Posterior wall | 1.142 (0.889–1.468) | 0.299 | ‐ | |
| Left atrium | 1.142 (1.053–1.238) | 0.001 | ‐ | |
| Mean gradient | 1.095 (1.040–1.153) | 0.001 | ‐ a | |
| Peak gradient | 1.083 (1.040–1.127) | <0.001 | 1.123 (1.057–1.192) | <0.001 |
| sPAP | 1.069 (1.020–1.121) | 0.005 | 1.101 (1.017–1.193) | 0.018 |
| QRS duration | 1.059 (1.007–1.113) | 0.027 | ‐ | |
| Intrinsic deflection | 1.121 (1.038–1.210) | 0.003 | 1.222 (1.085–1.377) | 0.001 |
| Romhilt‐Estes score | 1.672 (1.325–2.109) | <0.001 | ‐ | |
| Cornell voltage | 4.906 (1.958–12.296) | 0.001 | 4.964 (1.106–22.277) | 0.036 |
| Strain pattern | 4.529 (1.650–12.431) | 0.003 | 5.536 (1.111–27.592) | 0.037 |
| Left axis deviation | 1.818 (0.712–4.645) | 0.212 | ||
| Hypertension | 1.842 (0.750–4.518) | 0.183 | ‐ | |
| Diabetes mellitus | 0.733 (0.293–1.837) | 0.508 | ‐ | |
| Heart failure | 7.583 (1.672–34.391) | 0.009 | 1.876 (1.010–4.983) | 0.042 |
| Atrial fibrillation | 2.222 (0.685–7.205) | 0.183 | ‐ | |
HR = hazard ratio; CI = confidence interval; LVEF = left ventricular ejection fraction; sPAP = systolic pulmonary artery pressure.
Not included in multivariable analyze in order to prevent multicollinearity.
Table 3.
Association of Severe Aortic Stenosis with Electrocardiographic Parameters
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variables | OR (95% CI) | P Value | OR (95% CI) | P Value |
| fQRS | 2.968 (1.082–13.547) | 0.035 | 3.895 (1.120–13.547) | 0.033 |
| QRS duration | 0.976 (0.939–1.014) | 0.218 | ‐ | |
| Intrinsic deflection | 0.995 (0.952–1.041) | 0.835 | ‐ | |
| Romhilt‐Estes score | 1.134 (0.949–1.356) | 0.166 | ‐ | |
| Cornell voltage | 0.778 (0.410–1.476) | 0.442 | ‐ | |
| Strain pattern | 3.545 (1.547–8.127) | 0.003 | 3.291 (1.048–10.329) | 0.041 |
| Left axis deviation | 1.815 (0.786–4.193) | 0.163 | ‐ | |
HR = hazard ratio; CI = confidence interval; fQRS = fragmented QRS.
DISCUSSION
In this study, we aimed to investigate the relationship of fQRS with electrocardiographic, echocardiographic, and clinical features of AS, and demonstrated for the first time that fQRS is independently associated with manifest heart failure, higher peak systolic transaortic gradient, and lower LVEF in patients with moderate and severe AS.
Determining the severity and progression of AS has critical importance in daily clinical practice. Although echocardiography is a more specific technique and gives quantitative information, ECG is more readily available and generally obtained before an echocardiography.13 Left ventricular hypertrophy (LVH), often with secondary ST‐T changes, is typical ECG finding of AS.14 Despite having good specificity, conventional ECG criteria have poor sensitivity in detecting LVH.15 Thus, absence of LVH on ECG does not preclude severe AS. In one study, Romhilt‐Estes score was shown to have 86% sensitivity and 81% specifity for LVH in aortic valve disease and it may be the ECG score of choice to detect LVH in AS patients.16 Left atrial enlargement, left or right axis deviation, atrial fibrillation and conduction anomalies such as right and left bundle block are other common ECG findings of AS.14 QRS duration is also important in AS. In SEAS study, it was shown that longer QRS duration is independently associated with poor prognosis, particularly the risk of sudden cardiac death.17 Significancy of fQRS in AS has not been studied extensively so far. In a study comparing 87 severe AS patients with age‐ and gender‐matched healthy controls, higher frequency of fQRS was detected in severe AS group.18
Fragmented QRS is a sign of myocardial scar and fibrosis.3, 4 Nonhomogenous ventricular activity caused by myocardial scar results in fQRS.19 Furthermore, in patients with left ventricular hypertrophy, fQRS has been suggested to be a result of an intraventricular conduction defect.20 Fibrosis is an early morphological change in patients with AS and it is a determinant of both diastolic and systolic dysfunction. Fibrosis and myocyte enlargement starts in early stages of AS with normal LVEF and elevated left ventricular end diastolic pressure (LVEDP).21 With worsening of fibrosis and myocyte degeneration, LVEDP increases more and later EF decreases.11 Fibrosis is also one of the structural substrates for arrhythmogenicity, thus playing a major role for sudden death and the progression to heart failure.22, 23 The amount of myocardial fibrosis has significant effect on long‐term survival in severe AS even after aortic valve replacement.24
The results of the present study suggest that fragmented QRS indicates the severity of AS, independent from echocardiographic left ventricular wall thicknesses and electrocardiographic indicators of left ventricular hypertrophy. Furthermore, while fQRS predicts severity of AS, traditional LVH criteria such as Cornell voltage and Romhilt‐Estes Score cannot. These results may be considered as a sign of that fQRS is better than electrocardiographic and echocardiographic LVH as an indicator of myocardial fibrosis caused by AS. Thus, we believe fQRS may have a role in determining the severity of AS. Larger trials that assess long‐term prognosis are needed to establish the exact role of fQRS in AS.
The present study has several limitations that should be taken into account when interpreting its results. First, it has a relatively low number of patients from only one center. Second, patients with other severe valvular heart diseases and patients with an LVEF lower than 50% were excluded from the study in order to avoid any possible confounding effect. As a consequence, the results of the study cannot be generalized to all AS patients.
REFERENCES
- 1. Das MK, Michael MA, Suradi H,et al. Usefulness of fragmented QRS on a 12‐lead electrocardiogram in acute coronary syndrome for predicting mortality. Am J Cardiol 2009. Dec 15;104(12):1631–1637. [DOI] [PubMed] [Google Scholar]
- 2. Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113:2495–2501. [DOI] [PubMed] [Google Scholar]
- 3. Das MK, Suradi H, Maskoun W, et al. Fragmented wide QRS on a 12‐lead ECG: A sign of myocardial scar and poor prognosis. Circ Arrhythmia Electrophysiol 2008;1:258–268. [DOI] [PubMed] [Google Scholar]
- 4. Weinberg SL, Reynolds RW, Rosenman RH, et al. Electrocardiographic changes associated with patchy myocardial fibrosis in the absence of confluent myocardial infarction: An anatomic correlative study. Am Heart J 1950;40(5):745–759. [DOI] [PubMed] [Google Scholar]
- 5. Pietrasik G, Goldenberg I, Zdzienicka J, et al. Prognostic significance of fragmented QRS complex for predicting the risk of recurrent cardiac events inpatients with Q‐wave myocardial infarction. Am J Cardiol 2007;100:583–586. [DOI] [PubMed] [Google Scholar]
- 6. Peters S, Trümmel M, Koehler B. QRS fragmentation in standard ECG as a diagnostic marker of arrhythmogenic right ventricular dysplasia‐cardiomyopathy. Heart Rhythm 2008. Oct;5(10):1417–1421. [DOI] [PubMed] [Google Scholar]
- 7. Morita H, Kusano KF, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 2008. Oct 21;118(17):1697–1704. [DOI] [PubMed] [Google Scholar]
- 8. Canpolat U, Kabakçi G, Aytemir K,et al. Fragmented QRS complex predicts the arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol 2013; 24:1260–6 [DOI] [PubMed] [Google Scholar]
- 9. Yuce M, Davutoglu V, Ozbala B, Ercan S, Kizilkan N, Akcay M, Sari I, Akkoyun C, Dogan A, Alici MH, Yavuz F.Fragmented QRS is predictive of myocardial dysfunction, pulmonary hypertension and severity in mitral stenosis. Tohoku J Exp Med 2010. Apr;220(4):279–283. [DOI] [PubMed] [Google Scholar]
- 10. Joint Task Force on the Management of Valvular Heart Disease of theEuropean Society of Cardiology (ESC): European Association for Cardio‐ThoracicSurgery (EACTS) , Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012. Oct;33(19):2451–2496.22922415 [Google Scholar]
- 11. Hein S, Arnon E, Kostin S, et al. Progression from compensated hypertrophy to failure in the pressure‐overloaded human heart: Structural deterioration and compensatory mechanisms. Circulation 2003;107:984–991. [DOI] [PubMed] [Google Scholar]
- 12. Romhilt DW, Estes EH Jr. A point‐score system for the ECG diagnosis of left ventricular hypertrophy. Am Heart J 1968. Jun;75(6):752–758. [DOI] [PubMed] [Google Scholar]
- 13. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons , Bonow RO et al. ACC/AHA 2006 Guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease): Developed in collaboration with the society of cardiovascular anesthesiologists: Endorsed by the society for cardiovascular angiography and interventions and the society of thoracic surgeons. Circulation 2006. Aug 1;114(5):e84–231. [DOI] [PubMed] [Google Scholar]
- 14. Maganti K, Rigolin VH, Sarano ME, et al. Valvular heart disease: Diagnosis and management. Mayo Clin Proc 2010;85(5):483–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reichek N, Devereux RB. Left ventricular hypertrophy: Relationship of anatomic, echocardiographic and electrocardiographic findings. Circulation 1981. Jun;63(6):1391–1398. [DOI] [PubMed] [Google Scholar]
- 16. Buchner S, Debl K, Haimerl J, et al. Electrocardiographic diagnosis of left ventricular hypertrophy in aortic valve disease: evaluation of ECG criteria by cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance 2009, 11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anders M. Greve, Eva Gerdts, Kurt Boman, et al.Impact of QRS duration and morphology on the risk of sudden cardiac death in asymptomatic patients with aortic stenosis. J Am Coll Cardiol 2012;59:1142–1149. [DOI] [PubMed] [Google Scholar]
- 18. Ağaç MT, Korkmaz L, Bektas H, et al. Increased frequency of fragmented QRS in patients with severe aortic valve stenosis. Med Princ Pract 2014;23(1):66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das MK, Suradi H, Maskoun W, et al. Fragmentedwide QRS on a 12‐lead ECG: A sign of myocardial scar and poor prognosis. Circ Arrhythmia Electrophysiol 2008;1:258–268. [DOI] [PubMed] [Google Scholar]
- 20. Surawicz B, et al. Chou's Electrocardiography in Clinical Practice. Philadelphia: Elsevier; 2008. [Google Scholar]
- 21. Krayenbuehl H, Hess OM, Monrad ES, et al. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation 1989;79:744–755. [DOI] [PubMed] [Google Scholar]
- 22. Villari B, Vassalli G, Monrad ES, et al. Normalization of diastolic dysfunction in aortic stenosis late after valve replacement. Circulation 1995;91:2353–2358. [DOI] [PubMed] [Google Scholar]
- 23. Assayag P, Carre F, Chevalier B, et al. Compensated cardiac hypertrophy: Arrhythmogenicity and the new myocardial phenotype 1. Fibrosis Cardiovasc Res 1997;34: 439–444. [DOI] [PubMed] [Google Scholar]
- 24. Milano AD, Faggian G, Dodonov M, et al: Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. J Thorac Cardiovasc Surg 2012;144:830–837. [DOI] [PubMed] [Google Scholar]


