Abstract
Background
J waves result mainly from an increased density of transient outward current (I to). Mechanical stretch to the heart activates multiple signal transduction pathways, in which I to may be involved. The purpose of this study was to test the hypothesis that mechanical contact of lung cancer with the heart may manifest J waves.
Methods
We reviewed 12‐lead electrocardiograms to examine whether J waves were associated with contact of lung cancer with the heart. J waves were defied as an elevation of ≥0.1 mV at the junction between QRS complex and ST segment with either notching or slurring morphology. The locational interaction between lung cancer and the heart was determined by computed tomography image.
Results
A total of 264 patients (176 men; mean 68.5 ± 10.7 years) with lung cancer were evaluated. The prevalence of J waves was 25.4% in the total population. J waves were present in 40 of 44 (90.9%) patients with the contact. In contrast, J waves were present in 25 of 220 (11.4%) patients without the contact. The sensitivity and specificity of the contact for J waves were 90.9% and 88.6%, respectively. The odds ratio of the contact with the heart to the presence of J waves was 78 (95% confidence interval 25.7–236.4). The appearance of J waves that coincided with the development of lung cancer was observed in 12 patients.
Conclusion
The presence of J waves was associated with the contact of lung cancer with the heart.
Keywords: contact, electrocardiography, heart, J waves, lung cancer
1. Introduction
J waves are manifest on electrocardiogram (ECG) as a small secondary R wave (R′) at the junction between QRS complex and ST segment or a slurred wave at the terminal portion of QRS complex. J waves were traditionally referred to as Osborne waves in hypothermic condition (Osborn, 1953). Recently, attention has been paid to J waves as an arrhythmogenic ECG marker in idiopathic ventricular fibrillation (VF; Haissaguerre et al., 2008), Brugada syndrome (Kawata et al., 2013), and myocardial ischemia (Antzelevitch & Yan, 2010; Naruse et al., 2012; Rudic et al., 2012). An experimental study (Badri, Patel, & Yan, 2015) demonstrated that transmural voltage gradient due to transient outward current (I to). I to is more prominent in the ventricular epicardium and the M cell than in the endocardium, mediating a spike‐and‐dome morphology of action potential. This contributed to the generation of J waves. The transmural electrical gradient, when pronounced, exerted to form phase 2 reentry, providing a fundamental mechanism regarding J wave‐associated ventricular arrhythmia.
Anecdotal reports (Asteriou, Lazopoulos, Giannoulis, Kalafatis, & Barbetakis, 2013; Nakazato, Ohmura, Shimada, & Daida, 2003) demonstrated that mediastinal tumor was associated with the appearance of Brugada‐type ECG pattern which was restored to a normal ECG pattern after the tumor disappeared. These reports suggest that mechanical compression to the heart may cause a Brugada‐type ECG pattern to manifest. Likewise, pectus excavatum was attributed to a Brugada‐type ECG pattern (Awad et al., 2013; Kataoka, 2002) Mechanical stretch on the myocardium alters biological regulation, including genetic expressions, intracellular signal transductions, and ionic currents (Hamill & Martinac, 2001). Since lung cancer occupies lesional volume in almost fixed space surrounded by the thorax, intrathoracic tumor may exert a mechanical force to the heart. However, whether or not J waves occur coincidently with lung cancer is unknown. We hypothesized that contact force by lung cancer to the heart may increase the density of I to, thus presenting J waves. The purpose of this study was to test this hypothesis.
2. Materials and Methods
2.1. Study population
A total of 264 patients (176 men; mean age, 68.5 ± 10.7 years) who suffered lung cancer were enrolled in this study. The lung cancer was primary in all patients of this study. The patients were admitted in the University Hospital of Shiga University of Medical Science. No patient had any heart disease on the hospital admission. Figure 1 depicts the number of patients who underwent 12‐lead ECG recording, computed tomography (CT) of the chest, or both before, on, and after the hospital admission. All patients underwent these examinations on the hospital admission. ECGs were recorded before the hospital admission in 11 of 264 patients. One hundred and three of 264 patients underwent chest CT and ECG recording after the hospital admission. None met the criteria for a typical ECG pattern associated with Brugada syndrome (i.e., coved‐type ST‐segment elevation in the right precordial leads). No patients had family history of sudden cardiac death. Medications that may affect J waves, such as β‐blocker and calcium antagonist, were not prescribed in any patients. In addition, patients who were prescribed antiarrhythmic drugs, psychotropic drugs, or anesthetics were not present. No one took alcohol or cocaine habitually. All patients were afebrile when they underwent ECG recording and CT imaging. The research protocol was approved by the Institutional Review Board of Shiga University of Medical Science (approval number: 19‐75).
Figure 1.
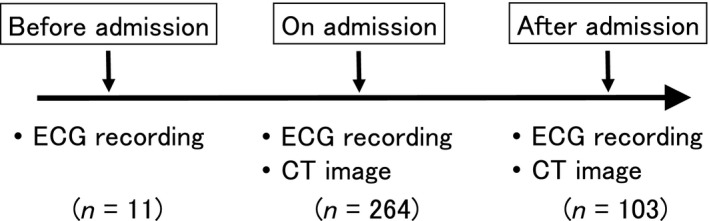
A schema depicting data collection in this study. Twelve‐lead electrocardiograms (ECGs) and chest‐computed tomography (CT) were recorded on hospital admission in all patients enrolled (n = 264). Eleven patients underwent ECG recording before hospital admission; while 103 patients underwent ECG recording and CT image after hospital admission
2.2. Data analysis
Electrocardiogram analysis was performed using software (MUSE7.1, GE Marquette Medical Systems, Inc., Milwaukee, WI, USA). The 12‐lead ECG was recorded at rest for 10 s at a sweep speed of 25 mm/s, calibrated to 1 mV/cm in the standard leads. The ECG data were digitally stored in a 12‐bit server computer with a sampling interval of 2 ms. The ECGs were recorded before, on, and after the hospital admission. The J wave was defined as the degree of the J‐point elevation with an amplitude of ≥0.1 mV. The morphology was either slurred (a smooth transition from the QRS complex to the ST segment) or notched (a positive deflection at the terminal QRS complex) in at least two conjugate leads. The sites of ECG leads presenting J wave were anterior precordial leads (V1 to V3), inferior leads (II, III, and aVF), lateral leads (I, aVL, and V4 to V6), or inferolateral leads (II, III, aVF, I, aVL, and V4 to V6). The J‐point amplitude was measured at the QRS‐ST junction in case of slurred morphology or the peak J‐point in the case of notched morphology. Two trained cardiologists independently evaluated 12‐lead ECGs for the presence of J wave with a blind technique. All ECGs containing J‐wave patterns were double‐checked. Patients with bundle branch block or intraventricular conduction disturbance were excluded from this study.
A 64‐slice computed tomography scan (Aquilion ONE, Toshiba Medical Systems, Corp., Otawara, Japan) was introduced to determine location of lung cancer and determine whether or not lung cancer contacted the heart with no contrast media. During the CT scan, patients were laid in a supine position and held their breath. The findings of CT images were interpreted in a blind manner to ECG findings.
2.3. Statistical analysis
Data were presented as mean ± standard deviation, number, or percentage. Group comparisons were made using t test or Mann–Whitney test, as appropriate. Categorical variables were compared using χ2 test. All statistical tests were two tailed, and a value of p < .05 was considered significant.
3. Results
3.1. Characteristics of study population
J waves were found in 67 of 264 patients, resulting in the overall prevalence of 25.4% in this study population. Table 1 shows the characteristics of J waves. The notched J waves were more prevalent than the slurred J waves. The J waves were most frequently present in inferior leads. In the patients presenting J waves, 44 (65.7%) patients showed contact of lung cancer with the heart on CT image. Figure 2 shows a representative case who presented J waves in leads II, III, and aVF. This patient was an 80‐year‐old male. There existed lung cancer in the right lower field on his chest X‐ray. Chest CT image showed that the lung cancer contacted the heart. Figure 3 shows a representative case who did not present J waves in any lead. This patient was a 63‐year‐old female. Lung cancer was situated near the hilum of the left lung on his chest X‐ray. Chest CT image revealed that the lung cancer was located away from the heart. Table 2 shows the relation between J waves and contact of lung cancer with the heart. J waves were observed in 40 of 44 (90.9%) of patients whose lung cancer contacted the heart. In contrast, J waves were present only in 25 of 220 (11.4%) patients whose lung did not contact the heart on CT image (p < .0001, by χ2 test). The sensitivity and specificity of the contact to the presence of J waves were 90.9% and 88.6%, respectively. The odds ratio of the contact with the heart to the presence of J waves was 78 (95% confidence interval 25.7–236.4).
Table 1.
Characteristics of J waves (n = 65)
| J‐wave morphology | ||
|---|---|---|
| Notching (n = 45) | Slurring (n = 20) | |
| Amplitude of J waves | ||
| ≥0.2 mV | 9 (20.0) | 8 (40.0) |
| Site of J waves | ||
| Inferior leads (II, III, aVF) | 34 (75.6) | 16 (80.0) |
| Lateral leads (I, aVL, V5,6) | 6 (13.3) | 0 (0) |
| Inferolateral leads (II, III, aVF, I, aVL, V5,6) | 2 (4.4) | 1 (5.0) |
| Right anterior leads (V1–3) | 3 (6.7) | 3 (15.0) |
Values are expressed as n (%).
Figure 2.
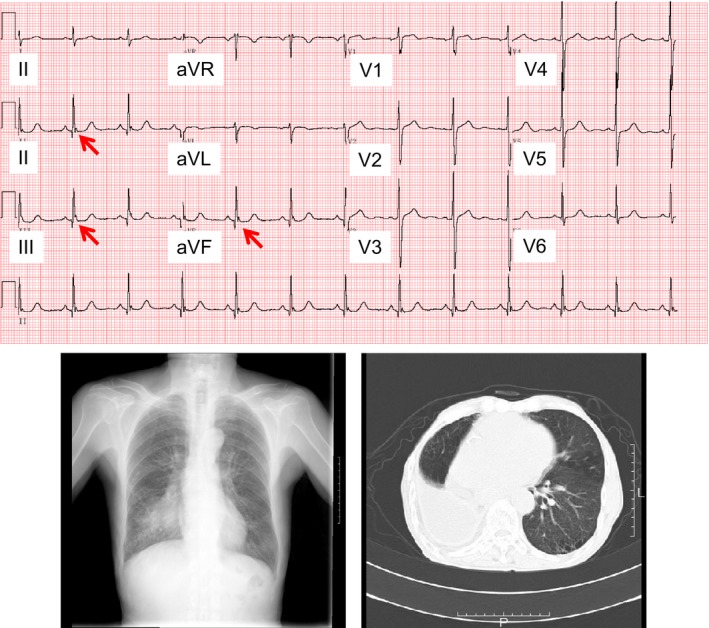
An 80‐year‐old man with notched J waves (arrows) in leads II, III, and aVF on 12‐lead ECG. Chest x‐ray shows that lung cancer is located in the right lower field. CT image demonstrates that lung cancer contacts the heart
Figure 3.
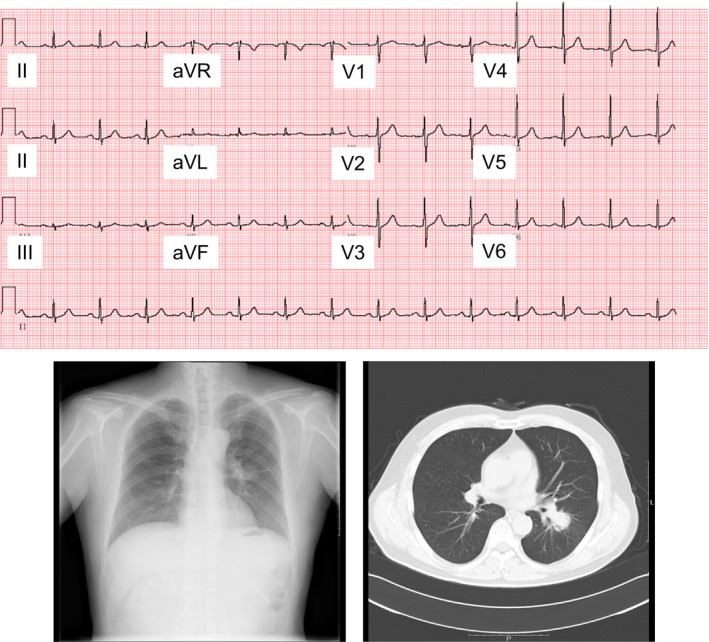
A 63‐year‐old woman without any J wave on 12‐lead ECG. Lung cancer is present near the left hilum of lung on chest x‐ray. CT image demonstrates that lung cancer exists apart from the heart
Table 2.
Relation between J waves and contact of lung cancer with the heart
| Contact (+, n = 44) | Contact (–, n = 220) | |
|---|---|---|
| J waves (+, n = 65) | 40 (90.9) | 25 (11.4) |
| J waves (–, n = 199) | 4 (9.1) | 195 (88.6) |
Values are expressed as n (%) in each 2 × 2 cell. In each cell, the number in parentheses indicates a ratio of patients with or without J waves in the presence of contact of lung cancer with the heart and that of patients with or without J waves in the absence of contact of lung cancer with the heart.
3.2. Locational interaction between J waves and lung cancer
Table 3 shows interaction between locations of lung cancer and ECG leads presenting J waves in patients whose lung cancer contacted the heart (n = 40). Most of J waves were present in inferior leads, when lung cancer existed in the right lung. Similarly, the majority of J waves were present in inferior leads, when lung cancer existed in the left lung.
Table 3.
Relation between location of lung cancer and ECG leads presenting J waves (n = 40)
| Location of lung cancer | ||
|---|---|---|
| Right lung (n = 20) | Left lung (n = 20) | |
| Inferior leads (II, III, aVF) | 14 (70.0) | 17 (85.0) |
| Lateral leads (I, aVL, V5,6) | 1 (5.0) | 1 (5.0) |
| Inferolateral leads (II, III, aVF, I, aVL, V5,6) | 2 (10.0) | 2 (10.0) |
| Right anterior leads (V1–3) | 3 (15.0) | 0 (0) |
Values are expressed as n (%).
3.3. Appearance of J wave
J waves appeared in eight of 11 patients who underwent ECG recording before the hospital admission and in four of 103 patients who underwent ECG recording after the hospital admission. Figure 4 shows a representative case whose J waves appeared with development of lung cancer. This patient was a 61‐year‐old woman. She did not show J waves before the hospital admission at the age of 59 years old (Figure 4a). She was admitted to our University Hospital for the treatment of lung cancer at the age of 61 years old. On the hospital admission, J waves appeared in leads II, III, and aVF (Figure 4b) and CT image showed contact of lung cancer with the heart. Figure 5 shows a representative case whose J waves appeared with exacerbation of lung cancer. This patient was a 57‐year‐old man. He was admitted to treat lung cancer. On the hospital admission, J waves were absent and the lung cancer did not contact with the heart (Figure 5a). However, 1 year after the hospital admission, the lung cancer worsened, contacting the heart. J waves were simultaneously present in leads II, III, and aVF (Figure 5b).
Figure 4.
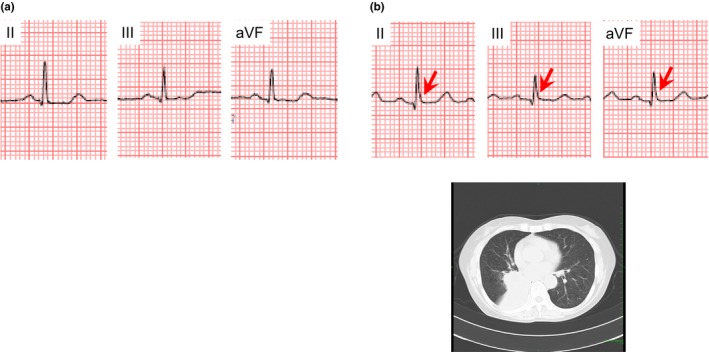
A 61‐year‐old woman who presented J waves coincidently with development of lung cancer. (a) No J wave was detected before the hospital admission. (b) J waves with slurring morphology (arrows) appeared in leads II, III, and aVF, when lung cancer contacted the heart on the hospital admission
Figure 5.
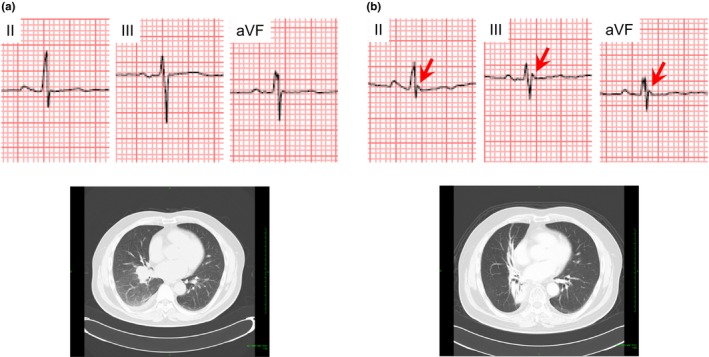
A 57‐year‐old man in which J waves appeared after hospital admission. (a) J waves were absent, when lung cancer did not contact the heart on the hospital admission. (b) J waves with notched morphology (arrows) appeared in lead II, III, and aVF, when lung cancer contacted the heart because of the exacerbation after the hospital admission
3.4. Follow‐up
We assessed whether patients with J waves developed life‐threatening events in 179 (67.8%) patients whose prognosis could be pursued after the hospital admission. During the mean follow‐up period of 1,016.4 ± 772.1 days, 49 patients died. No patients developed life‐threatening events due to cardiac origin. Although CT image and ECG recording were taken during the follow‐up period in 103 patients, disappearance of J waves could not be seen in patients who had J waves on the hospital admission, because ECG recording was not taken in patients whose lung cancer was resolved after the treatments such as surgical resection and/or chemotherapy.
4. Discussion
Osborne first reported J waves in hypothermic dogs in 1953 (Osborn, 1953). J waves have recently been recognized in patients with hypothermia as secondary depolarizations with small amplitude at the terminal portion of QRS complex (Okada, Nishimura, Yoshino, Kimura, & Ogino, 1983; Thompson, Rich, Chmelik, & Nelson, 1977) Coexistence of J waves with early repolarization suggested that J waves are involved in repolarizing process of the membrane potential (Gussak, Bjerregaard, Egan, & Chaitman, 1995). Yan and Antzelevitch (1996) performed an elegant experiment showing a significant causal relationship of transmural voltage gradient during phase 1 of action potential with J waves on ECG. Hence, they proposed a critical role of I to in generating J waves. In this study, we for the first time found the association of J waves with lung cancer. The major findings are as follows: (i) the prevalence of J waves was more frequent in patients with lung cancer contacting the heart than in patients with lung cancer apart from the heart, (ii) the appearance of J waves was related to the development of lung cancer, and (iii) no life‐threatening events derived from cardiac origin occurred in patients with J waves during the follow‐up. These results provide the first evidence in support of the hypothesis that an occurrence of mechanical contact force on the ventricular myocardium accentuates the manifestation of J waves.
4.1. J waves and lung cancer
J‐wave manifestation was attributed to various factors, including anoxia, injury current, acidosis, delayed ventricular depolarization, and early ventricular repolarization. Presence of action potential notch during phase 1 that is more prominent in the epicardium than in the endocardium results in the transmural repolarization gradient, presenting J waves at a junction between QRS complex and ST segment (Yan & Antzelevitch, 1996). The regional difference in the contribution of I to was verified with patch‐clamp techniques (Liu, Gintant, & Antzelevitch, 1993). Therefore, the shape and the amplitude of J waves depend on (i) the transmural distribution of action potential notch amplitude, (ii) the relative time course of the early phases of action potential at different sites within the ventricular wall, (iii) the propagation sequence of activation in the ventricle, and (iv) the conduction time across the ventricular wall.
Tarin, Farre, Rubio, Tunon, and Castro‐Dorticos (1999) reported a Brugada‐like ECG in patients with metastatic tumor in the mediastinal space that compressed the right ventricular outflow tract. In their case, the tumor‐induced mechanoelectric transduction might affect ionic channels and gap junction conductance in the right ventricular outflow tract, presumably giving rise to the rSR′ pattern and the ST‐segment elevation in the right precordial leads. Disappearance of these ECG abnormalities that occurred after surgical removal of the tumor suggests the possible effect of mechanoelectric transduction on J waves. In Brugada syndrome, a coved‐type ST‐segment elevation in the right precordial leads is mainly due to the transmural repolarization gradient from the endocardium to the epicardium caused by increased I to in the right ventricular outflow tract. The transient outward current that accentuates the action potential results in the prolongation of action potentials of the epicardium in the region of the right ventricular outflow tract, causing negative T wave in the same leads. We (Miyamoto, Hayashi, Ito, & Horie, 2011) experienced a case with anterior mediastinal tumor which surrounded the right ventricular outflow tract appeared to be associated with ST‐segment elevation, conduction disturbance, and abnormal repolarization in the right precordial leads. This study demonstrated that appearance of J waves was observed coincidently with development of lung cancer. This phenomenon may result from the reverse process of the above‐mentioned anecdotal reports (Miyamoto et al., 2011; Tarin et al., 1999).
4.2. Possible mechanisms of J waves in lung cancer
I to density is attributed to gene expression. The molecular basis for the transmural distribution of I to has been proposed. The transmural gradient of I to in the dog has been ascribed to a transmural distribution of (i) KCND3 gene (Kv4.3) encoding the subunit of the I to channel, (Zicha et al., 2004), (ii) KChIP2, a subunit that coassembles with Kv4.3, (Rosati et al., 2001), and (iii) IRX5, a transcriptional factor regulating KCND3 (Costantini et al., 2005; Rosati et al., 2001). Although we did not investigate whether gene mutation was present in this study population, it might be speculated that the mechanical stretch through the contact force of lung cancer to the heart could affect gene expression, altering I to availability. In addition, the mechanical stretch to the myocardium may modulate stretch‐activated ion channel availability (Peyronnet, Nerbonne, & Kohl, 2016). Furthermore, the mechanical stretch may increase Ca2+ current, which may result in an increase in Ca2+‐sensitive I to. Besides, I to increases when myocardial ischemia occurs. Given the contact force to the epicardial myocardium results in disturbance of microcirculation, regional ischemia may cause I to to increase.
Another mechanism of J waves might be due to intraventricular conduction delay (Antzelevitch et al., 2016). In Brugada syndrome, fibrosis and reduced connexin expression in the right ventricular outflow tract renders conduction slow and discontinuous, which leads to arrhythmogenesis (Nademanee et al., 2015). This scenario is supported by the evidence that application of radiofrequency ablation to the epicardium in the right ventricular outflow tract reduced arrhythmic events as well as ameliorated ST‐segment elevation in the right precordial leads (Nademanee et al., 2011). Besides the right ventricular outflow tract, conduction delay present somewhere else in the ventricle may give rise to J waves. Contact of the heart with lung cancer or the diaphragm may generate slow conduction in the ventricle, contributing to manifestation of J waves.
The locational interaction of J waves with lung cancer deserves noting. As shown in Table 3, the J waves were most prevalent in the inferior leads. This finding is different from an actual contact site of lung cancer with the heart. It is speculated that the diaphragm may play a role in generating J waves in this study. When the heart is compressed by lung cancer from the upper part, the heart may shift downward. Under this pathologic situation, the inferior wall attaching to the diaphragm may exert contact force, which could give rise to manifesting J waves. In addition, I to may be abundant in the inferior wall of the ventricle compared to other sites of the left ventricle.
To date, J waves have been reported to be a cautious marker indicating possibility of life‐threatening arrhythmia. Despite the high prevalence rate of J waves in this study population, no life‐threatening arrhythmic events occurred. We do not know the reason exactly; but, a possible explanation is that the follow‐up period of this study was too short as compared to previous studies (Haissaguerre et al., 2008; Olson, Viera, Soliman, Crow, & Rosamond, 2011; Rollin et al., 2012; Sinner et al., 2010; Tikkanen et al., 2009).
4.3. Study limitations
There are some limitations in this study. Fist, most results of this study were obtained mainly from the cross‐sectional observation. Second, though no patient suffered from life‐threatening arrhythmia, whether or not J waves observed in patients with lung cancer are arrhythmogenic remains further to be investigated. Therefore, we have to continue to assess the prognosis of this study population.
5. Conclusions
A spatial dispersion of repolarization caused by heterogeneous expression of I to may be caused by the contact dynamics of lung cancer in this study. This study provided a novel clinical entity of J waves.
Conflict of Interest
All authors declare no conflict of interest related to this study.
Acknowledgments
We thank Seiichi Fujisaki and Tatsumi Uchiyama (GE Yokokawa Medical System Co.) for technical assistance. We also thank Yoshihisa Fujisawa, Takako Ishigaki, Hiromi Izumi, Chihiro Ohkuni, Yuriko Sawada, and Shoko Shimizu (Shiga University of Medical Science Hospital) for ECG recording.
Hayashi H, Wu Q, Horie M. The relationship between J waves and contact of lung cancer with the heart. Ann Noninvasive Electrocardiol. 2017;22:e12433 10.1111/anec.12433
Funding information
There was no funding source related to this study.
References
- Antzelevitch, C. , & Yan, G. X. (2010). J Wave syndromes. Heart Rhythm: The Official Journal of the Heart Rhythm Society, 7, 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch, C. , Yan, G. X. , Ackerman, M. J. , Borggrefe, M. , Corrado, D. , Guo, J. , … Wilde, A. A. (2016). J‐Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge. Heart Rhythm: The Official Journal of the Heart Rhythm Society, 13, e295–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asteriou, C. , Lazopoulos, A. , Giannoulis, N. , Kalafatis, I. , & Barbetakis, N. (2013). Brugada‐like ECG pattern due to giant mediastinal lipoma. Hippokratia, 17, 368–369. [PMC free article] [PubMed] [Google Scholar]
- Awad, S. F. , Barbosa‐Barros, R. , Belem Lde, S. , Cavalcante, C. P. , Riera, A. R. , Garcia‐Niebla, J. , & Baranchuk, A. (2013). Brugada phenocopy in a patient with pectus excavatum: Systematic review of the ECG manifestations associated with pectus excavatum. Annals of Noninvasive Electrocardiology: The Official Journal of the International Society for Holter and Noninvasive Electrocardiology, 18, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri, M. , Patel, A. , & Yan, G. (2015). Cellular and ionic basis of J‐wave syndromes. Trends in Cardiovascular Medicine, 25, 12–21. [DOI] [PubMed] [Google Scholar]
- Costantini, D. L. , Arruda, E. P. , Agarwal, P. , Kim, K. H. , Zhu, Y. , Zhu, W. , … Bruneau, B. G. (2005). The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell, 123, 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussak, I. , Bjerregaard, P. , Egan, T. M. , & Chaitman, B. R. (1995). ECG phenomenon called the J wave. History, pathophysiology, and clinical significance. Journal of Electrocardiology, 28, 49–58. [DOI] [PubMed] [Google Scholar]
- Haissaguerre, M. , Derval, N. , Sacher, F. , Jesel, L. , Deisenhofer, I. , de Roy, L. , … Clémenty, J. (2008). Sudden cardiac arrest associated with early repolarization. New England Journal of Medicine, 358, 2016–2023. [DOI] [PubMed] [Google Scholar]
- Hamill, O. P. , & Martinac, B. (2001). Molecular basis of mechanotransduction in living cells. Physiological Reviews, 81, 685–740. [DOI] [PubMed] [Google Scholar]
- Kataoka, H. (2002). Electrocardiographic patterns of the Brugada syndrome in 2 young patients with pectus excavatum. Journal of Electrocardiology, 35, 169–171. [DOI] [PubMed] [Google Scholar]
- Kawata, H. , Morita, H. , Yamada, Y. , Noda, T. , Satomi, K. , Aiba, T. , … Shimizu, W. (2013). Prognostic significance of early repolarization in inferolateral leads in Brugada patients with documented ventricular fibrillation: A novel risk factor for Brugada syndrome with ventricular fibrillation. Heart Rhythm: The Official Journal of the Heart Rhythm Society, 10, 1161–1168. [DOI] [PubMed] [Google Scholar]
- Liu, D. W. , Gintant, G. A. , & Antzelevitch, C. (1993). Ionic bases for electrophysiological distinctions among epicardial, midmyocardial, and endocardial myocytes from the free wall of the canine left ventricle. Circulation Research, 72, 671–687. [DOI] [PubMed] [Google Scholar]
- Miyamoto, A. , Hayashi, H. , Ito, M. , & Horie, M. (2011). Remission of abnormal conduction and repolarization in the right ventricle after chemotherapy in patients with anterior mediastinal tumor. Journal of Cardiovascular Electrophysiology, 22, 350. [DOI] [PubMed] [Google Scholar]
- Nademanee, K. , Raju, H. , de Noronha, S. V. , Papadakis, M. , Robinson, L. , Rothery, S. , … Behr, E. R. (2015). Fibrosis, connexin‐43, and conduction abnormalities in the Brugada syndrome. Journal of the American College of Cardiology, 66, 1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nademanee, K. , Veerakul, G. , Chandanamattha, P. , Chaothawee, L. , Ariyachaipanich, A. , Jirasirirojanakorn, K. , … Ngarmukos, T. (2011). Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation, 123, 1270–1279. [DOI] [PubMed] [Google Scholar]
- Nakazato, Y. , Ohmura, T. , Shimada, I. , & Daida, H. (2003). Brugada‐like precordial ST elevation on ECG by anterior mediastinal infective mass lesion. Indian Pacing and Electrophysiology Journal, 3, 184. [PMC free article] [PubMed] [Google Scholar]
- Naruse, Y. , Tada, H. , Harimura, Y. , Hayashi, M. , Noguchi, Y. , Sato, A. , … Aonuma, K. (2012). Early repolarization is an independent predictor of occurrences of ventricular fibrillation in the very early phase of acute myocardial infarction. Circulation. Arrhythmia and Electrophysiology, 5, 506–513. [DOI] [PubMed] [Google Scholar]
- Okada, M. , Nishimura, F. , Yoshino, H. , Kimura, M. , & Ogino, T. (1983). The J wave in accidental hypothermia. Journal of Electrocardiology, 16, 23–28. [DOI] [PubMed] [Google Scholar]
- Olson, K. A. , Viera, A. J. , Soliman, E. Z. , Crow, R. S. , & Rosamond, W. D. (2011). Long‐term prognosis associated with J‐point elevation in a large middle‐aged biracial cohort: The ARIC study. European Heart Journal, 32, 3098–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn, J. J. (1953). Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. The American Journal of Physiology, 175, 389–398. [DOI] [PubMed] [Google Scholar]
- Peyronnet, R. , Nerbonne, J. M. , & Kohl, P. (2016). Cardiac mechano‐gated ion channels and arrhythmias. Circulation Research, 118, 311–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollin, A. , Maury, P. , Bongard, V. , Sacher, F. , Delay, M. , Duparc, A. , … Ruidavets, J. B. (2012). Prevalence, prognosis, and identification of the malignant form of early repolarization pattern in a population‐based study. American Journal of Cardiology, 110, 1302–1308. [DOI] [PubMed] [Google Scholar]
- Rosati, B. , Pan, Z. , Lypen, S. , Wang, H. S. , Cohen, I. , Dixon, J. E. , & McKinnon, D. (2001). Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. The Journal of Physiology, 533, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudic, B. , Veltmann, C. , Kuntz, E. , Behnes, M. , Elmas, E. , Konrad, T. , … Schimpf, R. (2012). Early repolarization pattern is associated with ventricular fibrillation in patients with acute myocardial infarction. Heart Rhythm: The Official Journal of the Heart Rhythm Society, 9, 1295–1300. [DOI] [PubMed] [Google Scholar]
- Sinner, M. F. , Reinhard, W. , Muller, M. , Beckmann, B. M. , Martens, E. , Perz, S. , … Kääb, S. (2010). Association of early repolarization pattern on ECG with risk of cardiac and all‐cause mortality: A population‐based prospective cohort study (MONICA/KORA). PLoS Medicine, 7, e1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarin, N. , Farre, J. , Rubio, J. M. , Tunon, J. , & Castro‐Dorticos, J. (1999). Brugada‐like electrocardiographic pattern in a patient with a mediastinal tumor. Pacing and Clinical Electrophysiology, 22, 1264–1266. [DOI] [PubMed] [Google Scholar]
- Thompson, R. , Rich, J. , Chmelik, F. , & Nelson, W. (1977). Evolutionary changes in the electrocardiogram of severe progressive hypothermia. Journal of Electrocardiology, 10, 67–70. [DOI] [PubMed] [Google Scholar]
- Tikkanen, J. T. , Anttonen, O. , Junttila, M. J. , Aro, A. L. , Kerola, T. , Rissanen, H. A. , … Huikuri, H. V. (2009). Long‐term outcome associated with early repolarization on electrocardiography. New England Journal of Medicine, 361, 2529–2537. [DOI] [PubMed] [Google Scholar]
- Yan, G. X. , & Antzelevitch, C. (1996). Cellular basis for the electrocardiographic J wave. Circulation, 93, 372–379. [DOI] [PubMed] [Google Scholar]
- Zicha, S. , Xiao, L. , Stafford, S. , Cha, T. J. , Han, W. , Varro, A. , & Nattel, S. (2004). Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. The Journal of Physiology, 561, 735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]


