Abstract
Background
Atrial fibrillation is a common cardiac arrhythmia with increasing prevalence in the aging population. It is a major cause of emergency department visits worldwide. Vernakalant, a relatively new antiarrhythmic drug with selectively preferential effects on the atrial tissue is currently used in many European countries for the termination of recent‐onset atrial fibrillation. Presently, the drug is still not approved by the United States Food and Drug Administration due to safety concerns. We evaluate the efficacy and safety of vernakalant for the conversion of recent‐onset atrial fibrillation or atrial flutter into normal sinus rhythm (NSR).
Methods
PubMed/MEDLINE (1993–2017), the Cochrane Central Register of Controlled Trials (2000–2017), and reference lists of relevant articles were searched for randomized controlled trials (RCTs) comparing vernakalant to a control drug and extracted subsequently.
Results
Nine RCTs were identified and included in the meta‐analysis. Pooled analysis of events extracted for a total of 1421 patients with recent‐onset atrial fibrillation showed a statistically significant increase in cardioversion within 90 minutes from drug infusion (Relative Risk [RR], 6.61; 95% Confidence Interval [CI], 2.78 – 15.71; p < .00001). In terms of adverse events, vernakalant was considered safe in comparison to control drugs (RR, 0.80; 95% CI, 0.61–1.05; p = .11).
Conclusion
Vernakalant is effective for rapid conversion of recent‐onset atrial fibrillation into NSR. However, although it showed a safe profile in terms of side effects in this analysis, we are still hesitant about this conclusion and few safety issues should be addressed within specific patients’ subgroups.
Keywords: atrial fibrillation/atrial arrhythmias, cardioversion, pharmacokinetics/dynamics, pharmacology
1. INTRODUCTION
Atrial fibrillation is a common cardiac dysrhythmia with increasing prevalence in the aging population. It remains one of the strongest risk factors for stroke and a major cause of cardiovascular morbidity worldwide (January et al., 2014; Kirchhof et al., 2016). Moreover, this arrhythmia is a frequent cause of hospital presentations and admissions, and is commonly encountered on daily basis especially in the emergency departments (Go et al., 2001; Friberg, Buch, Scharling, Gadsbøll, & Jensen, 2003). Conversion of recent onset atrial fibrillation into normal sinus rhythm (NSR) is essentially important for reducing the risk of hemodynamic instability, atrial remodeling, and the rate of hospitalizations (Conde & Baranchuk, 2014). Conversion into NSR can be achieved either pharmacologically or by direct‐current (DC) electrical cardioversion. While DC electrical cardioversion is more effective and restores NSR instantaneously, it necessitates the use of general anesthesia. Also, it can be associated with psychological stress for the patient along with multiple adverse events such as skin burns, heart block, and pacemaker malfunction (if present) Hanley, Robinson, & Kowey, 2016; Van Gelder, Tuinenburg, Schoonderwoerd, Tieleman, & Crijns, 1999; Conde, Lalor, Rodriguez, Elissamburu, & Marcelo, 2013).
Although antiarrhythmic drug therapy is a cornerstone in the treatment of cardiac arrhythmias, the development of new antiarrhythmics has remained slow despite substantial efforts given. This is most likely due to our limited understanding of the complex interplay between various ionic currents and their definitive role in arrhythmogenesis (Hanley et al., 2016; Kumar & Zimetbaum, 2013). Currently available antiarrhythmic drugs are limited by incomplete efficacy for maintaining NSR along with their extensive side effects profiles such as life‐threatening ventricular arrhythmias and extracardiac toxicities (Camm, 2012; Tsuji & Dobrev, 2013).
Vernakalant is the first antiarrhythmic drug with selectively preferential effects on the atrial tissue. This atrial‐selectivity causes a prolongation in the effective refractory period of the atria with minimal effects on the ventricles (Tsuji & Dobrev, 2013; Dorian et al., 2007; Dobrev, Hamad, & Kirkpatrick, 2010). Vernakalant is hepatically metabolized by cytochrome P450 (CYP 2D6) with a half‐life of approximately 3 hr, and can reach up to 8.5 hr in poor metabolizers (Savelieva, Graydon, & Camm, 2014; Mao, Wheeler, Clohs, Beatch, & Keirns, 2009). The mean plasma concentration of vernakalant peaks at the end of its ten‐minutes infusion and decreases abruptly afterwards. No dose adjustment for age, gender, or renal function is required. Moreover, it is unlikely for vernakalant to cause any major drug‐drug interactions given its rapid distribution, short half‐life and lack of significant effect on CYP3A4 (Savelieva et al., 2014; Mao et al., 2009).
2. METHODS
2.1. Search strategy
In this meta‐analysis, all prospective randomized controlled trials (RCTs) testing the efficacy and safety of vernakalant versus a control drug for conversion of recent‐onset atrial fibrillation or atrial flutter into NSR were identified. An online database search using Cochrane Central Register of Controlled Trials and PubMed/MEDLINE for all relevant trials published since 1993 onward till the date of last search: July 1, 2017 was conducted. English language restriction was applied to the search. Keywords and MeSH terms used included: “atrial fibrillation,” “atrial flutter,” “vernakalant,” “RSD1235,” “clinical trials,” and “randomized controlled trials.” Meeting abstracts or other gray literature weren't included. In addition, a manual search of secondary sources which included reference lists of initially reviewed articles was performed.
2.2. Study selection
Abstracts of trials meeting the following inclusion criteria were considered for review: (i) randomized controlled trial; (ii) studies that compared vernakalant versus a control drug; (iii) patient population with atrial fibrillation or atrial flutter. In addition, full‐text articles of abstracts that were unclear with respect to meeting the inclusion criteria were retrieved for further analysis. A PRISMA (Moher, Liberati, Tetzlaff, & Altman, 2009) flow diagram of the trials’ selection is illustrated in Figure 1. Nine prospective randomized controlled trials were included in this meta‐analysis: The CRAFT trial (Roy et al., 2004); the ACT I trial (Roy et al., 2008); the ACT II trial (Kowey et al., 2009); the ACT III trial (Pratt et al., 2010); the AVRO trial (Camm et al., 2011); the Scene II trial (Camm et al., 2012); Simon et al. trial (Simon et al., 2016); Beatch and Mangal trial (Beatch & Mangal, 2016); and the Asia‐Pacific trial (Beatch, Bhirangi, Juul‐Moller, & Rustige, 2016).
Figure 1.
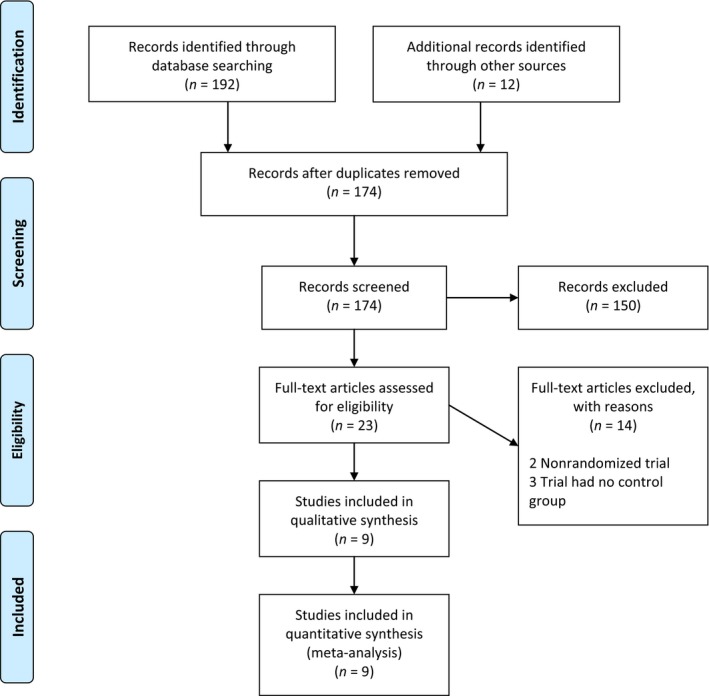
PRISMA 2009 flow diagram
2.3. Data abstraction
Dichotomous data type was extracted, i.e. the number of events and participants in the experimental and the control groups were identified. Relevant baseline characteristics of patients were extracted and presented in Table 1.
Table 1.
Characteristics of the included trials
| CRAFT | ACT I | ACT II | ACT III | AVRO | Scene II | Simon et al. | Beatch and Mangal | Asia‐Pacific trial | |
|---|---|---|---|---|---|---|---|---|---|
| Year | 2004 | 2008 | 2009 | 2010 | 2011 | 2012 | 2016 | 2016 | 2016 |
| Design | Randomized, double‐blind, placebo‐controlled | Randomized, double‐blind, placebo‐controlled | Randomized, double‐blind, placebo‐controlled | Randomized, double‐blind, placebo‐controlled | Randomized, double‐blind, active‐controlled | Randomized, double‐blind, placebo‐controlled | Randomized, controlled | Randomized, placebo controlled | Randomized, double‐blind, placebo‐controlled |
| No. of patients | 56 | 336 | 161 | 262 | 232 | 54 | 100 | 197 | 111 |
| AF duration | 3–72 hr | 3 hr–45 days | 3–72 hr | 3 hr–45 days | 3–48 hr | 3–48 hr (atrial flutter) | No longer than 48 hr | 3 hr–7 days | 3 hr–7 days |
| Primary endpoint | Proportion of patients who converted to NSR within 30 min | Proportion of patients who converted to NSR within 90 min | Proportion of patients who converted to NSR within 90 min | Proportion of patients who converted to NSR within 90 min | Proportion of patients who converted to NSR within 90 min | Proportion of patients who converted to NSR within 90 min | Time to conversion of atrial fibrillation to NSR | Proportion of patients who converted to NSR within 90 min | Proportion of patients who converted to NSR within 90 min |
| Age, mean (SD), y | 64 | 62 (13) | 68 (7) | 62 (11) | 63 (11) | 68 (11) | 57 (15) | 62 (13) | 60 (13) |
| Gender, male/female | 34/22 | 234/336 | 121/40 | 178/84 | 146/86 | 36/16 | 68/32 | 121/76 | 67/44 |
| No. CAD (%) | NR | 68 (20) | 129 (80) | 31(12) | 52 (22) | NR | 7 (7) | 30 (15) | 11 (10) |
| No. CHF (%) | Excluded | 50 (15) | 51 (32) | 52 (20) | 46 (20) | NR | 99 (99) | Excluded | 8 (7) |
| Independent clinical events committee | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
2.4. Study outcome
The primary outcome of interest in this meta‐analysis was the proportion of patients who converted to NSR within 90 minutes following drug infusion, i.e. testing the efficacy of rapid conversion from atrial fibrillation or atrial flutter into NSR. Secondary outcomes of this meta‐analysis included vernakalant safety in patients, which is characterized by the proportion of patients who developed adverse events, hypotension, nonsustained ventricular tachycardia, or death. Adverse events of interest included events prespecified in each trial as a safety endpoint and are considered serious enough or treatment‐emergent warranting immediate medical attention or drug discontinuation.
2.5. Data synthesis and analysis
A meta‐analysis was conducted from the extracted data using Review Manager (RevMan, version 5.4; Cochrane). Pooled risk ratios (RRs) were generated along with their 95% confidence intervals (CIs). To account for between‐studies heterogeneity, the random effects model was used. A p value of <.05 was predefined to indicate statistical significance. Moreover, heterogeneity was assessed using chi‐square test and the I 2 test, with considerable heterogeneity defined as I 2 > 50%.
3. RESULTS
As summarized in Figure 1, and after removing duplicates, the search yielded 174 potentially pertinent articles. Of them, 9 prospective randomized controlled trials were identified and included in the meta‐analysis; Table 1. A total of 1,421 patients with recent‐onset atrial fibrillation were included. Of them, 824 received vernakalant and 597 received control drug. In the vernakalant arm 372 patients converted into NSR within 90 min from drug infusion while only 51 patients who received the control drug converted into NSR. Pooled analysis of events extracted showed a statistically significant result in favor of vernakalant (RR, 6.61; 95% CI, 2.78–15.71; p < .00001); Figure 2. For patients with recent‐onset atrial flutter, only three trials studied the effect of vernakalant in comparison to control drug with 87 patients included. Of them, 39 received vernakalant and 28 received a control drug; only two patients from the vernakalant arm and one patient from the control arm converted into NSR. Pooled analysis of events extracted for the 87 patients did not yield a statistically significant result (RR, 0.80; 95% CI, 0.14–4.73; p = .81); Figure 3.
Figure 2.
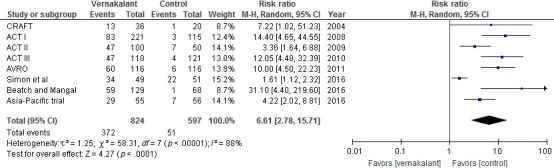
Conversion into NSR within 90 min in patients with atrial fibrillation treated with vernakalant versus control
Figure 3.

Conversion into NSR within 90 min in patients with atrial flutter treated with vernakalant versus control
In terms of adverse events, vernakalant was considered safe in comparison to control drugs (RR, 0.80; 95% CI, 0.61–1.05; p = .11); Figure 4. Hypotensive events were also analyzed in each group and showed no difference among them (RR, 1.51; 95% CI, 0.62–3.68; p = .37); Figure 5. For episodes of nonsustained ventricular tachycardia, there was no difference between the two groups (RR, 0.76; 95% CI, 0.40–1.45; p = .11); Figure 6. Likewise, death events were not statistically significant between the two groups (RR, 2.05; 95% CI, 0.53–7.97; p = .3); Figure 7.
Figure 4.

Adverse events in patients treated with vernakalant versus control
Figure 5.

Hypotension events in patients treated with vernakalant versus control
Figure 6.
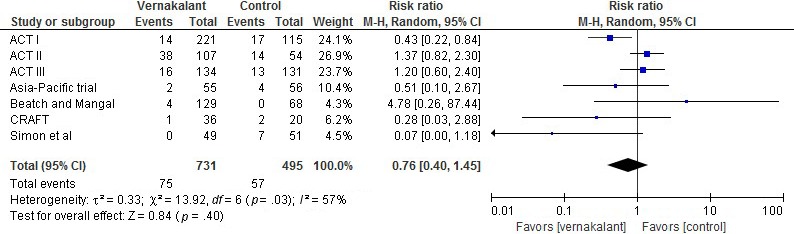
Episodes of nonsustained ventricular tachycardia in patients treated with vernakalant versus control
Figure 7.

Death events in patients treated with vernakalant versus control
4. DISCUSSION
Vernakalant is a relatively new antiarrhythmic agent with predominant properties on atrial electrophysiology. It has been used in many European countries for pharmacological cardioversion of new‐onset atrial fibrillation. At physiological heart rates, the block of atrial sodium (Na+) channels by vernakalant is relatively weak due to rapid unbinding kinetics from the channel. While at higher heart rates, its affinity for activated Na+ channels becomes greater (Tsuji & Dobrev, 2013; Savelieva et al., 2014; Fedida, 2007). Hence, effects on Na+ channels are rate and voltage dependent, i.e. it will likely exert an enhanced inhibitory effect at rapid rates and depolarized potentials as in the fibrillating atria (Conde & Baranchuk, 2014). Moreover, vernakalant inhibits the early activating potassium (K+) channels IKur and IK,ACh. These currents are specific to the atrium and are the ones responsible for prolongation of the atrial refractory period (Fedida, 2007; Fedida et al., 2005; Camm, 2014) Consequently, this atrial selectivity along with the minimal effects on the ventricles explain the low proarrhythmic risk for torsades de pointes (TdP) arrhythmia with vernakalant use (Dobrev et al., 2010; Dorian et al., 2007). At present, vernakalant is only available as intravenous formulation. Oral formulation of vernakalant was initially developed and evaluated in one randomized placebo‐controlled trial for the prevention of atrial fibrillation recurrence after cardioversion. (Torp‐Pedersen et al., 2011) In this trial, patients were randomized to receive 150, 300, or 500 mg of vernakalant vs placebo twice daily for up to 90 days. Only vernakalant 500 mg twice daily was effective and safe for prevention of atrial fibrillation after electrical cardioversion with a median time to first recurrence of >90 days versus 29 days for placebo (p = .0275). Afterwards, the sponsor of vernakalant decided that reluctance from the Food and Drug Administration (FDA) to license the intravenous form of vernakalant rendered further development of the oral form unattractive (Camm, 2014).
According to this meta‐analysis, vernakalant was effective in patients with recent‐onset atrial fibrillation. However, for atrial flutter patients it wasn't. The explanation for its lack of effect is unclear yet. It is possible that more selective and potent blockade of the potassium current, especially IKr, as seen with ibutilide or dofetilide, is required for effective conversion of atrial flutter into NSR (Camm et al., 2012). Moreover, this lack of effect could be related to the lower atrial rate with atrial flutter compared to atrial fibrillation. It is also interesting to observe that atrial flutter has been reported after vernakalant infusion. Although most of the reported cases where self‐limited, some required electrical cardioversion (Roy et al., 2004; Simon et al., 2016; Beatch & Mangal, 2016; Cosin‐Sales et al., 2016; Roy et al., 2008). Nevertheless, there are few described cases of atrial flutter with 1:1 atrioventricular (AV) conduction after administration of vernakalant for atrial fibrillation (Franzini, Muller‐Burri, & Shah, 2014; de Riva‐Silva et al., 2012). The mechanism behind this is also uncertain. It is possible that slowing of atrial conduction due to Na+ channels blocking could have favored the conversion into atrial flutter.
With respect to safety issues, vernakalant has been mainly evaluated within hours to days from drug administration. The most common adverse events were mild to moderate in nature and included dysgeusia, sneezing, and paresthesia, which are probably secondary to Na+ channel blockade in the central nervous system (Savelieva et al., 2014). Currently, the drug is still not approved for use in the United States as the FDA continues to have further questions about the safety of the drug (Camm, 2014). The FDA had concerns about drug‐induced hypotension as well as safety issues in patients with heart failure and acute coronary syndromes. Furthermore, the FDA requested another large trial to be conducted in 2008 to address the drug's safety. The ACT 5 trial was designed for this purpose; however, it was terminated early after a case of cardiogenic shock in a patient who received vernakalant (Camm, 2014).
In the included trials of this meta‐analysis, the reported adverse events were not homogenous across the studies. Moreover, not all the trials specified exactly the adverse events of interest. In addition, none of the included trials specified whether there are certain subgroups of patients who are at higher risk for developing adverse events. For example, it was unclear whether the adverse events happened mainly in heart failure patients or not, especially events of hypotension. Thus, more safety data needs to be collected while taking into consideration the variable comorbidity profiles in the population.
CONFLICT OF INTEREST
The authors have no conflict of interest.
Akel T, Lafferty J. Efficacy and safety of intravenous vernakalant for the rapid conversion of recent‐onset atrial fibrillation: A meta‐analysis. Ann Noninvasive Electrocardiol. 2018;23:e12508 10.1111/anec.12508
REFERENCES
- Beatch, G. N. , Bhirangi, K. , Juul‐Moller, S. , & Rustige, J. (2016). Efficacy and safety of vernakalant for cardioversion of recent‐onset atrial fibrillation in the Asia‐Pacific region: A phase 3 randomized controlled trial. Journal of Cardiovascular Pharmacology, 69, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatch, G. N. , & Mangal, B. (2016). Safety and efficacy of vernakalant for the conversion of atrial fibrillation to sinus rhythm; a phase 3b randomized controlled trial. BMC Cardiovascular Disorders, 16, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm, J. (2012). Antiarrhythmic drugs for the maintenance of sinus rhythm: Risks and benefits. International journal of cardiology, 155, 362–371. [DOI] [PubMed] [Google Scholar]
- Camm, A. J. (2014). The vernakalant story: How did it come to approval in Europe and what is the delay in the U.S.A?. Current Cardiology Reviews, 10, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm, A. , Capucci, A. , Hohnloser, S. , Torp‐Pedersen, C. , Gelder, I. , Mangal, B. , & Beatch, G. . (2011). A randomized active‐controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent‐onset atrial fibrillation. Journal of the American College of Cardiology, 57, 313–321. [DOI] [PubMed] [Google Scholar]
- Camm, A. , Toft, E. , Torp‐Pedersen, C. , Vijayaraman, P. , Juul‐Moller, S. , Ip, J. , … Wyse, D. (2012). Efficacy and safety of vernakalant in patients with atrial flutter: A randomized, double‐blind, placebo‐controlled trial. Europace: European pacing, arrhythmias, and cardiac electrophysiology Journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology, 14, 804–809. [DOI] [PubMed] [Google Scholar]
- Conde, D. , & Baranchuk, A. (2014). Vernakalant for the conversion of atrial fibrillation: The new kid on the block? Annals of Noninvasive Electrocardiology, 19, 299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde, D. , Lalor, N. , Rodriguez, L. , Elissamburu, P. , & Marcelo, T. (2013). Vernakalant versus electrical cardioversion in recent‐onset atrial fibrillation. International Journal of Cardiology, 168, 4431–4432. [DOI] [PubMed] [Google Scholar]
- Cosin‐Sales, J. , Loscos, A. , Peiro, A. , Sorando, M. R. , Buendia, F. , & Ruescas, L. (2016). Real‐world Data on the Efficacy of Vernakalant for Pharmacological Cardioversion in Patients With Recent‐onset Atrial Fibrillation. Revista Española de Cardiología, 69, 619–620. [DOI] [PubMed] [Google Scholar]
- Dobrev, D. , Hamad, B. , & Kirkpatrick, P. . (2010). Vernakalant. Nature Reviews. Drug Discovery, 9:915–916. [DOI] [PubMed] [Google Scholar]
- Dorian, P. , Pinter, A. , Mangat, I. , Korley, V. , Cvitkovic, S. S. , & Beatch, G. N. (2007). The effect of vernakalant (RSD1235), an investigational antiarrhythmic agent, on atrial electrophysiology in humans. Journal of cardiovascular pharmacology, 50, 35–40. [DOI] [PubMed] [Google Scholar]
- Fedida, D. (2007). Vernakalant (RSD1235): A novel, atrial‐selective antifibrillatory agent. Expert Opinion on Investigational Drugs, 16, 519–532. [DOI] [PubMed] [Google Scholar]
- Fedida, D. , Orth, P. M. , Chen, J. Y. , Lin, S. , Plouvier, B. , Jung, G. , … Beatch, G. N. (2005). The mechanism of atrial antiarrhythmic action of RSD1235. Journal of Cardiovascular Electrophysiology, 16, 1227–1238. [DOI] [PubMed] [Google Scholar]
- Franzini, C. , Muller‐Burri, S. A. , & Shah, D. C. (2014). Atrial flutter with 1: 1 atrioventricular conduction after administration of vernakalant for atrial fibrillation. Europace, 16, 3. [DOI] [PubMed] [Google Scholar]
- Friberg, J. , Buch, P. , Scharling, H. , Gadsbøll, N. , & Jensen, G. B. (2003). Rising rates of hospital admissions for atrial fibrillation. Epidemiology, 14, 666–672. [DOI] [PubMed] [Google Scholar]
- Go, A. S. , Hylek, E. M. , Phillips, K. A. , Chang, Y. , Henault, L. E. , Selby, J. V. , & Singer, D. E. (2001). Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA, 285, 2370–2375. [DOI] [PubMed] [Google Scholar]
- Hanley, C. M. , Robinson, V. M. , & Kowey, P. R. (2016). Status of Antiarrhythmic Drug Development for Atrial Fibrillation. Circulation: Arrhythmia and Electrophysiology, 9:e002479. [DOI] [PubMed] [Google Scholar]
- January, C. T. , Wann, L. S. , Alpert, J. S. , Calkins, H. , Cigarroa, J. E. , Cleveland, J. C. , … Field, M. E. (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation, 130, e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhof, P. , Benussi, S. , Kotecha, D. , Ahlsson, A. , Atar, D. , Casadei, B. , … Hendriks, J. (2016). 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European heart journal, 37, 2893–2962. [DOI] [PubMed] [Google Scholar]
- Kowey, P. , Dorian, P. , Mitchell, L. , Pratt, C. , Roy, D. , Schwartz, P. , … Toft, E. . (2009) Vernakalant hydrochloride for the rapid conversion of atrial fibrillation after cardiac surgery: A randomized, double‐blind, placebo‐controlled trial. Circulation Arrhythmia and electrophysiology, 2, 652–659. [DOI] [PubMed] [Google Scholar]
- Kumar, K. , & Zimetbaum, P. J. (2013). Antiarrhythmic drugs 2013: State of the art. Current cardiology reports, 15, 1–8. [DOI] [PubMed] [Google Scholar]
- Mao, Z. L. , Wheeler, J. J. , Clohs, L. , Beatch, G. N. , & Keirns, J. (2009). Pharmacokinetics of Novel Atrial‐Selective Antiarrhythmic Agent Vernakalant Hydrochloride Injection (RSD1235): influence of CYP2D6 Expression and Other Factors. The Journal of Clinical Pharmacology, 49, 17–29. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Annals of internal medicine, 151, 264–269. [DOI] [PubMed] [Google Scholar]
- Pratt, C. , Roy, D. , Torp‐Pedersen, C. , Wyse, D. , Toft, E. , Juul‐Moller, S. , … Drenning, D. . (2010). Usefulness of vernakalant hydrochloride injection for rapid conversion of atrial fibrillation. The American journal of cardiology, 106, 1277–1283. [DOI] [PubMed] [Google Scholar]
- de Riva‐Silva, M. , Montero‐Cabezas, J. M. , Salgado‐Aranda, R. , López‐Gil, M. , Fontenla‐Cerezuela, A. , & Arribas‐Ynsaurriaga, F. (2012). 1: 1 atrial flutter after vernakalant administration for atrial fibrillation cardioversion. Revista Española de Cardiología (English Edition), 65, 1062–1064. [DOI] [PubMed] [Google Scholar]
- Roy, D. , Pratt, C. , Torp‐Pedersen, C. , Wyse, D. , Toft, E. , Juul‐Moller, S. , … Camm, A. (2008). Vernakalant hydrochloride for rapid conversion of atrial fibrillation: A phase 3, randomized, placebo‐controlled trial. Circulation, 117, 1518–1525. [DOI] [PubMed] [Google Scholar]
- Roy, D. , Pratt, C. M. , Torp‐Pedersen, C. , Wyse, D. G. , Toft, E. , Juul‐Moller, S. , … Coutu, B. (2008). Vernakalant hydrochloride for rapid conversion of atrial fibrillation. Circulation, 117, 1518–1525. [DOI] [PubMed] [Google Scholar]
- Roy, D. , Rowe, B. H. , Stiell, I. G. , Coutu, B. , Ip, J. H. , Phaneuf, D. , … Beatch, G. N. (2004). A randomized, controlled trial of RSD1235, a novel anti‐arrhythmic agent, in the treatment of recent onset atrial fibrillation. Journal of the American College of Cardiology, 44, 2355–2361. [DOI] [PubMed] [Google Scholar]
- Savelieva, I. , Graydon, R. , & Camm, A. J. (2014). Pharmacological cardioversion of atrial fibrillation with vernakalant: Evidence in support of the ESC Guidelines. Europace, 16, 162–173. [DOI] [PubMed] [Google Scholar]
- Simon, A. , Niederdoeckl, J. , Skyllouriotis, E. , Schuetz, N. , Herkner, H. , Weiser, C. , … Spiel, A. O. (2016). Vernakalant is superior to ibutilide for achieving sinus rhythm in patients with recent‐onset atrial fibrillation: A randomized controlled trial at the emergency department. Europace, 19, 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp‐Pedersen, C. , Raev, D. H. , Dickinson, G. , Butterfield, N. N. , Mangal, B. , & Beatch, G. N. (2011). A randomized placebo‐controlled study of vernakalant (oral) for the prevention of atrial fibrillation recurrence post‐cardioversion. Circulation: Arrhythmia and Electrophysiology, 4, 637–643. [DOI] [PubMed] [Google Scholar]
- Tsuji, Y. , & Dobrev, D. (2013). Safety and efficacy of vernakalant for acute cardioversion of atrial fibrillation: An update. Vascular Health and Risk Management, 9, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelder, I. C. , Tuinenburg, A. E. , Schoonderwoerd, B. S. , Tieleman, R. G. , & Crijns, H. J. (1999). Pharmacologic versus direct‐current electrical cardioversion of atrial flutter and fibrillation. The American journal of cardiology, 84, 147–151. [DOI] [PubMed] [Google Scholar]


