Abstract
Background
Myotonic dystrophy type 1 (DM1) generates missplicing of the SCN5A gene, encoding the cardiac sodium channel (Nav1.5). Brugada syndrome, which partly results from Nav1.5 dysfunction and causes increased VF occurrence, can be unmasked by ajmaline. We aimed to investigate the response to ajmaline challenge in DM1 patients and its potential impact on their sudden cardiac death risk stratification.
Methods
Among 36 adult DM1 patients referred to our institution, electrophysiological study and ajmaline challenge were performed in 12 patients fulfilling the following criteria: (1) PR interval >200 ms or QRS duration >100 ms; (2) absence of complete left bundle branch block; (3) absence of permanent ventricular pacing; (4) absence of implantable cardioverter‐defibrillator (ICD); (5) preserved left‐ventricular ejection fraction >50%; and (6) absence of severe muscular impairment. Of note, DM1 patients with ajmaline‐induced Brugada pattern (BrP) were screened for SCN5A.
Results
In all the 12 patients studied, the HV interval was <70 ms. A BrP was unmasked in three patients but none carried an SCN5A mutation. Ajmaline‐induced sustained ventricular tachycardia occurred in one patient with BrP, who finally received an ICD. The other patients did not present any cardiac event during the entire follow‐up (15 ± 4 months).
Conclusion
Our study is the first to describe a high prevalence of ajmaline‐induced BrP in DM1 patients. The indications, the safety, and the implications of ajmaline challenge in this particular setting need to be determined by larger prospective studies.
Keywords: myotonic dystrophy type 1, Brugada syndrome, ajmaline challenge, sodium channels, cardiac arrhythmia
Myotonic dystrophy type 1 (DM1) is an autosomal dominant neuromuscular disorder due to an abnormal accumulation of a (CTG)n triplet repeat in the untranslated 3’ region of the gene encoding dystrophia myotonica protein kinase (DMPK).1 DM1 can be associated with various clinical manifestations including myotonia, muscle weakness, respiratory insufficiency, and cardiac rhythm disturbances, such as complete atrioventricular block, sustained ventricular tachycardia (VT), or ventricular fibrillation (VF).2 Cardiac rhythm disturbances are of major concern, as they can lead to sudden cardiac death (SCD) in up to one‐third of DM1 patients.3 Accordingly, considerable efforts have been made in order to identify patients at high risk of SCD. Hence, electrocardiographic and electrophysiological criteria have been described, mainly based on the analysis of the progressive cardiac conduction defects that can affect the DM1 population.3, 4 When such criteria are met, permanent prophylactic pacing is currently recommended.5 However, since DM1 patients can also develop VT or VF, additional parameters specifically focused on ventricular arrhythmias are needed to assess SCD risk in this population.
DMPK CTG‐expansion leads to the nuclear accumulation of mutant messenger ribonucleic acids (mRNAs) generating aberrant alternative splicing of numerous pre‐mRNAs.6 Of interest, a missplicing of SCN5A, the most frequently implicated gene in Brugada syndrome (BrS),7 has recently been demonstrated in DM1.8 Moreover, in comparison to the generally healthy European population, a 50‐fold higher prevalence of Brugada pattern (BrP) has been described in a retrospective ECG screening of 914 DM1 patients.8 This substantial prevalence of BrP could partly explain the occurrence of SCD in DM1, since BrS is associated to an increased risk of VF. However, as no ajmaline test or systematic use of V1–V2 leads at the third intercostal space have been performed in DM1 patients so far, BrP prevalence might still be underestimated in this population and its impact on the SCD occurrence unknown.
In this study, we sought to prospectively assess the specific response to ajmaline challenge in a series of DM1 patients and describe its impact on the SCD risk stratification of this specific population.
METHODS
Patient Population
This prospective study, which conforms to the guiding principles of the Declaration of Helsinki, was approved by our local institutional committee on human research. Written informed consent was obtained from all patients. All adult patients admitted from 2005 to 2013 in our institution with DM1 confirmed by genetic were screened. In accordance with the recent European Society of Cardiology guidelines, patients whose progressive conduction disturbances needed to be evaluated by an electrophysiological study were selected. Furthermore, as repolarization had to be reliably analyzable during ajmaline challenge, pacemaker (PM) recipients with permanent ventricular pacing or patients presenting with complete left bundle branch block were also excluded. Finally, patients with an impaired left ventricular ejection fraction (LVEF) and overt clinical congestive heart failure were also excluded, as these conditions are contraindications to ajmaline administration. Of note, for ethical reasons, ajmaline challenge was neither performed in DM1 patients having already beneficiated from an implantable cardioverter‐defibrillator (ICD) nor in patients with severe neuromuscular and respiratory impairment owing to their high vulnerability and poor prognosis.
Overall, DM1 patients were included when they fulfilled all the following criteria: (1) PR interval >200 ms or QRS duration >100 ms;4, 5 (2) absence of complete left bundle branch block >120 ms; (3) absence of permanent ventricular pacing; (4) absence of ICD prior to the ajmaline test; (5) preserved LVEF >50%; and (6) absence of severe muscular impairment, defined as a score of five on a five‐points rating scale.9 The patients were numbered from 1 to 12. Each family was represented by a letter from –a to –i , in order to highlight the kinship ties between patients.
Clinical Evaluation
Physical Examination
All patients underwent complete physical examination in order to disclose congestive heart failure. The severity of muscular weakness was scored by a standardized five‐points muscular impairments rating scale (Table 1).9
Table 1.
Baseline Clinical Characteristics of Individual Patients
| Patient‐Family | 1‐a | 2‐b | 3‐c | 4‐c | 5‐d | 6‐e | 7‐f | 8‐g | 9‐h | 10‐h | 11‐i | 12‐d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age‐sex | 34‐M | 49‐M | 29‐F | 18‐M | 43‐F | 39‐M | 38‐F | 46‐F | 29‐M | 65‐M | 54‐M | 44‐F |
| Muscular impairmenta | 2 | 3 | 1 | 1 | 1 | 2 | 3 | 2 | 2 | 1 | 1 | 1 |
| Heart failureb | No | No | No | No | No | No | No | No | No | No | No | No |
| Syncope | No | No | No | No | No | No | No | No | No | No | No | No |
| Aborted SCD | No | No | No | No | No | No | No | No | No | No | No | No |
| SCD‐BrS family history | No | No | No | No | No | No | No | No | No | No | No | No |
| Pacemaker | No | Yes | No | No | No | No | No | No | No | No | No | No |
| ICD | No | No | No | No | No | No | No | No | No | No | No | No |
A score of 1 indicates no clinical muscular impairment, 2 minimal signs without distal weakness except for digit flexor, 3 distal weakness without proximal weakness except for elbow extensors, 4 moderate proximal weakness, and 5 severe proximal weakness;
New York Heart Association (NYHA) functional class II to IV.
M = male; F = female; SCD = sudden cardiac death; ICD = implantable cardioverter‐defibrillator.
Electrocardiogram and Holter
PR interval and QRS duration were automatically calculated on the 12‐lead surface electrocardiograms, recorded at 25 mm/s. Repolarization abnormalities were systematically screened in leads V1 to V3. Only the coved type pattern (type 1), defined by an initial ST elevation >2 mm followed by a negative T wave, in at least one lead from V1 to V3, was considered conclusive. The saddle‐back pattern (type 2), precisely defined by the consensus report, was considered suspicious but not conclusive.7 Acute conduction disorders and ventricular arrhythmias were systematically disclosed by a 24‐hour Holter monitoring.
Echocardiography
Transthoracic echocardiograms were recorded before ajmaline test, using a ViVid nine system (GE Healthcare, Milwaukee, WI, USA). LVEF was calculated by left ventricular (LV) biplane Simpson's method. The LV dimensions and mass were analyzed using two‐dimensional echocardiogram‐directed M‐mode. The right ventricular (RV) function was estimated by the tricuspid annular plane systolic excursion (TAPSE) method, using two‐dimensional echocardiogram‐directed M‐mode.
Cardiac Computed Tomography
Coronary imaging by multislice‐computed tomography was performed before ajmaline challenge was done in order to exclude ischemic cardiomyopathy and confirm the absence of manifest structural heart disease.
Ajmaline Challenge
Ajmaline was intravenously administered at the dose of 1 mg/kg for 5 minutes, under constant digital ECG monitoring (LabSystem PRO, Bard EP, Lowell, Massachusetts), in the electrophysiological laboratory.7 V1 and V2 leads were simultaneously recorded from the standard fourth intercostal space (then named V1–V2) and from the third intercostal space (then named V1 h–V2 h), as this two‐tiered approach for V1–V2 leads proves highly sensitive for the BrP detection.10, 11 After a baseline acquisition, ECG monitoring was performed every minute during ajmaline infusion, then during the 10 minutes following the end of infusion in case of negative test or until ECG completely normalized in case of positive test. Ajmaline challenge was considered positive if a BrP developed in at least one right precordial lead placed in a standard or superior position.7 The second consensus report recommends that the intravenous administration of ajmaline should be halted when BrP develops, ventricular arrhythmias occur or QRS widens up to 130% of baseline or goes beyond this value. Therefore, we took into account the occurrence of sustained ventricular arrhythmias (sVA), which was defined by VT lasting >30 seconds or VF. Furthermore, negative tests were classified according to whether or not the QRS widening was ≥130% of baseline.
Electrophysiological Study
Based on the recently proven benefit of an invasive strategy and the potential deleterious impact of ajmaline on the impulse propagation in this category of patients, described as having a natural history of progressive conduction defect, an electrophysiological study was systematically performed before ajmaline challenge.4, 5 A quadripolar nondeflectable catheter was introduced through the femoral vein and placed under fluoroscopic guidance at the His bundle position. The HV interval was then measured at baseline. Subsequently, the catheter was advanced to the RV apex and used as a backup temporary pacing electrode during ajmaline challenge, according to the recommendations of the Second Consensus Conference for patients at high risk of drug‐induced complete atrioventricular block.7
Molecular Genetics
Genomic DNA was extracted from peripheral lymphocytes isolated from the patients. Analysis of the CTG repeat sequence in the 3’ untranslated region of the DMPK was performed with the use of polymerase chain reaction (PCR). DM1 was diagnosed when the size of the CTG expansion measured with Southern blot technique was ≥50 triplets.1 Patients with ajmaline‐induced BrP or excessive QRS widening were systematically screened for the SCN5A gene, which encodes the Nav1.5 voltage gated cardiac sodium channel. All SCN5A coding exons were amplified by PCR using primers designed with intronic flanking sequences. PCR and direct sequencing were used to identify any specific mutation in the index patient and the family members.12
Follow‐Up
Patients were clinically evaluated every 6 months. At each visit, symptoms assessment was followed by complete physical examination and 12‐lead ECG acquisition. Finally, device interrogation was performed in patients with implantable loop‐recorder, PM, or ICD.
Statistical Analysis
Data are presented as mean ± standard deviation.
RESULTS
Population Characteristics
Among 36 adult DM1 patients followed in our institution since 2005, 12 patients presenting the predefined inclusion criteria underwent ajmaline challenge and electrophysiological study from July 2012 to June 2013 (Fig. 1). All patients had a genetically confirmed DM1. The mean age of the cohort was 41 ± 13 years and 58% were men. No patients experienced previous syncope or aborted SCD. No family history of BrS or SCD was reported. Of note, one patient included in our series had a PM. However, the PM was implanted for sinus node dysfunction, with a very low percentage of ventricular pacing (<1%). Patient clinical characteristics are summarized in Table 1. The mean PR interval was 190 ± 25 ms and the mean QRS duration was 108 ± 11 ms. One patient (2‐b) had a spontaneous saddle‐back pattern at baseline. No transient complete atrioventricular block or VT was documented during the 24‐hour Holter monitoring. Transthoracic echocardiography excluded ventricular dysfunction, as the mean LVEF was 63% ± 7% and the mean TAPSE was 21 ± 4 mm. Two patients had a moderate LV hypertrophy with an LV mass index >100 g/m2. No coronary artery disease was identified by cardiac computed tomography. Patient electrocardiographic and echocardiographic characteristics are listed in Table 2. It is worth noting that a familial bind was found, two by two, in six patients (patient 3‐c is patient 4‐c's sister, patient 5‐d is patient 12‐d's sister and patient 9‐h is patient 10‐h's son).
Figure 1.
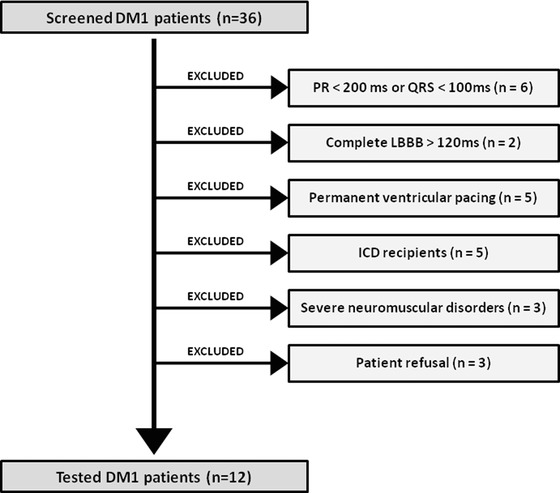
Flow of patients from the initial screening to the final selection for electrophysiological study and ajmaline test.
Table 2.
Individual Electrocardiographic and Echocardiographic Characteristics at Inclusion
| Patient‐Family | 1‐a | 2‐b | 3‐c | 4‐c | 5‐d | 6‐e | 7‐f | 8‐g | 9‐h | 10‐h | 11‐i | 12‐d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR interval (ms) | 152 | 217 | 182 | 195 | 201 | 183 | 194 | 156 | 191 | 246 | 172 | 190 |
| QRS duration (ms) | 102 | 102 | 112 | 110 | 95 | 135 | 100 | 101 | 116 | 97 | 118 | 111 |
| Saddle‐back pattern | No | Yes | No | No | No | No | No | No | No | No | No | No |
| Coved pattern | No | No | No | No | No | No | No | No | No | No | No | No |
| VTa | No | No | No | No | No | No | No | No | No | No | No | No |
| SND | No | Yes | No | No | No | No | No | No | No | No | No | No |
| High‐degree AVB | No | No | No | No | No | No | No | No | No | No | No | No |
| EF (%) | 63 | 55 | 61 | 55 | 62 | 67 | 76 | 63 | 57 | 59 | 64 | 73 |
| EDD (mm/m2) | 28 | 27 | 25 | 25 | 27 | 27 | 27 | 29 | 28 | 34 | 27 | 26 |
| LV mass index (g/m2) | 59 | 70 | 68 | 51 | 58 | 70 | 52 | 49 | 74 | 138 | 133 | 55 |
| TAPSE (mm) | 25 | 27 | 26 | 26 | 16 | 28 | 19 | 23 | 19 | 21 | 22 | 25 |
VT was defined by the succession of >4 premature ventricular complexes.
VT = ventricular tachycardia; SND = sinus node dysfunction; AVB = atrioventricular block; EF = ejection fraction; EDD = end‐diastolic diameter; LV = left ventricular; TAPSE = tricuspid annular plane systolic excursion; LA = left atrial.
Electrophysiological Study, Ajmaline Challenge, and Genetic Screening
All antiarrhythmic drugs were ceased at least >5 half‐life before electrophysiological study and ajmaline challenge. The mean baseline HV interval was 57 ± 9 ms. As the baseline HV interval never exceeded 70 ms, no prophylactic PM were implanted after the electrophysiological study.4, 5 Ajmaline response was divided into three groups (Fig 2., panels 1 and 2). A typical BrP occurred in three (25%) patients, one of whom (3‐c) developed a marked QRS widening followed by an sVA (Fig. 2, panel 3). Four other patients presented an important QRS widening ≥130% of baseline, with an equal repartition between right bundle branch block and left bundle branch block pattern. The other five (42%) patients had no ECG modifications. Ajmaline challenge was stopped in six (50%) patients before reaching the theoretical dose, due to BrP occurrence in one patient and a QRS widening ≥130% in five patients. The mean QRS widening was 139% ± 28% with regard to the baseline. SCN5A mutation screening was negative for the three patients with unmasked BrP. Interestingly, neither patients with BrP nor with QRS widening ≥130% had any familial bind within their respective group. EP study, ajmaline test, and genetic screening parameters are shown in Table 3.
Figure 2.
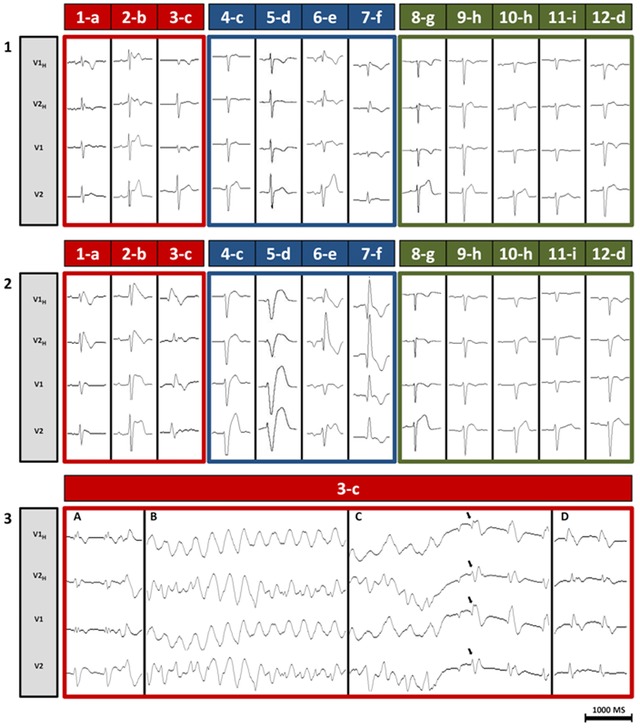
Standard and high V1–V2 ECG‐leads recorded during ajmaline test respectively from the fourth (V1–V2) and the third (V1H–V2H) intercostal space. (Panel 1) Recording at baseline, before ajmaline injection. All patients were in sinus rhythm and none presented with a spontaneous BrP. (Panel 2) Recording during ajmaline administration. Red‐framed patients developed a BrP. Blue‐framed patients displayed QRS widening ≥130% of baseline. Green‐framed patients had no substantial electrocardiographic modifications. (Panel 3) Detailed recording of patient 3‐c. (A) At 5 minutes from the beginning of ajmaline injection, sudden QRS widening associated with premature ventricular complexes. QRS narrowing is not obtained despite isoproterenol infusion at low dose (0.05 mg/H). (B) At 10 minutes, appearance of a sustained polymorphic ventricular tachycardia at 180 bpm, poorly tolerated, with patient close to losing consciousness. (C) At 11 minutes, sinus rhythm resumes spontaneously (black arrows) before external defibrillation could be done, unmasking a marked BrP. The patient is conscious and hemodynamically stable. (D) At 15 minutes, a typical BrP is observed.
Table 3.
Results of Electrophysiological Study, Ajmaline Test, and SCN5A Screening
| Patient‐Family | 1‐a | 2‐b | 3‐c | 4‐c | 5‐d | 6‐e | 7‐f | 8‐g | 9‐h | 10‐h | 11‐i | 12‐d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HV interval (ms) | 54 | 56 | 58 | 54 | 68 | 55 | 52 | 65 | 48 | 66 | 38 | 64 |
| Ajmaline dose (%)a | 100 | 80 | 60 | 80 | 60 | 60 | 80 | 100 | 100 | 100 | 100 | 100 |
| QRS widening (%)b | 124 | 115 | 169 | 179 | 189 | 147 | 153 | 126 | 107 | 125 | 126 | 106 |
| Drug‐induced BrP | Yes | Yes | Yes | No | No | No | No | No | No | No | No | No |
| Drug‐induced sVA | No | No | Yes | No | No | No | No | No | No | No | No | No |
| SCN5A mutation | No | No | No | No | No | No | No | ‐ | ‐ | ‐ | ‐ | ‐ |
Results are given in % of the theoretical dose of ajmaline, based on the recommendations of the Second Consensus Conference on Brugada Syndrome (0.2 mg/kg per min for 5 minutes).
Results are given in % of the baseline QRS.
NA = not available.
Drug‐Induced Sustained Ventricular Arrhythmia
One patient (3‐c), a 29‐year‐old woman who presented rare episodes of palpitations over the past few years, developed sVA during the ajmaline challenge (Fig. 2, panel 3). This polymorphic VT was a source of hemodynamic instability. However, before any external defibrillation could be done, sinus rhythm resumed spontaneously, unmasking a typical BrP. At that time, no data on the prognostic value of sVA during ajmaline challenge were available yet.13 Moreover, the patient was strongly reluctant to receive an ICD. Therefore, we jointly agreed to implant a loop‐recorder (Reveal, Medtronic, Minneapolis, MN, USA). Nonetheless, the patient was clearly informed that the detection of any spontaneous VT would lead to ICD implantation.
Follow‐Up
After a mean follow‐up of 15 ± 4 months, none of the 11 patients without drug‐induced sVA died suddenly or experienced any symptoms.
In contrast, 1 month after the loop‐recorder implantation, as the patient with drug‐induced sVA was dancing late at night after alcohol intake, she experienced a brief episode of palpitations with a faintness sensation. The control of the loop‐recorder documented a fast (220 bpm), disorganized, and sustained (40 seconds) VT, which spontaneously converted into sinus rhythm. The combination of unmasked BrP with spontaneous VT fulfilled the criteria for BrS. The patient was finally implanted with an ICD. The subsequent 9‐months follow‐up period has been uneventful.
DISCUSSION
High Prevalence of Unmasked BrP in DM1 Patients
To the best of our knowledge, this study is the first to describe BrP unmasking by ajmaline challenge in DM1. In this setting, the combination of ajmaline challenge with a two‐tiered V1–V2 approach proved highly sensitive, as 3 (25%) out of 12 DM1 patients developed a BrP without any familial bind. This result strengthens the observation of Wahbi et al. who retrospectively identified, among the 914 patients of their DM1 Heart Registry, 7 patients (0.8%) with a spontaneous BrP, while the prevalence observed in an apparently healthy European population is 0.017%.8 Furthermore, the absence of SCN5A mutation in our DM1 patients presenting a BrP during ajmaline challenge (a powerful sodium channel blocker) supports the recent hypothesis that Nav1.5 channel function alteration may play a role, as a result of SCN5A missplicing.8 Interestingly, one study previously analyzed the impact of ajmaline on DM1 patients, but no BrP could be identified as it was published prior to the first BrS description.14 An additional explanation could be the presence of structural cardiac abnormalities in DM1, with focal fibrosis and fatty infiltration notably affecting the right ventricle.15, 16 Histological remodeling is also described in the right ventricular outflow tract of BrS patients, leading to depolarization disturbances and conduction delays.17, 18 Therefore, ajmaline‐induced BrP in DM1 patients might be the outcome of subtle yet relevant tissue modifications mimicking those described in BrS.
Of note, ajmaline challenge has been performed so far in populations selected on the basis of criteria reinforcing the pretest sensitivity, such as symptoms (unexplained syncope, aborted cardiac arrest), suspicious ECG (“saddle‐back” pattern, documented VT), or evocative family history (BrS, SCD). For this reason, the overall percentage of positive ajmaline challenge in these high‐risk populations ranges from 17% to 39%.11, 19 Interestingly, our DM1 population could have been classified at low risk of developing a drug‐induced BrP, since only patient 2‐b (“saddle‐back” pattern) met one of these high‐risk criteria. However, we observed an unexpectedly high prevalence of unmasked BrP (25%) in DM1 patients. Hence, our study adds DM1 to arrhythmogenic right ventricular cardiomyopathy and Chagas disease, as a condition which can harbor the Brugada substrate.
Ajmaline Challenge as a Potential Tool for SCD Risk Stratification in DM1
Evaluation of SCD risk in DM1 has been based on the analysis of the conduction system impairment so far. Thus, a recent study showed that a systematic electrophysiological study was necessary in DM1 patients with PR interval >200 ms or QRS duration >100 ms.4 Moreover, prophylactic PM in case of HV interval ≥70 ms proved to be effective for decreasing the incidence of SCD.5 However, in Groh's series, one‐third of the SCD was related to fatal ventricular arrhythmias, of which one‐third occurred in PM recipients.3 This finding points out the need for complementary tools to determine in which cases ICD should be preferred to PM.
Of interest, based on current criteria, our DM1 patients would have been considered at low risk of SCD, since their HV interval was <70 ms. However, three patients developed an ajmaline‐induced BrP and were finally classified at higher risk of SCD. In practice, they have benefited from advices with prophylactic aim, such as lowering of body temperature in case of fever, limitation of alcohol intake, and avoidance of specific drugs listed on http://www.brugadadrugs.org. Moreover, one of them (3‐c) developed a sustained and hemodynamically unstable VT. This patient is the first described case of ajmaline‐induced sVA in DM1, since the VF reported by Otten et al. was initiated by a programmed electrical stimulation performed during a negative pharmacological provocation with procainamide.20 Patient 3‐c required further investigation with the implantation of a loop‐recorder. After the detection of a spontaneous sustained VT, she was finally implanted with an ICD.
These findings highlight the potential interest of the ajmaline challenge as an additional tool for SCD risk stratification in the DM1 population.
The Need for a Tailored Approach
Ajmaline is recognized to depress ventricular propagation by decreasing Purkinje fibers impulse velocity.21 Hence, up to one‐third of the patients with ajmaline‐induced high‐degree atrioventricular block will develop a complete atrioventricular block before 3 years.22 Therefore, ajmaline challenge is still indicated to explore syncope in patients with bundle branch block and PM implantation is recommended when a second‐ or third‐degree atrioventricular block is induced.5 Caution must be paid, however, as DM1 generates a markedly reduced distal conduction reserve,23 which could be rapidly depleted by sodium channel blockade. To date, the only available data about ajmaline impact on the distal cardiac conduction in DM1 are provided by our study and Komajda's series,14 representing a total of 24 patients. Interestingly, none of them developed complete atrioventricular block during ajmaline administration. In our case series, this result was in total accordance with the electrophysiological study and no PM was implanted. Nevertheless, in 5 (42%) out of our 12 patients, ajmaline infusion was stopped before reaching the theoretical dose, due to QRS widening ≥130%. Of note, this result is by far more frequent than the 1.2% observed in Veltmann's series.19 Moreover, among the 24 DM1 patients, 1 (4.2%) experienced sVA, whereas a recent large series comprising 1043 patients tested with ajmaline only found 9 (0.9%) cases of sVA.13 These findings show that side effects during ajmaline challenge may not be anecdotal in DM1, and should be taken into account.
The key points lie in the appropriate selection of the DM1 candidate for sodium channel blockade and the utmost caution while performing this challenge. Hence, exclusion of DM1 patients with marked conduction defects at baseline such as major bundle branch block (QRS duration >150 ms) or documented transient complete atrioventricular block might appear reasonable. Furthermore, the systematic introduction of a diagnostic catheter into the RV before ajmaline challenge could be useful in order to provide temporary pacing in case of induced complete atrioventricular block. Finally, avoidance of prolonged half‐time class I antiarrhythmic drugs (flecainide, procainamide) and slower administration of ajmaline (10 rather than 5 minutes) should be considered in this particular setting, in order to respectively limit their side effects duration and manage as promptly as possible the occurrence of serious induced cardiac rhythm disturbances. Larger prospective studies are needed, however, to assess the safety and define the appropriate modality of ajmaline challenge in DM1.
Study Limitations
First, our study is based on selected DM1 patients with slight conduction defects on the surface ECG. This section of DM1 population may not be fully representative of all possible forms of DM1 cardiac manifestations. Thus, although higher than previously assumed, the exact prevalence of unmasked BrP in the entire DM1 population is still difficult to assess. Second, regarding the small sample size and the absence of extended follow‐up, our case series cannot draw firm conclusion on the prognostic value of ajmaline challenge in terms of SCD risk in DM1. However, our findings could pave the way toward larger prospective studies.
CONCLUSION
Our study is the first to describe a high prevalence of ajmaline‐induced BrP in DM1 patients. It also emphasizes the potential interest of ajmaline challenge in their SCD risk stratification. Larger prospective studies are needed to assess the indications, the safety, and the clinical significance of ajmaline challenge in DM1 patients.
Disclosures: Agustín Bortone is a consultant to Biosense‐Webster, Inc. The other authors have no disclosures.
REFERENCES
- 1. Brook JD, McCurrach ME, Harley HG, et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992;68:799–808. [DOI] [PubMed] [Google Scholar]
- 2. Harper PS, van Engelen BG, Eymard B, et al. 99th European Neuromuscular Centre International Workshop: Myotonic dystrophy: Present management, future therapy. Neuromuscul Disord 2002;12:596–599. [DOI] [PubMed] [Google Scholar]
- 3. Groh WJ, Groh MR, Saha C, et al. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med 2008;358:2688–2697. [DOI] [PubMed] [Google Scholar]
- 4. Wahbi K, Meune C, Porcher R, et al. Electrophysiological study with prophylactic pacing and survival in adults with myotonic dystrophy and conduction system disease. JAMA 2012;307:1292–1301. [DOI] [PubMed] [Google Scholar]
- 5. Brignole M, Auricchio A, Baron‐Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;15:1070–1118. [DOI] [PubMed] [Google Scholar]
- 6. Kanadia RN, Johnston KA, Mankodi A, et al. A muscleblind knockout model for myotonic dystrophy. Science 2003;302(5652):1978–1980. [DOI] [PubMed] [Google Scholar]
- 7. Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005;111:659–670. [DOI] [PubMed] [Google Scholar]
- 8. Wahbi K, Algalarrondo V, Becane HM, et al. Brugada syndrome and abnormal splicing of SCN5A in myotonic dystrophy type 1. Arch Cardiovasc Dis 2013;106:635–643. [DOI] [PubMed] [Google Scholar]
- 9. Mathieu J, Boivin H, Meunier D, et al. Assessment of a disease specific muscular impairment rating scale in myotonic dystrophy. Neurology 2001;56:336–340. [DOI] [PubMed] [Google Scholar]
- 10. Veltmann C, Papavassiliu T, Konrad T, et al. Insights into the location of type 1 ECG in patients with Brugada syndrome: Correlation of ECG and cardiovascular magnetic resonance imaging. Heart Rhythm 2012;9:414–421. [DOI] [PubMed] [Google Scholar]
- 11. Govindan M, Batchvarov VN, Raju H, et al. Utility of high and standard right precordial leads during ajmaline testing for the diagnosis of Brugada syndrome. Heart 2010;96:1904–1908. [DOI] [PubMed] [Google Scholar]
- 12. Wang Q, Li Z, Shen J, et al. Genomic organization of the human SCN5A gene encoding the cardiac sodium channel. Genomics 1996;34:9–16. [DOI] [PubMed] [Google Scholar]
- 13. Conte G, Sieira J, Sarkozy A, et al. Life‐threatening ventricular arrhythmias during ajmaline challenge in patients with Brugada syndrome: Incidence, clinical features, and prognosis. Heart Rhythm 2013;10:1869–1874. [DOI] [PubMed] [Google Scholar]
- 14. Komajda M, Frank R, Vedel J, et al. Intracardiac conduction defects in dystrophia myotonica. Electrophysiological study of 12 cases. Br Heart J 1980;43:315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hermans MC, Faber CG, Bekkers SC, et al. Structural and functional cardiac changes in myotonic dystrophy type 1: A cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2012;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vignaux O, Lazarus A, Varin J, et al. Right ventricular MR abnormalities in myotonic dystrophy and relationship with intracardiac electrophysiologic test findings: Initial results. Radiology 2002;224(1):231–235. [DOI] [PubMed] [Google Scholar]
- 17. Ohkubo K, Watanabe I, Okumura Y, et al. Right ventricular histological substrate and conduction delay in patients with Brugada syndrome. Int Heart J 2010;51:17–23. [DOI] [PubMed] [Google Scholar]
- 18. Nademanee K, Veerakul G, Chandanamattha P, et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 2011;123:1270–1279. [DOI] [PubMed] [Google Scholar]
- 19. Vetmann C, Wolpert C, Sacher F, et al. Response to intravenous ajmaline: A retrospective analysis of 677 ajmaline challenges. Europace 2009;11:1345–1352. [DOI] [PubMed] [Google Scholar]
- 20. Otten RF, Scherschel JA, Lopshire JC, et al. Arrhythmia exacerbation after sodium channel blockade in myotonic dystrophy type 1. Muscle Nerve 2009;40:901–902. [DOI] [PubMed] [Google Scholar]
- 21. Obayashi K, Mandel WJ. Electrophysiological effects of ajmaline in isolated cardiac tissue. Cardiovasc Res 1976;10:20–24. [DOI] [PubMed] [Google Scholar]
- 22. Gronda M, Magnani A, Occhetta E, et al. Electrophysiological study of atrio‐ventricular block and ventricular conduction defects. Prognostic and therapeutical implications. G Ital Cardiol 1984;14:768–773. [PubMed] [Google Scholar]
- 23. Babuty D, Fauchier L, Tena‐Carbi D, et al. Is it possible to identify infrahissian cardiac conduction abnormalities in myotonic dystrophy by non‐invasive methods? Heart 1999;82:634–637. [DOI] [PMC free article] [PubMed] [Google Scholar]


