Abstract
Background
Brugada syndrome (BrS) is defined as presenting of type‐1 Brugada pattern (BrP). BrS can also be induced by fever. This study demonstrated a highest prevalence of fever‐induced BrS ever reported.
Method
During May 2014, febrile (oral temperature ≥ 38 °C) and nonfebrile patients underwent standard and high leads (V1 and V2 at 2nd intercostal space) electrocardiogram. Risk factor and cardiac symptoms were recorded. Patients with a persistent of type‐1 BrP after fever had subsided were excluded. The prevalence of BrS, type‐2 BrP and early repolarization pattern (ERP) were demonstrated.
Results
A total of 401 patients, 152 febrile, and 249 nonfebrile, were evaluated. BrS was identified in six febrile patients (five males and one female) and two males in nonfebrile patients. The study demonstrated higher prevalence of BrS in febrile group compared to nonfebrile group (4.0% vs 0.8%, respectively, P = 0.037). Among fever‐induced BrS patients, three patients (50.0%) experienced cardiac symptoms before and at the time of presentation and two patients (33.3%) had history of first‐degree relative sudden death. No ventricular arrhythmia was observed. All of type‐1 BrP disappeared after fever had subsided. We found no difference in prevalence of type‐2 BrP in febrile and nonfebrile group (2.0% vs 2.8%, respectively, P > 0.05) as well as ERP (3.3% vs 6.4%, respectively, P > 0.05).
Conclusions
Our study showed a highest prevalence of fever induced BrS ever reported. A larger study of prevalence, risk stratification, genetic test and management of fever‐induced BrS should be done, especially in an endemic area.
Keywords: brugda syndrome, fever, prevalence
Brugada syndrome (BrS) is a heritable disease associated with ventricular fibrillation or aborted sudden cardiac death (SCD) characterized by ST‐segment elevation in right precordial leads (V1and V2) in the absence of ischemia, electrolyte disturbance or other structural heart diseases.1, 2 According to the endemic area in Asian and Southeast Asian countries, the prevalence of BrS has been reported in recent years, especially in Thailand, Philippines, Japan, and Singapore varying from 0.5 to 1 per 1000.3, 4 In a recent study, the prevalence of BrS in febrile patients surprisingly increased approximately 20 times higher than afebrile group.5 The pathophysiology of fever induced BrS is not yet known but believed to be due to the depolarization and repolarization abnormalities explained by temperature dependence on both of wild‐type cardiac sodium channel and mutated SCN5A cardiac sodium channel.6, 9 However, the prevalence of fever‐induced BrS remains inconclusive. In this study, the prevalence of fever‐induced BrS was thus evaluated.
BrS is an autosomal dominant inherited disease with variable penetrance.1 Over 70 mutations have been reported. However, about 20% of cases have SCN5A mutation that is described by accelerated inactivation of sodium channels.7 Men are at risk eight to nine times more than woman to express the phenotype of mutation that leads to develop BrS.10 BrS is reported up to 20% of SCDs in particular normal structural heart patients.9,11 Type‐1 (coved pattern) BrP is described by initial ST elevation ≥2 mm, then slowly descending, with concave or rectilinear down slopping, and necessary followed by a negative T wave in V1 or V2.1 The new type‐2 BrP (saddle back), from the recent consensus, which is the combination of type‐2 and type‐3 BrP is described as high takeoff r’ followed by a convex ST elevation ≥5 mm with positive or flat T wave in V2.1 According to the revised diagnosed criteria, BrS should be diagnosed when (1) ST segment elevation with type‐1 morphology ≥2 mm in ≥1 lead in right precordial leads occurring either spontaneously or after provocative Class I antiarrhythmic drug test or (2) ST segment elevation with type‐2 or type‐3 in ≥1 right precordial leads when Class I antiarrhythmic provocative drug test induces a type‐1 morphology.2 The clinical manifestations of BrS include ventricular fibrillation (VF) or aborted SCD (usually at night), syncope, nocturnal agonal respiration, palpitations or chest discomfort. The symptoms usually present in the fourth decade of life11 and the mean age of sudden death is 41 ±15 years.10
A large number of cases of BrS unmasked by fever was reported in last decades; moreover, fever has also been suggested as a trigger of malignant ventricular arrhythmias.5 Certainly, fever precipitated malignant arrhythmias in 18% of patients that presented with cardiac arrest in symptomatic BrS.12 Fever is now accepted to be a factor inducingBrS.5
This study reported that the prevalence of type‐1 BrS in febrile population is 20 times higher than afebrile group, which disclosed the real prevalence of BrS up to 2% including asymptomatic carrier during febrile stage. Therefore, in an endemic area of BrS such as in Asian countries, the prevalence of BrS including asymptomatic carrier might be higher than previously reported.
MATERIALS AND METHODS
During May 2014, we conducted our study at Buriram Hospital's emergency department. We included all adults (≥15 years old) and febrile patients (defined as body temperature ≥38 °C by oral temperature). Afebrile patients (defined as body temperature ≤38 °C oral temperature) are randomly selected as controlled group. Patients who agreed to participate were informed and gave consent. We excluded patients who did not agree to participate in the study. Patients with history of structural heart disease, patients with myocardial infarction, and newly diagnosed structural heart disease were also excluded. Every febrile patient presented in the department underwent standard and high‐lead electrocardiography (ECG), in which V1 and V2 precordial electrodes were placed at second intercostal space instead of third intercostal space in standard electrocardiography. A control group of afebrile patients was selected randomly from the same emergency department during the same period. The control group underwent the same standard ECG and high‐lead ECG measurement. Patients’ demographic data, past medical history, previous cardiac symptoms, cardiac risk factors, and causes of fever were recorded using standard questionnaires. The ECGs were reviewed by two electrophysiology experts (TN and TW) for the possibilities of type I, II, and III BrP. BrP was diagnosed by using latest criteria.13 Moreover, early repolarization pattern (ERP) was also reviewed by recent criteria.14 In febrile group with suspected BrP, we also recorded their ECGs after their fever had subsided to confirm fever‐induced BrP. Drug challenge test was not done.The study was approved by ethics committee of Faculty of Medicine, Ramathibodi Hospital, Mahidol University.
Patients with type I, II BrP underwent further evaluation including echocardiography to look for underlying structural heart disease and regular follow up with experts. We encouraged the patients to bring their family for ECG.
STATIC ANALYSIS
Fisher's exact test was used to compare the prevalence of BrS between febrile and afebrile group. Age difference between febrile and nonfebrile group was also be evaluated by t‐test. The chi‐square test was used to compare the gender difference between fever and nonfever. To calculate confidence interval of the prevalence, we used two‐sided confidence interval for a single proportion method including continuity correction.15
RESULT
A total of 416 patients were enrolled in the study including all of 158 febrile patient and randomly selected 258 nonfebrile patients presented from the emergency departments, Buriram Hospital. Fifteen patients were excluded because of history of structural heart disease (N = 7), acute myocardial infarction (N = 6), no ECG during nonfebrile state (N = 1) and persisting BrP during nonfebrile state (N = 1). Therefore, 401 patients, 152 febrile and 249 nonfebrile, were evaluated. There is no difference in mean age between febrile and nonfebrile group respectively (54.8 ± 19.6 and 51.2 ± 18.0, P > 0.05). No significant difference is seen between gender and body temperature (P > 0.05). BrS was identified in six febrile patients (five males and one female) and two males in nonfebrile patients. The study demonstrated higher prevalence of BrS in febrile group compared to nonfebrile group (4.0% vs 0.8%, respectively, P < 0.05). Therefore, in our study, type 1 BrP was seen five times more common in febrile state compared to nonfebrile state. The estimate 95% confident interval was 1.9–8.8% for patients with fever and 0.1–3.2% for patients without fever. Mean age of febrile BrS was younger compared to nonfebrile patients; however, it is not statistically significant (48.2 ± 25.2 vs 54.8 ± 20.0 years, P > 0.05). Specifically in male group, the prevalence of BrS was higher in febrile male compared to nonfebrile. This is, however, not statistically significant (5.3% vs 1.3%, P > 0.05).
All of the patients presented with fever‐induced type‐1 BrP (Fig. 1) were admitted with 24 hours ECG monitored until fever subsided. Mean temperature of fever‐induced BrS was 38.8 ± 0.8 °C. Among fever‐induced BrS patients, three patients (50.0%) experienced cardiac symptoms before and at the time of presentation, and two patients (33.3%) had history of first‐degree relative sudden death. No ventricular arrhythmias was observed in any patient during admission. All of type‐1 BrP had disappeared after fever subsided (Fig. 1). The characteristics of Type‐1 BrP are summarized in Table 1. Most of the patients (five of six) were presented with fever because of an infection including acute pharyngitis, cholangitis and gastroenteritis. There was only one patient that presented with the process of inflammation from appendicitis. He successfully underwent appendectomy without any complications or arrhythmic events during the operation.
Figure 1.
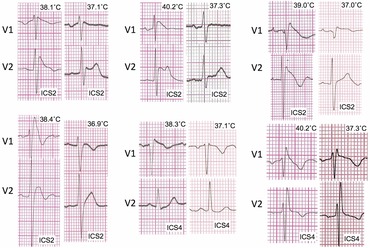
ECG representing type one BrP of six patients diagnosed fever‐induced BrS. Temperature is located at the right upper angle of each ECG. ICS (Inter Costal Space) ECG placement is located at right lower angle of each ECG.
Table 1.
Characteristic of Type‐1 BrP Patients Including Age, Gender, Temperature, Fever Etiology, Echocardiogram, Nonfebrile ECG, and Cardiac Symptoms
| Age | Fever | Nonfebrile | Cardiac | |||||
|---|---|---|---|---|---|---|---|---|
| No. | (years) | Gender | Temperature | Etiology | Echocardiogram | ECG | Symptoms | Remarks |
| 1 | 19 | Male | 38.4 °C | Pharyngitis | Normal | Normal | Chest Pain | SCD in father, patient refused to perform EPS |
| 2 | 20 | Male | 38.6 °C | Pharyngitis | Normal | RBBB | – | – |
| 3 | 82 | Male | 39.0 °C | Appendicitis | Normal | Normal | – | SCD in brother, EPS was not advised due to elderly |
| 4 | 50 | Male | 40.2 °C | Cholecystitis | Normal | Normal | Chest tightness | – |
| 5 | 50 | Male | 38.1 °C | Gastroenteritis | Normal | Type‐2 BrP | Palpitation | – |
| 6 | 68 | Female | 38.3 °C | UTI | Normal | Normal | – | – |
EPS = electrophysiology study; RBBB = right bundle branch block; UTI = urinary tract infection.
In nonfebrile group, two nonfebrile patients were presenting in the emergency department because of motorcycle accident and swelling from nephrotic syndrome. None of the patients was using any drug that could potentiate BrP ECG.
We found no difference in prevalence of type‐2 BrP in febrile and nonfebrile group (2.0% vs 2.8%, respectively, P > 0.05). No type‐3 BrP was discovered. Prevalence of ERP is not different between the febrile and the nonfebrile group (3.3% vs 6.5%, respectively, P > 0.05). Interestingly, ERP is found more frequent in males than females in both febrile (5.3% vs 0% respectively, P < 0.01) and nonfebrile groups (9.8% vs 1.0%, respectively, P < 0.01).
Two high‐risk patients with history of first‐degree relative SCD were advised to undergo Electrophysiology Study (EPS) and install intracardiac defibrillator. However, the patients refused.
Four of six BrS in febrile patients were diagnosed only by using high leads placement (Fig. 1). Moreover, all of two BrS in nonfebrile patient were diagnosed only by using high leads placement. In our study, six of eight patients (75%) were diagnosed only by high leads placement.
All of the patients had experienced episodes of fever before; however, they could not remember the association between prior episodes of fever and related cardiac symptoms.
FOLLOW‐UP
Because all of the patients are living in the remote area of Thailand, they refused to come to the hospital despite strong advice to follow up. However, they were followed up by telephone at one year after the diagnosis, they are living well without any cardiac event or death.
DISCUSSION
Fever has been concerned as an inducing factor of BrS and exacerbating ventricular arrhythmias. There is only one study reporting the prevalence of fever‐induced BrS, which is 2%.5 Interestingly, there was no Southeast Asian population in the study. This is the first study of fever‐induced BrS from an endemic area, Buriram province, in northeast region of Thailand. Our study demonstrated the highest prevalence of fever‐induced BrS ever reported. The prevalence of fever‐induced BrS was up to 4.0% in febrile population and even higher up to 5.3% in febrile male.
Moreover, one third of the patients had a history of first‐degree relative sudden death and half of the patients had symptoms of BrS, which could be defined as symptomatic BrS. This is in contrast to a previous study in which none of the fever‐induced BrS patients had symptoms or history of first‐degree relative sudden death.5 Due to higher rate of first‐degree relative SCD in fever‐induced BrS from our study, we suspected that, in the endemic area such as northeast region of Thailand, the genetic mutation might play a role in this different phenomenon. A large‐scale study of fever‐induced BrS including genetic test should be pursued.
However, in the absence of fever, every BrP ECG disappeared during nonfebrile state. Interestingly, one of the patients had ECG transformed into type‐2 BrP, and another one had ECG transformed into right bundle branch block. This pattern of transformation has also been reported in several studies and case reports.16
Interestingly, six of eight patients (75%) in our study were only diagnosed by using high lead placement. Because of high sensitivity to detect BrP of high leads ECG placement,17 it could explain why our study could detect more BrP ECG than the previous study.5 In our study, all of the BrP ECG were independently confirmed by two cardiac electro‐physiologists and reevaluated after fever subsided. All of the BrP disappeared. We could confirm that the BrP in our patients was diagnosed precisely without false positive results.
LIMITATION
For the limitations of our study, we conducted the study only in one hospital from the northeast region of Thailand, and only the patients that went to the emergency department were recruited. Thus, the result might not describe the real prevalence of BrS in northeast region of Thailand. First‐degree relative SCD in the family of one patient might be a false positive, because it was an unexplained car accident; however, the history fit with the definition of SCD previously described.18 Moreover, we had excluded one patient who died of severe sepsis before fever subsided. Therefore, we did not have a conclusion if this patient would be diagnosed as fever‐induced BrS or not. The prevalence of fever induced BrS might be even higher than 4.0%.
Information of genetic mutation was limited in this study, because SCN5A and others gene mutation were not performed. A future study of genetic mutation should be done, because no conclusion about genetic variation in particular fever‐induced BrS patients are reported.
CONCLUSIONS
Our study demonstrated a highest prevalence of fever‐induced BrS ever reported. Fever Induced BrS is more common than previously suspected. A larger multicenter and multidepartment study of prevalence, genetic mutation, risk stratification, and management of fever induced BrS should be done, especially in an endemic area.
Acknowledgments
The authors would like to thank Dr. Vijj Kasemsup, M.D., Ph.D., and Dr. Somkiat Leelasithorn, M.D., Department of Community Medicine, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand, for statistical consultations.
Author contributions: PR, TN: conception and research design; PR, WV, AC, NK, TN, AP, TW, TJ, SR: performed study; PR, WV, AC, NK, AP: data analysis; PR, WV, AC, NK, AP: drafted manuscript; PR, TN: edited and revised manuscript.
Financial Support: None.
Conflict of Interest: None to declare.
REFERENCES
- 1. Bayes de LA, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: A consensus report. J Electrocardiol 2012;45:433–442. [DOI] [PubMed] [Google Scholar]
- 2. Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–1963. [DOI] [PubMed] [Google Scholar]
- 3. Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada Syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005;111:659–670. [DOI] [PubMed] [Google Scholar]
- 4. Sidik NP, Quay CN, Loh FC, et al. Prevalence of Brugada sign and syndrome in patients presenting with arrhythmic symptoms at a Heart Rhythm Clinic in Singapore. Europace 2009;11:650–656. [DOI] [PubMed] [Google Scholar]
- 5. Adler A, Topaz G, Heller K, et al. Fever‐induced Brugada pattern: How common is it and what does it mean? Heart Rhythm 2013;10:1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilde AA, Postema PG, Di Diego JM, et al. The pathophysiological mechanism underlying Brugada syndrome: Depolarization versus repolarization. J Mol Cell Cardiol 2010;49:543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Probst V, Wilde AA, Barc J, et al. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ Cardiovasc Genet 2009;2:552–557. [DOI] [PubMed] [Google Scholar]
- 8. Chockalingam P, Clur SA, Breur JM, et al. The diagnostic and therapeutic aspects of loss‐of‐function cardiac sodium channelopathies in children. Heart Rhythm 2012;9:1986–1992. [DOI] [PubMed] [Google Scholar]
- 9. Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res 1999;85:803–809. [DOI] [PubMed] [Google Scholar]
- 10. Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: Report of the second consensus conference. Heart Rhythm 2005;2:429–440. [DOI] [PubMed] [Google Scholar]
- 11. Benito B, Brugada J, Brugada R, et al. Brugada syndrome. Rev Esp Cardiol 2009;62:1297–1315. [DOI] [PubMed] [Google Scholar]
- 12. Amin AS, Meregalli PG, Bardai A, et al. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Int Med 2008;149:216–218. [DOI] [PubMed] [Google Scholar]
- 13. Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. J Arrhyth 2014;30:1–28. [Google Scholar]
- 14. Tikkanen JT, Huikuri HV. Characteristics of "malignant" vs "benign" electrocardiographic patterns of early repolarization. J Electrocardiol 2015;48:390–4. doi: 10.1016/j.jelectrocard.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 15. Newcombe RG Two‐sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med 1998;17:857–872. [DOI] [PubMed] [Google Scholar]
- 16. Rattanawong P, Ngarmukos T, Wisaratapong T. Discovering Brugada syndrome during preoperative evaluation. J Anesth 2015; Jun 29:480. [DOI] [PubMed] [Google Scholar]
- 17. Govindan M, Batchvarov VN, Raju H, et al. Utility of high and standard right precordial leads during Ajmaline testing for the diagnosis of Brugada syndrome. Heart 2010;96:1904–1908. [DOI] [PubMed] [Google Scholar]
- 18. Priori SG, Aliot E, Blomstrom‐Lundqvist C, et al. Task force on sudden cardiac death of the European Society of Cardiology. Eur Heart J 2001;22:1374–1450. [DOI] [PubMed] [Google Scholar]


