Abstract
Mid‐ventricular obstructive hypertrophic cardiomyopathy (MVOHCM) is an uncommon type of HCM. LV apical aneurysms are present in more than 20% MVOHCM cases and has been identified as an independent predictor of potentially lethal arrhythmic events, including non‐sustained or sustained ventricular tachycardia (VT), and ventricular fibrillation (VF), as well as SCD. Although the pathogenesis of LVA remains unknown, but it has been suggested that apical aneurysm may be secondary to the increased after‐load and high apical pressure arising from significant pressure gradient of the midventricular obstruction. The scarred rim of the aneurysm and the adjacent areas of LV myocardial fibrosis and consequent apical oxygen‐demand mismatch may be responsible for the formation of apical aneurysm. Recent electrophysiologic studies have demonstrated that the aneurysmal rim forms the primary culprit arrhythmogenic substrate for generation of monomorphic ventricular tachycardia leading to SCD, but the clinical significance of the size of aneurysm in relation to SCD remains unsettled. We summarized the clinical features of the patients with MVOHCM and apical aneurysms. Appropriate therapeutic interventions include ICD implantation, and early surgical intervention for gradient relief may be undertaken to relief the MVO.
Keywords: apical aneurysm, hypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a genetic condition characterized by a wide spectrum of morphologies and natural history. Mid‐ventricular obstructive (MVO) hypertrophic cardiomyopathy (MVOHCM) is an uncommon type of HCM (Maron et al., 2003, 2014), characterized by the presence of a pressure gradient between the apical and basal chambers of the left ventricle (LV) (Maron et al., 2008). Patients with MVOHCM experience more frequent episodes of sudden cardiac death (SCD) and malignant ventricular tachyarrhythmias compared to those with HCM without mid‐ventricular obstruction (Cai et al., 2014). In clinical practice, MVOHCM is often accompanied by an apical aneurysm (Tengiz, Ercan, & Turk, 2006; Tse & Ho, 2003). Indeed, LV apical aneurysms are present in more than 20% MVOHCM cases and have been identified as an independent predictor of potentially lethal arrhythmic events, including non‐sustained or sustained ventricular tachycardia (VT), and ventricular fibrillation (VF), as well as SCD (Cui, Suo, Zhao, Li, & Liu, 2016; Mörner, Johansson, & Henein, 2011). Interestingly, Sato et al. described a case of MVOHCM presented with VF storm in association with acute myocardial infarction (AMI) and a LV apical aneurysm despite the presence of normal coronary arteries (Sato et al., 2007). The clinical features of the patients with MVOHCM and apical aneurysms are summarized in Table 1.
Table 1.
Case reports on ventricular tachycardia associated with MVOHCM
| Case report | Sato (Cui et al., 2016) | Efthimiadis et al. (Sato et al., 2007) | Pérez‐Riera et al. (Efthimiadis et al., 2009) | Dilaveris et al. (Pérez‐Riera et al., 2016) | Gao et al. (Yan et al., 2015) | Petrou et al. (Liu et al., 2017) | Shimahara et al. (Petrou et al., 2014) | Shah et al. (Shimahara et al., 2015) |
|---|---|---|---|---|---|---|---|---|
| Age(year)/sex | 58/M | 63/F | 53/M | 80/F | 54/M | 69/F | 44/M | 57/M |
| PG (mm Hg) | 64 | ‐ | 91 | ‐ | 60 | 120 | ‐ | ‐ |
| Arrhythmia | VF、NSVT | NSVT | SMVT | SMVT | SMVT | SMVT | VT | VT |
| Origin of VT | ‐ | ‐ | LV apical | LV | LV | LV | LVAA border | apical scar |
| ST‐T abnormality |
Negative ST elevation |
Negative T |
negative T ST elevation |
Negative | Negative | ‐ | ||
| Apical aneurysm | + | + | 4.4 cm | + | + | + | + | + |
| Mural thrombus | + | ‐ | ‐ | ‐ | + | ‐ | ‐ | ‐ |
| CAG/CTA | Normal | ‐ | Normal | Normal | Normal | ‐ | Normal | |
| Left ventriculogram | None | None | + | + | + | + | None | None |
| CMR | + | + | None | None | + | None | None | None |
| Therapy | declined | ICD | ICD | ICD | Surgerya | ICD | Surgeryb | ICD/Surgeryb |
| Treatments | β‐blocker | ‐ | β‐blocker | None | Metoprolol amiodarone | ‐ | β‐blocker amiodarone | None |
| Follow‐up | 6 months | 2 years | ‐ | ‐ | 18 months | ‐ | 2 years | 6 months |
| Prognosis | ECG abnormalities persisted | died due to heart failure | ‐ | ‐ | clinically stable and free from arrhythmic recurrence | ‐ | No cardiac events |
one (inappropriate) discharge from the ICD |
CAG: coronary artery angiography; CTA: computed tomographic angiography; ICD: implantable cardioverter defibrillator; LV: left ventricular; negative: T‐wave inversions in precordial leads; NSVT: non‐sustained ventricular tachycardia; PG: pressure gradient; SMVT: sustained monomorphic ventricular tachycardia.
Myectomy of the inappropriate hypertrophy myocardium and removal of ventricular thrombus and excision of the apical aneurysm.
Apical aneurysmectomy, left ventricular reconstruction, and cryoablation at the rim of the aneurysm were performed; Left ventriculogram, showed mid‐ventricular obstruction with a similar appearance of an hourglass shape and an apical aneurysm; CMR, cardiac magnetic resonance.
A number of case reports concerning MVOHCM accompanied by apical aneurysms have been associated with VT, and have a worse prognosis (Dilaveris et al., 2017; Efthimiadis et al., 2009; Pérez‐Riera, Barbosa‐Barros, Lucca, Viana, & de Abreu, 2016). The scarred rim of the apical aneurysm and the associated extensive areas of myocardial fibrosis have been regarded as arrhythmogenic substrates for the origination of malignant ventricular tachyarrhythmias. Ventricular tachycardia is a rare complication of HCM and more likely to occur in MVOHCM with apical aneurysm. Apical aneurysms can be identified by echocardiography with the use of contrast agents, but can be reliably identified by cardiac magnetic resonance imaging, in which late gadolinium enhancement is present owing to the presence of myocardial scarring and fibrosis (Figure 1).
Figure 1.
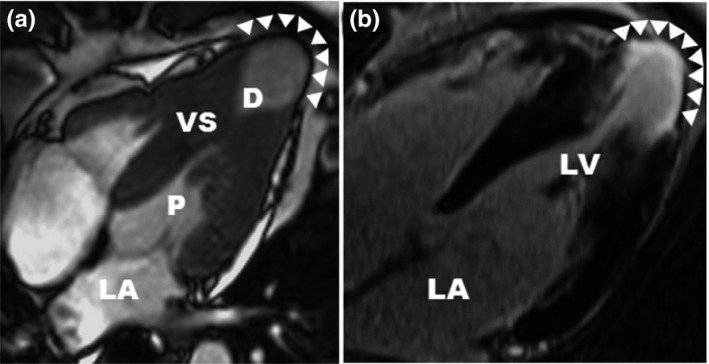
cardiac magnetic resonance (cited from Rowin et al) (a) CMR demonstrates medium‐size apical aneurysm (3.2 cm; arrowheads) with associated hourglass shaped LV chamber producing distinct proximal (P) and distal (D) chambers. (b) LGE localized to the aneurysm rim (arrowheads)
Recent studies have evaluated the long‐term outcomes of the patients with MVOHCM and apical aneurysms (Table 2). In HCM cohorts, MVO has been identified as an independent predictor of HCM‐related mortality, especially the composite endpoint of SCD and potentially lethal arrhythmic events (Minami et al., 2011). Furthermore, a large case series by Rowin et al. found that the presence of LV apical aneurysms represents a high‐risk subgroup associated with a number of adverse complications such higher incidences of VT/VF and out‐of‐hospital cardiac arrest requiring ICD implantation (Rowin et al., 2017). Ventricular tachyarrhythmias accounted for 38% of hospitalizations in HCM patients, and sustained monomorphic VT occurred in patients with MVOHCM and abnormal wall motion of the LV apex (Furushima et al., 2010). Efthimiadis et al. confirmed that MVOHCM was associated with unfavorable prognosis in terms of end‐stage HCM, sudden death, and lethal arrhythmic events (Efthimiadis et al., 2013). Yan et al. showed that MVOHCM is associated with cardiac death in Chinese population (Yan et al., 2015). One‐half of these patients experienced major cardiovascular events, and 20% developed an apical aneurysm, which was significantly associated with arrhythmia events. These data warrant attention for early recognition and appropriate intervention of MVOHCM. Liu et al systematic review and meta‐analysis suggested that non‐sustained ventricular tachycardia (nsVT) was the strongest predictor for cardiovascular death (13.02%, 95% CI 3.60%–25.91%), while MVO was the strongest predictor for all‐cause death and sudden cardiac death (16.44%, 95% CI 7.45%–31.55%, respectively) in HCM patients (Liu, Li, Berger, Johns, & Gao, 2017).
Table 2.
Case series on ventricular tachycardia in patients with MVOHCM
| Maron et al. (Maron et al., 2008) | Minami et al. (Dilaveris et al., 2017) | Furushima et al. (Minami et al., 2011) | Rowin et al. (Rowin et al., 2017) | Efthimiadis et al. (Efthimiadis et al., 2013) | Yan et al. (Yan et al., 2015) | |
|---|---|---|---|---|---|---|
| Subjects (n) | 19a | 46 | 12 | 93 (34)a | 34 | 60 |
| Age (years) | 55.6 ± 12.7 | 53.2 ± 14.7 | 56 ± 11.4 | 56 ± 13 | 50.1 ± 16.6 | 40.2 ± 15 |
| M/sex (n) | 13 (68.4%) | 28 (60.9%) | 11 (91.7%) | 64 (68.8%) | 16 (47.1%) | 45 (75%) |
| Family history of SCD (n) | 6 (13%) | 11 (11.8%) | 6 (10%) | |||
| Arrhythmia | 5 (26.3%) NSVT | 14 (30.4%) NSVT | 12 (100%) SMVT | 33 (35.5%) NSVT | 7 (20.6%) NSVT | 19 (31.7%) NSVT |
| LVWT (mm) | 17.9 ± 3.2 | 19.1 ± 4.3 | 19 ± 5 | 24 ± 4.4 | ||
| LVAA (n) | 19 (100%) | 13 (28.3%) | 93 | 9 (26.5%) | 12 (20%) | |
| PG (mmHg) | 74 ± 42 | 45.9 ± 14.7 | 44 ± 26 | 65.6 ± 31.1 | ||
| follow‐up (yr) | 4.1 ± 3.7 | 10.4 ± 8.2 | 9.8 ± 6.9 months | 4.4 ± 3.2 | 7.6 ± 7.7 | |
| Progressive HF (n) | 5 (26.3%) | 6 (13%) | 5 (15%) | 4 (11.8%) | 10 (16.7%) | |
| Stroke or thromboembolic(n) | 2 (10.5%) | 5 (10.9%) | 5 (5.3%) | 3 (8.8%) | 4 (6.7%) | |
| Treatments | ||||||
| Beta‐blockers | 35 (76.1%) | 80 | 25 (73.5%) | |||
| CCBs | 14 (30.4%) | 36 (38.7%) | 4 (11.7%) | |||
| Amiodarone | 0 | 18 | 6 (17.6%) | |||
| Warfarin | 13 (28.3%) | 39 (41.9%) | 9 (26.5%) | 6 (10%) | ||
| ICD (n) | 3 (15.7%) | 10 (21.7%) | 10 (83.3%) | 56 (60.2%) | 6 (17.6%) | 4 (6.7%) |
| Other | 7b | 26c | ||||
HF: heart failure; ICD: implantable cardioverter defibrillator; LVAA: left ventricular apical aneurysm; LVWT: maximum left ventricular wall thickness; N CCB: calcium channel blocker; SCD: sudden cardiac death; SMVT: sustained monomorphic ventricular tachycardia; SVT: non‐sustained ventricular tachycardia.
Maron et al identified 28 patients with left ventricular apical aneurysms, 19 patients showed an “hourglass” contour with mid‐ventricular hypertrophy. Rowin et al studied 93 Patients of HCM With LV Apical Aneurysms, 34 of which (37%) had intraventricular mid‐cavity pressure gradients (44 ± 26 mm Hg).
Radiofrequency VT ablation ((n = 7, 7.5%).
alcohol septal ablation (n = 12, 20.0%), surgical myotomy‐myectomy (n = 12, 20%) and dual‐chamber pacemaker implantation(n = 2, 3.3%).
Although the pathogenesis of LVA remains unknown, but it has been suggested that apical aneurysm may be secondary to the increased after load and high apical pressure arising from significant pressure gradient of the mid‐ventricular obstruction. Other proposed explanations include small‐vessel disease with decreased coronary flow reserve, squeezing of the coronary artery from increased systolic myocardial wall stress in the hypertrophic segment, decreased coronary perfusion pressure due to mid‐ventricular obstruction and coronary artery spasm. The scarred rim of the aneurysm and the adjacent areas of LV myocardial fibrosis and consequent apical oxygen‐demand mismatch may be responsible for the formation of apical aneurysm. Recent electrophysiologic studies have demonstrated that the aneurysmal rim forms the primary culprit arrhythmogenic substrate for generation of monomorphic ventricular tachycardia leading to SCD, but the clinical significance of the size of aneurysm in relation to SCD remains unsettled. Therefore, we propose that MVOHCM with apical aneurysm should be considered as a new type of arrhythmogenic cardiomyopathy.
When antiarrhythmic agents with beta‐blockers and amiodarone fail to control VT, different interventions can be used. Surgical treatment can be performed to relieve the obstruction and reduce the apical aneurysm (Gao et al., 2011) and implantable cardioverter defibrillator (ICD) can be inserted (Petrou, Kyrzopoulos, Sbarouni, Tsiapras, & Voudris, 2014). ICD implantation has proven to be highly effective for preventing sudden cardiac death due to ventricular arrhythmias. A recent study reported that 37.2% of mid‐ventricular obstruction with LVA patients had defibrillators implanted, mainly for primary prevention and based on the team discretion that those patients share a high‐risk profile of SCD and that 25.7% of patients with ICD received appropriate shock therapy (Elsheshtawy et al., 2018). However, ICD implantation does not prevent recurrence of arrhythmia nor does it address ongoing heart failure. Furthermore, ICD shock might contribute to rehospitalization and myocardial injury, and backup ventricular pacing may impair LV function.
Surgical options such as transapical myectomy and cryoablation (Shimahara, Kobayashi, Fujita, & Sato, 2015), the apical rim is believed to be the critical slow conduction zone in the reentry of VT (Pérez‐Riera et al., 2016). However, the narrow aneurysmal neck forms a formidable obstacle for the catheter advancement through the narrow aneurysmal neck and catheter ablation is not always successful and cannot treat intracavitary obstruction. Therefore, combined with cryoablation from the epicardial and endocardial surface can be effective (Osawa, Fujimatsu, Takai, & Suzuki, 2011). Moreover, apical aneurysmectomy, myectomy, and subendocardial resection 24 have been shown to prevent future episodes of VT. Given that the mechanism of VT was his apical scar and that resection of the scar tissue and obliteration of the aneurysmal pouch would resolve the ventricular arrhythmias. Surgical myectomy of the inappropriate hypertrophy myocardium and excision of the apical aneurysm were considered to be a salvage option. (Elsheshtawy et al., 2018; Gao et al., 2011; Osawa et al., 2011; Petrou et al., 2014; Shah, Schaff, Abel, & Gersh, 2011; Shimahara et al., 2015) However, currently there are insufficient data as to the optimum timing for surgical approach.
In summary, ventricular arrhythmias are the most common complications in a patient with MVOHCM and are associated with the presence of apical aneurysms. The presence of apical aneurysm is a strong risk factor for lethal arrhythmic events, including non‐sustained or sustained ventricular tachycardia (VT), and ventricular fibrillation (VF), as well as SCD. Appropriate therapeutic interventions include ICD implantation, and early surgical intervention for gradient relief may be undertaken to relief the MVO.
CONFLICTS OF INTEREST
None declared.
Cui L, Tse G, Zhao Z, Bazoukis G, Letsas KP, Panagiotis K, Roever L, Li G, Liu T. Mid‐ventricular obstructive hypertrophic cardiomyopathy with apical aneurysm: An important subtype of arrhythmogenic cardiomyopathy. Ann Noninvasive Electrocardiol. 2019;24:e12638 10.1111/anec.12638
Contributor Information
Gary Tse, Email: tseg@cuhk.edu.hk.
Tong Liu, Email: liutongdoc@126.com.
REFERENCES
- Cai, C. , Duan, F. J. , Yang, Y. J. , Guo, X. , Liu, Y. , Liu, Y. , … Fan, C. . (2014). Comparison of the prevalence, clinical features, and long‐term outcomes of midventricular hypertrophy vs apical phenotype in patients with hypertrophic cardiomyopathy. Canadian Journal of Cardiology, 30, 441–447. 10.1016/j.cjca.2013.10.005 [DOI] [PubMed] [Google Scholar]
- Cui, L. , Suo, Y. , Zhao, Y. , Li, G. , & Liu, T. (2016). Mid‐Ventricular Obstructive Hypertrophic Cardiomyopathy and Apical Aneurysm Mimicking Acute ST‐Elevation Myocardial Infarction. Annals of Noninvasive Electrocardiology, 21, 98–101. 10.1111/anec.12284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilaveris, P. , Aggeli, C. , Synetos, A. , Skiadas, I. , Antoniou, C. K. , Tsiamis, E. , & Tousoulis, D. (2017). Sustained ventricular tachycardia as a first manifestation of hypertrophic cardiomyopathy with mid‐ventricular obstruction and apical aneurysm in an elderly female patient. Annals of Noninvasive Electrocardiology, 22, e12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efthimiadis, G. K. , Pagourelias, E. D. , Parcharidou, D. , Gossios, T. , Kamperidis, V. , Theofilogiannakos, E. K. , … Styliadis, I. H. (2013). Clinical characteristics and natural history of hypertrophic cardiomyopathy with midventricular obstruction. Circulation Journal, 77, 2366–2374. 10.1253/circj.CJ-12-1561 [DOI] [PubMed] [Google Scholar]
- Efthimiadis, G. K. , Pliakos, C. , Pagourelias, E. D. , Parcharidou, D. G. , Spanos, G. , Paraskevaidis, S. , … Parcharidis, G. (2009). Hypertrophic cardiomyopathy with midventricular obstruction and apical aneurysm formation in a single family: Case report. Cardiovasc Ultrasound, 7, 26 10.1186/1476-7120-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsheshtawy, M. O. , Mahmoud, A. N. , Abdelghany, M. , Suen, I. H. , Sadiq, A. , & Shani, J. (2018). Left ventricular aneurysms in hypertrophic cardiomyopathy with midventricular obstruction: A systematic review of literature. Pacing and Clinical Electrophysiology, 41, 854–865. 10.1111/pace.13380 [DOI] [PubMed] [Google Scholar]
- Furushima, H. , Chinushi, M. , Iijima, K. , Sanada, A. , Izumi, D. , Hosaka, Y. , & Aizawa, Y. (2010). Ventricular tachyarrhythmia associated with hypertrophic cardiomyopathy: Incidence, prognosis, and relation to type of hypertrophy. Journal of Cardiovascular Electrophysiology, 21, 991–999. 10.1111/j.1540-8167.2010.01769.x [DOI] [PubMed] [Google Scholar]
- Gao, X. J. , Kang, L. M. , Zhang, J. , Dou, K. F. , Yuan, J. S. , & Yang, Y. J. (2011). Mid‐ventricular obstructive hypertrophic cardiomyopathy with apical aneurysm and sustained ventricular tachycardia: A case report and literature review. Chin Med J (Engl), 124, 1754–1757. [PubMed] [Google Scholar]
- Liu, Q. , Li, D. , Berger, A. E. , Johns, R. A. , & Gao, L. . (2017). Survival and prognostic factors in hypertrophic cardiomyopathy: A meta‐analysis. Scientific Reports, 7, 11957 10.1038/s41598-017-12289-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron, B. J. , McKenna, W. J. , Danielson, G. K. , Kappenberger, L. J. , Kuhn, H. J. , Seidman, C. E. , … Torbicki, A. . (2003). College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Journal of the American College of Cardiology, 42, 1687–1713. 10.1016/S0735-1097(03)00941-0 [DOI] [PubMed] [Google Scholar]
- Maron, B. J. , Ommen, S. R. , Semsarian, C. , Spirito, P. , Olivotto, I. , & Maron, M. S. (2014). Hypertrophic cardiomyopathy: Present and future, with translation into contemporary cardiovascular medicine. Journal of the American College of Cardiology, 64, 83–99. 10.1016/j.jacc.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Maron, M. S. , Finley, J. J. , Bos, J. M. , Hauser, T. H. , Manning, W. J. , Haas, T. S. , … Maron, B. J. (2008). Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation, 118, 1541–1549. 10.1161/CIRCULATIONAHA.108.781401 [DOI] [PubMed] [Google Scholar]
- Minami, Y. , Kajimoto, K. , Terajima, Y. , Yashiro, B. , Okayama, D. , Haruki, S. , … Hagiwara, N. (2011). Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. Journal of the American College of Cardiology, 57, 2346–2355. 10.1016/j.jacc.2011.02.033 [DOI] [PubMed] [Google Scholar]
- Mörner, S. , Johansson, B. , & Henein, M. (2011). Arrhythmogenic left ventricular apical aneurysm in hypertrophic cardiomyopathy. International Journal of Cardiology, 151, e8–e9. [DOI] [PubMed] [Google Scholar]
- Osawa, H. , Fujimatsu, T. , Takai, F. , & Suzuki, H. (2011). Hypertrophic cardiomyopathy with apical aneurysm: Left ventricular reconstruction and cryoablation for ventricular tachycardia. Gen Thorac Cardiovasc Surg, 59, 354–358. 10.1007/s11748-010-0695-7 [DOI] [PubMed] [Google Scholar]
- Pérez‐Riera, A. R. , Barbosa‐Barros, R. , de Lucca, A. A. , Viana, M. J. , & de Abreu, L. C. (2016). Mid‐ventricular Hypertrophic Obstructive Cardiomyopathy with Apical Aneurysm Complicated with Syncope by Sustained Monomorphic Ventricular Tachycardia. Annals of Noninvasive Electrocardiology, 21, 618–621. 10.1111/anec.12377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou, E. , Kyrzopoulos, S. , Sbarouni, E. , Tsiapras, D. , & Voudris, V. (2014). Mid‐ventricular hypertrophic obstructive cardiomyopathy complicated by an apical aneurysm, presenting as ventricular tachycardia. J Cardiovasc Ultrasound, 22, 158–159. 10.4250/jcu.2014.22.3.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowin, E. J. , Maron, B. J. , Haas, T. S. , Garberich, R. F. , Wang, W. , Link, M. S. , & Maron, M. S. (2017). Hypertrophic Cardiomyopathy With Left Ventricular Apical Aneurysm: Implications for Risk Stratification and Management. Journal of the American College of Cardiology, 69, 761–773. [DOI] [PubMed] [Google Scholar]
- Sato, Y. , Matsumoto, N. , Matsuo, S. , Yoda, S. , Kunimoto, S. , & Saito, S. (2007). Mid‐ventricular hypertrophic obstructive cardiomyopathy presenting with acute myocardial infarction. Texas Heart Institute Journal, 34, 475–478. [PMC free article] [PubMed] [Google Scholar]
- Shah, D. K. , Schaff, H. V. , Abel, M. D. , & Gersh, B. J. (2011). Ventricular tachycardia in hypertrophic cardiomyopathy with apical aneurysm. Annals of Thoracic Surgery, 91, 1263–1265. 10.1016/j.athoracsur.2010.09.041 [DOI] [PubMed] [Google Scholar]
- Shimahara, Y. , Kobayashi, J. , Fujita, T. , & Sato, S. (2015). Transapical myectomy and surgical cryoablation for refractory ventricular tachycardia due to hypertrophic cardiomyopathy with apical aneurysm. European Journal of Cardio‐Thoracic Surgery, 48, 334–335. 10.1093/ejcts/ezu351 [DOI] [PubMed] [Google Scholar]
- Tengiz, I. , Ercan, E. , & Turk, U. O. (2006). Percutaneous myocardial ablation for left mid‐ventricular obstructive hypertrophic cardiomyopathy. International Journal of Cardiovascular Imaging, 22, 13–18. 10.1007/s10554-005-5295-8 [DOI] [PubMed] [Google Scholar]
- Tse, H. F. , & Ho, H. H. (2003). Sudden cardiac death caused by hypertrophic cardiomyopathy associated with midventricular obstruction and apical aneurysm. Heart, 89, 178 10.1136/heart.89.2.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, L. R. , Zhao, S. H. , Wang, H. Y. , Duan, F. , Wang, Z. , Yang, Y. , … Fan, C. (2015). Clinical characteristics and prognosis of 60 patients with midventricular obstructive hypertrophic cardiomyopathy. Journla of Cardiovascular Medicine, 16, 751–760. 10.2459/JCM.0000000000000163 [DOI] [PubMed] [Google Scholar]


