Abstract
Background
Fragmented QRS complexes (fQRS) were associated with left ventricular mass (LVM) in hypertensive patients. Our study aimed to investigate the association between fQRS and left ventricular hypertrophy (LVH) in hypertensive patients.
Methods
Two hundred thirty‐six hypertensive patients were divided into fQRS group and non‐fQRS group. fQRS were defined as the presence of an additional R wave, notching in the R or S wave, or the presence of >1 R' in two contiguous leads. Echocardiography was used to detect LVH.
Results
Patients with fQRS had higher levels of LVM than patients without fQRS (181.55 ± 65.64 g vs. 149.21 ± 35.08 g, P < 0.001). Receiver operating characteristic curves showed areas under the curve was 0.62 for fQRS (95% CI 0.54–0.69, P = 0.003). In univariate analyses, the presence of fQRS on ECG was positively associated with LVM. Multiple regression analyses found fQRS was associated with LVM, independently.
Conclusion
fQRS is a common electrocardiographic phenomenon in patients with hypertension. Although the diagnostic value for LVH is limited, the presence of fQRS on ECG is associated with a higher risk for worse LVH.
Keywords: fragmented QRS, hypertension, left ventricular hypertrophy, left ventricular mass
Fragmented QRS complexes (fQRS) are novel electrocardiographic signals, which include various RSR' patterns with different morphologies of the QRS complexes on resting 12‐lead electrocardiography (ECG). Various RSR' patterns include an additional R wave (R') or notching of the R wave or S wave, or the presence of >1 R' in two contiguous leads, corresponding to the abnormal cardiac depolarization.1 Previous studies have shown that fQRS might be a predictor of poor clinical outcomes in patients with coronary artery disease (CAD), dilated cardiomyopathy, hypertrophic cardiomyopathy, or structure heart disease.2, 3, 4, 5
Hypertension is an important risk factor for cardiovascular disease and has become a major global burden on public health.6 In 2002, one‐sixth of all Chinese adults were found to be hypertensive.7 Hypertension could lead to target organ damage, such as renal damage, cardiac damage, and vascular damage. Left ventricular hypertrophy (LVH) is a common cardiac damage and one of the most important risk factors of worse cardiovascular prognosis in patients with hypertension. Pathological changes present in patients with hypertensive LVH include an increase in the size of cardiomyocyte, alteration in the extracellular matrix, and cardiac fibrosis.8 The pathological features would lead to abnormal electric conduction among cardiomyocytes, which may be presented as fQRS on surface ECG. A previous study about the prognostic value of fQRS in patients with acute myocardial infarction found that hypertension was associated with fQRS by multivariate logistic regression analysis.9 Another small size study also found that hypertensive patients with fQRS had higher index of left ventricular mass (LVM) than patients without fQRS.10
In view of the possible relationship between fQRS and hypertension, the goal of the study was to investigate the association between fQRS and LVH in hypertensive patients. For this purpose, we analyzed the results of ECG and echocardiography in patients with hypertension.
METHODS
Study Population
From August 2012 to December 2013, we conducted a retrospective study on hypertensive patients with negative results of coronary angiography (CAG) at affiliated People's Hospital of Jiangsu University. The study protocol was approved by the affiliated People's Hospital of Jiangsu University's Ethics Committee. Hypertension was defined as a blood pressure ≥140/90 mmHg or the use of antihypertension medications. Exclusion criteria included patients with CAG proved CAD, structure heart disease, systolic heart failure, chronic renal dysfunction, history of pacemaker, or any abnormal characteristic on the ECG (included atrial fibrillation, abnormal Q wave, bundle branch block, Wolff–Parkinson–White syndrome and so on). All patients were evaluated by medical history, clinical presentation, examination recording, and clinical biochemistry.
ECG Analysis
An electronically recorded 12‐lead ECG at rest (filter range, 0.15–100 Hz; AC filter, 60 Hz, 25 mm/s, and 10 mm/mV) was obtained within the first 24 hours after admission. fQRS complexes were defined as the presence of an additional R wave (R'), notching in the R or S wave, or the presence of >1 R' in two contiguous leads in patients with QRS duration ≤120 ms. However, patients with a typical bundle‐brunch block pattern or incomplete right bundle‐branch block pattern were excluded.1 The electrocardiogram was analyzed by two independent readers blinded to the echocardiogram and clinical findings.
Echocardiography Assessment
All patients underwent two‐dimensional transthoracic echocardiography. The echocardio‐grapher was blinded to the clinical data. Measurements included left atrium diameter (LAD), interventricular septal thickness (IVST), left ventricular posterior wall thickness (LVPWT), left ventricular end diastolic/systolic dimension (LVEDD/LVESD). LVM was calculated as 0.8 × (1.04 × [IVST + LVEDD + LVPWT3 − LVEDD3] + 0.6 g),11 and was used to assess the severity of LVH. In addition, we also evaluated the systolic function by left ventricular ejection fraction (LVEF) and the diastolic function by the ratio of peak of early diastolic transmitral flow to peak of early diastolic annular velocity (E/E' ratio).
Statistical Analysis
Descriptive data for continuous variables were presented as mean ± standard deviation. Categorical data were summarized using frequency counts and percentages. For continuous variables, normal distribution was evaluated with Kolmolgorov‐Smirnov test. The continuous variables were compared using the Student's t test (if homogeneity of variances was assumed) or the Mann‐Whitney U test (if homogeneity of variances was not met). For categorical clinical variables, differences between groups were evaluated with the chi‐square or Fisher's exact test when appropriate. A P value of ≤0.05 was taken as significance. Furthermore, receiver operating characteristic (ROC) curve was performed to investigate the value of fQRS in differentiating LVH in hypertensive patients. In addition, Spearman correction coefficients were used to evaluate correlations between LVM and other variables. Subsequently, analysis was undertaken through multiple linear regressions to investigate a set of independent variables predictors of LVM. All analyses were conducted with SPSS for Windows 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographics and Clinical Data
A final cohort of 236 patients (138 males, mean age 61 years) was analyzed. The ECG findings showed that fQRS were presented 36.4% in the patients enrolled. In the patients with fQRS, there were 60 patients with fQRS in the inferior leads, and 26 patients with fQRS in the anterior leads. The baseline characteristics of the patients were shown in Table 1. The patients in the fQRS group had longer history of hypertension (9.67 ± 5.98 years vs. 7.87 ± 7.66 years, P < 0.001), higher proportion of treatment with beta‐blockers (20.9% vs. 8.0%, P = 0.004) and diuretics (32.6% vs. 19.3%, P = 0.022), higher levels of serum creatinine (82.34 ± 19.03 umol/L vs. 73.44 ± 14.47 umol/L, P < 0.001), but lower proportion of diabetes (9.3% vs. 23.1%, P = 0.007) than patients in the non‐fQRS group. No significant differences were observed in age, male proportion, history of smoking, classification of hypertension, other antihypertension medications, and prevalence of blood pressure control between the two groups.
Table 1.
Comparison of Baseline Characteristics between the Two Groups
| fQRS Group | Non‐fQRS Group | ||
|---|---|---|---|
| Characteristics | (n = 86, 36.4%) | (n = 150, 63.6%) | P Value |
| Age (years) | 62.35 ± 12.44 | 60.05 ± 9.90 | 0.17 |
| Male gender, n (%) | 54(62.8) | 84(56.0) | 0.31 |
| Diabetes mellitus, n (%) | 8(9.3) | 37(23.1) | 0.007 |
| Smoking, n (%) | 33(38.4) | 71(47.3) | 0.18 |
| TC (mmol/L) | 4.02 ± 0.74 | 4.30 ± 0.98 | 0.07 |
| LDL (mmol/L) | 2.15 ± 0.59 | 2.29 ± 0.75 | 0.12 |
| Creatinine (umol/L) | 82.34 ± 19.03 | 73.44 ± 14.47 | <0.001 |
| Length of hypertension (y) | 9.67 ± 5.98 | 7.87 ± 7.66 | 0.002 |
| Classification of hypertension | 0.27 | ||
| Class 1, n (%) | 7(8.1) | 10(6.7) | |
| Class 2, n (%) | 27(31.4) | 63(42.0) | |
| Class 3, n (%) | 52(60.5) | 77(51.3) | |
| Medications for hypertension | |||
| ACEI/ARB, n (%) | 40(46.5) | 52(34.7) | 0.073 |
| CCB, n (%) | 50(58.1) | 39(26.0) | <0.001 |
| Beta‐blockers, n (%) | 18(20.9) | 12(8.0) | 0.004 |
| Diuretics, n (%) | 28(32.6) | 29(19.3) | 0.022 |
| Others, n (%) | 14(16.3) | 34(22.7) | 0.24 |
| Patients with controlled BP, n (%) | 49(57) | 87(58) | 0.88 |
fQRS, fragmented QRS; TC, total cholesterol; LDL, low density lipoprotein; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; BP, blood pressure.
Echocardiography Results
Patients in the fQRS group had higher levels of IVST (11.38 ± 2.08 mm vs. 10.50 ± 1.67 mm, P = 0.002) and LVM (181.55 ± 65.64 g vs. 149.21 ± 35.08 g, P < 0.001) than patients in the non‐fQRS group, but the difference of LVPWT between the two group was not significant (8.93 ± 1.27 mm vs. 8.68 ± 0.89 mm, P = 0.063). The patients in the fQRS group also had larger LVEDD (48.33 ± 6.60 mm vs. 45.46 ± 3.47 mm, P = 0.015), LVESD (32.23 ± 7.75 mm vs. 29.67 ± 5.77 mm, P = 0.005), and LAD (35.35 ± 6.10 mm vs. 32.51 ± 5.47 mm, P < 0.001) than patients in the non‐fQRS group.
We also evaluated the systolic and diastolic cardiac function by the echocardiography. The results showed that patients in the fQRS group had worse systolic and diastolic cardiac function than patients in the non‐fQRS (LVEF 62.95 ± 10.68 vs. 66.38 ± 5.47, P = 0.006; fractional shortening 35.44 ± 4.74 vs. 36.98 ± 1.96, P = 0.014; E/E' 10.21 ± 1.62 vs. 9.39 ± 1.30, P < 0.001, Table 2).
Table 2.
Comparison of the Echocardiographic Measurements between the Two Groups
| fQRS Group | Non‐fQRS Group | ||
|---|---|---|---|
| Measurements | (n = 86, 36.4%) | (n = 150, 63.6%) | P Value |
| IVST (mm) | 11.38 ± 2.08 | 10.50 ± 1.67 | 0.002 |
| LVPWT (mm) | 8.93 ± 1.27 | 8.68 ± 0.89 | 0.063 |
| LVEDD (mm) | 48.33 ± 6.60 | 45.46 ± 3.47 | 0.015 |
| LVESD (mm) | 32.23 ± 7.75 | 29.67 ± 5.77 | 0.005 |
| LAD (mm) | 35.35 ± 6.10 | 32.51 ± 5.47 | <0.001 |
| FS (%) | 35.44 ± 4.74 | 36.98 ± 1.96 | 0.014 |
| LVEF (%) | 62.95 ± 10.68 | 66.38 ± 5.47 | 0.006 |
| E/E' ratio | 10.21 ± 1.62 | 9.39 ± 1.30 | <0.001 |
| LVM (g) | 181.55 ± 65.64 | 149.21 ± 35.08 | <0.001 |
| LVH n (%) | 46 (43.5) | 45(30.0) | <0.001 |
fQRS, fragmented QRS; IVST, interventricular septal thickness; LVPWT, left ventricular posterior wall thickness; LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; LAD, left atrium diameter; FS, fractional shortening; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; LVH, left ventricular hypertrophy; E/E’ ratio, the ratio of peak of early diastolic transmitral flow to peak of early diastolic annular velocity.
The Diagnostic Value of fQRS for LVH
We defined LVH as IVST or/and LVPWT ≥ 12 mm. There was higher proportion of patients with LVH in the fQRS group under this definition (43.5% vs. 30.0%, P < 0.001, Table 2). The presence of fQRS on ECG had high specificity (72%) for identifying LVH in patients with hypertension, but low sensitivity (51%). The positive and negative predictive values of fQRS for LVH were 53% and 70%, respectively. Receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of fQRS for LVH. The area under the curve was 0.62 (95% CI 0.54–0.69, P = 0.003, Fig. 1).
Figure 1.
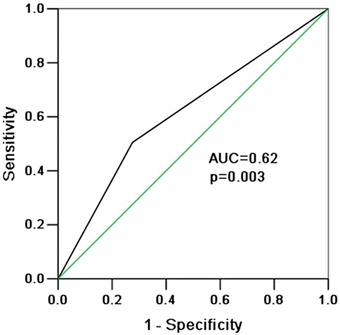
ROC curve was conducted to evaluate the diagnostic value of fQRS for LVH. The area under the curve (AUC) was 0.62 (95% CI 0.54–0.69, P = 0.003).
Association between fQRS and the Severity of LVH
LVM was used to assess the severity of LVH in hypertensive patients. Patients with fQRS on ECG had higher levels of LVM than patients without fQRS. To further investigate the association between fQRS and the severity of LVH, Spearman correction coefficients and multiple linear regressions were used. In univariate analyses, the presence of fQRS on ECG was positively associated with LVM (r = 31, P < 0.001, Table 3). Multiple regression analyses revealed that presence of fQRS on ECG was associated with LVM independently (Table 4).
Table 3.
Correction between Left Ventricular Mass and Other Variables
| Parameter | R | P Value |
|---|---|---|
| fQRS | 0.31 | < 0.001 |
| Male gender | −0.22 | < 0.001 |
| Age | 0.03 | 0.33 |
| Length of hypertension | 0.18 | 0.002 |
| Classification of hypertension | 0.11 | 0.05 |
| Treatment with ACEI/ARB | −0.07 | 0.14 |
| Treatment with beta‐blockers | 0.04 | 0.29 |
| Treatment with CCB | 0.14 | 0.014 |
| Treatment with diuretics | 0.15 | 0.009 |
| Treatment with other drugs | −0.06 | 0.17 |
| Blood pressure control | −0.24 | < 0.001 |
| Smoking | 0.10 | 0.07 |
| Diabetes mellitus | 0.02 | 0.40 |
| Creatinine | 0.33 | < 0.001 |
| Total cholesterol | −0.19 | 0.002 |
| Low density lipoprotein | −0.12 | 0.04 |
fQRS, fragmented QRS; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker.
Table 4.
Multivariable Linear Regression Analyses
| Variables | Beta Coefficients | 95% CI | P Value |
|---|---|---|---|
| fQRS | 0.22 | 10.75 to 35.80 | < 0.001 |
| Creatinine | 0.25 | 0.39 to 1.11 | < 0.001 |
| Blood pressure control | −0.20 | −32.30 to −8.56 | 0.001 |
| Length of hypertension | 0.17 | 0.37 to 2.01 | 0.005 |
fRQS, fragmented QRS; CI, confidence interval.
DISCUSSION
This study demonstrated that fQRS was a common electrocardiographic phenomenon in patients with hypertension. Patients with fQRS had higher risks of LVH, systolic, and diastolic heart dysfunction. ROC curve showed that fQRS had value in distinguishing patients with LVH. Although the diagnostic value was limited, multiple linear regression analysis found that presence of fRQS on ECG was associated with LVM independently, which was used to assess the severity of LVH.
fQRS was described as a marker of myocardial scar in patients with CAD. The presence of fQRS might be related to the electrophysiological features of viable myocardial tissue islands in the myocardial scar. The islands of chronically ischemic myocardium displayed slow conduction, which would be responsible for inhomogeneous activation of the left ventricular. This altered the depolarization of ventricular, which was probably represented fragmentation in the QRS complex on the ECG.1 However, some other studies found that fQRS was also related to acute coronary syndrome, Brugada syndrome, dilated cardiomyopathy, hypertrophy cardiomyopathy, and left ventricular noncompaction cardiomyopathy. And the formation of fQRS might be related by inhomogeneous activation of ventricles and myocardial conduction delay because of myocardial scar, ischemia, fibrosis, or abnormal iron channels.3, 4, 12, 13, 14
The formation of fQRS in patients with hypertension might be related to the LVH. An exaggerated accumulation of collagen type I and III fibers within the myocardial interstitium and surrounding intramural coronary arteries and arterioles have been described in hypertensive patients with LVH.15 Microvascular disease and endothelial dysfunction were also apparent in hypertensive heart disease. In addition, increased arterial stiffness and the concomitant fall in central diastolic blood pressure were characteristics of macrovascular remodeling in patients with long‐standing hypertension.16 The falling of central diastolic blood pressure would decrease coronary perfusion and contribute to myocardial ischemia. The fibrosis and myocardial ischemia might promote inhomogeneous activation of ventricles, and lead to the formation of fQRS on ECG.
In addition, hypertension‐related myocardial remodeling was also accompanied by cell‐to‐cell gap junction alterations. It has been shown that the expression of connexin 43 was decreased in spontaneous hypertensive rats with LVH.17 Another animal study found enhanced neoformation of side‐to‐side type and internalization of end‐to‐end type of gap junctions prevailed in the myocardium of rats with hypertension.18 The remodeling of gap junction in hypertensive hearts might also promote inhomogeneous activation of ventricles and the formation of fQRS.
Similar to other ECG criteria for diagnosing LVH19, the fQRS also had some limitations for detection of LVH, one of which was the low sensitivity. In our study, the presence of fQRS on ECG had a poor sensitivity of 51% for detection of LVH. This may be related to the complicated mechanisms of LVH in hypertensive patients. In addition to the fibrosis and myocardial ischemia, the pathological changes present in patients with LVH also include the increase in the size of cardiomyocyte and other changes. The formation of fQRS might be related to the alteration in the extracellular matrix and cardiac fibrosis, and just was the tip of the iceberg. However, univariate analyses showed that the presence of fQRS on ECG was associated with LVM positively, and multiple linear regression analyses found that the fQRS was associated with LVM independently. All these results indicated that fQRS was associated with a significantly higher risk for worse LVH. This may be more important to hypertensive patients. LVH is a marker of cardiovascular risk, and the risk increases with the increasing of severity of LVH.20 Therefore, the presence of fQRS on ECG may be associated with high risk of cardiovascular events in patients with hypertension.
In addition, our study also found that patients with fQRS had higher risk of left ventricular systolic and diastolic dysfunction, which was similar to previous study.21 This might be related to the higher degree of myocardial fibrosis and myocardial ischemia in the fQRS group.
Study Limitations
There are several limitations of the present study. The relatively small sample size might influence the relationship between fQRS and hypertension. In addition, this is a retrospective study and has some disadvantages comparing to prospective study. Furthermore, the exact mechanism of fQRS in patients with hypertension is unknown at present. The clinical significance of fQRS in patients with hypertension needs further prospective studies.
CONCLUSION
The present study suggests that fQRS is a common electrocardiographic phenomenon in patients with hypertension. Although the presence of fQRS on ECG has limited diagnostic value for detection of LVH, fQRS is associated with a significantly higher risk for worse LVH.
Acknowledgments
We thank all of our colleagues working in the Department of Cardiology, the affiliated People's Hospital of Jiangsu University.
Disclosure: There are no conflicts of interests to disclose.
REFERENCES
- 1. Das MK, Khan B, Jacob S, et al. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation 2006;113(21):2495–2501. [DOI] [PubMed] [Google Scholar]
- 2. Guo R, Zhang J, Li Y, et al. Prognostic significance of fragmented QRS in patients with non‐ST elevation myocardial infarction: Results of a 1‐year, single‐center follow‐up. Herz 2012;37(7):789–795. [DOI] [PubMed] [Google Scholar]
- 3. Sha J, Zhang S, Tang M, et al. Fragmented QRS is associated with all‐cause mortality and ventricular arrhythmias in patient with idiopathic dilated cardiomyopathy. Ann Noninvasive Electrocardiol 2011;16(3):270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Femenia F, Arce M, Van Grieken J, et al. Fragmented QRS as a predictor of arrhythmic events in patients with hypertrophic obstructive cardiomyopathy. J Interv Card Electrophysiol 2013;38(3):159–165. [DOI] [PubMed] [Google Scholar]
- 5. Yuce M, Davutoglu V, Ozbala B, et al. Fragmented QRS is predictive of myocardial dysfunction, pulmonary hypertension and severity in mitral stenosis. Tohoku J Exp Med 2010;220(4):279–283. [DOI] [PubMed] [Google Scholar]
- 6. Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001;104:2746–2753. [DOI] [PubMed] [Google Scholar]
- 7. Wu Y, Huxley R, Li L, et al. Prevalence, awareness, treatment, and control of hypertension in China: Data from the China National Nutrition and Health Survey 2002. Circulation 2008;118(25):2679–2686. [DOI] [PubMed] [Google Scholar]
- 8. Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest 2007;117(3):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lorgis L, Jourda F, Hachet O, et al. Prognostic value of fragmented QRS on a 12‐lead ECG in patients with acute myocardial infarction. Heart Lung 2013;42(5):326–331. [DOI] [PubMed] [Google Scholar]
- 10. Kadi H, Kevser A, Ozturk A, et al. Fragmented QRS complexes are associated with increased left ventricular mass in patients with essential hypertension. Ann Noninvasive Electrocardiol 2013;18(6):547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janardhanan R, Kramer CM. Imaging in hypertensive heart disease. Expert Rev Cardiovasc Ther 2011;9(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo R, Li Y, Xu Y, et al. Significance of fragmented QRS complexes for identifying culprit lesions in patients with non‐ST‐elevation myocardial infarction: A single‐center, retrospective analysis of 183 cases. BMC Cardiovasc Disord 2012;12:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morita H, Kusano KF, Miura D, et al. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation 2008;118:1697–1704. [DOI] [PubMed] [Google Scholar]
- 14. Ning XH, Tang CM, Chen KP, et al. The prognostic significance of fragmented QRS in patients with left ventricular noncompaction cardiomyopathy. Can J Cardiol 2012;28(4):508–514. [DOI] [PubMed] [Google Scholar]
- 15. Diez J. Diagnosis and treatment of myocardial fibrosis in hypertensive heart disease. Circ J 2008;72(Suppl A):A8–A12. [DOI] [PubMed] [Google Scholar]
- 16. Raman SV. The hypertensive heart. An integrated understanding informed by imaging. J Am Coll Cardiol 2010;55(2):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bacharova L, Plandorova J, Klimas J, et al. Discrepancy between increased left ventricular mass and “normal” QRS voltage is associated with decreased connexin 43 expression in early stage of left ventricular hypertrophy in spontaneously hypertensive rats. J Electrocardiol 2008;41(6):730–734. [DOI] [PubMed] [Google Scholar]
- 18. Fialova M, Dlugosova K, Okruhlicova L, et al. Adaptation of the heart to hypertension is associated with maladaptive gap junction connexin‐43 remodeling. Physiol Res 2008;57(1):7–11. [DOI] [PubMed] [Google Scholar]
- 19. Ang D, Lang C. The prognostic value of the ECG in hypertension: Where are we now? J Hum Hypertens 2008;22(7):460–467. [DOI] [PubMed] [Google Scholar]
- 20. Olsen MH, Wachtell K, Bella JN, et al. Albuminuria predicts cardiovascular events independently of left ventricular mass in hypertension: A LIFE substudy. J Hum Hypertens 2004;18(6):453–459. [DOI] [PubMed] [Google Scholar]
- 21. Canga A, Kocaman SA, Durakoglugil ME, et al. Relationship between fragmented QRS complexes and left ventricular systolic and diastolic functions. Herz 2013;38(6):665–670. [DOI] [PubMed] [Google Scholar]


