Abstract
In left bundle branch block (LBBB), the ventricles are activated in a sequential manner with alterations in left ventricular mechanics, perfusion, and workload resulting in cardiac remodeling. Underlying molecular, cellular, and interstitial changes manifest clinically as changes in size, mass, geometry, and function of the heart. Cardiac remodeling is associated with progressive ventricular dysfunction, arrhythmias, and impaired prognosis. Clinical and diagnostic notions about LBBB have evolved from a simple electrocardiographic alteration to a critically important finding affecting diagnostic and clinical management of many patients. Advances in cardiac magnetic resonance imaging have significantly improved the assessment of patients with LBBB and provided additional insights into pathophysiological mechanisms of left ventricular remodeling. In this review, we will discuss the epidemiology, etiologies, and electrovectorcardiographic features of LBBB and propose a classification of the conduction disturbance.
Keywords: anatomy, classification, epidemiology, etiology, left bundle branch block
1. INTRODUCTION
Left bundle branch block (LBBB) is a dromotropic disorder, which has gained increasing attention as a critical diagnostic tool for patient selection for cardiac resynchronization therapy (CRT). In LBBB, the right ventricle (RV) is activated before the left ventricle (LV), which results in changes in LV mechanics, perfusion, and workload. With time, this abnormal activation can lead to cardiac remodeling with reduced cardiac function, which can be deleterious for patients with otherwise structurally abnormal hearts.
2. EPIDEMIOLOGY
2.1. Young subjects with idiopathic LBBB and older subjects with primary disease of the intraventricular conduction system
Age: LBBB in children, teenagers, and young adults (<35 years) is unusual. It is observed in severe obstructive hypertrophic cardiomyopathy (HCM) treated with septal myectomy (Perez‐Riera et al., 2013; Riera, Cano, Cano, Gimenez, & de Padua Fleury Neto, L. A., & Sousa, J. E., 2002).
The mean age at LBBB diagnosis is relatively high, and the incidence increases progressively with advancing age. Hypertension, coronary artery disease (CAD), left ventricular hypertrophy (LVH), ST‐T abnormalities, and an increased cardiothoracic ratio are associated with LBBB.
LBBB is a predictor of increased mortality in heart failure (HF) patients independently of age, gender, and underlying disease (Imanishi et al., 2006).
Sex: In postmenopausal women, QRS duration (QRSd) is an independent predictor of incidental HF only in LBBB, with more pronounced risk at QRS ≥ 140 ms (Zhang et al., 2013).
Race: Hispanics with systolic HF have an increased prevalence of LBBB (Hebert et al., 2012).
Genetic background: Connexin 43 polymorphism within the ventricular muscle distal to the specialized conduction system may be important for LBBB development. Additionally, bundle branch block (BBB) is associated with an increased risk of sudden cardiac death (SCD). Reduced levels of connexin 40 are associated with BBB and reduced levels of connexin 43 are associated with increased risk of ventricular arrhythmias (Ladenvall et al., 2015).
ECG signs of left atrial abnormality: Significantly diagnostic of LVH in the presence of LBBB (Mehta, Jain, Mehta, & Billie, 2000).
The presence of LBBB has no adverse prognostic significance in subjects without evidence of structural heart disease (Rodstein, Gubner, Mills, Lovell, & Ungerleider, 1951). In 67,375 asymptomatic U.S. Air Force cadets, Lamb found LBBB in 13 subjects who had no evidence of heart disease (Lamb, Kable, & Averill, 1960).
In the Framingham Study population, new LBBB occurred mostly in people with a history of hypertension, cardiomegaly, CAD, or a combination of these; 48% developed clinical CAD or congestive HF. In men, the appearance of LBBB contributed independently to an increased risk of mortality. Comparison with age‐ and sex‐matched control subjects free from LBBB confirmed that in the general adult population, newly acquired LBBB, is most often a hallmark of hypertension or CAD or both (Schneider, Thomas, Kreger, McNamara, & Kannel, 1979).
2.2. Prevalence
The prevalence of LBBB was 0.43% for men and 0.28% for women in a randomly selected population study (age 33–71 years) conducted in Iceland from 1967 to 1977 (Hardarson et al., 1987).
2.3. Incidence
In the general population, the incidence of LBBB was 3.2 per 10,000/year for men and 3.7 per 10,000/year for women. In comparison with the control group, the LBBB patients had an increased LV diameter (Hardarson et al., 1987).
3. LBBB ETIOLOGIES
Hypertension: Hypertensive patients have an increased risk for LVH. LBBB identifies individuals with worse global and regional LV systolic function and impaired LV relaxation independently of the degree of LVH by echocardiography (Li et al., 2004).
Acute coronary syndrome (ACS): Detection of ACS in the presence of LBBB continues to be a challenge despite criteria proposed by Sgarbossa et al. and others. Serial ECGs and comprehensive ECG analysis may aid in the diagnostic workup (Madias, 2002).
Chronic myocardial infarction (MI) (Meric, Halilovic, Barakovic, & Kabil, 2004)
Dilated cardiomyopathy (Blanc, Fatemi, Bertault, Baraket, & Etienne, 2005)
Takotsubo cardiomyopathy (TCM): At presentation, LBBB was present in eight (9%) of 84 consecutive patients, who met the diagnostic criteria for TCM. Patients with LBBB tended to be older, and they had higher peak creatine kinase‐MB values (Parodi et al., 2009).
Transcatheter aortic valve implantation (TAVI): 30%–50% of patients develop new LBBB. TAVI‐induced LBBB is an independent predictor of mortality (Houthuizen et al., 2012).
Lenègre disease: Probst et al. demonstrated that hereditary Lenègre disease is caused by a haploinsufficiency mechanism, with a splicing mutation in the SCN5A gene, leading to a progressive cardiac conduction defect, which in combination with aging leads to this dromotropic disturbance (Probst et al., 2003).
Sclerosis of the left side of the cardiac skeleton: Lev disease (Bharati et al., 1975).
Cardiac interventions: Complete LBBB (CLBBB) is the rule after septal myectomy/myotomy in HCM (Perez‐Riera et al., 2013).
Left ventricular non‐compaction: The most common dromotropic disturbance is LBBB (40%) (Akhbour et al., 2015).
Neuromuscular disease: Of 1,828 gene mutation carriers of myotonic dystrophy type 1, LBBB was present in 5.7% (Petri, Vissing, Witting, Bundgaard, & Kober, 2012).
Myocarditis (Chien, Liang, Lin, Lin, & Huang, 2008)OR:
Aortic valve disease (Poels et al., 2014)
Mitral valve disease (Silva, Khuri, Barbee, Fontenot, & Cheirif, 1996)
Perinatal exposure to HIV type 1 (Diogenes et al., 2005)
Acute pulmonary embolism (rare) (Kasmani, Okoli, Mohan, Casey, & Ledrick, 2009)
Congenital aortic stenosis (Glancy & Pothineni, 2015).
Primary amyloidosis (Bellavia et al., 2009).
4. ANATOMIC CONSIDERATIONS
The first portion of the left‐sided His system is the penetrating bundle, which is characterized by longitudinal systematization and a length of 75 mm. The second portion is the branching bundle of His that bifurcates at the crest of the muscular septum into the right and left bundle branch (RBB and LBB). The LBB runs to the left as an increasingly broad sheet of cells made up of multiple fine fascicles. Reaching the wall of the LV, the sheet heads toward the apex in the subendocardial layer of the muscular septum.
LBB trunk: Length of 10 mm, the diameter is 5 mm in its onset, and 9 mm at the end (reverse trapezoid shape), the cells are formed by Purkinje fibers. The blood is supplied by:
Branches of the posterior descending coronary artery, which in 85%–90% of hearts is a distal branch of the right coronary artery (RCA).
Branches of left anterior descending coronary artery (LAD).
After a few centimeters, the LBB divides into three groups of fibers (Figure 1):
Left anterior fascicle (LAF):is distributed in the base of the anterolateral papillary muscle (ALPM). The LAF has an extension of 35 mm, diameter of 3 mm. The cells are formed by Purkinje fibers.
Left posterior fascicle (LPF): is distributed in the base of the posteromedial papillary muscle (PMPM), basal inferior region of the septum and inferobasal and lateral wall of the LV. Isolated left posterior fascicular block (LPFB) is very rare.
Left septal fascicle (LSF): has a very variable origin and morphologies and is distributed in the apical and centroseptal region and low interventricular septum (IVS). The LSF originates the first 10–20 ms electrical vector (Penaloza & Tranchesi, 1955).
Figure 1.
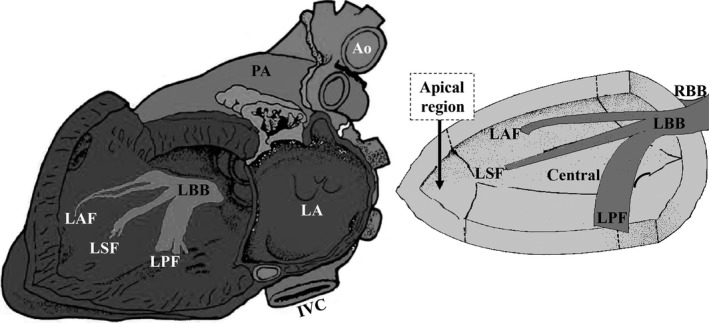
The three fascicles of the left His system in the left sagittal view. Ao: Aorta; IVC: Inferior Vena Cava; LA: Left Atrium; LBB: Left Bundle Branch; LAF: Left Anterior Fascicle; LSF: Left Septal Fascicle; LPF: Left Posterior Fascicle; PA: Pulmonary Artery; RBB: Right Bundle Branch
4.1. The Durrer concept
Durrer et al (Durrer et al., 1970) demonstrated, using 870 intramyocardial electrodes in isolated human hearts, that three endocardial areas are synchronously excited from 0 to 5 ms after the start of the LV activity potential. The first LV areas excited were:
High on the anterior paraseptal wall just below the attachment of the ALPM where the LAF ends;
Central on the left surface of the IVS;
Posterior paraseptal about one third of the distance from the apex to the base near the base of the PMPM where the LPF ends.
Thus, the only vector that manifests is the one dependent on the LSF, located in the center of the left side of the IVS, which originates the first septal vector.
5. VENTRICULAR ACTIVATION SEQUENCE
In LBBB, the ventricles are activated sequentially. The RV is activated before the LV, which produces a wide and notched QRS. The normal direction of septal depolarization is reversed (RV– LV), as the impulse spreads first to the RV via the RBB and then to the LV via slow activation of the septum. This sequential ventricular activation extends the QRSd to ≥120 ms and eliminates the normal initial septal q‐waves in the lateral leads. The overall direction of depolarization produces monophasic wide R‐waves in the lateral leads (I, V5–V6) and concomitant deep rS or QS‐waves from V1 to V3.
6. ECG CRITERIA
Supraventricular heart rhythm
QRSd ≥ 120 ms, >100 ms in children from 4 to 16 years of age, and >90 ms in children <4 years of age (Surawicz et al., 2009)
Frequent left axis deviation
rS or QS in V1–V2
Broad monophasic R‐wave in the lateral leads with “M”‐shape or a notched, monophasic R‐wave with plateau or occasionally RS or Rs pattern in V5 and V6
The absence of initial q‐waves in the lateral leads I, V5–V6. Small q‐waves are allowed in aVL
R‐wave peak time ≥60 ms in V5–V6
Poor R‐wave progression in the right precordial leads
Almost constantly QS complex in aVR
The ST‐segment and T‐wave vectors are opposite to the greater deflection of the QRS: positive from V1 to V3 and negative in the lateral leads. These are secondary repolarization abnormalities with wide QRS‐ST‐T angle and normal ventricular gradient. Figure 2 represents ventricular repolarization in uncomplicated LBBB. Secondary alteration of ventricular repolarization is observed with QRS/ST‐T angle near 180º. The ST segment depression is convex upward followed by negative asymmetrical T‐wave in the lateral leads and ST segment concave upward followed by positive asymmetric T‐wave in the right leads (Surawicz, 1988). Figure 3 shows the mechanisms behind the notching at the apex of the R‐wave in the lateral leads.
Figure 2.
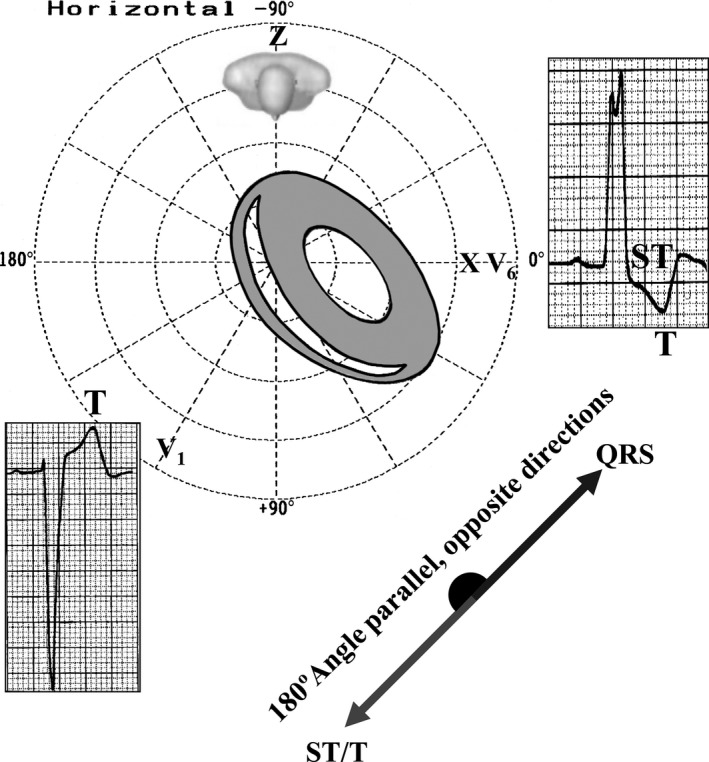
Secondary alteration of repolarization in uncomplicated CLBBB
Figure 3.
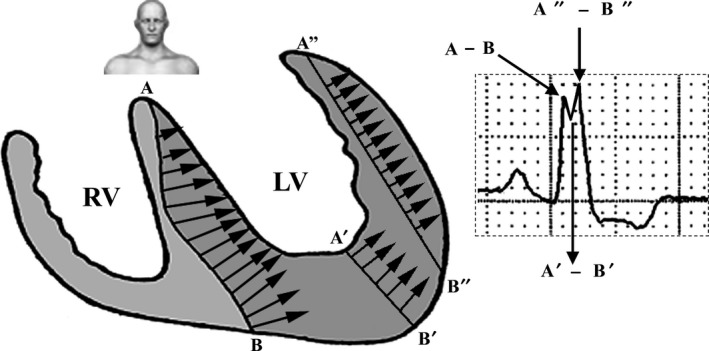
Monophasic R‐wave of slow recording with notching or slurring in the lateral leads I, aVL, V5, and V6. Septal depolarization from right to left makes a wide A–B wave front; however, when the stimulus reaches the central portion of the LV (cavity), it suffers a marked decrease in wavefront width (A′–B′) responsible for the notch in the apex of R‐wave. Next, the wavefront reaches the LV free wall increasing again the width of the wavefront (A″–B″), responsible for the second apex of R‐wave. In severe LVH of the free wall, this second apex presents a higher voltage relative to the first one
6.1. Incomplete LBBB (ILBBB)
ILBBB is less common than CLBBB. Conduction is preserved but subnormal in the LBB. Thus, the initial depolarization of the LV occurs via impulses spreading from the RV, but after a while the impulse passes the block in the LBB and executes the remaining ventricular depolarization. Hence, the initial QRS complex resembles LBBB, but QRSd is <120 ms. The presence of LVH is the rule in ILBBB.
6.2. Electrocardiographic criteria for ILBBB
QRSd between 110 and 119 ms in adults, between 90 and 100 ms in children 8–16 years of age, and between 80 and 90 ms in children <8 years of age.
R‐wave peak time in left leads >60 ms.
The absence of q‐wave in left leads.
Notched ascending limb of R‐wave in I, aVL, V5–V6.
7. VECTORCARDIOGRAPHIC (VCG) CRITERIA FOR CLBBB
7.1. Horizontal plane (HP)
Narrow, long QRS loop, and with morphology usually in the shape of eight; the QRSd is ≥120 ms; the QRS loop shape is elongated and narrow; the main body of the QRS loop is located posteriorly and to the left within the range from ‐ 90° to ‐ 40°, with clockwise (CW) inscription; maximal vector of the QRS located in the left posterior quadrant (between –40º and −80º) and of increased magnitude (>2 mV); main portions of the QRS loop of CW rotation. Counterclockwise (CCW) rotation may indicate parietal CLBBB or LBBB complicated with lateral MI or severe LVH; the efferent limb (II) is located to the right in relation to afferent limb (III and IV); conduction delay in the mid and terminal portion; increased magnitude of the max QRS vector (>2 mV); T‐loop is directed rightward and anteriorly with CCW recording. The CW rotation of the T‐loop in this plane suggests CLBBB complicated with infarction or LVH.
7.2. Frontal plane (FP)
10 ms vector directed to the left and inferiorly; rarely to the left and superiorly; QRS loop of CCW rotation or in eight; QRS loop with characteristic middle final delay; direction of maximal vector usually between +30º and –30º; T‐loop is opposite to the QRS and of CCW rotation (Figure 4b).
Figure 4.
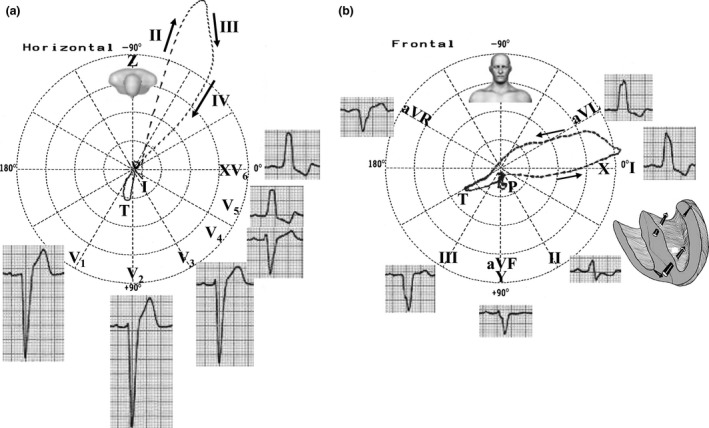
(a) ECG/VCG correlation of CLBBB and the four vectors of depolarization in LBBB in the HP; (b) ECG/VCG correlation in the FP
7.3. Right Sagittal Plane (RSP)
Vector of initial 10 ms to the front and below (or to back); QRS loop of CW rotation in the RSP or CCW in the left sagittal plane (LSP), but rarely rotates in eight; QRS loop with characteristic middle final delay; direction of maximal vector of posterior orientation (between +150° and −175°); the T‐loop is opposite to the QRS loop and the rotation is CW (RSP) or CCW (LSP).
8. ELECTROCARDIOGRAPHIC CLASSIFICATION CRITERIA FOR LBBB
-
According to the degree
-
Criteria (currently more used in the literature):
ILBBB (QRSd from 90 to 119 ms)
CLBBB (QRSd ≥120 ms in adults).
Strauss’ strict criteria: QRSd ≥140 ms for men and ≥130 ms for women, along with mid‐QRS notching or slurring in ≥2 contiguous leads. These new criteria are currently used for CRT (Strauss, Selvester, & Wagner, 2011).
-
Mexican School criteria (Sodi, Bisteni, & Medrano, 1964):
First‐degree LBBB;
Second‐degree LBBB (first degree and second degree correspond to ILBBB);
Third‐degree LBBB or CLBBB.
-
Spanish School criteria. Global left ventricular blocks (Bayés de Luna, 1998):
Advanced or third‐degree LBBB (≥120 ms),
-
Non‐advanced global left ventricular blocks:
First‐degree LBBB (partial) corresponds to types I and II of the Mexican school: isolated R in V6 with more or less slurring but QRSd <120 ms.
Intermittent or second degree LBBB (ventricular aberrancy).
-
-
According to the topography
Predivisional (90% of cases): QRSd between 120–170 ms.
-
Postdivisional (10% of cases)
Fascicular: by unequal dromotropic involvement of divisions or fascicles of the LBB: LAF, LPF, and sometimes LSF (Demoulin & Kulbertus, 1972; Uhley, 1972). Two challenging issues are: why does the initial ventricular activation (10 ms) occur in three points of the left septal surface and not in two (to be expected if the left His system was functionally bifascicular) and how to explain the cases of LBBB divisional blocks (LAFB + LPFB) that present q‐waves in the lateral leads, outshining the typical electrocardiographic pattern of LBBB? Mauricio Rosenbaum called them "left intraventricular blocks without changes in the initial part of the QRS," and in his classical book, states that these cases are "hard to explain” (Rosenbaum, Elizari, & Lazzari, 1967). In 1970, Medrano et al (Medrano, Brenes, Micheli, & Sodi‐Pallares, 1970) proposed that in these cases, the fibers of the septal division would originate before the location or area of block in the posteroinferior or anterosuperior divisions. As a result, middle–septal activation is preserved (1AM vector) and is responsible for those q‐waves in the lateral leads concealing the LBBB pattern. Totally blocking both the anterosuperior and posteroinferior divisions does not result in LBBB, as should be expected if only two left fascicles exist. Figure 5 illustrates an atypical LBBB with initial q‐waves in the left leads.
Parietal, global Purkinjean, diffuse intraventricular, intramyocardial, or intramural (in the Purkinje‐muscle union). It is characterized by wider QRS, QRS loop of clockwise rotation in the HP and pan conduction delay of the QRS loop. In general, this represents greater myocardial involvement.
-
According to steadiness
Permanent or definitive: most of the cases.
-
Intermittent, transient, episodic, or second‐degree LBBB that could be:
-
Rate‐dependent intermittent LBBB
Tachycardia‐dependent or “phase 3” LBBB: it occurs when an impulse arrives at tissues that are still refractory caused by incomplete repolarization. Transient LBBB is less common than transient RBBB (only 25% of phase 3 aberration is of the LBBB type).
Bradycardia‐dependent, d eceleration ( bradycardia ) d ependent aberrancy (DDA) or “phase 4” LBBB: Rosenbaum et al. (1973) showed that bradycardia‐dependent intermittent BBB is related to hypopolarization of the involved fascicle in the presence of spontaneous diastolic depolarization.
Concealed conduction (Issa, Miller, & Zipes, 2012): aberration caused by concealed transseptal conduction that occurs in several situations including perpetuation of aberrant conduction during tachyarrhythmias, unexpected persistence of acceleration‐dependent aberration, and alteration of aberration during atrial bigeminal rhythm.
Intermittent LBBB independent from heart rate. Mechanisms: Mobitz type I; Mobitz type II by Wenckebach phenomenon; and by significant hypopolarization.
-
According to electrical axis of the QRS complex in the FP
Figure 5.
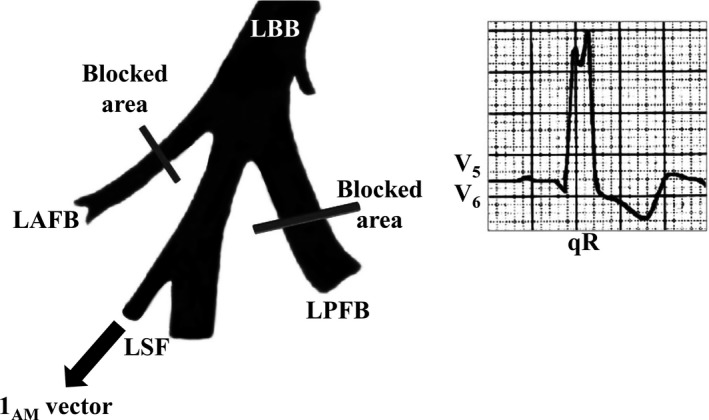
Fascicular LBBB is rarely possible qR pattern in lateral leads because the LSF arises from LBBB trunk. LAFB: Left Anterior Fascicular Block; LBB: Left Bundle Branch; LPFB: Left Posterior Fascicular Block; LSF: Left Septal Fascicle
QRS axis not deviated: between −29º and + 60º (65%–70%); QRS axis with extreme deviation: between −30º and −90º (~25%) (Parharidis et al., 1997); QRS axis deviated to the right: between + 60º and + 90º (~4%); QRS axis with extreme deviation to the right: beyond + 90º (<1%). “Paradoxical type of Lepeschkin” (Lepeschkin, 1951). The mechanism of production of this ECG pattern appears to be diffuse conduction system involvement in advanced myocardial disease (Nikolic & Marriott, 1985). The majority of subjects had dilated cardiomyopathy with biventricular enlargement (Childers, Lupovich, Sochanski, & Konarzewska, 2000).
9. CAUSES THAT DETERMINE PARADOXICAL CLBBB
CLBBB associated with RVH or severe cardiomyopathy with biventricular enlargement or diffuse advanced myocardial disease (>98% of cases); fascicular CLBBB (LAFB + LPFB) with a higher degree of block in the LPF. In the presence of AF LBBB with intermittent right axis deviation, it is explained by an additional LPFB accompanying predivisional LBBB (Patane, Marte, & Di Bella, 2008; Patane, Marte, Dattilo, & Sturiale, 2012); LBBB in Wegener granulomatosis (Khurana, Mazzone, & Mandell, 2000); CLBBB associated with lateral infarction (free wall of LV); CLBBB with accidental exchange of limb electrodes; CLBBB associated with true dextrocardia (Salazar & Lej, 1978).
10. CONCLUSION
In this review, we describe current aspects of epidemiology, etiology, anatomy, and ECG and VCG in complete and incomplete, permanent and transient LBBB. Finally, we present a classification of the LBBB taking into account several aspects: according to the degree, topography, steadiness, and QRS electrical axis.
Knowledge of these factors may help in the appropriate therapeutic approaches in the various clinical scenarios, where this dromotropic disorder is present.
CONFLICTS OF INTEREST
None.
Pérez‐Riera AR, Barbosa‐Barros R, de Rezende Barbosa MPC, et al. Left bundle branch block: Epidemiology, etiology, anatomic features, electrovectorcardiography, and classification proposal. Ann Noninvasive Electrocardiol. 2019;24:e12572 10.1111/anec.12572
REFERENCES
- Akhbour, S. , Fellat, I. , Fennich, N. , Abdelali, S. , Doghmi, N. , Ellouali, F. , & Cherti, M. (2015). Electrocardiographic findings in correlation to magnetic resonance imaging patterns in African patients with isolated ventricular noncompaction. The Anatolian Journal of Cardiology, 15(7), 550–555. 10.5152/akd.2014.5577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayés de Luna, A. (1998). Clinical electrocardiography: A textbook (2nd ed.). Armonk: , NY: Futura Publishing Company Inc. [Google Scholar]
- Bellavia, D. , Pellikka, P. A. , Abraham, T. P. , Al‐Zahrani, G. B. , Dispenzieri, A. , Oh, J. K. , … Miller, F. A. (2009). 'Hypersynchronisation' by tissue velocity imaging in patients with cardiac amyloidosis. Heart, 95(3), 234–240. 10.1136/hrt.2007.140343 [DOI] [PubMed] [Google Scholar]
- Bharati, S. , Lev, M. , Dhingra, R. C. , Chuquimia, R. , Towne, W. D. , & Rosen, K. M. (1975). Electrophysiologic and pathologic correlations in two cases of chronic second degree atrioventricular block with left bundle branch block. Circulation, 52(2), 221–229. 10.1161/01.CIR.52.2.221 [DOI] [PubMed] [Google Scholar]
- Blanc, J. J. , Fatemi, M. , Bertault, V. , Baraket, F. , & Etienne, Y. (2005). Evaluation of left bundle branch block as a reversible cause of non‐ischaemic dilated cardiomyopathy with severe heart failure. A new concept of left ventricular dyssynchrony‐induced cardiomyopathy. Europace, 7(6), 604–610. 10.1016/j.eupc.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Chien, S. J. , Liang, C. D. , Lin, I. C. , Lin, Y. J. , & Huang, C. F. (2008). Myocarditis complicated by complete atrioventricular block: Nine years' experience in a medical center. Pediatrics & Neonatology, 49(6), 218–222. 10.1016/S1875-9572(09)60014-0 [DOI] [PubMed] [Google Scholar]
- Childers, R. , Lupovich, S. , Sochanski, M. , & Konarzewska, H. (2000). Left bundle branch block and right axis deviation: A report of 36 cases. Journal of Electrocardiology, 33, 93–102. 10.1054/jclc.2000.20326 [DOI] [PubMed] [Google Scholar]
- Demoulin, J. C. , & Kulbertus, H. E. (1972). Histopathological examination of concept of left hemiblock. British Heart Journal, 34(8), 807–814. 10.1136/hrt.34.8.807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogenes, M. S. , Succi, R. C. , Machado, D. M. , Moises, V. A. , Novo, N. F. , & Carvalho, A. C. (2005). Cardiac longitudinal study of children perinatally exposed to human immunodeficiency virus type 1. Arquivos Brasileiros De Cardiologia, 85(4), 233–240. 10.1590/S0066-782X2005001700002 [DOI] [PubMed] [Google Scholar]
- Durrer, D. , van Dam, R. T. , Freud, G. E. , Janse, M. J. , Meijler, F. L. , & Arzbaecher, R. C. (1970). Total excitation of the isolated human heart. Circulation, 41(6), 899–912. 10.1161/01.CIR.41.6.899 [DOI] [PubMed] [Google Scholar]
- Glancy, D. L. , & Pothineni, K. R. (2015). ECG of the month: A forty‐year‐old woman with a history of a cardiac operation at age 5 years. Journal of the Louisiana State Medical Society, 167(4), 196–197. [PubMed] [Google Scholar]
- Hardarson, T. , Arnason, A. , Eliasson, G. J. , Palsson, K. , Eyjolfsson, K. , & Sigfusson, N. (1987). Left bundle branch block: Prevalence, incidence, follow‐up and outcome. European Heart Journal, 8(10), 1075–1079. 10.1093/oxfordjournals.eurheartj.a062172 [DOI] [PubMed] [Google Scholar]
- Hebert, K. , Quevedo, H. C. , Tamariz, L. , Dias, A. , Steen, D. L. , Colombo, R. A. , … Arcement, L. M. (2012). Prevalence of conduction abnormalities in a systolic heart failure population by race, ethnicity, and gender. Ann Noninvasive Electrocardiol, 17(2), 113–122. 10.1111/j.1542-474X.2012.00492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthuizen, P. , Van Garsse, L. A. , Poels, T. T. , de Jaegere, P. , van der Boon, R. M. , Swinkels, B. M. , … Prinzen, F. W. (2012). Left bundle‐branch block induced by transcatheter aortic valve implantation increases risk of death. Circulation, 126(6), 720–728. 10.1161/CIRCULATIONAHA.112.101055 [DOI] [PubMed] [Google Scholar]
- Imanishi, R. , Seto, S. , Ichimaru, S. , Nakashima, E. , Yano, K. , & Akahoshi, M. (2006). Prognostic significance of incident complete left bundle branch block observed over a 40‐year period. American Journal of Cardiology, 98(5), 644–648. 10.1016/j.amjcard.2006.03.044 [DOI] [PubMed] [Google Scholar]
- Issa, Z. F. , Miller, J. M. , & Zipes, D. P. (2012). Clinical arrhythmology and electrophysiology: A companion to braunwald’s heart disease (2nd ed.). Philadelphia, PA: Elsevier Health Sciences. [Google Scholar]
- Kasmani, R. , Okoli, K. , Mohan, G. , Casey, K. , & Ledrick, D. (2009). Transient left bundle branch block: An unusual electrocardiogram in acute pulmonary embolism. American Journal of the Medical Sciences, 337(5), 381–382. 10.1097/MAJ.0b013e3181907b25 [DOI] [PubMed] [Google Scholar]
- Khurana, C. , Mazzone, P. , & Mandell, B. (2000). New onset left bundle branch block with right axis deviation in a patient with Wegener's granulomatosis. Journal of Electrocardiology, 33(2), 199–201. 10.1016/S0022-0736(00)80077-1 [DOI] [PubMed] [Google Scholar]
- Ladenvall, P. , Andersson, B. , Dellborg, M. , Hansson, P. O. , Eriksson, H. , Thelle, D. , & Eriksson, P. (2015). Genetic variation at the human connexin 43 locus but not at the connexin 40 locus is associated with left bundle branch block. Open Heart, 2(1), e000187 10.1136/openhrt-2014-000187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, L. E. , Kable, K. D. , & Averill, K. H. (1960). Electrocardiographic findings in 67,375 asymptomatic subjects. V. Left bundle branch block. American Journal of Cardiology, 6, 130–142. 10.1016/0002-9149(60)90042-4 [DOI] [PubMed] [Google Scholar]
- Lepeschkin, E. (1951). Modern electrocardiography (Vol. I). The P‐Q‐R‐S‐T‐U Complex, Baltimore, MD: Williams & Wilkins Company. [Google Scholar]
- Li, Z. B. , Wachtell, K. , Okin, P. M. , Gerdts, E. , Liu, J. E. , Nieminen, M. S. , … Devereux, R. B. (2004). Association of left bundle branch block with left ventricular structure and function in hypertensive patients with left ventricular hypertrophy: The LIFE study. Journal of Human Hypertension, 18(6), 397–402. 10.1038/sj.jhh.1001709 [DOI] [PubMed] [Google Scholar]
- Madias, J. E. (2002). Serial ECG recordings via marked chest wall landmarks: An essential requirement for the diagnosis of myocardial infarction in the presence of left bundle branch block. Journal of Electrocardiology, 35(4), 299–302. 10.1054/jelc.2002.35845 [DOI] [PubMed] [Google Scholar]
- Medrano, G. A. , Brenes, C. , De Micheli, A. , & Sodi‐Pallares, D. (1970). Simultaneous block of the anterior and posterior subdivisions of the left branch of the bundle of His (biphasic block), and its association with the right branch block (triphasic block). Experimental and clinical electrocardiographic study. Archivos Del Instituto De Cardiologia De Mexico, 40(6), 752–770. [PubMed] [Google Scholar]
- Mehta, A. , Jain, A. C. , Mehta, M. C. , & Billie, M. (2000). Usefulness of left atrial abnormality for predicting left ventricular hypertrophy in the presence of left bundle branch block. American Journal of Cardiology, 85(3), 354–359. 10.1016/S0002-9149(99)00746-8 [DOI] [PubMed] [Google Scholar]
- Meric, M. , Halilovic, E. , Barakovic, F. , & Kabil, E. (2004). Coronary disease and left branch block. Medicinski Arhiv, 58(5), 288–291. [PubMed] [Google Scholar]
- Nikolic, G. , & Marriott, H. J. (1985). Left bundle branch block with right axis deviation: A marker of congestive cardiomyopathy. Journal of Electrocardiology, 18(4), 395–404. 10.1016/S0022-0736(85)80022-4. [DOI] [PubMed] [Google Scholar]
- Parharidis, G. , Nouskas, J. , Efthimiadis, G. , Styliadis, J. , Gemitzis, K. , Hatzimiltiadis, S. , … Tsifodimos, D. (1997). Complete left bundle branch block with left QRS axis deviation: Defining its clinical importance. Acta Cardiologica, 52(3), 295–303. [PubMed] [Google Scholar]
- Parodi, G. , Salvadori, C. , DelPace, S. , Bellandi, B. , Carrabba, N. , & Gensini, G. F. , …Tuscany Registry of Tako‐Tsubo C (2009). Left bundle branch block as an electrocardiographic pattern at presentation of patients with Tako‐tsubo cardiomyopathy. Journal of Cardiovascular Medicine, 10(1), 100–103. 10.2459/JCM.0b013e32831a6a26 [DOI] [PubMed] [Google Scholar]
- Patane, S. , Marte, F. , Dattilo, G. , & Sturiale, M. (2012). Acute myocardial infarction and left bundle branch block with changing axis deviation. International Journal of Cardiology, 154(3), e47–e49. 10.1016/j.ijcard.2009.03.128 [DOI] [PubMed] [Google Scholar]
- Patane, S. , Marte, F. , & Di Bella, G. (2008). Atrial fibrillation with left bundle branch block and intermittent right axis deviation during acute myocardial infarction. International Journal of Cardiology, 127(1), e1–e2. 10.1016/j.ijcard.2007.01.022 [DOI] [PubMed] [Google Scholar]
- Penaloza, D. , & Tranchesi, J. (1955). The three main vectors of the ventricular activation process in the normal human heart. I. Its Significance. American Heart Journal, 49(1), 51–67. 10.1016/0002-8703(55)90052-1 [DOI] [PubMed] [Google Scholar]
- Perez‐Riera, A. R. , de Lucca, A. A. , Barbosa‐Barros, R. , Yanowitz, F. G. , de Cano, S. F. , Cano, M. N. , & Palandri‐Chagas, A. C. (2013). Value of electro‐vectorcardiogram in hypertrophic cardiomyopathy. Annals of Noninvasive Electrocardiology, 18(4), 311–326. 10.1111/anec.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri, H. , Vissing, J. , Witting, N. , Bundgaard, H. , & Kober, L. (2012). Cardiac manifestations of myotonic dystrophy type 1. International Journal of Cardiology, 160(2), 82–88. 10.1016/j.ijcard.2011.08.037 [DOI] [PubMed] [Google Scholar]
- Poels, T. T. , Houthuizen, P. , Van Garsse, L. A. , Maessen, J. G. , de Jaegere, P. , & Prinzen, F. W. (2014). Transcatheter aortic valve implantation‐induced left bundle branch block: Causes and consequences. Journal of Cardiovascular Translational Research, 7(4), 395–405. 10.1007/s12265-014-9560-x [DOI] [PubMed] [Google Scholar]
- Probst, V. , Kyndt, F. , Potet, F. , Trochu, J. N. , Mialet, G. , Demolombe, S. , Mathu, C. , & Le Marec, H. (2003). Haploinsufficiency in combination with aging causes SCN5A‐linked hereditary Lenegre disease. Journal of the American College of Cardiology, 41(4), 643–652. 10.1016/S0735-1097(02)02864-4 [DOI] [PubMed] [Google Scholar]
- Riera, A. R. , de Cano, S. J. , Cano, M. N. , Gimenez, V. M. , & de Padua Fleury Neto, L. A., & Sousa, J. E., (2002). Vector electrocardiographic alterations after percutaneous septal ablation in obstructive hypertrophic cardiomyopathy. Possible Anatomic Causes. Arq Bras Cardiol, 79(5), 466–475. 10.1590/S0066-782X2002001400004 [DOI] [PubMed] [Google Scholar]
- Rodstein, M. , Gubner, R. , Mills, J. P. , Lovell, J. F. , & Ungerleider, H. E. (1951). A mortality study in bundle branch block. Archives of Internal Medicine, 87(5), 663–668. 10.1001/archinte.1951.03810050039004 [DOI] [PubMed] [Google Scholar]
- Rosenbaum, M. B. , Elizari, M. V. , & Lazzari, J. O. (1967). Los Hemibloqueos. Buenos Aires, Argentina: Editorial Paidos. [Google Scholar]
- Rosenbaum, M. B. , Elizari, M. V. , Lazzari, J. O. , Halpern, M. S. , Nau, G. J. , & Levi, R. J. (1973). The mechanism of intermittent bundle branch block: Relationship to prolonged recovery, hypopolarization and spontaneous diastolic depolarization. Chest, 63(5), 666–677. 10.1378/chest.63.5.666 [DOI] [PubMed] [Google Scholar]
- Salazar, J. , & Lej, F. A. (1978). Electrocardiographic changes following surgical repair of ostium primum defect. Acta Cardiologica, 33(1), 55–61. [PubMed] [Google Scholar]
- Schneider, J. F. , Thomas, H. E. Jr , Kreger, B. E. , McNamara, P. M. , & Kannel, W. B. (1979). Newly acquired left bundle‐branch block: The Framingham study. Annals of Internal Medicine, 90(3), 303–310. 10.7326/0003-4819-90-3-303 [DOI] [PubMed] [Google Scholar]
- Silva, J. A. , Khuri, B. , Barbee, W. , Fontenot, D. , & Cheirif, J. (1996). Systolic excursion of the mitral annulus to assess septal function in paradoxic septal motion. American Heart Journal, 131(1), 138–145. 10.1016/S0002-8703(96)90062-9 [DOI] [PubMed] [Google Scholar]
- Sodi, D. , Bisteni, A. , & Medrano, G. (1964). Electrocardiografia y vectorcardiografia deductivas (Vol. 1). Mexico, DF: La Prensa Médica Mexicana. [Google Scholar]
- Strauss, D. G. , Selvester, R. H. , & Wagner, G. S. (2011). Defining left bundle branch block in the era of cardiac resynchronization therapy. American Journal of Cardiology, 107(6), 927–934. 10.1016/j.amjcard.2010.11.010 [DOI] [PubMed] [Google Scholar]
- Surawicz, B. (1988). ST‐T abnormalities In MacFarlane P. W., & Lawrie T. D. V. (Eds.), Comprehensive electrocardiology (pp. 511–563). New York, NY: Pergamon Books Ltd. [Google Scholar]
- Surawicz, B. , Childers, R. , Deal, B. J. , Gettes, L. S. , Bailey, J. J. , Gorgels, A. , Mathu, C. , & Heart Rhythm, S. (2009). AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part III: Intraventricular conduction disturbances: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. Journal of the American College of Cardiology, 53(11), 976–981. 10.1016/j.jacc.2008.12.013 [DOI] [PubMed] [Google Scholar]
- Uhley, H. N. (1972). Some controversy regarding the peripheral distribution of the conduction system. American Journal of Cardiology, 30(8), 919–920. 10.1016/0002-9149(72)90022-7 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. M. , Rautaharju, P. M. , Soliman, E. Z. , Manson, J. E. , Martin, L. W. , Perez, M. , Mathu, C. , & Prineas, R. J. (2013). Different patterns of bundle‐branch blocks and the risk of incident heart failure in the Women's Health Initiative (WHI) study. Circulation: Heart Failure, 6(4), 655–661. 10.1161/CIRCHEARTFAILURE.113.000217 [DOI] [PMC free article] [PubMed] [Google Scholar]


