Abstract
Background
P‐wave duration, its dispersion and signal‐averaged ECG, are currently used markers of vulnerability to atrial fibrillation (AF). However, since tangential atrial currents are better detectable at the body surface as magnetic than electric signals, we investigated the accuracy of magnetocardiographic mapping (MCG), recorded in unshielded clinical environments, as predictor of AF occurrence.
Methods
MCG recordings, in sinus rhythm (SR), of 71 AF patients and 75 controls were retrospectively analyzed. Beside electric and magnetic P‐wave and PR interval duration, two MCG P‐wave subintervals, defined P‐dep and P‐rep, were measured, basing on the point of inversion of atrial magnetic field (MF). Eight parameters were calculated from inverse solution with “Effective Magnetic Dipole (EMD) model” and 5 from “MF Extrema” analysis. Discriminant analysis (DA) was used to assess MCG predictive accuracy to differentiate AF patients from controls.
Results
All but one (P‐rep) intervals were significantly longer in AF patients. At univariate analysis, three EMD parameters differed significantly: in AF patients, the dipole‐angle‐elevation angular speed was lower during P‐dep (p < 0.05) and higher during P‐rep (p < 0.001) intervals. The space‐trajectory during P‐rep and the angle‐dynamics during P‐dep were higher (p < 0.05), whereas ratio‐dynamics P‐dep was lower (p < 0.01), in AF. At DA, with a combination of MCG and clinical parameters, 81.5% accuracy in differentiating AF patients from controls was achieved. At Cox‐regression, the angle‐dynamics P‐dep was an independent predictor of AF recurrences (p = 0.037).
Conclusions
Quantitative analysis of atrial MF dynamics in SR and the solution of the inverse problem provide new sensitive markers of vulnerability to AF.
Keywords: atrial fibrillation, discriminant analysis, inverse solution, magnetocardiography, surface cardiac mapping
1. INTRODUCTION
Atrial Fibrillation (AF) is one of the main causes of cardiovascular mortality and it is related to an increased risk of stroke, heart failure (Wang et al., 2003) and death, especially when self‐terminating paroxysmal AF (PAF) events are asymptomatic (Brachmann et al., 2016). Projections of AF prevalence demonstrated a constant yearly rise, increasing from 700,000 patients in 2010 to between 1.3 and 1.8 million patients with AF in the United Kingdom by 2060 (Chugh et al., 2014; Lane, Skjøth, Lip, Larsen, & Kotecha, 2017).
Alterations of atrial conduction and/or refractoriness and increased left atrium (LA) size are related to both genesis and maintenance of AF (Jurkko et al., 2010; Sarvari et al., 2016) with reciprocal relationship between AF and electrophysiological and/or structural remodelling due to atrial cardiomyopathy (Goette et al., 2017).
In patients with non‐permanent AF, P‐wave (PW) duration, PW amplitude, PW dispersion and parameters derived from signal‐averaged ECG analysis, are most used markers of abnormal interatrial conduction and vulnerability to AF recurrence (Filos, Chouvarda, Tachmatzidis, & Vassilikos, 2017; Lehtonen et al., 2017; Park et al., 2016; Pérez‐Riera et al., 2016; Tse et al., 2018). However, the predictive value of P‐wave duration (PWD) is less significant if inter‐atrial block (IAB) is only partial. Given the high incidence of silent‐AF and cryptogenic strokes, there is a growing interest for new methods to non‐invasively identify early risk markers for AF occurrence and/or recurrence.
Among them, magnetocardiographic mapping (MCG) can be more sensitive to early alteration of atrial electrophysiology, because ECG and MCG are sensitive to different configurations of the source current and mostly tangential atrial currents are better detectable as magnetic than electric signals at the body surface (Baule & McFee, 1970; Kim & Ahn, 2012; Siltanen, 1989).
In fact, compared to body surface potential measurements, which reflect the flux of the primary current distribution whereas the magnetic measurements are associated with the curl of the same source. Therefore, a vortex type loop current would be undetectable in ECG measurements but generates a measurable magnetic field. Moreover, unlike ECG, MCG is also not affected by conductivity variations caused by the lungs, pericardial effusion, muscles, and by the skin electrode interference.
Among different analytic approaches used to evaluate the predictive value of MCG for AF occurrence and recurrence, pseudo‐current reconstruction was reported as sensitive method to non‐invasively differentiate inter‐atrial conduction patterns in normal subjects as well as in patients with PAF (Jurkko et al., 2009; Koskinen et al., 2005; Lehto et al., 2009; Mäntynen et al., 2007; Sato et al., 2012). Delayed atrial conduction along in the Bachmann’s bundle (BB), prevalence of inter‐atrial conduction at the fossa ovalis (FO), or multisite inter‐atrial conduction pattern were associated with PAF (Jurkko et al., 2010).
All previous MCG studies were carried out in magnetically shielded rooms, providing optimal signal‐to‐noise ratio but, beside costs, not practical for routine ambulatory clinical application of the method. Since the present trend instead is toward the development of mobile non‐cryogenic instrumentations to carry unshielded MCG at patient bedside (Ghasemi‐Roudsari et al., 2017; Mooney et al., 2016), this retrospective study aimed to preliminarily assess the accuracy of ambulatory atrial MCG, recorded in an unshielded hospital laboratory for interventional electrophysiology, to predict PAF events, using parameters derived from automatic analysis of atrial magnetic field (MF) dynamics and of the three‐dimensional (3D) spatial dynamics of the atrial electromagnetic vector (EMV) calculated after solution of the inverse problem with the Effective Magnetic Dipole (EMD) model. Our hypotheses were that reliable MCG of atrial activity is feasible also in unshielded hospital ambulatory with a sensitivity adequate for clinical quantitative assessment of markers of AF vulnerability.
2. METHODS
2.1. Patients
The design of the study consisted of a retrospective collection of 71 patients with PAF (Kirchhof et al., 2016) and 75 normal controls (most of them sport practitioners), selected to yield as much as possible similar age distribution in both groups.
Inclusion criteria were the availability of: (a) at least one ECG‐documented episode of PAF preceding the MCG recordings; (b) a 12‐lead ECG taken in sinus rhythm (SR); (c) Two MCG recordings in SR, repeated sequentially to check for reproducibility; (d) exhaustive clinical records including history, cardiovascular risk factors, clinical work‐out according to good clinical practice and antiarrhythmic drugs (AAD) therapy; (e) a follow‐up period of at least 6 months; and (f) written informed consent to the anonymized retrospective use of clinical and MCG data for research purpose.
A trans‐thoracic echocardiogram (TTE) was not required as inclusion criteria. However, most of patients and controls had undergone a TTE within the 3 months before MCG recording. Patients with MCG recorded only after electric cardioversion or radiofrequency ablative treatment were excluded from this study. Other exclusion criteria were the presence of implanted devices or other ferromagnetic contaminants inducing artefact and the inability to stay comfortably supine for the duration of the MCG scan.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local review board.
2.2. Magnetocardiographic mapping
MCG was ambulatorily performed with a 36‐channel system (CardioMag Imaging Inc., Schenectady, NY, USA) in an unshielded laboratory equipped for interventional electrophysiology, measuring the z‐component of cardiac MF with direct current superconducting quantum interference device (DC‐SQUID) sensors, coupled to second order axial gradiometers with a 50–70 mm baseline, enclosed in a cylindrical cryostat cooled with liquid helium. The intrinsic sensitivity of the system was about 30 fT/Hz, above 1 Hz in the frequency range of clinical interest. Signals were recorded, in the supine position, with a Windows NT‐based acquisition system (24‐bits A/D conversion, 1 kHz sampling frequency, recording bandwidth: DC‐250 Hz), from an area of 20 × 20 cm of the anterior chest wall. MCG is typically recorded in sinus rhythm for 90 s (Fenici, Brisinda, & Meloni, 2005). However, for high‐resolution analysis of atrial activity, a continuous recording of 5 min was preferred. MCG data were accepted only if averaged signals of all 36 channels were free from artifacts.
2.3. Signal post‐processing and intervals definition
Post‐processing was performed with the CardioMag Windows NT‐based proprietary software. It consisted of digital filtering (low‐pass at 40 Hz without selective COMB filter of power line 50 Hz noise), time averaging to optimize the signal‐to‐noise ratio, and automatic reconstruction of time‐variant MF dynamics.
MCG waveforms were combined in the “butterfly” mode. The reference baseline was automatically defined within the T–P interval, usually about 50 ms before the onset of the PW (in SR). Electric PWD and PR interval duration were assessed from standard 12‐leads ECG. The magnetic PW onset was automatically defined by the software when the magnetic atrial signal exceeded the average level of baseline noise by at least three‐times. However, interactive analysis of the MF distribution of atrial depolarization (with one millisecond resolution) was also used to refine the onset of atrial depolarization (Figure 1). Due to the overlap of atrial repolarization MF component on magnetic PW, the offset of magnetic PW was arbitrarily chosen to coincide with the end of PW in reference ECG lead D2.
Figure 1.
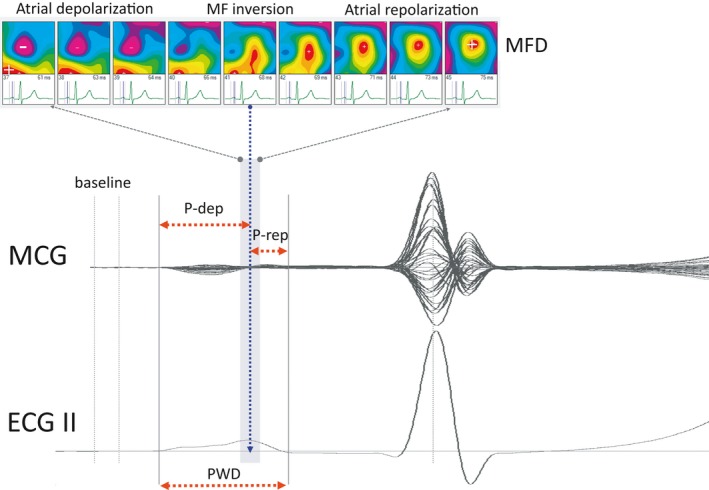
Schematic representation of atrial MCG measurements (PWD: P wave duration; MFD: magnetic field distribution; P‐dep and P‐rep: as explained in the text)
2.4. Magnetic field map animation and orientation time course
Atrial MF distribution was dipolar during both depolarization and repolarization. From visual analysis of the time‐variant dynamics of MF distribution during the PW, a point of polarity inversion of atrial MF was observed after the peak of the PW (Figure 1). Based on that point of inversion, two sub‐intervals were defined within the magnetic PW, which we arbitrarily named: PW depolarization (P‐dep) and PW repolarization (P‐rep).
2.5. Inverse solution and quantitative assessment of MF dynamics
After qualitative assessment based on visual inspection, quantitative analysis of atrial MF dynamics was automatically performed with a patented software tool (Bakharev, 2011), consisting of: (a) calculation of the time‐variant dynamics of atrial MF extrema; and (b) calculation of the spatial dynamics of the EMV component after solution of the inverse problemwith the EMD model. Thirteen parameters were automatically calculated:
-
Atrial MF Extrema dynamics (5 parameters):
Two Angle Extrema, maximum (Angle Extrema 1) and minimum (Angle Extrema 2), defined as α angle between a line through the poles and a horizontal line, the origin set to plus pole;
The Angle Dynamics (α angle rotation in each interval of 30 ms);
The Distance Dynamics (dynamic change of the distance between the poles ±);
The Ratio Dynamics,(MF strength ratio between the poles ±).
-
The three‐dimensional EMD vector (EMDV) components:
XY, XZ, YZ, space trajectory (dipole component dynamics);
Dipole angle azimuth (the angle between the projection of 3D average EMDV on XY plane and the x‐axis, being the origin of the axes the vertex);
Dipole angle elevation(the angle between the projection of 3D average EMDV on XZ plane and the x‐axis, being the origin of the axes the vertex);
Dipole Angle Azimuth derivative (angular speed of dipole angle azimuth);
Dipole Angle Elevation derivative (angular speed of dipole angle elevation).
2.6. Statistical analysis
Continuous data were expressed as mean ± standard deviation (SD). Differences between groups were examined using parametric student T test or non‐parametric Mann–Whitney U test. Categorical variables were rated with Chi‐square test. A p‐value <0.05 was considered statistically significant. Linear correlation and Cox regression were applied to study PAF data.
Discriminant analysis (DA) was used to identify MF parameters differentiating PAF patients from controls. The discriminant functions used by linear DA were built up as a linear combination of the variables that seek to maximize the differences between the two groups. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated, too.
All statistical tests were carried out with SPSS (version 21.0, SPSS, Inc., Chicago, IL, USA).
3. RESULTS
3.1. Clinical characteristics
The demographic and clinical characteristics of the patients and controls are summarized in Table 1.
Table 1.
Demographic and echocardiographic characteristics of examined population
| Variable | Patients with PAF (n = 71) | Controls (n = 75) | p |
|---|---|---|---|
| Age (years) | 58.4 ± 15.2 | 50.0 ± 15.29 | 0.001 |
| BMI (kg/m2) | 26.09 ± 3.85 | 24.30 ± 3.54 | 0.004 |
| Sex (Man) | 51 (72%) | 39 (52%) | 0.032 |
| Hypertension | 43 (61%) | 31 (41%) | 0.02 |
| Mellitus diabetes | 6 (8%) | 6 (8%) | n.s. |
| Mitral regurgitationa | 33 (46%) | 19 (25%) | 0.008 |
| Smoking habitb | 8 (11%) | 12 (16%) | n.s. |
| Dyslipidaemias | 27 (38%) | 30 (40%) | n.s. |
| Ischemic heart disease | 7 (14%) | 0 (0%) | 0.001 |
| LA size (mm) | 38.7 ± 6.4 | 34.8 ± 5.7 | 0.007 |
| CHADS2 score (0–6) | 0 (32%), 1 (51%), 2 (9%), 3(4%), 4 (4%) | 0 (65%), 1 (30%), 2 (5%) | <0.001 |
Notes. BMI: Body Mass Index; CHADS2: Congestive heart failure history, Hypertension, Age ≥ 75 years, Diabetes mellitus, Previous stroke or TIA; LA: Left atrium; PAF: Paroxysmal atrial fibrillation.
Above mild regurgitation at Echocardiogram.
Active smokers >5 cigarettes daily.
Differences between groups regarding gender distribution, body mass index (BMI), CHADS2 score, presence of hypertension, previous ischemic heart disease (IHD), echocardiographic larger left atrium size and presence of mitral regurgitation were statistically significant.
In addition, hypertension and mitral regurgitation were risk factor for AF (ODD Ratio 2.18 and 2.56 respectively, I.C. 95%). Forty‐seven patients were under AAD therapy. CHADS2 score ≥2 was also a risk factor for AF (ODD Ratio 3.61 ‐ I.C. 95%).
3.2. Atrial intervals duration in PAF group and controls
At univariate analysis, all but one (P‐rep) measured intervals were significantly prolonged in PAF patients compared with controls (Table 2).
Table 2.
Measured intervals (all patients)
| Variable | Patients with PAF (n = 71) | Controls (n = 75) | p |
|---|---|---|---|
| ECG P‐wave duration (PWDe) ‐ (ms) | 105.7 ± 13.5 | 100.2 ± 11.7 | 0.009 |
| Magnetic P‐wave duration (PWDm) ‐ (ms) | 98.6 ± 14.3 | 89.9 ± 11.3 | <0.001 |
| P‐wave depolarization (P‐dep) ‐ (ms) | 69.7 ± 13.3 | 64.7 ± 10.8 | 0.013 |
| P‐wave repolarization (P‐rep) ‐ (ms) | 28.9 ± 13.3 | 25.3 ± 10.2 | n.s. |
| ECG PR (PRe) ‐ (ms) | 176.4 ± 26.1 | 162.4 ± 27.4 | 0.002 |
| Magnetic PR (PRm) ‐ (ms) | 173.2 ± 27.9 | 151.5 ± 26.0 | <0.001 |
Note. PAF: Paroxysmal atrial fibrillation; PR: PR interval.
However, when the comparison was done excluding patients under chronic treatment with AAD, although all intervals of the PAF patients were longer, differences (except for MCG PR) between the groups were not statistically significant.
3.3. Quantitative analysis
At univariate analysis, among atrial MF Extrema parameters, only the Angle dynamics P‐dep and the Ratio dynamics P‐dep were significantly different between two groups (Table 3).
Table 3.
EMV and Extrema parameters
| Variable | Patients with PAF (n = 71) | Controls (n = 75) | p |
|---|---|---|---|
| Angle dynamics P‐dep (°) | 174.25 ± 82.42 | 142.55 ± 81.40 | 0.021 |
| Ratio dynamics P‐dep | 1.53 ± 1.59 | 2.45 ± 2.87 | 0.003 |
| Dipole angle azimuth angular speed P‐dep (degree/ms) | 0.20 ± 1.70 | −16.12 ± 119.84 | n.s. |
| Dipole angle elevation angular speed P‐dep (degree/ms) | −0.19 ± 0.43 | −0.31 ± 0.33 | 0.044 |
| Dipole angle azimuth angular speed P‐rep (degree/ms) | 5.77 ± 6.54 | 6.26 ± 7.77 | n.s. |
| Dipole angle elevation angular speed P‐rep (degree/ms) | −0.46 ± 1.15 | 0.16 ± 0.92 | <0.001 |
| XY P‐rep (cm) | 5.22 ± 2.68 | 4.72 ± 2.28 | n.s. |
| YZ P‐rep (cm) | 5.92 ± 2.43 | 5.18 ± 2.03 | 0.047 |
| XZ P‐rep (cm) | 5.81 ± 2.71 | 5.23 ± 2.01 | n.s. |
| Max space trajectory P‐rep (cm) | 9.89 ± 4.28 | 8.87 ± 3.40 | n.s. |
Note. EMV: Effective magnetic vector; PAF: paroxysmal atrial fibrillation; P‐dep: P‐wave depolarization; P‐rep: P‐wave repolarization.
The spatial dynamics of the atrial magnetic dipole can be quantitatively described, with time‐resolution of 1 ms, by the Dipole Angle Azimuth and the Dipole Angle Elevation of the EMV, calculated after inverse solution. Three EMV parameters differed significantly between PAF patients and controls (Table 3):
The magnitude of dipole angle elevation angular speed (ElAngSpeed), calculated during the P‐dep subinterval was lower in PAF than in controls (−0.19 ± 0.43 degree/ms vs. −0.31 ± 0.33 degree/ms, respectively; p < 0.05);
The magnitude of dipole angle elevation angular speed (ElAngSpeed), calculated during P‐rep was higher in PAF (−0.46 ± 1.15 degree/ms vs. 0.16 ± 0.92 degree/ms, respectively; p < 0.001);
The Space trajectory during P‐rep was higher (YZP‐rep p < 0.05) in PAF.
At DA, the combination of all the above MCG parameters in the formula:
provided 80.8% (76% cross‐correlated) discriminant accuracy (Sensitivity 76%, Specificity 76%, PPV 75%, NPV 77%) between PAF patients (F1 > 0) and controls (F1 < 0). Adding CHADS2 score to this statistical model, discriminant accuracy increased from 80.8% to 81.5% (sensitivity 77%).
The discriminant accuracy of MCG was practically unchanged (79%, 76% cross‐validate), also when PAF patients in AAD therapy were excluded from the analysis.
If AF patients were subdivided basing on P‐rep interval duration, with a cut‐off of ≥40 ms a subgroup of 16 patients was identified, which parameters increased the discriminant accuracy from healthy controls to 96.7% (92.3 cross‐correlated).
3.4. Comparison between discriminant accuracy of ECG and MCG predictors of AF
Differences in discriminant accuracy of ECG and MCG parameters, alone or in combination, as function of the individual CHADS2 score were also calculated and are summarized in Table 4.
Table 4.
Discriminant Accuracy ECG and MCG parameters or combination
| ECG intervals | MCG intervals | MCG parameters | DAc (cross‐validated) |
|---|---|---|---|
| PWD | 65.8% (63.7) | ||
| PWD, PR | 65.8% (64.4) | ||
| PWD, PR | PWD | 70.0% (68.5) | |
| PWD, PR | PWD, PR | 76.7% (76.0) | |
| PWD, PR | PWD, PR | Extrema + EMV | 81.5% (77.4) |
Note. DAc: Discriminant accuracy; EMV: Effective magnetic vector; PR: PR interval; PWD: P‐wave duration.
3.5. Predictors of AF‐recurrence
During an average follow‐up period of 24 ± 5.2 months, 36 patients had recurrence of PAF. At the multivariate logistic Cox regression, the Angle dynamics P‐dep was an independent predictor of AF‐recurrence (p = 0.037).
4. DISCUSSION
Already in the nineties, Frustaci et al. demonstrated structural abnormalities such as chronic inflammatory infiltrates, foci of myocyte necrosis and focal replacement fibrosis, in right atrial septal biopsies of patients with “lone AF” (Frustaci et al., 1997). Nowadays AF is considered expression of an atrial cardiomyopathy defined as “any complex of structural, architectural, contractile or electrophysiological changes affecting the atria with the potential to produce clinically‐relevant manifestations” (Goette et al., 2017). Diabetes, obesity, IHD, hypertension, dyslipidemias and AF itself (Wijffels, Kirchhof, Dorland, & Allessie, 1995) induce atrial remodeling that contributes to the maintenance, progression and stabilization of AF.
Being the pathogenic mechanisms of AF potentially different in individual patients, the most efficient treatment and early preventive strategies are not yet clearly defined. The maintenance of SR and the prevention of new‐AF episodes obtained with AAD showed an efficacy of only 50%–60% (Camm, 2012) and no strong evidence has been provided so far of the efficacy of radiofrequency (RF) ablation as the first‐line treatment (Cosedis Nielsen et al., 2012; Hakalahti, Biancari, Nielsen, & Raatikainen, 2015; Morillo et al., 2014; Wazni et al., 2005). On the other hand, it has been clearly demonstrated that the management of cardiovascular risk‐factors and promotion of healthy lifestyles (Fioravanti, Brisinda, Sorbo, & Fenici, 2015; Pathak et al., 2015) is efficient and should represent the first‐line therapy to prevent AF recurrences and to improve patients quality of life.
Since early identification of silent AF decreases risk of thromboembolic events, beside prolonged ECG monitoring with loop recorders (Brachmann et al., 2016). ECG detection of IAB with different methods is widely used. However, although some studies suggested increased risk of developing new onset AF when IAB is observed, others did not find a similar association and it is still unclear the extent to which partial IAB contributes to the risk of new‐onset AF or recurrences (Cotter et al., 2013; Gul et al., 2017; Tse et al., 2018). A systematic review and meta‐analysis has shown that IAB is a significant predictor of new onset AF, with hazard ratio (HR) of 2.42, and of AF recurrence after ablation (HR: 2.59; Tse et al., 2018). Wu et al. showed that IAB in SR and CHADS2 score independently and synergistically predicted new‐onset AF (Wu et al., 2016). A positive predictive accuracy of 79% in separating patients with PAF from control subjects has been reported with ECG measurement of PWD using a P maximum value of 106 ms (Pérez‐Riera et al., 2016).
Beside ECG, there is growing evidence that contactless multi‐channel MCG can provide additional information (Fenici et al., 2005; Yamada & Yamaguchi, 2005).
Indeed, although experience is still limited to only few institution, contactless MCG is one of the most promising technology for non‐invasive cardiac electro‐anatomical imaging and accurate localization of arrhythmogenic substrates (Brisinda, Venuti, Sorbo, & Fenici, 2013; Fenici & Brisinda, 2006; Fenici, Brisinda, Venuti, & Sorbo, 2013; Kwong, Leithäuser, Park, & Yu, 2013). Several studies, all carried out in magnetically shielded rooms, have demonstrated the usefulness of MCG to study AF (Jurkko et al., 2009, 2010; Kim & Ahn, 2012; Kim, Kim, Lee, & Ahn, 2007; Koskinen et al., 2005; Lehto et al., 2009; Lim et al., 2007; Mäntynen et al., 2007; Nakai et al., 2008; Sato et al., 2012; Yamada & Yamaguchi, 2005; Yamada, Tsukada, Miyashita, Kuga, & Yamaguchi, 2003; Yoshida et al., 2015). The reproducibility of automatically measured terminal high‐frequency component of signal averaged atrial magnetic signals was evaluated in PAF patients compared with controls (Koskinen et al., 2005). MCG provides non‐invasive detection of inter‐atrial conduction pathways (Jurkko et al., 2009; Mäntynen et al., 2007) and has shown that susceptibility to PAF is associated with propagation from the right to the LA via margin of FO or multiple pathways (Jurkko et al., 2009, 2010; Mäntynen et al., 2007). 3D‐spectral analysis with a 64‐channel MCG was used to preoperatively detect areas of AF dominant frequency (DF; Nakai et al., 2008; Yoshida et al., 2015). Finally, an increase in right atrial MF strength was a predictor of AF recurrence after RF ablation (Sato et al., 2012).
Since costs and operational difficulty related to mandatory need of heavy electromagnetic shielding and cryogenic instrumentations has significantly impaired the clinical application of the MCG, the present trend is toward the development of novel non‐cryogenic recording systems to perform MCG at the patient’s bedside with mobile unshielded instruments (Ghasemi‐Roudsari et al., 2017; Mooney et al., 2016). However, clinical experience with MCG of AF patients performed in unshielded hospital environments was very limited, so far (Fenici & Brisinda, 2007a, 2007b). The present study aiming to preliminary assess the feasibility of unshielded MCG of atrial activity and its predictive value in identifying patients at risk new‐onset or recurrence AF, provided the following new information:
First, it was confirmed that MCG is feasible in an unshielded hospital laboratory for clinical electrophysiology with sensitivity good enough to reliably investigate atrial activity of patients with non‐ permanent AF.
Second, although in our study cohort ECG intervals (PWD and PR) even in combination with CHADS2had much lower discrimination accuracy in separating patients with PAF from control subjects. If MCG intervals simultaneously measured from the same dataset were included, discriminant accuracy increased only mildly (from 65.8% to 70%; Table 4).
Third, visual analysis of atrial MF dynamics showed a reproducible inversion of MF polarity already during the descending limb of the PW (Figure 2a), consistent with dominance of the shallower early right atrial repolarization MF overlapping the deeper left atrial depolarization sources, interpretation supported by previous simultaneous MCG and right atrial monophasic action potential recording (Fenici & Brisinda, 2007a,2007b). Because of that MF configuration, we arbitrarily named the second PW subinterval P‐rep. However, after subtracting average atrial repolarization MF (i.e. the MF averaged at 50% of the PR interval), a residual PW MF distribution consistent with atrial depolarization was unmasked (Figure 2b), and the overall PW EMD localization shows a trajectory moving from right to left and to the back, thus consistent with depolarization from the right to the left atrium (Figure 2c).
Figure 2.
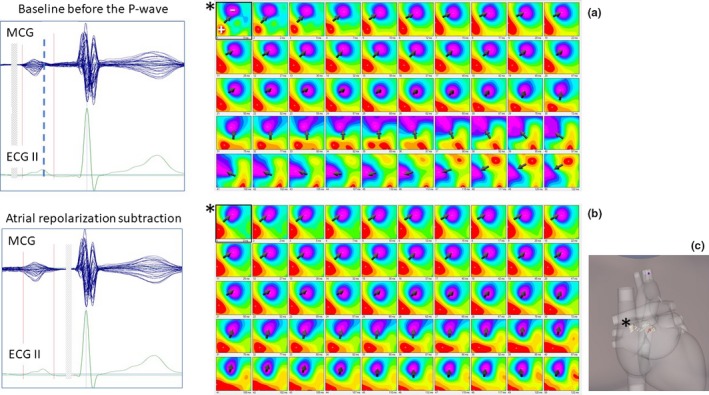
(a) Time‐variant dynamics of the MF distribution during the whole P‐wave. Typical inversion of the MF polarity is evident during the P‐wave descending limb (thick dashed line). (b) After subtraction of atrial repolarization MF, residual MF distribution consistent with the underlying atrial depolarization is unmasked. (c) Inverse EMD localization within the 3D heart model shows a trajectory consistent with atrial depolarization from the right (* PW onset) to the left atrium
By including in the DA the parameters of PW Extrema MF dynamics and the EMV parameters, automatically calculated after inverse solution with the EMD model, the discriminant accuracy in differentiating AF patients (with or without AAD therapy) increased from 76.7% to 81.5%, with the same sensitivity (77%) of echocardiographic evaluation of LA mechanical dispersion and of left ventricular global longitudinal strain to identify PAF patients (Sarvari et al., 2016).
Interestingly, discriminant accuracy obtained with MCG data of a subgroup of AF patients, screened with on P‐rep interval cut‐off ≥40 ms, increased to above 90%.
This suggests that a significant alteration of MCG parameters calculated during the descending limb of the PW is very sensitive marker for AF vulnerability. Among them, the YZ P‐rep and the ElAngSpeed P‐rep are indices of delayed activation of the LA (i.e. IAB; Conte et al., 2017; Jurkko et al., 2010; Pérez‐Riera et al., 2016; Tse et al., 2018), however, it is not possible at the moment to exclude or quantify a concomitant effect of atrial repolarization abnormality.
Finally, it is noteworthy that one MCG parameter (Angle dynamics P‐dep) was an independent predictor of AF‐recurrence during the follow‐up at the multivariate logistic Cox regression (p = 0.037).
4.1. Limitation of the study
A first unquestionable limitation of this study is the relatively small number of investigated patients. Therefore, we arbitrarily decided not to exclude any cases (e.g. patients with ischemic heart disease), which determines that the study groups were significantly different in several relevant clinical factors.
This however, partially due to the retrospective design of the study, cannot detract the interest for the results, since to the best of our knowledge this is the first study attempting a quantitative assessment and predictive accuracy of MCG parameters obtained by the inverse solution of atrial MF introducing them in a multifactorial predictive model. Future and perspective studies are needed to confirm our preliminary results and evaluate possible mechanistic differences related to underlying pathology.
A second factor limiting the number of recruited patients has been the exclusion of patients with implanted devices such as pacemakers or defibrillators. This past limitation to clinical use of MCG is going to be soon overcome with the present development of devices compatible with magnetic resonance imaging.
5. CONCLUSION
Previous MCG studies of atrial activity were carried out in magnetically shielded rooms (MSR), providing optimal signal‐to‐noise ratio but, beside costs, not practical for routine ambulatory clinical application of the method. Such limitation can be overcome, as demonstrated in the present study, using unshielded MCG mapping system with second‐order gradiometers configuration and advanced real‐time electronic noise suppression. Although MCG signals are cleaner if acquired in MSR, yet also with shielded MCG signal‐averaging is still used to improve the signal‐to‐noise ratio. Thus, the prevalent difference between shielded and unshielded MCG is the number of seconds averaged. In our study clinically useful information for quick clinical assessment of AF risk occurrence/recurrence were obtained by averaging 90–300 s of MCG signals in SR. Furthermore, the development of novel, non‐cryogenic instrumentations based on magnetic optical sensors, or other technology working without electromagnetic shielding, is foreseen to further simplify widespread clinical use of MCG with significantly reduction of costs.
6. UNCITED REFERENCE
Mooney et al. (2016)2016.
Guida G, Sorbo AR, Fenici R, Brisinda D. Predictive value of unshielded magnetocardiographic mapping to differentiate atrial fibrillation patients from healthy subjects. Ann Noninvasive Electrocardiol. 2018;23:e12569 10.1111/anec.12569
REFERENCES
- Bakharev, A. (2011). Ischemia identification, quantification and partial localization in MCG ‐ EP 1 349 494 B1.
- Baule, G. M. , & McFee, R. (1970). The magnetic heart vector. American Heart Journal, 79(2), 223–236. 10.1016/0002-8703(70)90312-1 [DOI] [PubMed] [Google Scholar]
- Brachmann, J. , Morillo, C. A. , Sanna, T. , Di Lazzaro, V. , Diener, H.‐C. , Bernstein, R. A. , … Passman, R. S. (2016). Uncovering atrial fibrillation beyond short‐term monitoring in cryptogenic stroke patients. Circulation: Arrhythmia and Electrophysiology, 9(1), e003333 10.1161/CIRCEP.115.003333 [DOI] [PubMed] [Google Scholar]
- Brisinda, D. , Venuti, A. , Sorbo, A. R. , & Fenici, R. (2013). Magnetocardiographic demonstration of complex ventricular preexcitation resulting in ablation failure. International Journal of Cardiology, 168(5), 5046–5048. 10.1016/j.ijcard.2013.07.215 [DOI] [PubMed] [Google Scholar]
- Camm, J. (2012). Antiarrhythmic drugs for the maintenance of sinus rhythm: Risks and benefits. International Journal of Cardiology, 155(3), 362–371. 10.1016/j.ijcard.2011.06.012 [DOI] [PubMed] [Google Scholar]
- Chugh, S. S. , Havmoeller, R. , Narayanan, K. , Singh, D. , Rienstra, M. , Benjamin, E. J. , … Murray, C. J. L. (2014). Worldwide epidemiology of atrial fibrillation: A global burden of disease 2010 study. Circulation, 129(8), 837–847. 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte, G. , Luca, A. , Yazdani, S. , Caputo, M. L. , Regoli, F. , Moccetti, T. , … Auricchio, A. (2017). Usefulness of P‐wave duration and morphologic variability to identify patients prone to paroxysmal atrial fibrillation. American Journal of Cardiology, 119(2), 275–279. 10.1016/j.amjcard.2016.09.043 [DOI] [PubMed] [Google Scholar]
- Cosedis Nielsen, J. , Johannessen, A. , Raatikainen, P. , Hindricks, G. , Walfridsson, H. , Kongstad, O. , … Hansen, P. S. (2012). Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. New England Journal of Medicine, 367(17), 1587–1595. 10.1056/NEJMoa1113566 [DOI] [PubMed] [Google Scholar]
- Cotter, P. E. , Martin, M. P. J. , Ring, L. , Warburton, E. A. , Belham, M. , & Pugh, P. J. (2013). Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology, 80(17), 1546–1550. 10.1212/WNL.0b013e31828f1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenici, R. , & Brisinda, D. (2006). Magnetocardiography provides non‐invasive three‐dimensional electroanatomical imaging of cardiac electrophysiology. International Journal of Cardiovascular Imaging, 22(3–4), 595–597. 10.1007/s10554-006-9076-9 [DOI] [PubMed] [Google Scholar]
- Fenici, R. , & Brisinda, D. (2007a). Bridging noninvasive and interventional electroanatomical imaging: Role of magnetocardiography. Journal of Electrocardiology, 40(1), S47–S52. 10.1016/j.jelectrocard.2006.10.032 17993328 [DOI] [Google Scholar]
- Fenici, R. , & Brisinda, D. (2007b). Magnetocardiography provides non‐invasive three‐dimensional electroanatomical imaging of cardiac electrophysiology. Anadolu Kardiyoloji Dergisi, 7, 23–28. 10.14744/AnatolJCardiol.2017.7556 [DOI] [PubMed] [Google Scholar]
- Fenici, R. , Brisinda, D. , & Meloni, A. M. (2005). Clinical application of magnetocardiography. Expert Review of Molecular Diagnostics, 5(3), 291–313. 10.1586/14737159.5.3.291 [DOI] [PubMed] [Google Scholar]
- Fenici, R. , Brisinda, D. , Venuti, A. , & Sorbo, A. R. (2013). Thirty years of clinical magnetocardiography at the Catholic University of Rome: Diagnostic value and new perspectives for the treatment of cardiac arrhythmias. International Journal of Cardiology, 168(5), 5113–5115. 10.1016/j.ijcard.2013.07.238 [DOI] [PubMed] [Google Scholar]
- Filos, D. , Chouvarda, I. , Tachmatzidis, D. , Vassilikos, V. , & Maglaveras, N. (2017). Computer methods and programs in biomedicine beat‐to‐beat P‐wave morphology as a predictor of paroxysmal atrial fibrillation. Computer Methods and Programs in Biomedicine, 151, 111–121. 10.1016/j.cmpb.2017.08.016 [DOI] [PubMed] [Google Scholar]
- Fioravanti, F. , Brisinda, D. , Sorbo, A. R. , & Fenici, R. (2015). BMI reduction decreases AF recurrence rate in a mediterranean cohort. Journal of the American College of Cardiology, 66(20), 2264–2265. 10.1016/j.jacc.2015.07.084 [DOI] [PubMed] [Google Scholar]
- Frustaci, A. , Chimenti, C. , Bellocci, F. , Morgante, E. , Russo, M. A. , & Maseri, A. (1997). Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation, 96(4), 1180–1184. 10.1161/01.CIR.96.4.1180 [DOI] [PubMed] [Google Scholar]
- Ghasemi‐Roudsari, S. , Al‐Shimary, A. , Varcoe, B. , Byrom, R. , Kearney, L. , & Kearney, M. (2017). A portable prototype magnetometer to differentiate ischemic and non‐ischemic heart disease in patients with chest pain. PLoS One, 13, 1–10. 10.1371/journal.pone.0191241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goette, A. , Kalman, J. M. , Aguinaga, L. , Akar, J. , Cabrera, J. A. , Chen, S. A. , … Nattel, S. (2017). EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Heart Rhythm, 14(1), e3–e40. 10.1016/j.hrthm.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul, E. E. , Pal, R. , Caldwell, J. , Boles, U. , Hopman, W. , Glover, B. , … Baranchuk, A. (2017). Interatrial block and interatrial septal thickness in patients with paroxysmal atrial fibrillation undergoing catheter ablation: Long‐term follow‐up study. Annals of Noninvasive Electrocardiology, 22(4), 1–5. 10.1111/anec.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakalahti, A. , Biancari, F. , Nielsen, J. C. , & Raatikainen, M. J. P. (2015). Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: Systematic review and meta‐analysis. Europace, 17(3), 370–378. 10.1093/europace/euu376 [DOI] [PubMed] [Google Scholar]
- Jurkko, R. , Mäntynen, V. , Lehto, M. , Tapanainen, J. M. , Montonen, J. , Parikka, H. , & Toivonen, L. (2010). Interatrial conduction in patients with paroxysmal atrial fibrillation and in healthy subjects. International Journal of Cardiology, 145(3), 455–460. 10.1016/j.ijcard.2009.05.064 [DOI] [PubMed] [Google Scholar]
- Jurkko, R. , Mäntynen, V. , Tapanainen, J. M. , Montonen, J. , Väänänen, H. , Parikka, H. , & Toivonen, L. (2009). Non‐invasive detection of conduction pathways to left atrium using magnetocardiography: Validation by intra‐cardiac electroanatomic mapping. Europace, 11(2), 169–177. 10.1093/europace/eun335 [DOI] [PubMed] [Google Scholar]
- Kim, D. , & Ahn, H. (2012). Current status and future of cardiac mapping in atrial fibrillation. Atrial Fibrillation ‐ Basic Research and Clinical, Applications, 6, 108–117. [Google Scholar]
- Kim, D. , Kim, K. , Lee, Y.‐H. , & Ahn, H. (2007). Detection of atrial arrhythmia in superconducting quantum interference device magnetocardiography; preliminary result of a totally‐noninvasive localization method for atrial current mapping. Interactive Cardiovascular and Thoracic Surgery, 6(3), 274–279. 10.1510/icvts.2006.142869 [DOI] [PubMed] [Google Scholar]
- Kirchhof, P. , Benussi, S. , Kotecha, D. , Ahlsson, A. , Atar, D. , Casadei, B. , … Zeppenfeld, K. (2016). 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace, 18(11), 1609–1678. 10.1093/europace/euw295 [DOI] [PubMed] [Google Scholar]
- Koskinen, R. , Lehto, M. , Väänänen, H. , Rantonen, J. , Voipio‐Pulkki, L. M. , Mäkijärvi, M. , … Toivonen, L. (2005). Measurement and reproducibility of magnetocardiographic filtered atrial signal in patients with paroxysmal lone atrial fibrillation and in healthy subjects. Journal of Electrocardiology, 38(4), 330–336. 10.1016/j.jelectrocard.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Kwong, J. S. W. , Leithäuser, B. , Park, J.‐W. , & Yu, C.‐M. (2013). Diagnostic value of magnetocardiography in coronary artery disease and cardiac arrhythmias: A review of clinical data. International Journal of Cardiology, 167, 1835–1842. 10.1016/j.ijcard.2012.12.056 [DOI] [PubMed] [Google Scholar]
- Lane, D. A. , Skjøth, F. , Lip, G. Y. H. , Larsen, T. B. , & Kotecha, D. (2017). Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. Journal of the American Heart Association, 6(5), e005155 10.1161/JAHA.116.005155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto, M. , Jurkko, R. , Parikka, H. , Mäntynen, V. , Väänänen, H. , Montonen, J. , … Laine, M. (2009). Reversal of atrial remodeling after cardioversion of persistent atrial fibrillation measured with magnetocardiography. PACE ‐ Pacing and Clinical Electrophysiology, 32(2), 217–223. 10.1111/j.1540-8159.2008.02205.x [DOI] [PubMed] [Google Scholar]
- Lehtonen, A. O. , Langén, V. L. , Puukka, P. J. , Kähönen, M. , Nieminen, M. S. , Jula, A. M. , & Niiranen, T. J. (2017). Incidence rates, correlates, and prognosis of electrocardiographic P‐wave abnormalities – A nationwide population‐based study. Journal of Electrocardiology, 50(6), 925–932. 10.1016/j.jelectrocard.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Lim, H. K. , Chung, N. , Kim, K. , Ko, Y.‐G. , Kwon, H. , Lee, Y.‐H. , & Park, Y. K. (2007). Can magnetocardiography detect patients with non‐ST‐segment elevation myocardial infarction? Annals of Medicine, 39(8), 617–627. 10.1080/07853890701538040 [DOI] [PubMed] [Google Scholar]
- Mäntynen, V. , Vitikainen, A. M. , Koskinen, R. , Mäkijärvi, M. , Toivonen, L. , & Montonen, J. (2007). Magnetocardiography is sensitive to differences in inter‐atrial conduction in patients with paroxysmal lone atrial fibrillation. International Congress Series, 1300, 508–511. 10.1016/j.ics.2006.12.081 [DOI] [Google Scholar]
- Mooney, J. W. , Ghasemi‐Roudsari, S. , Banham, E. R. , Symonds, C. , Pawlowski, N. , & Varcoe, B. T. H. (2016). A portable diagnostic device for cardiac magnetic field mapping. Biomedical Physics & Engineering Express, 3, 015008 10.1088/2057-1976/3/1/015008 [DOI] [Google Scholar]
- Morillo, C. A. , Verma, A. , Connolly, S. J. , Kuck, K. H. , Nair, G. M. , Champagne, J. , … Natale, A. (2014). Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of paroxysmal atrial fibrillation (RAAFT‐2). JAMA, 311(7), 692 10.1001/jama.2014.467 [DOI] [PubMed] [Google Scholar]
- Nakai, K. , Oka, T. , Okabayashi, H. , Tsuboi, J. , Fukuhiro, Y. , Fukushima, A. , … Yoshizawa, M. (2008). Three‐dimensional spectral map of atrial fibrillation by a 64‐channel magnetocardiogram. Journal of Electrocardiology, 41(2), 123–130. 10.1016/j.jelectrocard.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Park, J. K. , Park, J. , Uhm, J. S. , Joung, B. , Lee, M. H. , & Pak, H. N. (2016). Low P‐wave amplitude (xxaaa0.1 mV) in lead i is associated with displaced inter‐atrial conduction and clinical recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Europace, 18(3), 384–391. 10.1093/europace/euv028 [DOI] [PubMed] [Google Scholar]
- Pathak, R. K. , Middeldorp, M. E. , Meredith, M. , Mehta, A. B. , Mahajan, R. , Wong, C. X. , … Sanders, P. (2015). Long‐term effect of goal directed weight management in an atrial fibrillation cohort: A Long‐term Follow‐Up Study (LEGACY Study) short. Journal of the American College of Cardiology, 65, 2159–2169. 10.1016/j.jacc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- Pérez‐Riera, A. R. , de Abreu, L. C. , Barbosa‐Barros, R. , Grindler, J. , Fernandes‐Cardoso, A. , & Baranchuk, A. (2016). P‐wave dispersion: An update. Indian Pacing and Electrophysiology Journal, 16(4), 126–133. 10.1016/j.ipej.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvari, S. I. , Haugaa, K. H. , Stokke, T. M. , Ansari, H. Z. , Leren, I. S. , Hegbom, F. , … Edvardsen, T. (2016). Strain echocardiographic assessment of left atrial function predicts recurrence of atrial fibrillation. European Heart Journal Cardiovascular Imaging, 17(6), 660–667. 10.1093/ehjci/jev185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Y. , Yoshida, K. , Ogata, K. , Inaba, T. , Tada, H. , Sekiguchi, Y. , … Aonuma, K. (2012). An increase in right atrial magnetic strength is a novel predictor of recurrence of atrial fibrillation after radiofrequency catheter ablation. Circulation Journal, 76(7), 1601–1608. 10.1253/circj.CJ-11-1419 [DOI] [PubMed] [Google Scholar]
- Siltanen, P. (1989). Magnetocardiography In Macfarlane T. L. N. Y. P. W. (Ed.), Comprehensive electrocardiology. Theory and practice in health and disease (Vol. 2, pp. 1405–1438). New York, NY: Pergamon Press. [Google Scholar]
- Tse, G. , Wong, C. W. , Gong, M. , Wong, W. T. , Bazoukis, G. , Wong, S. H. , … Baranchuk, A. (2018). Predictive value of inter‐atrial block for new onset or recurrent atrial fibrillation: A systematic review and meta‐analysis. International Journal of Cardiology, 250, 152–156. 10.1016/j.ijcard.2017.09.176 [DOI] [PubMed] [Google Scholar]
- Wang, T. J. , Larson, M. G. , Levy, D. , Vasan, R. S. , Leip, E. P. , Wolf, P. A. , & Benjamin, E. J. (2003). Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham heart study. Circulation, 107(23), 2920–2925. 10.1161/01.CIR.0000072767.89944.6E [DOI] [PubMed] [Google Scholar]
- Wazni, O. M. , Marrouche, N. F. , Martin, D. O. , Verma, A. , Bhargava, M. , Saliba, W. , & Natale, A. (2005). Radiofrequency ablation vs antiarrhythmic drugs as first‐line treatment of symptomatic atrial fibrillation: A randomized trial. JAMA: the Journal of the American Medical Association, 293, 2634–2640. 10.1001/jama.293.21.2634 [DOI] [PubMed] [Google Scholar]
- Wijffels, M. C. , Kirchhof, C. J. , Dorland, R. , & Allessie, M. A. (1995). Atrial fibrillation begets atrial fibrillation. Circulation Journal, 92, 1954–1968. 10.1161/01.CIR.92.7.1954 [DOI] [PubMed] [Google Scholar]
- Wu, J.‐T. , Wang, S.‐L. , Chu, Y.‐J. , Long, D.‐Y. , Dong, J.‐Z. , Fan, X.‐W. , & Yang, C.‐K. (2016). Usefulness of a combination of interatrial block and a high CHADS2 score to predict new onset atrial fibrillation. International Heart Journal, 57(5), 580–585. 10.1536/ihj.15-505 [DOI] [PubMed] [Google Scholar]
- Yamada, S. , Tsukada, K. , Miyashita, T. , Kuga, K. , & Yamaguchi, I. (2003). Noninvasive, direct visualization of macro‐reentrant circuits by using magnetocardiograms: Initiation and persistence of atrial flutter. Europace, 5(4), 343–350. 10.1016/S1099-5129(03)00081-3 [DOI] [PubMed] [Google Scholar]
- Yamada, S. , & Yamaguchi, I. (2005). Magnetocardiograms in clinical medicine: Unique information on cardiac ischemia, arrhythmias, and fetal diagnosis. Internal Medicine (Tokyo, Japan), 44(1), 1–19. 10.1021/ie040220b [DOI] [PubMed] [Google Scholar]
- Yoshida, K. , Ogata, K. , Inaba, T. , Nakazawa, Y. , Ito, Y. , Yamaguchi, I. , … Aonuma, K. (2015). Ability of magnetocardiography to detect regional dominant frequencies of atrial fibrillation. Journal of Arrhythmia, 31(6), 345–351. 10.1016/j.joa.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


