Abstract
Background
Antazoline is an old antihistaminic and new antiarrhythmic agent with unknown mechanisms of action which recently has been shown to effectively terminate atrial fibrillation. The aim of study was to examine the effects of antazoline on hemodynamic and ECG parameters.
Methods
Antazoline was given intravenously in three 100 mg boluses to 10 healthy volunteers (four males, mean age 40 + 11 years). Hemodynamic and ECG parameters were measured using impedance cardiography [systolic (sBP), diastolic (dBP), mean (mBP) blood pressure, stroke volume (SV), cardiac output (CO), total peripheral resistance (TPR) and heart rate (HR), P wave, PR interval, QRS complex, QT and corrected QT (QTcF) interval]. Plasma concentration of antazoline was also measured.
Results
Antazoline caused significant prolongation of P wave, QRS as well as QT and QTcF (101 ± 10 vs 110 ± 16 ms, p < .05, and 101 ± 12 vs 107 ± 12 ms, p < .05, 399 ± 27 vs 444 ± 23 ms, p < .05, and 403 ± 21 vs 448 ± 27 ms, p < .05, respectively). Also, a significant decrease in SV was noted (94.9 ± 21.8 vs 82.4 ± 19.6 ml, p < .05). A significant correlation between changes in plasma drug concentration and changes in CO, HR, and dBP was found.
Conclusions
Antazoline impairs slightly hemodynamics, significantly reducing SV. Significant prolongation of P wave and QRS duration corresponds to drug‐induced prolongation of conduction, whereas QT prolongation represents drug‐induced prolongation of repolarization.
Keywords: antazoline, antazoline concentration, antiarrhythmic agent, atrial fibrillation, hemodynamic and electrocardiographic effects
1. Introduction
Antazoline is an old first‐generation antihistaminic and antiallergic agent with antiarrhythmic and anticholinergic properties. The mechanisms of antiarrhythmic action of the drug remain unknown. Data from literature show that antazoline is very effective in termination of atrial fibrillation (AF) (Farkowski et al., 2015, 2016; Piotrowski et al., 2014), but the drug is not listed in the formal guidelines because there are no published randomized controlled trials confirming its efficacy and safety (Camm et al., 2010, 2012; January et al., 2014) However, in some countries, it is registered and widely used for termination of AF.
Despite the fact that the drug has been known for a long time, the studies evaluating drug‐induced ECG and hemodynamic effects as well as pharmacokinetic profile of antazoline have not been conducted. The dosing of the drug remains empirical and side effects have not been systematically evaluated. In general, antazoline has been administered to patients with AF and no significant organic heart disease in rapid intravenous boluses. The optimal dosing and time to sinus rhythm restoration have not been well established.
Drug‐induced changes in hemodynamic parameters may be noninvasively measured by impedance cardiography (ICG) which accuracy was confirmed in studies comparing this method with invasive measurements (Cybulski et al., 2004; Fortin et al., 2006; Gimbel, 2005; Zgoła et al., 2014) The ICG also allows continuous monitoring of ECG. (Zgoła et al., 2014).
The aim of this study was to examine the effects of antazoline on hemodynamic and ECG parameters in relation to the plasma levels of the drug. This report is a second part of the ELEPHANT study [ELEctrophysiological, pharmacokinetic, and hemodynamic effects of PHenazolinum (ANTazoline mesylate)], which evaluates the pharmacokinetic profile of antazoline and its impact on hemodynamic, ECG and electrophysiological parameters. The ELEPHANT I was completed and showed relative fast drug elimination and relatively high volume of distribution of antazoline (Giebułtowicz, Piotrowski et al., 2016; Giebułtowicz, Kojro et al., 2016).
2. Methods
2.1. Study group
The study protocol was approved by the Local Ethics Committee of the Postgraduate Medical School, Warsaw, Poland (No. 17/PB/2014). Ten healthy volunteers—members of electrophysiology team in our institution (six women and four men, mean age 41 ± 11 years), without structural heart disease, were enrolled to this study after giving a written informed consent. One subject had hypertension. The mean height, weight and BMI were 1.7 ± .1 m, 71.4 ± 13.7 kg and 24.4 ± 3.4 kg m−2, respectively. Echocardiographic parameters were within normal limits: mean left atrial dimension was 33 ± 4 mm, left ventricular ejection fraction—65 ± 2%, and ventricular septal wall thickness—10 ± 2 mm. Laboratory parameters were normal in all subjects.
The exclusion criteria were lack of written informed consent, age < 18 years, known allergy to antazoline, presence of significant heart disease such as heart failure with NYHA class > II or left ventricular ejection fraction (LVEF) < 40%, uncontrolled hypertension or documented coronary artery disease. Other contraindications included cardiac rhythm other than sinus, systolic blood pressure (sBP) < 90 mmHg, presence of liver or kidney failure or/and chronic obstructive pulmonary disease or/and diabetes mellitus, active inflammatory process, and in women—a positive pregnancy test or the presence of menstruation.
2.2. Administration of antazoline
Antazoline mesylate (Phenazolinum, Polfa, Poland) was given intravenously in three consecutive boluses of 100 mg each, injected over 1 min with 2 min intervals between boluses. Thus, the final cumulative target dose of antazoline was 300 mg. According to the protocol, injection of antazoline could have been stopped immediately if significant side effects occurred. This drug regimen was chosen based on previous clinical observations suggesting that to achieve optimal drug efficacy duration of injections and intervals between boluses should be short and that cumulative doses exceeding 300 mg did not significantly increase drug efficacy.
2.3. Measurements of hemodynamic and electrocardiographic parameters
The hemodynamic and ECG parameters were measured using device for continuous hemodynamic and autonomic assessment (Task Force Monitor Systems, CNSystems, Austria) which allows noninvasive examination of various hemodynamic and ECG parameters by measuring changes in the transthoracic electrical impedance. A detailed methodology has been described elsewhere (Zgoła et al., 2014).
All the recordings and drug injections were performed in quiet environment in the electrophysiology laboratory. The ICG electrodes were placed at both sides of the thorax and at the neck base. The ECG electrodes were positioned in standard locations. After 10 min allowed for stabilization of hemodynamic parameters and sympatho‐vagal balance, the ICG and ECG recordings were started and followed by antazoline injections (Zgoła et al., 2014).
The ICG and ECG parameters were recorded continuously. For the statistical analysis, short‐term periods of ICG and ECG recordings were used, registered just before drug administration, 2 min after each bolus of the drug and 4, 6, 8, 10, 30, and 60 min after third (last) antazoline bolus administration. They consisted of 30 consecutive cardiac cycles recorded in these prespecified time points and the average of these measurements was used in the final analysis. Care was taken to include only good‐quality signals and all the artifacts were excluded after a visual assessment of the recordings. The following ICG parameters were measured:
-
1
Stroke volume (SV) [ml], calculated using the formula:
VEPT × (dZmax/Z0) × LVET, where VEPT is the part of the electrically participating thoracic volume, calculated from weight, height, age and gender, dZmax is systolic amplitude [Ohm s−1], Z0 stands for total thoracic impedance [Ohm], and LVET is the left ventricular ejection time [ms].
-
2
Cardiac output (CO) [L min−1], calculated using the formula:
CO = SV × heart rate.
-
3
Total peripheral resistance (TPR) [dyn × s cm−5] calculated using the formula:
(mBP−CVP)/CO × 80, where mBP is the mean arterial blood pressure [mmHg] and CVP is central venous pressure.
Finger pletysmography was also used to obtain beat‐to‐beat systolic (sBP), diastolic (dBP) and mean (mBP) blood pressure values.
The ECG parameters were also recorded and analyzed using the Task Force Monitor system. The analyzed parameters included heart rate (HR), P wave, PR interval, QRS complex, QT and corrected (according to the Fridericia formula) QT (QTcF) interval durations. All the measurements were performed using electronic calipers at a paper speed of 100 mm s−1.
2.4. Methods of measurement of concentration of antazoline
2.4.1. Chemicals
The reference standard of antazoline mesylate was a gift from Polfa Warszawa S.A (Wola, Poland). Xylomethazoline was purchased from Sigma‐Aldrich (St. Louis, MO, USA). The solvents, HPLC gradient grade methanol, formic acid 98%, ant ethyl acetate were purchased from Merck (Darmstadt, Germany). Ultrapure water was obtained from the Millipore water purification system (Milli‐Q, Billerica, MA, USA).
2.4.2. Sample analysis
Approximately 2 ml blood samples were collected (sodium citrate as an anticoagulant, 3.2%) and plasma concentration of antazoline was measured 2 min following each bolus and 4, 6, 8, 10, 30, and 60 min after last drug injection. Immediately after collection, blood samples were centrifuged at 2000 g for 15 min at room temperature and plasma samples were aliquoted and stored at −80°C until analysis. The concentration of the antazoline was measured by Liquid Chromatography Tandem Mass Spectrometry (LC‐MS/MS) with xylomethazoline as an internal standard (Giebułtowicz, Piotrowski et al., 2016; Giebułtowicz, Kojro et al., 2016).
2.5. Statistical analysis
Since continuous variables were normally distributed the results are presented as the arithmetic mean (± SD) and the differences between examined parameters were evaluated by one‐way repeated‐measures analysis of variance (ANOVA). Because the assumption of sphericity was violated, Geisser‐Greenhouse correction was applied. If an analysis of variance yielded statistically significant value of G‐G preplanned comparisons were performed. We focused on a few specific comparisons rather than on every possible comparison so we preplanned hypothesis a priori. The simple contrast was used, where the baseline measures were the reference levels. For the calculation of correlation between concentration of antazoline as well as hemodynamic and ECG parameters Pearson's correlation coefficient was used. Statistical analysis was carried out using the SAS version 9.2 statistical system. Plots were created with the STATISTICA suite of analytic software. A p value < .05 was considered significant.
3. Result
3.1. Side effects
The drug was well tolerated by all volunteers. Side effects included transient heat sensation in the throat and lower abdomen as well as funny taste. These mild symptoms occurred shortly after injection and disappeared within 2 min in eight subjects. Longer lasting side effects included vertigo that lasted up to 3 hr after last dose of antazoline and was present in an upright position in five volunteers.
3.2. Drug‐induced ECG changes
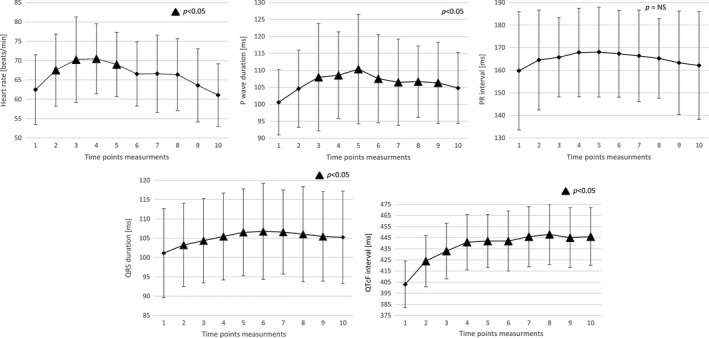
Antazoline significantly accelerated HR with a maximum value achieved 2 min after third dose of antazoline (62 ± 9 at baseline vs 70 ± 11 beats min−1—maximal value, p = .0141). The increase in HR remained significant up to 4 min from the final drug bolus (p = .0027). Afterwards HR returned steadily to the baseline values.
The P‐wave duration significantly increased starting from 2 min after second dose of the drug and remained significantly longer than the baseline value up to 30 min after the last dose of antazoline (101 ± 10 at baseline vs 110 ± 16 ms—maximal value, p = .0432, and for trend p = .0448).
The PR interval also increased with a maximum value between 2 and 4 min after third dose of antazoline, however, these changes did not reach significant difference compared with baseline measurements.
The QRS interval started to be significantly longer from the second minute after the first dose of the drug and remained significantly prolonged up to 60 min from the last dose of antazoline (p = .0325). The maximal QRS prolongation was recorded between 4 and 8 min after the last dose (101 ± 12 at baseline vs 107 ± 11 – 12 ms ‐ maximal value, p < .0001, p = .0086, p = .0008, respectively, and for trend p = .0325).
The QTcF interval also significantly increased 2 min after the first dose of antazoline and remained significantly longer than baseline value up to 60 min after the last dose of the drug (403 ± 21 at baseline vs 448 ± 27 ms—maximal value) (p < .0001). Between 2 and 60 min after the last dose of the drug, the values of QTcF were relatively stable.
The mean values of ECG parameters measured at prespecified time points are shown in Table 1 and in Figure 1, whereas drug‐induced ECG changes (Δ—change from baseline) are shown in Figure 2.
Table 1.
Mean values of ECG parameters measured at prespecified time points
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| HR (beats min−1) | 62 ± 9 | 68 ± 9 | 70 ± 11 | 70 ± 9 | 69 ± 8 | 67 ± 8 | 67 ± 10 | 66 ± 9 | 64 ± 9 | 61 ± 8 |
| P (ms) | 101 ± 10 | 105 ± 11 | 108 ± 16 | 109 ± 13 | 110 ± 16 | 108 ± 13 | 107 ± 13 | 107 ± 11 | 106 ± 12 | 105 ± 10 |
| PR (ms) | 160 ± 26 | 165 ± 22 | 166 ± 18 | 168 ± 20 | 168 ± 20 | 167 ± 19 | 166 ± 20 | 165 ± 18 | 163 ± 23 | 162 ± 24 |
| QRS (ms) | 101 ± 12 | 103 ± 11 | 104 ± 11 | 105 ± 11 | 107 ± 11 | 107 ± 12 | 107 ± 11 | 106 ± 12 | 105 ± 12 | 105 ± 12 |
| QTcF (ms) | 403 ± 21 | 424 ± 23 | 433 ± 25 | 441 ± 25 | 442 ± 24 | 442 ± 27 | 446 ± 27 | 448 ± 27 | 445 ± 27 | 446 ± 26 |
HR, heart rate; P, P‐wave duration; PR, PR interval; QRS, QRS duration; QTcF, corrected QT interval calculated using the Fridericia formula.
Figure 1.
Mean ± SD values of examined ECG parameters at prespecified time points
Figure 2.
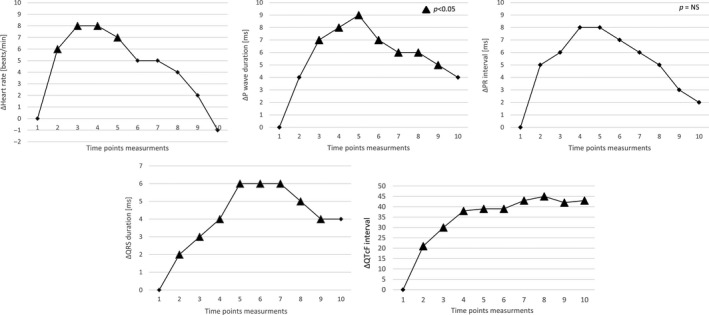
Relative drug‐induced ECG changes (Δ—change from baseline)
3.3. Drug‐induced changes in hemodynamic parameters
The changes in blood pressure values (sBP, dBP, and mBP) were not significant when compared with baseline values (p = .3292, p = .2243, p = .3746, respectively). There was a trend toward a decrease in BP values immediately after antazoline injection and a trend toward an increase in BP values starting from the second time point measurement.
The SV started to be significantly lower from the second minute after the second bolus of antazoline and this change lasted up to 10 min following last dose (94.9 ± 21.8 vs 82.4 ± 19.6 ml, p = .0082, and for trend p = .0489). After that SV slightly increased but it did not return to the baseline values.
The CO rapidly decreased after the first bolus of the drug and quickly returned to baseline, however, these changes were not significant when compared with baseline values (p = .2366).
The TPR slightly increased with maximal values reached between 30 and 60 min following last drug injection but the differences were not significant (p = .2912).
The mean values of hemodynamic parameters measured at prespecified time points are shown in Figure 3, whereas drug‐induced changes in hemodynamic parameters changes (Δ—change from baseline) are shown in Figure 4.
Figure 3.
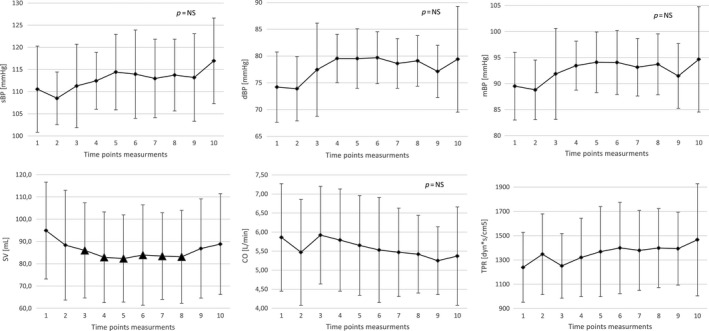
Mean ± SD values of examined hemodynamic parameters at prespecified time points. sBP, systolic blood pressure; dBP, diastolic blood pressure; mBP, mean blood pressure; SV, stroke volume; CO, cardiac output; TPR, total peripheral resistance
Figure 4.
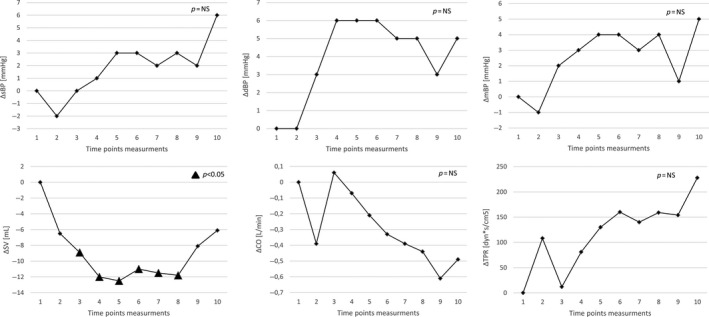
Relative drug‐induced hemodynamic parameters changes (Δ—change from baseline)
3.4. Correlation between antazoline‐induced changes and drug plasma concentration
A significant positive correlation between changes in antazoline concentration at prespecified time point measurements (Δ) in CO (r = .83, p = .012), HR (r = .79, p = .02) and dBP (r = .72, p = .042) was found. The values of Pearson correlation coefficients for changes in concentration of antazoline and ΔQTcF (negative correlation, r = −.52), TPR (negative correlation, r = −.63), mBP (positive correlation, r = .56), PR (positive correlation, r = .53), and SV (negative correlation, r = −.52) reached moderate levels due to small number of measurement points (n = 8) and adopted statistical significance (p < .05) was not reached (p values were within the range .09–.19). For the other parameters no correlation was found.
Changes in antazoline concentration and prolongation of QTcF values are shown in Figure 5, whereas the scatter plot with antazoline concentrations versus change from baseline QTcF (∆QTcF) is shown in Figure 6. The other correlation curves are shown in Figure 7.
Figure 5.
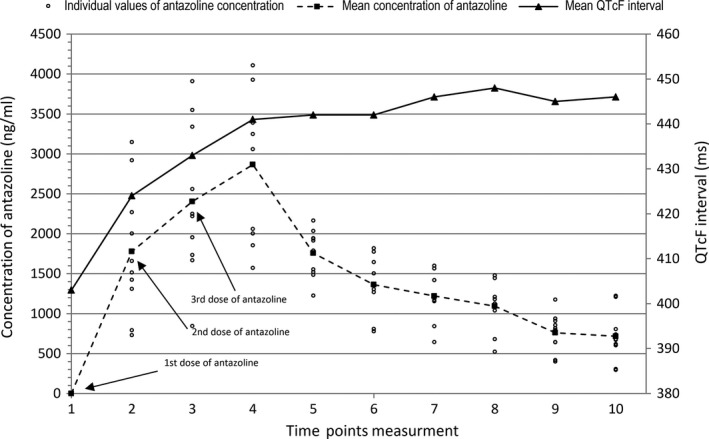
Comparison between the concentration of antazoline (mean and individual values) and QTcF interval
Figure 6.
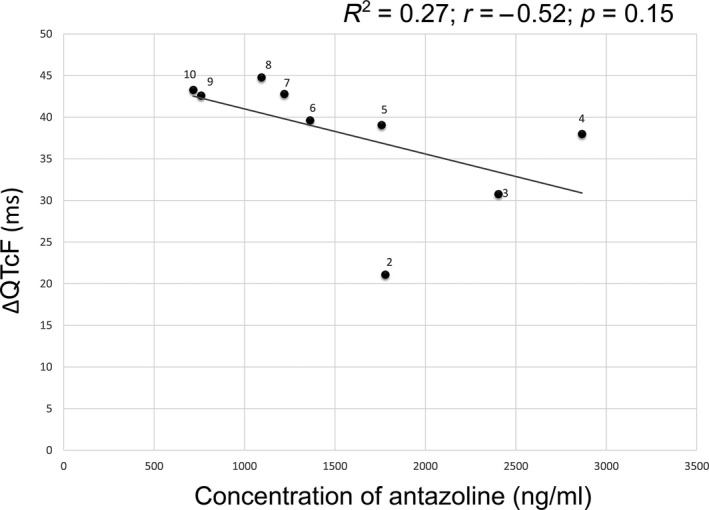
Scatter plot with mean antazoline concentrations versus change‐from‐baseline QTcF (∆QTcF). Numbers depict time point measurements
Figure 7.
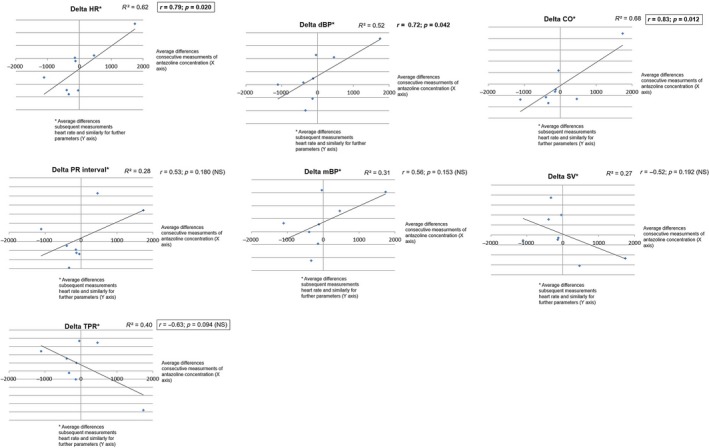
Correlation curves between plasma concentration of antazoline and changes in ECG and hemodynamic parameters. HR, heart rhythm; dBP, diastolic blood pressure; CO, cardiac output; SV, stroke volume; mBP, mean blood pressure; TPR, total peripheral resistance
4. Discussion
To our knowledge, this is the first study describing antazoline‐induced ECG and hemodynamic effects in healthy subjects. This study showed that in healthy volunteers antazoline exerts significant effects on several ECG parameters and has minor to moderate effects on hemodynamic parameters.
Antazoline has been known for a long time as an antihistaminic drug. In the past, few studies evaluated efficacy and safety of antazoline for termination of supraventricular arrhythmias. (Balsam et al., 2015; Farkowski et al., 2015, 2016; Piotrowski et al., 2014; Srzednicki & Sadowski, 1990) However, the results of these studies are limited and difficult to compare because antazoline was given in variable doses and to patients with various types of supraventricular arrhythmias as well as different duration of arrhythmia. Moreover, some authors attempted to use antazoline for ventricular arrhythmias (Reynolds, Baird, & Clifford, 1964; Shah, Vaidya, & Doshi, 1972).
Recently, new important data on antazoline have been published. First, the results of the first randomized study were announced (the AnPAF Study). The drug was significantly more effective than placebo in terminating AF (conversion rate from AF to sinus rhythm was 72% vs 10% of placebo, p < .0001) and the safety profile was excellent. (Farkowski et al., 2015) Second, in the another study antazoline occurred more effective than propafenone in acute termination of AF (72% vs 55%, p = .0022). (Farkowski et al., 2016) Third, an experimental study performed on rabbit hearts showed that antazoline completely eliminated inducibility of AF (100% success rate), whereas flecainide was effective in only 46% of cases (Frommeyer et al., 2015).
Furthermore, the first ELEPHANT I substudy which examined the pharmacokinetic profile of single bolus of antazoline, showed relative fast drug elimination with a terminal elimination half‐life of 2.29 hr and relatively high volume of distribution (Giebułtowicz, Piotrowski et al., 2016).
Also some other studies confirmed high acute success rate of antazoline in terminating AF in various clinical situations (Piotrowski et al., 2014; Reynolds et al., 1964; Srzednicki & Sadowski, 1990).
Moreover, another study showed that antazoline inhibited hERG current, however, this effect was less pronounced as compared to quinidine. Thus, antazoline might carry lower cardiac risk than quinidine and might be safer than quinidine for termination of AF (Badyra et al., 2016).
In spite of the above mentioned clinical and experimental data, little is known about drug‐induced ECG changes and hemodynamic effects. Our study showed that the drug is safe in healthy persons. Changes in hemodynamic parameters suggest slightly negative inotropic effect which may occur important in patients with organic heart disease, especially with reduced left ventricular function. Drug‐induced reduction in stroke volume may also be in part due to an increase in heart rate following drug administration. This fact may restrict the use of the drug only to patients with AF without significant heart disease. It may be speculated that in patients with organic heart disease drug‐induced QT and SV changes may occur important. Further studies in this group of patients are needed.
Antazoline is described as an antihistaminic agent with antiarrhythmic quinidine‐like properties, however, drug‐induced ECG changes suggest multiple antiarrhythmic actions of antazoline. The drug has never been included in the Vaughan–Williams classification. (Hassan, Al Suwaidi, & Salam, 2013) Prolongation of the P wave, PR, and QRS durations suggests possible sodium channel blockade, whereas increase in QT interval duration may represent drug‐induced prolongation of repolarization caused by inhibition of potassium channels. These drug actions are not simultaneous suggesting that shortly after drug injection termination of AF may be caused mainly by blocking sodium channel, whereas later on prolongation of repolarization and inhibition of potassium channels may be more important for antiarrhythmic drug properties. It corresponds with a well‐known clinical observation that in some patients with AF restoration of sinus rhythm is achieved very rapidly, within few minutes from drug injection, whereas in others a delayed drug effect is seen and AF termination takes place after several minutes from drug administration.
The relationship between plasma level and examined parameters showed strong positive correlation for HR, PR, and dBP, whereas negative correlation for SV and TPR was found. On the basis of these results, we can speculate that the degree of blockage of sodium channels is high at high concentrations of antazoline, whereas at low plasma concentrations of antazoline the degree of blockage of potassium channels is increased. This may suggest the presence of active metabolites of antazoline or its intracellular activity (see Figure 5). Further studies assessing impact of antazoline on ion channels in myocardium are needed.
The fact that there is a significant correlation between drug plasma level and changes in some ECG or hemodynamic parameters suggests that measurements of antazoline plasma level may occur important in clinical practice for predicting drug efficacy or side effects.
4.1. Limitations of the study
This study has several limitations. First, the number of volunteers was relatively small and all subjects had no heart disease. Thus, the results of our study should not be extrapolated on patients with arrhythmias or cardiac disorders. Second, the dose of antazoline and mode of injection were chosen arbitrarily, based on clinical experience. Thus, our results may not be applicable to other antazoline regimens. Thirdly, there are some limitations of ICG itself. Although the method has been validated, it is based on indirect measurements and some parameters such as TPR, CO are calculated based on the values of other indices like SV, BP and heart rate. In our study, we did not use any other invasive or noninvasive method for evaluation of hemodynamic status. Thus, conclusions regarding of drug‐induced negative inotropic effects are limited. Due to relatively low number of measured time points some correlations did not reach statistical significance and only trend could be observed. Finally, we limited our analysis to 60 min after the final antazoline dose, thus the question how long the QT interval remains prolonged following drug injection remains unanswered.
5. Conclusion
Antazoline seems to be relatively safe antiarrhythmic agent in healthy humans. Whether it also holds true for patients with heart disease needs to be examined in future studies. Based on ECG findings it may be speculated that antazoline causes both prolongation of conduction and prolongation of repolarization, thus it possess multi ion‐channel actions which are responsible for its antiarrhythmic properties.
Conflict of Interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Piotrowski R, Giebułtowicz J, Baran J, et al. Antazoline—insights into drug‐induced electrocardiographic and hemodynamic effects. Results of the ELEPHANT II substudy. Annals of Noninvasive Electrocardiology. 2017;22:e12441 10.1111/anec.12441
Funding information
This study was funded by the research grant from the Postgraduate Medical School, Warsaw, Poland No 501‐1‐1‐10‐14‐14.
References
- Badyra, B. , Lisowski, B. , Borusewicz, M. , Piotrowski, R. , Kułakowski, P. , Wiśniowska, B. , & Polak, S . (2016). Interactions of antazoline with human ether‐à‐go‐go‐related gene channels. Kardiologia polska (Abstract Supplement), 74, 374–375. [Google Scholar]
- Balsam, P. , Koźluk, E. , Peller, M. , Piątkowska, A. , Lodziński, P. , Kiliszek, M. , … Opolski, G . (2015). Antazoline for termination of atrial fibrillation during the procedure of pulmonary veins isolation. Advances in Medical Sciences, 60, 231–235. [DOI] [PubMed] [Google Scholar]
- Camm, A. J. , Kirchhof, P. , Lip, G. Y. , Schotten, U. , Savelieva, I. , Ernst, S. , … Rutten, F. H . (2010). Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). European Heart Journal, 31, 2369–2429. [DOI] [PubMed] [Google Scholar]
- Camm, A. J. , Lip, G. Y. , De Caterina, R. , Savelieva, I. , Atar, D. , Hohnloser, S. H. , … Kirchhof, P . (2012). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. European Heart Journal, 33(21), 2719–2747. [DOI] [PubMed] [Google Scholar]
- Cybulski, G. , Koźluk, E. , Michalak, E. , Niewiadomski, W. , & Piątkowska, A . (2004). Holter‐type impedance cardiography device. A system for continuous and non‐invasive monitoring of cardiac haemodynamics. Kardiologia polska, 61, 138–146. [PubMed] [Google Scholar]
- Farkowski, M. , Maciag, A. , Chwyczko, T. , Beckowski, M. , Syska, P. , Kowalik, I. , … Szwed, H . (2015). Clinical efficacy of antazoline in rapid cardioversion of paroxysmal atrial fibrillation: A single centre, randomized, double blind, placebo controlled study. European Heart Journal (Abstract Supplement), 36, 906. [Google Scholar]
- Farkowski, M. M. , Maciąg, A. , Żurawska, M. , Pytkowski, M. , Kowalik, I. , Woźniak, J. , … Szwed, H . (2016). Comparative effectiveness and safety of antazoline‐based and propafenone‐based strategies for pharmacological cardioversion of short‐duration atrial fibrillation in the emergency department. Polskie Archiwum Medycyny Wewnetrznej, 24(126), 381–387. [DOI] [PubMed] [Google Scholar]
- Fortin, J. , Habenbacher, W. , Heller, A. , Hacker, A. , Grüllenberger, R. , Innerhofer, J. , … Wach, P . (2006). Non‐invasive beat‐to‐beat cardiac output monitoring by an improved method of transthoracic bioimpedance measurement. Computers in Biology and Medicine, 36, 1185–1203. [DOI] [PubMed] [Google Scholar]
- Frommeyer, G. , Sterneberg, M. , Dechering, D. G. , Kaese, S. , Bögeholz, N. , Pott, C. , … Eckardt, L . (2015). Effective suppression of atrial fibrillation by the antihistaminic agent antazoline: First experimental insights into a novel antiarrhythmic agent. European Heart Journal (Abstract Supplement), 36, 1069. [DOI] [PubMed] [Google Scholar]
- Giebułtowicz, J. , Kojro, G. , Piotrowski, R. , Kułakowski, P. , & Wroczyński, P . (2016). Cloud‐point extraction is compatible with liquid chromatography coupled to electrospray ionization mass spectrometry for the determination of antazoline in human plasma. Journal of Pharmaceutical and Biomedical Analysis, 128, 294–301. [DOI] [PubMed] [Google Scholar]
- Giebułtowicz, J. , Piotrowski, R. , Baran, J. , Kułakowski, P. , & Wroczyński, P . (2016). Application of a novel liquid chromatography/tandem mass spectrometry method for the determination of antazoline in human plasma: Result of ELEPHANT‐I [ELEctrophysiological, pharmacokinetic and haemodynamic effects of PHenazolinum (ANTazoline mesylate)] human pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis, 123, 113–119. [DOI] [PubMed] [Google Scholar]
- Gimbel, J. R. (2005). Method and demonstration of direct confirmation of response to cardiac resynchronization therapy via preimplant temporary biventricular pacing and impedance cardiography. American Journal of Cardiology, 96, 874–876. [DOI] [PubMed] [Google Scholar]
- Hassan, O. F. , Al Suwaidi, J. , & Salam, A. M. Anti‐Arrhythmic Agents in the Treatment of Atrial Fibrillation. Journal of Atrial Fibrillation, 2013; 6 (1); 43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January, C. T. , Wann, L. S. , Alpert, J. S. , Calkins, H. , Cigarroa, J. E. , Cleveland Jr, J. C. , … Yancy, C. W . (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation, 130, 2071–2104. [DOI] [PubMed] [Google Scholar]
- Piotrowski, R. , Kryński, T. , Baran, J. , Futyma, P. , Stec, S. , & Kułakowski, P . (2014). Antazoline for rapid termination of atrial fibrillation during ablation of accessory pathways. Cardiology Journal, 21, 299–303. [DOI] [PubMed] [Google Scholar]
- Reynolds, E. W. Jr , Baird, W. M. , & Clifford, M. E. (1964). A clinical trial of antazoline in the treatment of arrhythmias. American Journal of Cardiology, 14, 513–521. [DOI] [PubMed] [Google Scholar]
- Shah, S. S. , Vaidya, C. H. , & Doshi, H. V. (1972). Antazoline in the treatment of cardiac arrhythmias. Postgraduate Medical Journal, 48(559), 304–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srzednicki, M. , & Sadowski, Z. (1990). Kulikowski A [Evaluation of the anti ‐ arrhythmia effectiveness of Phenazolinum Polfa in paroxysmal atrial fibrillation]. Polski Tygodnik Lekarski, 45, 924–927. [PubMed] [Google Scholar]
- Zgoła, K. , Kułakowski, P. , Czepiel, A. , Świątkowski, M. , Makowska, E. , Błachnio, E. , … Misiewicz, M . (2014). Haemodynamic effects of etomidate, propofol and electrical shock in patients undergoing implantable cardioverter‐defibrillator testing. Kardiologia Polska, 72(8), 707–715. [DOI] [PubMed] [Google Scholar]


